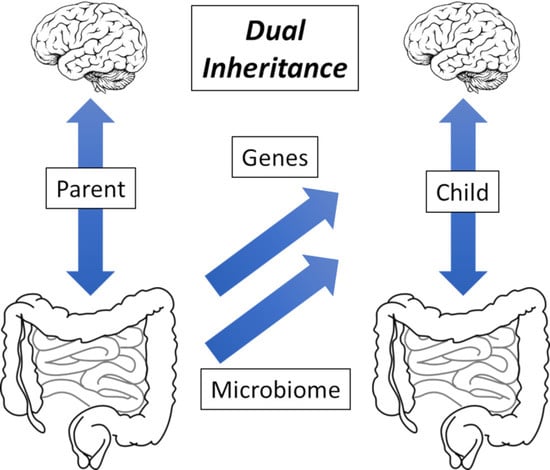
On the Inheritance of Microbiome-Deficiency: Paediatric Functional Gastrointestinal Disorders, the Immune System and the Gut–Brain Axis
Abstract
:1. Functional Gastrointestinal Disorders: Non-Communicable Disease
2. Investigating the Cause of Disease: Denis Burkitt and Dysbiosis
3. Summary of Microbiome-Related Concepts and Terminology
4. Evolution: Lynn Margulis and Carl Woese; A Vertebrate Holobiont
5. Epidemiology: David Strachan and David Barker; An Infant Origins Hypothesis
6. A Microbiome-Health Hypothesis: “Handshaking” and the Birth Process
6.1. Natural Birth: Efficient Microbial Transfer
6.2. Delivery by Sterile Caesarean Section: Limited Microbiota Transfer
7. A Microbiome-Health Hypothesis: Peristaltic Control and Antigen Recognition
8. Current Understanding of the Neonate Immune System
9. The Nature of the Microbiome: Mobile Genetic Elements
10. The Degradation of the Microbiome: The Role of Heavy Metal Pollution
10.1. Diet-Induced Extinctions
10.2. Antibiotics
10.3. Heavy Metal Toxins
11. FGI Disorders and Non-Communicable Disease: Drawing the Threads Together
11.1. The Healthy Animal: A Virtuous Circle
11.2. The Lack of Handshaking: A Potential Cause of Paediatric FGI Disorders
12. Loss of Microbiome Function: When Is a Holobiont Not a Holobiont?
13. The Search for a Standard Microbiome: Birth Microbes
14. The Search for Semiochemical Function: An Ingestible Sensor
15. Future Prevention of Paediatric FGID: A Birth Probiotic
15.1. Food
15.2. Antibiotics
15.3. Heavy Metal Toxins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Haregu, T.N.; Byrnes, A.; Singh, K.; Sathish, T.; Pasricha, N.; Wickramasinge, K.; Thankappan, K.R.; Oldenburg, B. A scoping review of non-communicable disease research capacity strengthening initiatives in low and middle income countries. Glob. Health Res. Policy 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Moore, J.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–148. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64. [Google Scholar] [CrossRef]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative review: Nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-processed foods and health outcomes: A narrative review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm Epigenetics and Influence of Environmental Factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The Seminal Microbiome in Health and Disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The Preconception Environment and Sperm Epigenetics. Andrology 2020, 8, 924–942. [Google Scholar] [CrossRef] [PubMed]
- Vallgårda, S. Why the concept “lifestyle diseases” should be avoided. Scand. J. Public Health 2011, 39, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. Microbiome-gut dissociation in the neonate: Obesity and coeliac disease as examples of microbiome-function deficiency disorder. Gastrointest. Disord. 2022, 4, 108–128. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef]
- Parker, S.; Palsson, O.; Sanders, D.S.; Simren, M.; Sperber, A.D.; Törnblom, H.; Urwin, H.; Whitehead, W.; Aziz, I. Functional gastrointestinal disorders and associated health impairment in individuals with celiac disease. Clin. Gastroenterol. Hepatol. 2022, 20, 1315–1325.e4. [Google Scholar] [CrossRef]
- Burkitt, D. A sarcoma involving the jaws in African children. Br. J. Surg. 1958, 46, 218–223. [Google Scholar] [CrossRef]
- Burkitt, D.P. Some diseases characteristic of modern western civilization. Br. Med. J. 1973, 1, 274–278. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S. Microbiome-gut dissociation: Investigating the origins of obesity. Gastrointest. Disord. 2021, 3, 156–172. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut microbiota: A missing link in psychiatry. World Psychiatry 2020, 19, 111–112. [Google Scholar] [CrossRef]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. The enclosed intestinal microbiome: Semiochemical signals from the Precambrian and their disruption by heavy metal pollution. Life 2022, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Street, B.; Palacios-Pérez, M. Microbiome-gut dissociation in the neonate: Autism-related developmental brain disease and the origin of the placebo effect. Gastrointest. Disord. 2022, 4, 291–311. [Google Scholar] [CrossRef]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Palacios-Pérez, M. Feeding our microbiota: Stimulation of the immune/semiochemical system and the potential amelioration of non-communicable diseases. Life 2022, 12, 1197. [Google Scholar] [CrossRef]
- Chernikova, D.; Yuan, I.; Shaker, M. Prevention of allergy with diverse and healthy microbiota: An update. Curr. Opin. Pediatr. 2019, 31, 418. [Google Scholar] [CrossRef] [PubMed]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92. [Google Scholar]
- Rosenburg, E.; Zilber-Rosenburg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Yong, E. I Contain Multitudes: The Microbes within Us and a Grander View of Life; Penguin Random House LLC.: London, UK, 2016; ISBN 978-1-784-70017-1. [Google Scholar]
- Gilbert, C.; Maumus, F. Multiple horizontal acquisitions of plant genes in the whitefly Bemisia tabaci. Genome Biol. Evol. 2022, 14, evac141. [Google Scholar] [CrossRef]
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [CrossRef] [PubMed]
- Budd, G.E. At the origin of animals: The revolutionary Cambrian fossil record. Curr. Genom. 2013, 14, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.A.; Fitzstevens, J.L.; Schmidt, V.T.; Enav, H.; Huus, K.; Mbong, M.; Adegite, B.R.; Zinsou, J.F.; Esen, M.; Velavan, T.; et al. Codiversification of gut microbiota with humans. Science 2022, 377, 1328–1332. [Google Scholar] [CrossRef]
- Stensvold, C.R.; van der Giezen, M. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol. 2018, 34, 369–377. [Google Scholar] [CrossRef]
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. mSystems 2018, 3, e00140. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: A missing link in gut microbiome studies. mSystems 2018, 3, e00201-17. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; De Benedetto, A. Atopic dermatitis in animals and in people: An update and comparative review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Eriksson, J.G. The fetal origins hypothesis–10 years on. BMJ 2005, 330, 1096–1097. [Google Scholar] [CrossRef]
- Almond, D.; Currie, J. Killing me softly: The fetal origins hypothesis. J. Econ. Perspect. 2011, 25, 153–172. [Google Scholar] [CrossRef]
- Sandercock, G.R.H.; Cohen, D.D. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: An allometric approach. J. Sci. Med. Sport 2019, 22, 201–205. [Google Scholar] [CrossRef]
- Ðuric, S.; Sember, V.; Starc, G.; Soric, M.; Kovac, M.; Jurak, G. Secular trends in muscular fitness from 1983 to 2014 among Slovenian children and adolescents. Scand. J. Med. Sci. Sport. 2021, 31, 1853–1861. [Google Scholar] [CrossRef]
- Mesa, D.M.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Cabero, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth—First 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. Toward a Theoretical Biology; The basic ideas of biology; Edinburgh University Press: Edinburgh, Scotland, 1968; pp. 1–32. [Google Scholar]
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and the epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Basic, M.; Bleich, A. Gnotobiotics: Past, present and future. Lab. Anim. 2019, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.L.; Fields, A.; Gee, D.G.; Gabard-Durnam, L.; Caldera, C.; Humphreys, K.L.; Goff, B.; Flannery, J.; Telzer, E.H.; Shapiro, M.; et al. Mind and gut: Associations between mood and gastrointestinal distress in children exposed to adversity. Dev. Psychopathol. 2020, 32, 309–328. [Google Scholar] [CrossRef]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016, 170, e162385. [Google Scholar] [CrossRef]
- Song, S.J.; Wang, J.; Martino, C.; Jiang, L.; Thompson, W.K.; Shenhav, L.; McDonald, D.; Marotz, C.; Harris, P.R.; Hernandez, C.D.; et al. Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med 2021, 2, 951–964.e5. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Anthony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Tun, H.M.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; Scott, J.A.; et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018, 172, 368–377. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Tejesvi, M.V.; Vänni, P.; Suokas, M.; Tossavainen, P.; Pirttilä, A.M.; Talvensaari-Mattila, A.; Nissi, R. Child type 1 diabetes associated with mother vaginal bacteriome and mycobiome. Med. Microbiol. Immunol. 2022, 211, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the development of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93. [Google Scholar]
- Chien, P. Global rising rates of caesarean sections. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Ye, J.; Moller, A.-B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, A.J.; Sim, K.; Deierl, A.; Kroll, S.; Brannigan, E.; Darby, J. “Vaginal seeding” of infants born by caesarean section. BMJ 2016, 352, i227. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Blanco-Míguez, A.; Manghi, P.; Asnicar, F.; Dubois, L.; Golzato, D.; Armanini, F.; Cumbo, F.; Huang, K.D.; Manara, S.; et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature 2023, 614, 125–135. [Google Scholar] [CrossRef]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.528202 (accessed on 23 April 2023). [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell-cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod cells: Emerging biology of the gut-brain sensory transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Biogenic amines: Signals between commensal microbiota and gut physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermùndez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2005, 29, 1395–1403. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Papaevangelou, V.; Castagnoli, R.; Winkler, S.; Xanthou, G.; Tsafaras, G.P.; Ntontsi, P. Advantages and Limitations of the Neonatal Immune System. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; da silva Lopes Salles, E.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Munguía-Fuentes, R.; Yam-Puc, J.C.; Silva-Sánchez, A.; Marcial-Juárez, E.; Gallegos-Hernández, I.A.; Calderón-Amador, J.; Randall, T.D.; Flores-Romo, L. Immunization of newborn mice accelerates the architectural maturation of lymph nodes, but AID-dependent IgG responses are still delayed compared to the adult. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Rousseaux, A.; Brosseau, C.; le Gall, S.; Piloquet, H.; Barbarot, S.; Bodinier, M. Human milk oligosaccharides: Their effects on the host and their potential as therapeutic agents. Front. Immunol. 2021, 12, 1791. [Google Scholar] [CrossRef]
- Brosseau, C.; Selle, A.; Duval, A.; Misme-Aucouturier, B.; Chesneau, M.; Brouard, S.; Cherbuy, C.; Cariou, V.; Bouchaud, G.; Mincham, K.T.; et al. Prebiotic supplementation during pregnancy modifies the gut microbiota and increases metabolites in amniotic fluid, driving a tolerogenic environment in utero. Front. Immunol. 2021, 12, 2857. [Google Scholar] [CrossRef] [PubMed]
- Adogony, V.; Respondek, F.; Biourge, V.; Rudeaux, F.; Delaval, J.; Bind, J.L.; Salmon, H. Effects of dietary scFOS on immunoglobulins in colostrums and milk of bitches. J. Anim. Physiol. Anim. Nutr. 2007, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, Y.; Zhou, J.; Huang, W.; Yan, J.; Tao, J.; Fan, Q.; Liu, Y.; Mei, D.; Yan, Q.; et al. Core fucosylation of maternal milk N-glycan evokes B cell activation by selectively promoting the L-fucose metabolism of gut bifidobacterium spp. and lactobacillus spp. mBio 2019, 10, e00128-19. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Zhang, L.; Abdel-Rahman, S.A.; Naik, S.P.; Gabr, M. Gut microbial-derived metabolites as immune modulators of T helper 17 and regulatory T cells. Int. J. Mol. Sci. 2023, 24, 1806. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front. Immunol. 2021, 12, 2702. [Google Scholar] [CrossRef]
- Pernomian, L.; Duarte-Silva, M.; Ribeiro De Barros Cardoso, C. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Griffith, F. The significance of pneumococcal types. J. Hyg. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; MacCleod, C.M.; McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Suttle, C.A. Viruses and nutrient cycles in the sea. BioScience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Hehemann, J.H.; Kelly, A.G.; Pudlo, N.A.; Martens, E.C.; Boraston, A.B. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc. Natl. Acad. Sci. USA 2012, 109, 19786–19791. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.A.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Shapiro, D.J.; Hicks, L.A.; Pavia, A.T.; Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–2009. J. Antimicrob. Chemother. 2014, 69, 234–240. [Google Scholar] [CrossRef]
- Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Effects of antibiotics upon the gut microbiome: A review of the literature. Biomedicines 2020, 8, 502. [Google Scholar] [CrossRef]
- Babakhanova, A.T.; Dzhumabekhov, A.T.; Zhao, A.V.; Kuandykov, Y.K.; Tanabayeva, S.B.; Fakhradiyev, I.R.; Nazarenko, Y.; Saliev, T.M. Impact of appendectomy on gut microbiota. Surg. Infect. 2021, 22, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Davison, T.F.; Freeman, B.M. Physiological aspects of growth promotion in poultry. Vet. Res. Commun. 1983, 7, 59–68. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.E.; El-Nobi, G.A.; El-Bouhy, Z.M. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogórek, K.; Korycińska, J. Blastocystis: How do specific diets and human gut microbiota affect its development and pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Bostock, J. Case of a periodical affection of the eyes and chest. Med. Chir. Trans. 1819, 10, 161–165. [Google Scholar] [CrossRef]
- Bostock, J. Of the catarrhus aestivus or summer catarrh. Med. Chir. Trans. 1828, 14, 437–446. [Google Scholar] [CrossRef]
- Corson, R. Fashions in Makeup: From Ancient to Modern Times; Peter Owen Ltd.: London, UK, 1972. [Google Scholar]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the Industrial Revolution. eLife 2020, 9, e49555. [Google Scholar] [CrossRef]
- Shao, M.; Zhu, Y. Long term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci. Rep. 2020, 10, 4453. [Google Scholar] [CrossRef]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The effects of essential and non-essential metal toxicity in the Drosophila melanogaster insect model: A review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H. The removal of lead from gasoline: Historical and personal reflections. Environ. Res. 2000, 84, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Swift, G.L.; Card, T.R.; Sanders, D.S.; Ludvigsson, J.F.; Bai, J.C. Psychological morbidity of celiac disease: A review of the literature. United Eur. Gastroenterol. J. 2015, 3, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Budu-Aggrey, A.; Joyce, S.; Davies, N.M.; Paternoster, L.; Munafò, M.R.; Brown, S.J.; Evans, J.; Sallis, H.M. Investigating the causal relationship between allergic disease and mental health. Clin. Exp. Allergy 2021, 51, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Bertua, B.; Martínez-Costa, C.; Collado, M.C. Perinatal nutrition: How to take care of the gut microbiota? Clin. Nutr. Exp. 2016, 6, 3–16. [Google Scholar] [CrossRef]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.-Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef]
- Jones, S.K.; McCarthy, D.M.; Vied, C.; Bhide, P.G. Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in amygdala. Proc. Natl. Acad. Sci. USA 2022, 119, e2213120119. [Google Scholar] [CrossRef]
- Reese, A.T.; Chadaideh, K.S.; Diggins, C.E.; Schell, L.D.; Beckel, M.; Callahan, P.; Ryan, R.; Thompson, M.E.; Carmody, R.N. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 2021, 10, e60197. [Google Scholar] [CrossRef]
- Bogatyrev, S.R.; Rolando, J.C.; Ismagilov, R.F. Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome 2020, 8, 19. Available online: https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-020-0785-4 (accessed on 23 April 2023). [CrossRef]
- Rühlemann, M.C.; Hermes, B.M.; Bang, C.; Doms, S.; Moitinho-Silva, L.; Thingholm, L.B.; Frost, F.; Degenhardt, F.; Wittig, M.; Kässens, J.; et al. Genome-wide association study in 8,956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat. Genet. 2021, 53, 147–155. [Google Scholar] [CrossRef]
- Ewald, D.R.; Sumner, S.J. Human microbiota, blood group antigens, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1413. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Microbe Profile: Akkermansia muciniphila: A conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology 2017, 163, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L.; Groisman, E.A. Novel “superspreader” bacteriophages promote horizontal gene transfer by transformation. mBio 2017, 8, e02115-16. [Google Scholar] [CrossRef] [PubMed]
- Lekunberri, I.; Subirats, J.; Borrego, C.M.; Balcazar, J.L. Exploring the contribution of bacteriophage to antibiotic resistance. Environ. Pollut. 2017, 220, 981–984. [Google Scholar] [CrossRef]
- Pratt, C.; Campbell, M.D. The effect of bifidobacterium on reducing symptomatic pain in patients with irritable bowel syndrome: A systematic review. Probiotics Antimicrob. Proteins 2020, 12, 834–839. [Google Scholar] [CrossRef]
- Hidalgo, G.; Marini, E.; Sanchez, W.; Contreras, M.; Estrada, I.; Comandini, O.; Buffa, R.; Magris, M.; Dominguez-Bello, M.G. The nutrition transition in the Venezuelan Amazonia: Increased overweight and obesity with transculturation. Am. J. Hum. Biol. 2014, 26, 710–712. [Google Scholar] [CrossRef]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarsón, Ó.; et al. The microbiome of uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef]
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross sectional cohort study. Lancet 2017, 389, 1730–1739. [Google Scholar] [CrossRef]
- Irimia, A.; Chaudhari, N.N.; Robles, D.J.; Rostowsky, K.A.; Maher, A.S.; Chowdhury, N.F.; Calvillo, N.F.; Ngo, V.; Gatz, M.; Mack, W.J.; et al. The indigenous South American Tsimane exhibit relatively modest decrease in brain volume with age despite high systemic inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2147–2155. [Google Scholar] [CrossRef]
- Ryan, C.R. Towards an ethics of reciprocity: Ethnobotanical knowledge and medicinal plants as cancer therapies. Humanities 2014, 3, 624–644. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Jheeta, S. Measuring microbiome effectiveness: A role for ingestible sensors. Gastrointest. Disord. 2020, 2, 3–11. [Google Scholar] [CrossRef]
- Wang, A.; Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Chauhan, S.; Gottlieb, K.T.; Konda, V.; Maple, J.T.; Murad, F.; Plau, P.R.; et al. Wireless capsule endoscopy. Gastrointest. Endosc. 2013, 78, 805–815. [Google Scholar] [CrossRef]
- Beardslee, L.A.; Banis, G.E.; Chu, S.; Liu, S.; Chapin, A.A.; Stine, J.M.; Pasricha, P.J.; Ghodossi, R. Ingestible sensors and sensing systems for minimally invasive diagnosis and monitoring: The next frontier in minimally invasive screening. ACS Sens. 2020, 5, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, D.; Shan, Y.; He, S.; Su, Z.; Liang, J.; Zheng, J.; Yang, Z.; Yang, H.; Xu, W.; et al. Edible and nutritive electronics: Materials, fabrications, components, and applications. Adv. Mater. Technol. 2020, 5, 2000100. [Google Scholar] [CrossRef]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 360, 915–918. [Google Scholar] [CrossRef]
- Traverso, G.; Ciccarelli, G.; Schwartz, S.; Hughes, T.; Boettcher, T.; Barman, R.; Langer, R.; Swiston, A. Physiologic status monitoring via the gastrointestinal tract. PLoS ONE 2015, 10, e0141666. [Google Scholar] [CrossRef]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]
- Indrio, F.; Neu, J.; Pettoello-Mantovani, M.; Marchese, F.; Martini, S.; Salatto, A.; Aceti, A. Development of the gastrointestinal tract in newborns as a challenge for an appropriate nutrition: A narrative review. Nutrients 2022, 14, 1405. [Google Scholar] [CrossRef]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- McConnell, J.R.; Wilson, A.I.; Stohl, A.; Arienzo, M.M.; Chellman, N.J.; Eckhardt, S.; Thompson, E.M.; Pollard, A.M.; Steffensen, J.P. Lead pollution recorded in Greenland ice indicates European emissions tracked plagues, wars and imperial expansion during antiquity. Proc. Natl. Acad. Sci. USA 2018, 115, 5726–5731. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.F.; Dixson, B.J. Venus figurines of the early paleolithic: Symbols of fertility or attractiveness? J. Anthropol. 2011, 2011, 569120. [Google Scholar] [CrossRef]
- Barbante, C.; Veysseyre, A.; Ferrari, C.; van der Velde, C.M.; Capodaglio, G.; Cescon, P.; Scarponi, G.; Boutron, C. Greenland snow evidence of large scale atmospheric contamination for platinum, palladium and rhodium. Environ. Sci. Technol. 2001, 35, 835–839. [Google Scholar] [CrossRef] [PubMed]




| Appendicitis | Coeliac Disease * | Coronary Heart Disease |
|---|---|---|
| Deep vein thrombosis | Diabetes, type 2 | Diverticular disease |
| Gall stones | Haemorrhoids | Hiatus hernia |
| Multiple Sclerosis * | Obesity | Pernicious anaemia * |
| Pulmonary embolism | Rheumatoid arthritis * | Thyrotoxicosis * |
| Tumours of the bowel * | Ulcerative colitis * | Varicose veins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Jheeta, S.; López-Cortés, G.I.; Street, B.; Fuentes, H.V.; Palacios-Pérez, M. On the Inheritance of Microbiome-Deficiency: Paediatric Functional Gastrointestinal Disorders, the Immune System and the Gut–Brain Axis. Gastrointest. Disord. 2023, 5, 209-232. https://doi.org/10.3390/gidisord5020018
Smith D, Jheeta S, López-Cortés GI, Street B, Fuentes HV, Palacios-Pérez M. On the Inheritance of Microbiome-Deficiency: Paediatric Functional Gastrointestinal Disorders, the Immune System and the Gut–Brain Axis. Gastrointestinal Disorders. 2023; 5(2):209-232. https://doi.org/10.3390/gidisord5020018
Chicago/Turabian StyleSmith, David, Sohan Jheeta, Georgina I. López-Cortés, Bernadette Street, Hannya V. Fuentes, and Miryam Palacios-Pérez. 2023. "On the Inheritance of Microbiome-Deficiency: Paediatric Functional Gastrointestinal Disorders, the Immune System and the Gut–Brain Axis" Gastrointestinal Disorders 5, no. 2: 209-232. https://doi.org/10.3390/gidisord5020018
APA StyleSmith, D., Jheeta, S., López-Cortés, G. I., Street, B., Fuentes, H. V., & Palacios-Pérez, M. (2023). On the Inheritance of Microbiome-Deficiency: Paediatric Functional Gastrointestinal Disorders, the Immune System and the Gut–Brain Axis. Gastrointestinal Disorders, 5(2), 209-232. https://doi.org/10.3390/gidisord5020018


.png)