Is Bartonella a Cause of Primary Sclerosing Cholangitis? A Case Study
Abstract
:1. Introduction
2. Case Presentation
- Iron deficiency anemia:
- ○
- Hemoglobin = 10.6 g/dL (reference range (RR): 13.5–17.7 g/dL);
- ○
- Hematocrit = 35% (RR: 37–49%);
- ○
- Ferritin = 14 ng/mL (RR: 47–356 ng/mL).
- Increased inflammatory markers:
- ○
- Leukocytosis WBC = 13,600/uL (RR: 4000–11,000/uL);
- ○
- ESR = 41 mm/hr (RR: 0–20 mm/hr);
- ○
- Faecal calprotectin = 188 mg/kg (RR: <50 mg/kg).
- Elevated liver enzymes in a cholestatic pattern:
- ○
- Alkaline phosphatase = 1117 U/L (RR: 98–448 U/L);
- ○
- Aspartate aminotransferase = 121 U/L (RR: 6–38 U/L);
- ○
- Alanine aminotransferase = 207 U/L (RR: 13–63 U/L);
- ○
- Gamma glutamyltransferase = 457 U/L (RR: 6–75 U/L).
- Immunologic testing:
- ○
- Total IgG = 1800 mg/dL (RR: 681–1648);
- ○
- Anti-tissue transglutaminase IgA = 1.9U/mL (RR: 0–19 U/mL);
- ○
- Anti-gliadin antibody IgA = 0.2 (U/mL) (RR: 0–20 U/mL);
- ○
- Antinuclear antibody (ANA) negative (RR: negative);
- ○
- Anticytoplasmic antibody negative (RR: negative);
- ○
- Smooth muscle antibody negative (RR: negative);
- ○
- Anti-liver kidney microsome antibody-1 = 0.8 U (RR: 0–20 U);
- ○
- Myeloperoxidase antibody <0.2 (RR: 0.0–0.9);
- ○
- C-anti-neutrophil cytoplasmic antibody = 1:640 (RR: <1:20).
- Thiopurine S-methyltransferase gene variants: none detected;
- Serological testing of IgG to Bartonella henselae and Bartonella quintana by enzyme linked immunofluorescence assay (ELISA) were both elevated at 1:256 (RR: non-reactive);
- Serum PCR testing for Bartonella species was negative;
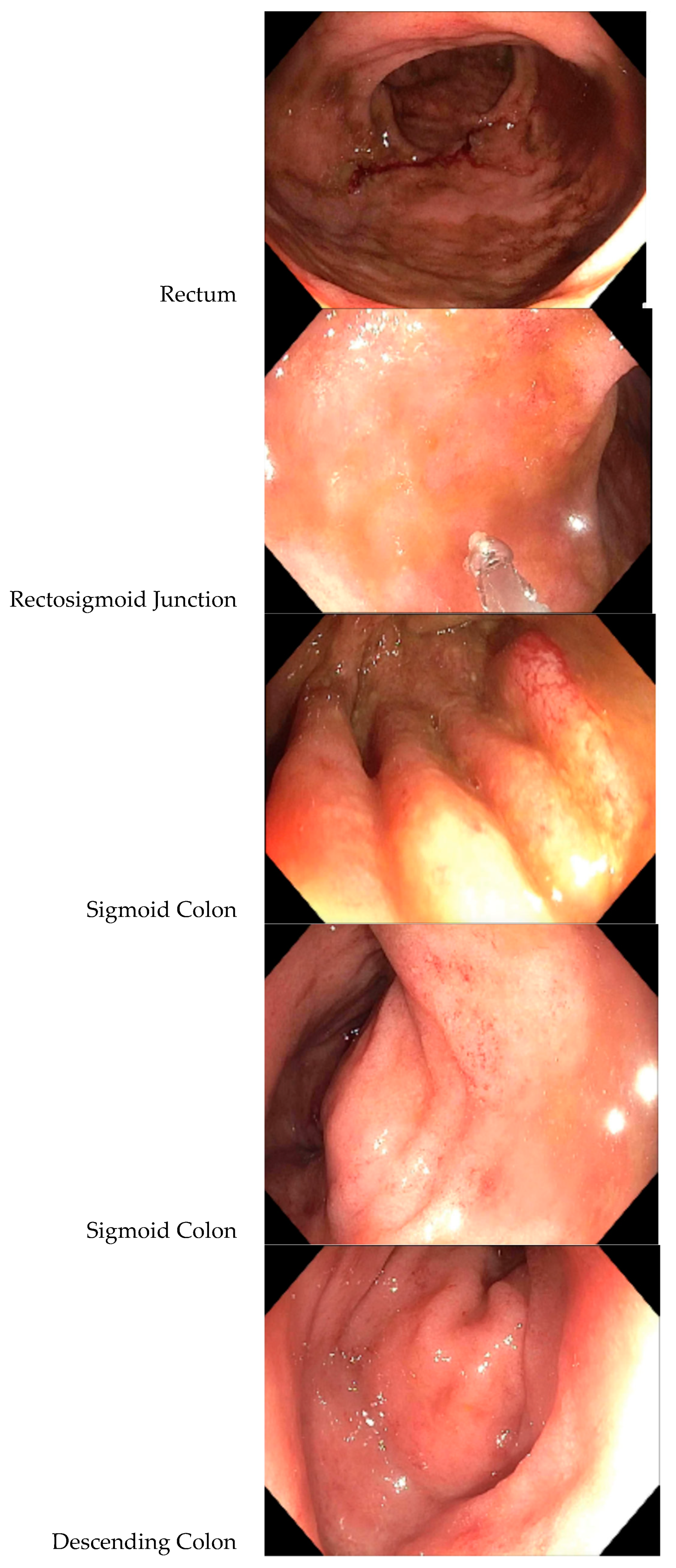
- A colonoscopy revealed a diffuse area of moderately congested, erythematous, friable and inflamed mucosa in the rectum, the recto-sigmoid colon, the sigmoid colon, the descending colon and at the splenic flexure (see Figure 1). A biopsy revealed mild active inflammation with mild architectural changes;
- An abdominal ultrasound showed dilation of the distal common bile duct measuring 7.3 mm; hepatic enlargement measuring 17.8 cm sagitally (RR: 8.7–13.7 cm); the liver was mildly echogenic; the remainder of the ultrasound was normal;
- An MRI with magnetic resonance cholangiopancreatography (MRCP) showed slight beading of the common bile duct, common hepatic duct, and proximal hepatic duct; the common bile duct was dilated to 10 mm in width (see Figure 2).
3. Discussion
4. Conclusions
Conflicts of Interest
Abbreviations
| PSC | Primary sclerosing cholangitis |
| LFT | Liver function test |
| HLA | Histocompatibility-complex |
| CSD | Cat scratch disease |
| EM | Erythema migrans |
| IgG | Immunoglobulin G |
| IFA | Indirect immunoflourescence assay |
| MRI | Magnetic resonance imaging |
| MRCP | Magnetic resonance cholangiopancreatography |
| ELISA | Enzyme linked immunoassay |
| CDC | Centers for Disease Control and Prevention |
| RR | Reference range |
| B. henselae | Bartonella henslae |
| B. quintana | Batronella quintana |
| B. burgdorferi | Borrellia burgdorferi |
| B. clarridgeiae | Bartonella clarridgeiae |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| TNF-α | Tumor necrosis factor-alpha |
References
- Goode, E.; Rushbrook, S. A review of the medical treatment of primary sclerosing cholangitis in the 21st century. Ther. Adv. Chronic Dis. 2015, 7, 68–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damman, J.; Rodriguez, E.; Ali, A.; Buness, C.; Cox, K.; Carey, E.; Lindor, K.D. Review article: The evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2018, 47, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massei, F.; Massimetti, M.; Messina, F.; Macchia, P.; Maggiore, G. Bartonella henselae and inflammatory bowel disease. Lancet 2000, 356, 1245–1246. [Google Scholar] [CrossRef]
- Reis, C.; Cote, M.; Le Rhun, D.; Lecuelle, B.; Levin, M.; Vayssier-Taussat, M.; Bonnet, S.I. Vector Competence of the Tick Ixodes ricinus for Transmission of Bartonella birtlesii. PLoS Negl. Trop. Dis. 2011, 5, e1186. [Google Scholar] [CrossRef] [Green Version]
- Cotté, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.-J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074–1080. [Google Scholar] [CrossRef]
- Liang, H.; Manne, S.; Shick, J.; Lissoos, T.; Dolin, P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine 2017, 96, e7116. [Google Scholar] [CrossRef] [PubMed]
- Bonato, G.; Cristoferi, L.; Strazzabosco, M.; Fabris, L. Malignancies in Primary Sclerosing Cholangitis—A Continuing Threat. Dig. Dis. 2015, 33, 140–148. [Google Scholar] [CrossRef]
- Cox, K.; Cox, K. Oral Vancomycin: Treatment of Primary Sclerosing Cholangitis in Children. J. Pediatric Gastroenterol. Nutr. 1997, 25, 479. [Google Scholar] [CrossRef]
- Davies, Y.; Cox, K.; Abdullah, B.; Safta, A.; Terry, A.; Cox, K. Long-term Treatment of Primary Sclerosing Cholangitis in Children With Oral Vancomycin: An Immunomodulating Antibiotic. J. Pediatric Gastroenterol. Nutr. 2008, 47, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Tabibian, J.; Weeding, E.; Jorgensen, R.; Petz, J.; Keach, J.; Talwalkar, J.; Lindor, K.D. Randomized clinical trial: Vancomycin or metronidazole in patients with primary sclerosing cholangitis—A pilot study. Aliment. Pharmacol. Ther. 2013, 37, 604–612. [Google Scholar] [CrossRef]
- Rahimpour, S.; Nassiri-Toosi, M.; Khalili, H.; Daryani, N.E.; Taromlou, M. A Triple Blinded, Randomized, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis: A Pilot Study. J. Gastrointest. Liver Dis. 2016, 25, 457–464. [Google Scholar]
- Dao, A.; Abidian, M.; Lestrange, A.; Matter, M.; Rangnekar, A.; Charabaty, A. Oral Vancomycion Induces and Maintains Remission of Ulcerative Colitis in the Subset of Patients with Associated Primary Sclerosing Cholangitis. Inflamm. Bowel Dis. Lett. 2019, 25, e90–e91. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-Z.; Reilly, C.R.; Steward-Harrison LCBalouch, F.; Muir, R.; Lewindon, P.J. Oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis-ulcerative colitis. Gut 2019, 68, 1533–1535. [Google Scholar] [CrossRef]
- Hey, P.; Lokan, J.; Johnson, P.; Gow, P. Efficacy of oral vancomycin in recurrent primary sclerosing cholangitis following liver transplantation. Br. Med. J. Case Rep. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buness, C.; Lindor, K.; Miloh, T. Oral Vancomycin Therapy in a Child with Primary Sclerosing Cholangitis and Severe Ulcerative Colitis. Pediatric Gastroenterol. Hepatol. Nutr. 2016, 19, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, Y.; Tsay, C.; Caccamo, D.; Cox, K.; Castillo, R.; Cox, K. Successful Treatment of Recurrent Primary Sclerosing Cholangitis after Orthotopic Liver Transplantation with Oral Vancomycin. Case Rep. Transplant. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabibian, J.; O’Hara, S.; Lindor, K. Primary sclerosing cholangitis and the microbiota: Current knowledge and perspectives on etiopathogenesis and emerging therapies. Scand. J. Gastroenterol. 2014, 49, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarbanel, D.; Seki, S.; Davies, Y.; Marlen, N.; Benavides, J.; Cox, K.; Nadeau, K.C.; Cox, K.L. Immunomodulatory Effect of Vancomycin on Treg in Pediatric Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. J. Clin. Immunol. 2012, 33, 397–406. [Google Scholar] [CrossRef] [Green Version]
- La Scola, B.; Raoult, D. Culture of Bartonella quintana and Bartonella henselae from Human Samples: A 5-Year Experience (1993 to 1998). J. Clin. Microbiol. 1999, 37, 1899–1905. [Google Scholar] [CrossRef] [Green Version]
- Sander, A.; Posselt, M.; Böhm, N.; Ruess, M.; Altwegg, M. Detection of Bartonella henselae DNA by Two Different PCR Assays and Determination of the Genotypes of Strains Involved in Histologically Defined Cat Scratch Disease. J. Clin. Microbiol. 1999, 37, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, M.; Herremans, M.; Verbakel, H.; Bergmans, A.; Roord, J.; van Dijken, P.; Peeters, M.F. Serological testing for Bartonella henselae infections in The Netherlands: Clinical evaluation of immunofluorescence assay and ELISA. Clin. Microbiol. Infect. 2007, 13, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Giladi, M.; Kletter, Y.; Avidor, B.; Metzkor-Cotter, E.; Varon, M.; Golan, Y.; Weinberg, M.; Riklis, I.; Ephros, M.; Leonard, S. Enzyme Immunoassay for the Diagnosis of Cat-Scratch Disease Defined by Polymerase Chain Reaction. Clin. Infect. Dis. 2001, 33, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.; Posselt, M.; Oberle, K.; Bredt, W. Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: Evaluation and comparison of two commercial serological tests. Clin. Diagn. Lab. Immunol. 1998, 5, 486–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billeter, S.; Levy, M.; Chomel, B.; Breitschwerdt, E. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med. Vet. Entomol. 2008, 22, 1–15. [Google Scholar] [CrossRef]
- Adelson, M.; Rao, R.; Tilton, R.; Cabets, K.; Eskow, E.; Fein, L.; Occi, J.L.; Mordechai, E. Prevalence of Borrelia burgdorferi, Bartonella spp; Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis Ticks Collected in Northern New Jersey. J. Clin. Microbiol. 2004, 42, 2799–2801. [Google Scholar] [CrossRef] [Green Version]
- Holden, K.; Boothby, J.; Kasten, R.; Chomel, B. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus Ticks from California, USA. Vector Borne Zoonotic Dis. 2006, 6, 99–102. [Google Scholar] [CrossRef]
- Halos, L.; Jamal, T.; Maillard, R.; Beugnet, F.; Le Menach, A.; Boulouis, H.; Vayssier-Taussat, M. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet. Res. 2005, 36, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Eskow, E.; Rao, R.; Mordechai, E. Concurrent Infection of the Central Nervous System by Borrelia burgdorferi and Bartonella henselae. Arch. Neurol. 2001, 58, 1357. [Google Scholar] [CrossRef] [Green Version]
- Podsiadly, E.; Chmielewski, T.; Tylewska-Wierzbanowska, S. Bartonella henselae and Borrelia burgdorferi Infections of the Central Nervous System. Ann. N. Y. Acad. Sci. 2003, 990, 404–406. [Google Scholar] [CrossRef]
- Rolain, J.; Brouqui, P.; Koehler, J.; Maguina, C.; Dolan, M.; Raoult, D. Recommendations for Treatment of Human Infections Caused by Bartonella Species. Antimicrob. Agents Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, M.; Schairer, J.; Madigan, G.; Ball, A. Bartonella henselae is associated with heartburn, abdominal pain, skin rash, mesenteric adenitis, gastritis and duodenitis. J. Pediatric Gastroenterol. Nutr. 2002, 35, 158. [Google Scholar]
- Maritsi, D.; Zarganis, D.; Metaxa, Z.; Papaioannou, G.; Vartzelis, G. Bartonella henselae Infection: An Uncommon Mimicker of Autoimmune Disease. Case Rep. Pediatrics 2013, 2013, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chiuri, R.; Matronola, M.; Di Giulio, C.; Comegna, L.; Chiarelli, F.; Blasetti, A. Bartonella henselae Infection Associated with Autoimmune Thyroiditis in a Child. Horm. Res. Paediatr. 2013, 79, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Van Audenhove, A.; Verhoef, G.; Peetermans, W.; Boogaerts, M.; Vandenberghe, P. Autoimmune haemolytic anaemia triggered by Bartonella henselae infection: A case report. Br. J. Haematol. 2001, 115, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, M.; Tsuneoka, H.; Tateishi, H.; Fujita, K.; Uchida, M. Bartonella Infection Associated with Systemic Juvenile Rheumatoid Arthritis. Clin. Infect. Dis. 2001, 32, e22–e23. [Google Scholar] [CrossRef] [Green Version]
- Cozzani, E.; Cinotti, E.; Ameri, P.; Sofia, A.; Murialdo, G.; Parodi, A. Onset of cutaneous vasculitis and exacerbation of IgA nephropathy after Bartonella henselae infection. Clin. Exp. Dermatol. 2011, 37, 238–240. [Google Scholar] [CrossRef]
- Hopp, L.; Eppes, S. Development of IgA nephritis following cat scratch disease in a 13-year-old boy. Pediatric Nephrol. 2004, 19, 682–684. [Google Scholar] [CrossRef]
- Giladi, M.; Maman, E.; Paran, D.; Bickels, J.; Comaneshter, D.; Avidor, B.; Varon-Graidy, M.; Ephros, M.; Wientroub, S. Cat-scratch disease-associated arthropathy. Arthritis Rheum. 2005, 52, 3611–3617. [Google Scholar] [CrossRef]
- Beard, C.B.; Nelson, C.A.; Mead, P.S.; Petersen, L.R.; Raoult, D.; Maggi, R.G.; Mozayeni, B.R.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; et al. Bartonella spp. Bacteremia and Rheumatic Symptoms in Patients from Lyme Disease–endemic Region. Emerg. Infect. Dis. 2012, 18, 1919–1921. [Google Scholar]
- Durey, A.; Kwon, H.; Im, J.; Lee, S.; Baek, J.; Han, S.; Kang, J.-S.; Lee, J.-S. Bartonella henselae infection presenting with a picture of adult-onset Still’s disease. Int. J. Infect. Dis. 2016, 46, 61–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockmeyer, B.; Schoerner, C.; Frangou, P.; Moriabadi, T.; Heuss, D.; Harrer, T. Chronic Vasculitis and Polyneuropathy due to Infection with Bartonella henselae. Infection 2007, 35, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Massei, F.; Gori, L.; Taddeucci, G.; Macchia, P.; Maggiore, G. Bartonella henslae infection associated with Guillain-Barre syndrome. Pediatric Infect. Dis. J. 2006, 25, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, E.; Sodini, F.; Boscarelli, G.; Nasca, G.; Branchi, M.; Pellegrini, G. Immune thrombocytopenic purpura as a complication of Bartonella henselae infection. Le Infezioni Medicina 2008, 2, 99–102. [Google Scholar]
- Baylor, P.; Garoufi, A.; Karpathios, T.; Lutz, J.; Mogelof, J.; Moseley, D. Transverse Myelitis in 2 Patients With Bartonella henselae Infection (Cat Scratch Disease). Clin. Infect. Dis. 2007, 45, e42–e45. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, E.; McBride, J.; Schmiederer, M.; Anderson, B. Role of Bartonella henselae in the etiology of Henoch-Schönlein purpura. Pediatric Infect. Dis. J. 2002, 21, 28–31. [Google Scholar] [CrossRef]
- Robinson, J.; Spady, D.; Prasad, E.; McColl, D.; Artsob, H. Bartonella seropositivity in children with Henoch-Schonlein purpura. BMC Infect. Dis. 2005, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, F.; Agmon-Levin, N.; Perricone, C. Genetic Factors of Autoimmune Diseases. J. Immunol. Res. 2016, 2016, 1–2. [Google Scholar] [CrossRef]
- Rashid, T.; Ebringer, A. Autoimmunity in Rheumatic Diseases Is Induced by Microbial Infections via Crossreactivity or Molecular Mimicry. Autoimmune Dis. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Granholm, N.; Cavallo, T. Autoimmunity, Polyclonal B-Cell Activation and Infection. Lupus 1992, 1, 63–74. [Google Scholar] [CrossRef]
- Lintner, K.; Wu, Y.; Yang, Y.; Spencer, C.; Hauptmann, G.; Hebert, L.; Atkinson, J.P.; Yu, Y. Early Components of the Complement Classical Activation Pathway in Human Systemic Autoimmune Diseases. Front. Immunol. 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayadas, T.; Tsokos, G.; Tsuboi, N. Mechanisms of Immune Complex–Mediated Neutrophil Recruitment and Tissue Injury. Circulation 2009, 120, 2012–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, A.; Massei, F.; Not, T.; Massimetti, M.; Bussani, R.; Maggiore, G. Systemic Bartonella henselae Infection with Hepatosplenic Involvement. J. Pediatric Gastroenterol. Nutr. 1999, 29, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kordick, D.; Brown, T.; Shin, K.; Breitschwerdt, E. Clinical and pathologic evaluation of chronic Bartonella henslae or Bartonella clarridgiae infection in cats. J. Clin. Microbiol. 1999, 37, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Christen, B.; Leung, D.; Vigo-Pelfrey, C. Serodiagnosis of Lyme borreliosis by western immunoblot: Reactivity of various significant antibodies against Borrelia burgdorferi. J. Clin. Microbiol. 1992, 30, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Surveillance Case Definitions|NNDSS [Internet]. Available online: https://wwwn.cdc.gov/nndss/case-definitions.html (accessed on 14 February 2020).
- Case Definitions for Infectious Conditions under Public Health Surveillance. In Morbidity and Mortality Weekly Report; CDC: Atlanta, GA, USA, 1997; p. 46(RR10).
- Breitschwerdt, E.; Sontakke, S.; Hopkins, S. Neurological Manifestations of Bartonellosis in Immunocompetent Patients: A Composite of Reports from 2005–2012. J. Neuroparasitol. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Sobraquès, M.; Maurin, M.; Birtles, R.; Raoult, D. In Vitro Susceptibilities of Four Bartonella bacilliformis Strains to 30 Antibiotic Compounds. Antimicrob. Agents Chemother. 1999, 43, 2090–2092. [Google Scholar] [CrossRef] [Green Version]
- Howden, B.; Smith, D.; Mansell, A.; Johnson, P.; Ward, P.; Stinear, T.; Davies, J.K. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol. 2008, 8, 39. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinderlehrer, D.A. Is Bartonella a Cause of Primary Sclerosing Cholangitis? A Case Study. Gastrointest. Disord. 2020, 2, 48-57. https://doi.org/10.3390/gidisord2010005
Kinderlehrer DA. Is Bartonella a Cause of Primary Sclerosing Cholangitis? A Case Study. Gastrointestinal Disorders. 2020; 2(1):48-57. https://doi.org/10.3390/gidisord2010005
Chicago/Turabian StyleKinderlehrer, Daniel A. 2020. "Is Bartonella a Cause of Primary Sclerosing Cholangitis? A Case Study" Gastrointestinal Disorders 2, no. 1: 48-57. https://doi.org/10.3390/gidisord2010005
APA StyleKinderlehrer, D. A. (2020). Is Bartonella a Cause of Primary Sclerosing Cholangitis? A Case Study. Gastrointestinal Disorders, 2(1), 48-57. https://doi.org/10.3390/gidisord2010005



