Evaluation and Management of Pediatric Feeding Disorder
Abstract
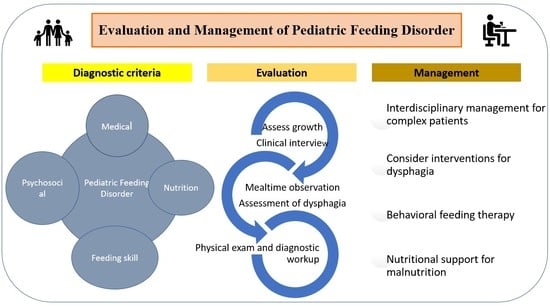
:1. Introduction
2. Pediatric Feeding Disorder (PFD)
3. Evaluation of PFD
3.1. Initial Evaluation
3.2. Clinical Interview
3.3. Feeding Questionnaires
3.4. Mealtime Observation
3.5. Physical Examination and Laboratory Studies
3.6. Assessment of Dysphagia
4. Management of PFD
4.1. Stepwise Approach to PFD
4.2. Interdisciplinary Management
4.3. Interventions for Dysphagia
4.4. Behavioral Treatment
4.5. Dietary Interventions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, G. Development of taste and food preferences in children. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Lupton, D. Food, the Body and the Self; Sage Publications: Thousand Oaks, CA, USA, 1996. [Google Scholar]
- Bentovim, A. The clinical approach to feeding disorders of childhood. J. Psychosom. Res. 1970, 14, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, B.W.C.; Leventhal, J.M.; McCarthy, P.L. Mothers’ perceptions of problems of feeding and crying behaviors: A prospective study. Am. J. Dis. Child 1985, 139, 269–272. [Google Scholar] [CrossRef]
- Gouge, A.L.; Ekvall, S.W. Diets of handicapped children: Physical, psychological, and socio- economic correlations. Am. J. Ment. Defic. 1975, 80, 149–157. [Google Scholar] [PubMed]
- Manikam, R.; Perman, J.A. Pediatric feeding disorders. J. Clin. Gastroenterol. 2000, 30, 34–36. [Google Scholar] [CrossRef]
- Perske, R. (Ed.) Mealtimes for Severely and Profoundly Handicapped Persons: New Concepts and Attitudes; University Park Press: Baltimore, MA, USA, 1977. [Google Scholar]
- Babbitt, R.L.; Hoch, T.A.; Coe, D.A. Behavioral feeding disorders. In Disorders of Feeding and Swallowing in Infants and Children; Tuchman, D.N., Walters, R.S., Eds.; Singular: San Diego, CA, USA, 1994; pp. 77–95. [Google Scholar]
- Dahl, M.; Sundelin, C. Feeding problems in an affluent society. Follow-up at four years of age in children with early refusal to eat. Acta Paediatr. 1992, 81, 575–579. [Google Scholar] [CrossRef]
- Marchi, M.; Cohen, P. Early childhood eating behaviors and adolescent eating disorders. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 112–117. [Google Scholar] [CrossRef]
- Silverman, A.H.; Tarbell, S. Feeding and vomiting problems in pediatric populations. In Handbook of Pediatric Psychology; Roberts, M.C., Steele, R.G., Eds.; Guilford Press: New York, NY, USA, 2009; pp. 429–445. [Google Scholar]
- Kovacic, K.; Rein, L.E.; Szabo, A.; Kommareddy, S.; Bhagavatula, P.; Goday, P. Pediatric feeding disorder: A nationwide prevalence study. J. Pediatr. 2021, 228, 126–131. [Google Scholar] [CrossRef]
- Silverman, A.H. Interdisciplinary care for feeding problems in children. Nutr. Clin. Pract. 2010, 25, 160–165. [Google Scholar] [CrossRef]
- Bithoney, W.G.; McJunkin, J.M.; Michalek, J.; Egan, H.; Snyder, J.; Munier, A. Prospective evaluation of weight gain in both nonorganic and organic failure-to-thrive children: An outpatient trial of a multidisciplinary team intervention strategy. J. Dev. Behav. Pediatr. 1989, 10, 27–31. [Google Scholar] [CrossRef]
- Budd, K.S.; McGraw, T.E.; Farbisz, R.; Murphy, T.B.; Hawkins, D.; Heilman, N.; Werle, M. Psychosocial concomitants of children’s feeding disorders. J. Pediatr. Psychol. 1992, 17, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Goday, P.S.; Huh, S.Y.; Silverman, A.; Lukens, C.T.; Dodrill, P.; Cohen, S.S.; Delaney, A.L.; Feuling, M.B.; Noel, R.J.; Gisel, E.; et al. Pediatric feeding disorder: Consensus definition and conceptual framework. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ammaniti, M.; Lucarelli, L.; Cimino, S.; D’Olimpio, F.; Chatoor, I. Feeding disorders of infancy: A longitudinal study to middle childhood. Int. J. Eat. Disord. 2012, 45, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rommel, N.; De Meyer, A.M.; Feenstra, L.; Veereman-Wauters, G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 75–84. [Google Scholar] [CrossRef]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar] [CrossRef]
- Benfer, K.A.; Weir, K.A.; Bell, K.L.; Ware, R.S.; Davies, P.S.; Boyd, R.N. Oropharyngeal dysphagia and gross motor skills in children with cerebral palsy. Pediatrics 2013, 131, e1553–e1562. [Google Scholar] [CrossRef]
- Dodrill, P. Feeding problems and oropharyngeal dysphagia in children. J. Gastroenterol. Hepatol. Res. 2014, 3, 1055–1060. [Google Scholar]
- Kumin, L.; Bahr, D.C. Patterns of feeding, eating, and drinking in young children with Down syndrome with oral motor concerns. Down Syndr. Q. 1999, 4, 1–8. [Google Scholar]
- De Vries, I.A.; Breugem, C.C.; Van der Heul, A.M.; Eijkemans, M.J.; Kon, M.; Mink van der Molen, A.B. Prevalence of feeding disorders in children with cleft palate only: A retrospective study. Clin. Oral. Investig. 2014, 18, 1507–1515. [Google Scholar] [CrossRef]
- Delaney, A.L.; Arvedson, J.C. Development of swallowing and feeding: Prenatal through first year of life. Dev. Disabil. Res. Rev. 2008, 14, 105–117. [Google Scholar] [CrossRef]
- Lefton-Greif, M.A.; Carroll, J.L.; Loughlin, G.M. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr. Pulmonol. 2006, 41, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.T.; Mageandran, S.D.; Mei, C. Incidence and clinical presentation of dysarthria and dysphagia in the acute setting following paediatric traumatic brain injury. Child Care Health Dev. 2010, 36, 44–53. [Google Scholar] [CrossRef]
- Mussatto, K.A.; Hoffmann, R.G.; Hoffman, G.M.; Tweddell, J.S.; Bear, L.; Cao, Y.; Brosig, C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics 2014, 133, e570–e577. [Google Scholar] [CrossRef] [Green Version]
- Bryant-Waugh, R.; Markham, L.; Kreipe, R.E.; Walsh, B.T. Feeding and eating disorders in childhood. Int. J. Eat. Disord. 2010, 43, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Farrow, C.V.; Coulthard, H. Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite 2012, 58, 842–846. [Google Scholar] [CrossRef] [Green Version]
- Phalen, J. Managing feeding problems and feeding disorders. Pediatr. Rev. 2013, 34, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.H. Behavioral Management of Feeding Disorders of Childhood. Ann. Nutr. Metab. 2015, 66, 33–42. [Google Scholar] [CrossRef]
- Berlin, K.S.; Davies, W.H.; Silverman, A.H.; Woods, D.W.; Fischer, E.A.; Rudolph, C.D. Assessing children’s mealtime problems with the Mealtime Behavior Questionnaire. Child Health Care 2010, 39, 142–156. [Google Scholar] [CrossRef]
- Archer, L.A.; Rosenbaum, P.L.; Streiner, D.L. The children’s eating behavior inventory: Reliability and validity results. J. Pediatr. Psychol. 1991, 16, 629–642. [Google Scholar] [CrossRef]
- Davies, W.H.; Ackerman, L.K.; Davies, C.M.; Vannatta, K.; Noll, R.B. About Your Child’s Eating: Factor structure and psychometric properties of a feeding relationship measure. Eat. Behav. 2007, 8, 457–463. [Google Scholar] [CrossRef]
- Musher-Eizenman, D.; Holub, S. Comprehensive feeding practices questionnaire: Validation of a new measure of parental feeding practices. J. Pediatr. Psychol. 2007, 32, 960–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.L.; Birch, L.L. Parents’ and children’s adiposity and eating style. Pediatrics 1994, 94, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.; Martel, C.; Porporino, M.; Zygmuntowicz, C. The Montreal Children’s Hospital Feeding Scale: A brief bilingual screening tool for identifying feeding problems. Paediatr. Child Health 2011, 16, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukens, C.T.; Linscheid, T.R. Development and validation of an inventory to assess mealtime behavior problems in children with autism. J. Autism Dev. Disord. 2008, 38, 342–352. [Google Scholar] [CrossRef]
- Derogatis, L.R. Symptom Checklist-90-Revised (SCL-90-R). Administration, Scoring, and Interpretation Manual; Clinical Psychometric Research: Towson, MD, USA, 1983; pp. 1–61. [Google Scholar]
- Abidin, R. Parenting Stress Index—Short Form; Psychological Assessment Resources: Odessa, FL, USA, 1995; pp. 26–45. [Google Scholar]
- Linscheid, T.J.; Budd, K.S.; Rasnake, L.K. Pediatric feeding problems. In Handbook of Pediatric Psychology; Roberts, M.C., Ed.; Gilford Press: New York, NY, USA, 2003; pp. 481–498. [Google Scholar]
- Barnard, K.E.; Hammond, M.; Booth, C.; Bee, H.; Spieker, S. Measurement and meaning of parent-child interaction. In Applied Developmental Psychology; Morrison, F., Lord, C., Keating, D., Eds.; Academic Press: New York, NY, USA, 1989; pp. 40–76. [Google Scholar]
- Mathisen, B.; Skuse, D.; Wolke, D.; Reilly, S. Oral-motor dysfunction and failure to thrive among inner-city infants. Dev. Med. Child Neurol. 1989, 31, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.R.; Patel, R.K.; Le Grice, B.; Shepherd, R.W. Children with persistent feeding difficulties: An observational analysis of the feeding interactions of problem and non-problem eaters. Health Psychol. 1993, 12, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Deane, M.G.; Burton, E.M.; Harlow, S.A.; Glover, A.S.; Deane, D.A.; Guill, M.F.; Hudson, V. Swallowing dysfunction in infants less than 1 year of age. Pediatr. Radiol. 2001, 31, 423–428. [Google Scholar] [CrossRef]
- Arslan, S.S.; Demir, N.; Karaduman, A.A. Both pharyngeal and esophageal phases of swallowing are associated with recurrent pneumonia in pediatric patients. Clin. Respir. J. 2018, 12, 767–771. [Google Scholar] [CrossRef]
- van den Engel-Hoek, L.; de Groot, I.J.; de Swart, B.J.; Erasmus, C.E. Feeding and swallowing disorders in pediatric neuromuscular diseases: An overview. J. Neuromuscul. Dis. 2015, 2, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Arvedson, J.C.; Lefton-Greif, M.A. Instrumental assessment of pediatric dysphagia. Semin. Speech Lang. 2017, 38, 135–146. [Google Scholar] [CrossRef]
- Milano, K.; Chatoor, I.; Kerzner, B. A Functional Approach to Feeding Difficulties in Children. Curr. Gastroenterol. Rep. 2019, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, V.K.; Dovey, T.M.; Martin, C.I.; Meyer, C. Identifying clinically relevant feeding problems and disorders. J. Child Health Care 2010, 14, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, M.E. Pediatric feeding problems: A behavior analytic approach to assessment and treatment. Behav. Anal. Today 2003, 4, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Kerzner, B.; Milano, K.; MacLean, W.C.; Berall, G.; Stuart, S.; Chatoor, I. A practical approach to classifying and managing feeding difficulties. Pediatrics 2015, 135, 344–345. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, J.O. Pediatric feeding disorders and severe developmental disabilities. Semin. Speech Lang. 2017, 38, 116–125. [Google Scholar] [CrossRef]
- Hojsak, I.; Bronsky, J.; Campoy, C.; Domellö, F.M.; Embleton, N.; Mis Fidler, N.; Hulst, J.; Indrio, F.; Lapillonne, A.; Mølgaard, C.; et al. Young child formula: A position paper by the ESPGHAN committee on nutrition. J. Pediatr. Gast. Nutr. 2018, 66, 177–185. [Google Scholar] [CrossRef]
- Golden, M.H. Evolution of nutritional management of acute malnutrition. Indian Pediatr. 2010, 47, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Finnane, J.M.; Jansen, E.; Mallan, K.M.; Daniels, L.A. Mealtime structure and responsive feeding practices are associated with less food fussiness and more food enjoyment in children. J. Nutr. Educ. Behav. 2017, 49, 11–18. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM 5®); American Psychiatric Association: Washington, DC, USA, 2013; pp. 557–586. [Google Scholar]
- Chatoor, I. Diagnosis and Treatment of Feeding Disorders in Infants, Toddlers, and Young Children; Zero to Three: Washington, DC, USA, 2009. [Google Scholar]
- Sant’Anna, A.M.; Hammes, P.S.; Porporino, M.; Martel, C.; Zygmuntowicz, C.; Ramsay, M. Use of cyproheptadine in young children with feeding difficulties and poor growth in a pediatric feeding program. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 674–678. [Google Scholar] [CrossRef]
- Benjasuwantep, B.; Chaithirayanon, S.; Eiamudomkan, M. Feeding problems in healthy young children: Prevalence, related factors and feeding practices. Pediatr. Rep. 2013, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Byrne, R.; Jansen, E.; Daniels, L. Perceived fussy eating in Australian children at 14 months of age and subsequent use of maternal feeding practices at 2 years. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, S.O.; Power, T.G.; Fisher, J.O.; Mueller, S.; Nicklas, T.A. Revisiting a neglected construct: Parenting styles in a child-feeding context. Appetite 2005, 44, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Volkert, V.M.; Scahill, L.; McCracken, C.E.; McElhanon, B. A systematic review and meta-analysis of intensive multidisciplinary intervention for pediatric feeding disorders: How standard is the standard of care? J. Pediatr. 2017, 181, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.K. Aspiration and swallowing dysfunction in pediatric patients. Infant. Child Adolesc. Nutr. 2011, 3, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.T.; Dodrill, P.; Ward, E.C. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst. Rev. 2012, 10, CD009456. [Google Scholar] [CrossRef] [PubMed]
- Gosa, M.M.; Schooling, T.; Coleman, J. Thickened liquids as a treatment for children with dysphagia and associated adverse effects. Infant. Child Adolesc. Nutr. 2011, 3, 344–350. [Google Scholar] [CrossRef]
- Clarke, P.; Robinson, M.J. Thickening milk feedings may cause necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F280. [Google Scholar] [CrossRef] [Green Version]
- Abrams, S.A. Be cautious in using thickening agents for preemies. AAP News 2011, 32, 23. [Google Scholar]
- Pados, B.F.; Park, J.; Thoyre, S.M.; Estrem, H.; Nix, W.B. Milk flow rates from bottle nipples used after hospital discharge. MCN Am. J. Matern. Child Nurs. 2016, 41, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Jackman, K.T. Go with the flow: Choosing a feeding system for infants in the neonatal intensive care unit and beyond based on flow performance. Newborn Infant Nurs. Rev. 2013, 13, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.; Kennedy, G.; Pring, T.; Hird, M.I. Improving bottle feeding in preterm infants: Investigating the elevated side-lying position. Infant 2007, 3, 354–358. [Google Scholar]
- Thoyre, S.M.; Holditch-Davis, D.; Schwartz, T.A.; Melendez Roman, C.R.; Nix, W.B. Coregulated approach to feeding preterm infants with lung disease. Nurs. Res. 2012, 61, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Thoyre, S.M.; Knafl, G.J.; Hodges, E.A.; Nix, W.B. Efficacy of semielevated side-lying positioning during bottle-feeding of very preterm infants. J. Perinat. Neonat. Nurs. 2014, 28, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, J.A.; Myers, L.R.; Moorhead, A.; Jacobs, S.E.; Ong, K.; Salo, F.; Murray, S.; Donath, S.; Davis, P.G. A randomised trial of two techniques for bottle feeding preterm infants. J. Paediatr. Child Health 2013, 49, 462–466. [Google Scholar] [CrossRef] [Green Version]
| A. A Disturbance in Oral Intake of Nutrients, Inappropriate for Age, Lasting at Least Two Weeks, and Associated with One or More of the Following: | |
| Medical dysfunction Nutritional dysfunction Feeding skill dysfunction | Cardiorespiratory compromise during oral feeding Aspiration or recurrent aspiration pneumonitis Malnutrition Specific nutrient deficiency or significantly restricted intake of one or more nutrients resulting from decreased dietary diversity Reliance on enteral feeds or oral supplements to sustain nutrition and/or hydration Need for texture modification of liquid or food Use of modified feeding position or equipment Use of modified feeding strategies |
| Psychosocial dysfunction | Active or passive avoidance behaviors by child when feeding or being fed Inappropriate caregiver management of child’s feeding and/or nutrition needs Disruption of social functioning within a feeding context Disruption of caregiver-child relationship associated with feeding |
| B. Absence of the cognitive processes consistent with eating disorders and pattern of oral intake is not due to a lack of food or congruent with cultural norms. | |
| System | Diseases |
|---|---|
| Oral, nasal, or pharyngeal disorders | Cleft lip, cleft palate |
| Macroglossia | |
| Micrognathia | |
| Cranio-facial syndromes | |
| Extensive dental disease | |
| Choanal atresia | |
| Chronic tonsillitis | |
| Aerodigestive disease | |
| Airway | Laryngeal clefts |
| Vocal cord/vocal fold paralysis | |
| Laryngomalacia, tracheomalacia | |
| Subglottic stenosis | |
| Reactive airway disease | |
| Gastrointestinal | Food allergies and food intolerances |
| Eosinophilic esophagitis | |
| Esophageal motility disorder (post-esophageal atresia or achalasia) | |
| Short bowel syndrome | |
| Feeding/volume intolerance of any cause | |
| Gastroparesis | |
| Gastroesophageal reflux disease | |
| Congenital anomalies of GI tract | |
| Cardio-pulmonary disorders | Any form of congenital heart disease (esp. hypoplastic left heart syndrome) and other conditions that result in staged single ventricle repair |
| Associated pulmonary hypertension | |
| Myocarditis and other causes of heart failure | |
| Bronchopulmonary dysplasia | |
| Chronic lung disease | |
| Any process resulting in chronic tachypnea | |
| Neurological, neuromuscular, developmental, and psychiatric disorders | Autism spectrum disorder |
| Disorders of motor control with hyper- or hypotonia (Cerebral palsy, Muscular dystrophies) | |
| Attention deficit/hyperactivity disorder | |
| Iatrogenic | Prolonged hospitalization with critical care support (e.g., ventilation, continuous positive airway pressure, high-flow, oxygen) |
| Invasive operative procedures affecting vital systems (e.g., tracheostomy, gastrostomy) | |
| Aversive feeding |
| Identify Red Flags | Evaluate for Signs of Oral Motor Dysfunction | Stabilize Nutrient Intake | Review Basic Feeding Guidelines |
|---|---|---|---|
| Aspiration Dysphagia Pain with feeding Vomiting and diarrhea Developmental delay Chronic cardio- respiratory symptoms Growth failure Nutrient deficiencies Force-feeding | Excessive drooling Poor postural control Abnormal muscle tone Excessive gagging or choking Failure to advance textures Difficulty with feeding milestones Difficulty managing food or liquid in mouth | Supplemental calories for growth failure Multi-nutrient supplementation for limited dietary variety Single nutrient supplementation for deficiency | Avoid mealtime distractions Maintain pleasant, neutral attitude while feeding Limit meal duration Provide 4–6 meals/snacks a day with water in between Serve age-appropriate foods Systematically offer new foods (8–15 times) Encourage self-feeding Tolerate age-appropriate mess |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmaraj, R.; Elmaoued, R.; Alkhouri, R.; Vohra, P.; Castillo, R.O. Evaluation and Management of Pediatric Feeding Disorder. Gastrointest. Disord. 2023, 5, 75-86. https://doi.org/10.3390/gidisord5010008
Dharmaraj R, Elmaoued R, Alkhouri R, Vohra P, Castillo RO. Evaluation and Management of Pediatric Feeding Disorder. Gastrointestinal Disorders. 2023; 5(1):75-86. https://doi.org/10.3390/gidisord5010008
Chicago/Turabian StyleDharmaraj, Rajmohan, Rasha Elmaoued, Razan Alkhouri, Pankaj Vohra, and Ricardo O. Castillo. 2023. "Evaluation and Management of Pediatric Feeding Disorder" Gastrointestinal Disorders 5, no. 1: 75-86. https://doi.org/10.3390/gidisord5010008
APA StyleDharmaraj, R., Elmaoued, R., Alkhouri, R., Vohra, P., & Castillo, R. O. (2023). Evaluation and Management of Pediatric Feeding Disorder. Gastrointestinal Disorders, 5(1), 75-86. https://doi.org/10.3390/gidisord5010008


.png)