Abstract
The aim of this study was to investigate the short-chain fatty acid (SCFA) activity of the gut microbiota of patients with metabolic-associated fatty liver disease (MAFLD). The level and spectrum of short-chain fatty acids (SCFAs) were determined via gas–liquid chromatography. Liver fibrosis was assessed using the FIB-4 index and elastography. Among 42 non-cirrhotic MAFLD patients, 24 had high fecal SCFA levels (group H) and 18 had low fecal SCFA levels (group L). Patients in group H had lower serum uric acid, total cholesterol, and LDL cholesterol levels but a higher BMI than those in group L. All patients in group L and only 37.9% of those in group H were found to have hypercholesterolemia. In patients with hypercholesterolemia, the level of SCFAs was lower than that in patients without hypercholesterolemia. Patients in group H had less liver fibrosis than patients in group L. A total of 50.0% of the patients in group H and 92.3% of those in group L had significant liver fibrosis (≥F2). Patients with significant liver fibrosis had lower levels of fecal SCFAs—particularly acetate and butyrate. The fecal SCFA levels were positively correlated with gamma-glutamyl transferase, total bilirubin levels, BMI, and platelet count and were negatively correlated with FIB-4, liver stiffness, serum total, and LDL cholesterol levels.
1. Introduction
Metabolic-associated fatty liver disease (MAFLD) is recognized as the most prevalent form of chronic liver disease, affecting one quarter of the global population [1,2,3]. The spectrum of MAFLD manifestations ranges from steatosis and steatohepatitis to MAFLD-related cirrhosis. Despite the increasing prevalence of MAFLD, the onset and progression of the disease remain unclear. This process probably depends on factors closely related to metabolic disorders, such as obesity, hypertension, dyslipidemia, and type 2 diabetes mellitus [1,2,3,4,5,6,7,8]. The main pathogenetic mechanisms of these diseases include inflammation, oxidative stress, insulin resistance, and dyslipidemia [3,4,5].
Several studies have suggested that abnormalities in the composition of the gut microbiota play an essential role in the development and progression of MAFLD. The involvement of several mechanisms has been discussed. The first is the modulation of bile acid synthesis, which is critical for fat absorption and affects glucose metabolism through the farnesoid X receptor [8]. The second is the activation of the innate immune system due to the translocation of bacterial components [1,2,3,4,5,6]. The third is the production of endogenous ethanol [2,8]; reduced choline metabolism alters very low-density lipoprotein metabolism and promotes inflammation [3,4,7]. Finally, there is a change in the production of short-chain fatty acids (SCFAs) [3,4,5,6,7,8,9].
It is well known that SCFAs, in addition to being the intestinal epithelium’s energy supply, perform various biological functions, such as immunity regulation, lipogenesis, and gluconeogenesis [3,6,9,10]. Observations of variations in the levels of SCFAs in patients with MAFLD have been presented in the literature, but the results are highly contradictory. Some studies have reported increased levels of SCFAs in the feces [11,12] and their positive correlation with body mass index (BMI) [13]. Other studies demonstrated that fecal SCFAs are decreased in patients with MAFLD and are negatively associated with body mass index (BMI) and waist circumference [14,15,16]. Therefore, the function of SCFAs in the human energy balance requires further investigation.
In this study, we analyzed the concentrations of SCFAs in the feces of MAFLD patients to better understand the protective function of these metabolites of the gut microbiota.
2. Results
None of the patients with MAFLD that were tested had normal fecal levels of total SCFAs: 24 were above normal and 18 were below normal. Based on these data, we divided the patients into a group with a total SCFA level above normal (the “Elevated” group) and a group with a total SCFA level below average (the “Decreased” group). The groups did not differ in age and sex distribution. Although patients with elevated levels of SCFA had a higher BMI, their serum levels of total cholesterol and LDL cholesterol and their uric acid levels were lower than those of patients with decreased levels of SCFAs. At the same time, there were no significant differences in the values of other biomarkers of metabolic syndrome (serum triglycerides and glucose levels) or in the serum levels of albumin, hemoglobin, C-reactive protein, and creatinine. The serum levels of bilirubin and gamma-glutamyl transferase and the platelet count were higher in patients with elevated fecal SCFA levels than in those with decreased levels. At the same time, in patients with elevated levels of SCFAs, the level of liver fibrosis—assessed by using the FIB-4 index and elastometry—was lower than that in patients with a decreased content of these acids in the feces (Table 1).

Table 1.
Main characteristics of patients with metabolic-associated liver disease with elevated and decreased fecal short-chain fatty acid (SCFA) levels.
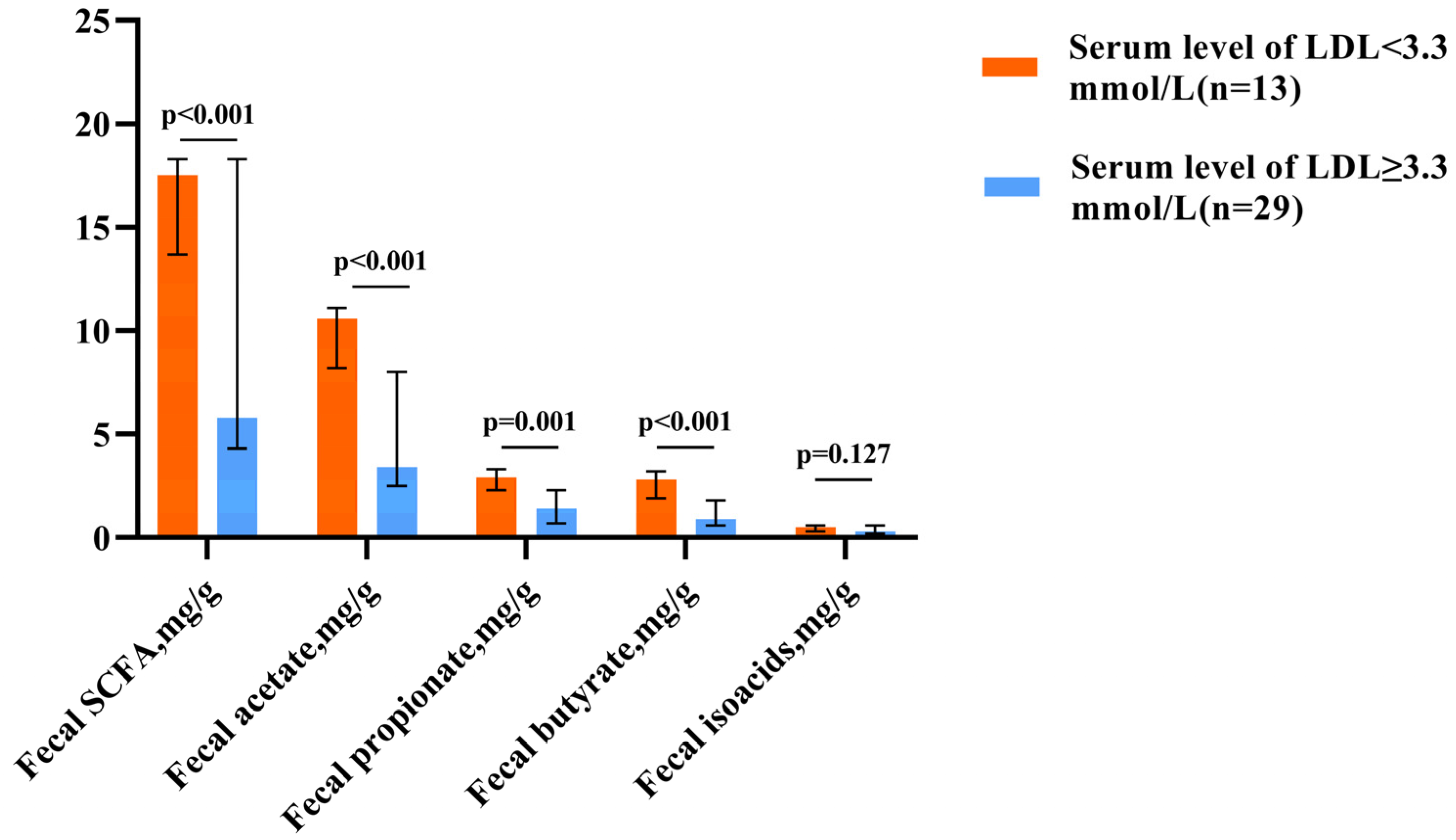
Hypercholesterolemia, which is defined as an LDL level above 3.3 mmol/L [17], was found in all patients with decreased fecal SCFA levels and only in 37.9% of patients with elevated levels (p < 0.001). Patients with hypercholesterolemia had lower SCFA levels than those of patients without this pathology (Figure 1).
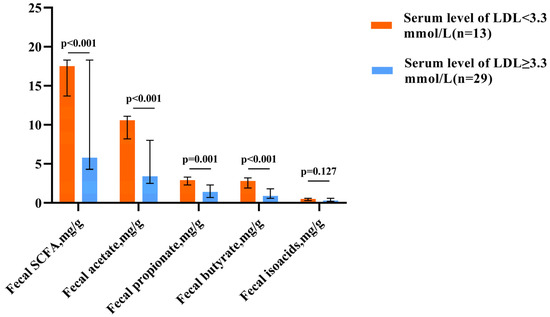
Figure 1.
Fecal SCFA levels in patients with normal and elevated LDL cholesterol levels. The column height shows the median and the bars indicate the interquartile range.
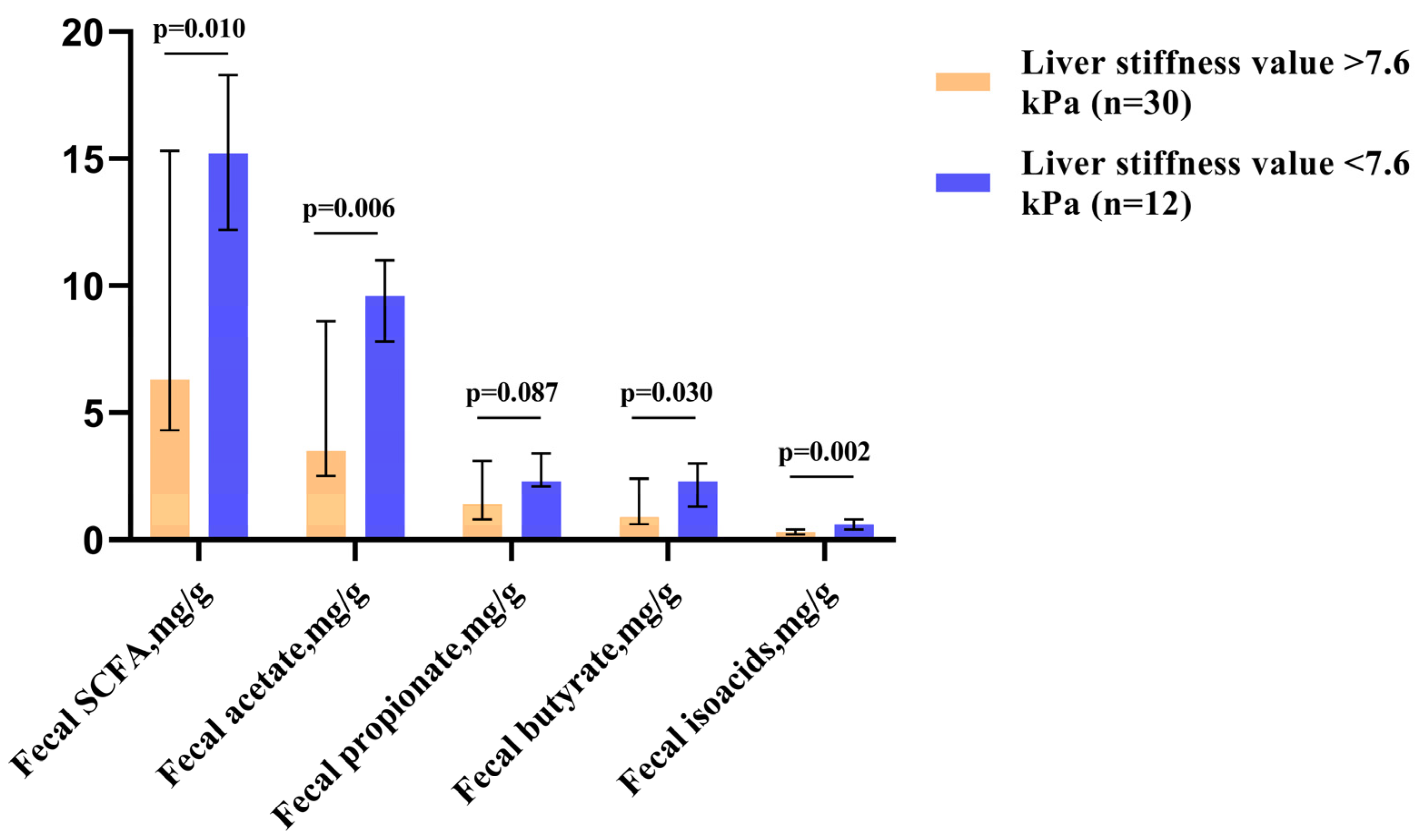
Among patients with elevated SCFA levels, patients with significant liver fibrosis (≥F2, which corresponded to liver stiffness values of >7.6 kPa [18]) accounted for 50.0%. In contrast, among those who had decreased SCFA levels in their feces, these patients accounted for 92.3% (p = 0.016). Patients with significant liver fibrosis had lower levels of fecal SCFAs—particularly acetate and butyrate (Figure 2).
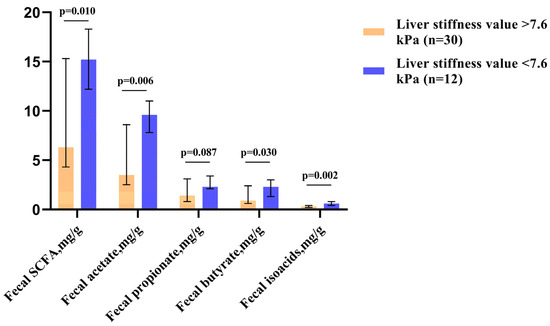
Figure 2.
Fecal SCFA levels in patients with significant liver fibrosis (≥F2, corresponding to liver stiffness values of >7.6 kPa) and without. The column height shows medians, and the bars indicate the interquartile range.
Fecal levels of SCFAs and their fractions were positively correlated with bilirubin levels, BMI, gamma-glutamyl transferase levels, and platelet count and negatively correlated with total cholesterol and LDL cholesterol levels and non-invasive indicators of liver fibrosis. These and other significant correlations are presented in Table 2.

Table 2.
Correlation matrix of fecal levels of short-chain fatty acids (SCFAs) and the main parameters of metabolic-associated fatty liver disease.
3. Discussion
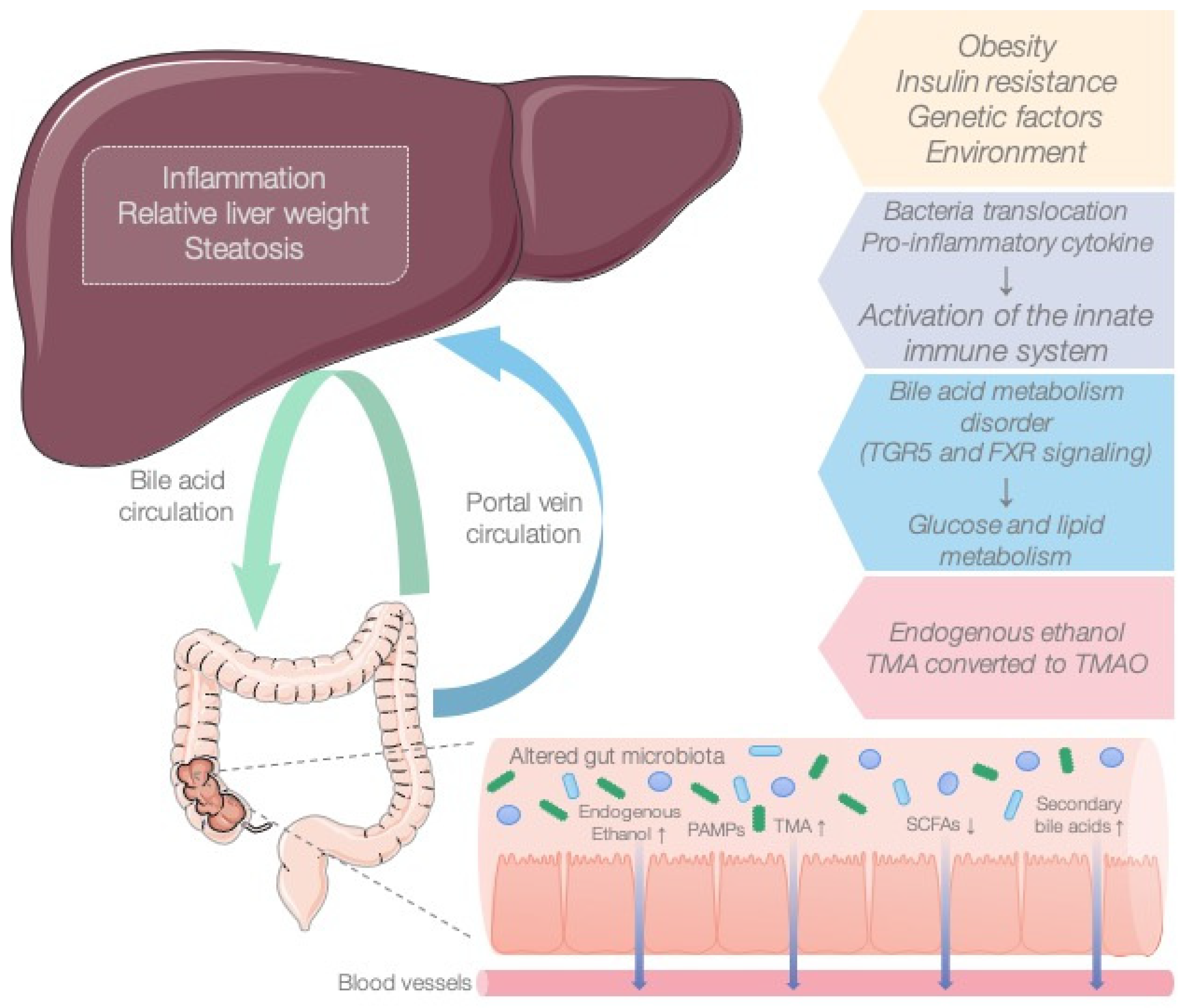
MAFLD is a global metabolic disease whose pathogenesis remains undefined, and effective therapeutic strategies are still lacking. In light of the concept of the gut–liver axis, gut microbiota have become an essential research topic in MAFLD. Numerous studies have demonstrated that the microbiota influence the onset and development of the disease (Figure 3) [19,20,21]. Bacterial metabolites such as SCFAs (acetate, propionate, butyrate) are the most common bacterial products derived from commensal bacterial fermentation of dietary fiber in the intestine. Their role in the development of MAFLD remains controversial. On the one hand, SCFAs provide the host with additional energy and play a key role in lipogenesis and gluconeogenesis. However, on the other hand, SCFAs increase satiety by activating free fatty acid receptors and preventing excess weight. Previously, we published a review in which we considered the association of SCFAs with the onset and progression of MAFLD [22,23].
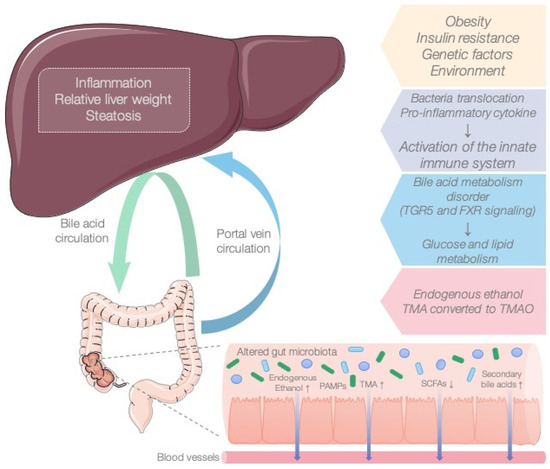
Figure 3.
The role of the microbiota in the pathogenesis of MAFLD.
In the current study, we found that patients with elevated levels of SCFAs had lower serum levels of total cholesterol, LDL cholesterol, and uric acid than patients with decreased SCFAs did. Nonetheless, these patients had a higher BMI and elevated serum levels of bilirubin, gamma-glutamyltransferase, and platelets in comparison with those with normal SCFAs. Hypercholesterolemia was found in all patients with normal fecal SCFAs but in only 37.9% of patients with elevated levels of these acids. In patients with hypercholesterolemia, the level of SCFAs was lower than that in patients without this pathology.
Our data confirm the results of studies that have found increased levels of SCFAs in obese individuals [13,24,25,26]. It has been reported that higher levels of SCFAs in feces are associated with a low diversity of microbiota, high intestinal permeability, and changes in the Firmicutes/Bacteroidetes ratio [13,24,25,26]. Backhed et al. discussed how changes in the metabolic activity of the microbiota may contribute to the progression of steatosis through inhibitory effects on regulators of peripheral lipid and glucose metabolism, such as AMP-activated protein kinase (AMPK) and angiopoietin-related protein 4 (ANGPTL4)/fasting-induced adipose factor (FIAF). The inhibition of FIAF results in increased lipoprotein lipase activity and fatty acid uptake into adipose tissue and the liver, whereas the inhibition of AMPK results in decreased peripheral fatty acid oxidation and adipose tissue accumulation [27]. Clinical and experimental studies have demonstrated that SCFAs can reduce serum levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol while increasing levels of GLP-1, PYY, and leptin [28,29,30].
To the best of our knowledge, we are the first to demonstrate that the level of liver fibrosis in patients with high SCFA levels—as measured using the FIB-4 index and elastography—was lower than that in patients with normal levels of these acids in the feces. A total of 50.0% of patients with elevated levels of SCFAs had significant liver fibrosis, whereas 92.3% of patients with decreased levels of SCFAs in the feces had significant liver fibrosis. Patients with significant liver fibrosis had lower levels of fecal SCFAs, particularly acetate.
SCFAs are quorum molecules that create dialogue between the microbiota and the host organism. Previously, SCFAs have been shown to reduce hepatic steatosis by activating AMP-activated protein kinase, inducing fatty acid oxidation gene expression, and inhibiting pro-inflammatory macrophage activation [31,32]. According to researchers, SCFAs also perform epigenetic regulation by inhibiting histone deacetylase and reducing the number of acetyl groups associated with chromatin, thereby preventing the progression of liver injury [33]. Another study showed that SCFAs can inhibit hepatic steatosis by activating the AMPK and PPAR signaling pathways and downregulating genes associated with lipid synthesis, such as sterol-regulatory-element-binding protein 1c (SREBP-1c), fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1), acetyl-CoA carboxylase-1 (ACC1), and liver X receptor (LXR) [34].
We hypothesize that the presence of the groups of MAFLD patients with different levels of SCFAs identified in our study may reflect different endotypes of MAFLD that can have different pathogeneses and different approaches to therapy. Further studies in a large patient population, including circulating and fecal SCFA studies, are needed to confirm our hypothesis.
The limitations of our study include the small number of patients, the study of only fecal SCFAs, and the lack of dietary analysis.
4. Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Sechenov University (No. 01-22 of 1 December 2022). This study was conducted from 10 December 2022 to 25 March 2023. The diagnosis of MAFLD was established based on a complex of clinical, laboratory, and instrumental data [35,36,37].
The criteria for inclusion for patients in this study were an age of 18–75 years and MAFLD.
All patients were tested for markers of viral hepatitis, autoimmune hepatitis, hemochromatosis (transferrin saturation with iron), and Wilson’s disease (indicators of copper metabolism) to rule out other causes of liver disease. MAFLD was diagnosed based on the presence of a fatty liver as measured via elastography, the absence of hepatotoxic alcohol consumption (<30 g/day for men and <20 g/day for women) [36], and the presence of symptoms of metabolic disorders (hyperglycemia and/or hyperlipidemia).
This study excluded patients who took drugs that affected the composition of the gut microbiota (probiotics, prebiotics, antibiotics, prokinetics, and PPIs) within the three months leading up to the start of this study, as well as those with concomitant diseases in which there was a change in the composition and metabolic function of the intestinal microbiota (except for diabetes mellitus, hypertension, and hyperuricemia in patients with MAFLD).
Of the total of 100 patients, 58 did not meet the criteria, due to alcoholic cirrhosis (n = 7), viral infection (n = 14), autoimmune disease (n = 9), mixed (n = 6) etiology, cancer (n = 7), and the use of drugs that affected the composition of the gut microbiota (n = 15).
We determined normal levels of SCFA in feces based on the results of the control group, which participated in our previously published study and was analyzed for the fecal SCFA level using the same method [38]. The control group consisted of healthy persons without complaints who visited our clinic for a prophylactic check-up. The control group (n = 20; age was 56 (52–59) years; male/female ratio was 9/11) did not consume drugs that affected the microbiota of the intestines. For the fecal SCFA level, the mean was 10.3 g/kg and the standard deviation was 0.65 g/kg, which made it possible to estimate the normal range, covering 95% of normal persons, to be 9.0–11.5 g/kg [38].
In all study participants, the absolute and relative content of acetic (C2), propionic (C3), and butyric (C4) acids; the level of isoacids (SCFA isomers); and the ratio of isoacids to unbranched acids (isoCn/Cn) were determined by using the following method. Fecal samples were collected from all study participants and kept at −80 °C until further analysis. After defrosting, a 0.1 g fecal sample was placed in a tube with a conical bottom; 2 mL of distilled water and 1 mL of calibration solution were added before it was mixed through shaking for 10 min. Further, 0.5 mL of 1 N HCl was added, and the mixture was then centrifuged at 5000 rpm for 10 min. Next, one microliter of supernatant was injected using a microsyringe into a Khromos GH-1000 gas chromatograph evaporator with a flame ionization detector equipped with a 36 m long quartz capillary column with an inner diameter of 0.32 mm and a stationary free fatty acid phase in the form of films that were 0.33 µm thick. The chromatograph operation mode was isothermal, with a thermostat temperature of 150 °C and an evaporator and detector temperature of 230 °C. The carrier gas was nitrogen, and the column inlet pressure was set to 1.8 atm. The carrier gas flow was 2.0 mL/min and the airflow was 300 mL/min. Chromatography took about 8 min. We determined the absolute content of individual acids in the mixture by calculating the areas of chromatographic peaks using the “triangle” method and by processing the chromatograms with a computer [39].
Liver stiffness and CAP measurements were determined using Fibroscan (iLivTouch FT 100, Wuxi Hisky Company, China) by experienced operators, and all patients were successively measured using both M and XL probes at the same measurement point. Liver tissue imaging was performed with the participant lying supine, with the right arm in maximum abduction. The tip of the probe transducer was placed on the skin between the rib bones at the level of the right lobe of the liver. The CAP is an average estimate of ultrasound attenuation at 3.5 MHz and is expressed in dB/m. The levels of CAP used to define the presence and degree of steatosis were as follows: the corresponding optimal cutoff values for >S0 (no steatosis), >S1 (mild steatosis), and >S2 (moderate steatosis) were 248, 268, and 280 dB/m, respectively, while a CAP score of >280 dB/m suggested S3 (severe steatosis). Liver stiffness was expressed by the median value (in kPa) of 10 measurements performed at depths between 25 and 65 mm. The cut-off value for defining the presence of fibrosis was a liver stiffness of >7.6 kPa [18,40,41,42].
We used non-invasive fibrosis scores (fibrosis-4 (FIB-4) index) to estimate the possibility of liver fibrosis. The FIB-4 index was calculated using the following formula: FIB-4 = (age × AST)/[PLT(×109/L) × (√ALT)]. Patients with an FIB-4 value of <1.30 were classified as having no or moderate fibrosis, while those with an FIB-4 value of >3.25 were defined as having extensive fibrosis or cirrhosis [37,38].
Statistical data were processed using the STATISTICA 10 software (StatSoft Inc., Tulsa OK, USA). The data are presented as the median [interquartile range]. The significance of differences between the two groups was assessed by using the Mann–Whitney method. Differences in categorical variables were determined by using Fisher’s exact test. Correlation analysis was performed by using the Spearman method. The differences were considered significant if the probability of making a Type I error was p < 0.05.
5. Conclusions
Two profiles of the metabolic activity of microbiota (with increased and decreased SCFA production) in patients with MAFLD were identified. These profiles were associated with different levels of liver fibrosis and serum cholesterol levels and may be correlated with the risk of progression of MAFLD to cirrhosis, hepatocellular carcinoma, and cardiovascular events. Further prospective studies are required to verify this hypothesis.
Author Contributions
Data curation, X.C. and R.M.; Writing—original draft, X.C., O.Z. and R.M.; Methodology, O.Z., E.P. and M.Z.; Visualization, M.R. and A.B.; Supervision, V.I.; Project administration, V.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Sechenov University (No. 01-22 of 1 December 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request from the corresponding author. The data are not publicly available because it is not recommended by the local ethics committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; Angelis, M.; Wang, H.; Di Palo, D.; Bonfrate, L.; Wang, D.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wei, W.; Tang, L.; Tian, Y.; Zhu, Y.; Luo, Y.; Liu, S. CONSORT-Characteristics and metabolic phenotype of gut microbiota in NAFLD patients. Medicine 2022, 101, e29347. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Lang, S.; Martin, A.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.; Krawczyk, M.; Nowag, A.; Scholz, C.; Kretzschmar, A.; et al. Phenotyping non-alcoholic fatty liver disease by the gut microbiota: Ready for prime time? J. Gastroenterol. Hepatol. 2020, 35, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Liu, Y.Y.; Lin, C.C.; Wang, C.C.; Wu, Y.J.; Yong, C.C.; Chen, K.D.; Chuah, S.K.; Yao, C.C.; Huang, P.Y.; et al. Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan. Nutrients 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fan, J.-G. Microbial metabolites in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef]
- May, K.S.; Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.; Chen, X.; Wang, J.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Sun, L.; Zhang, W. The Molecular and Mechanistic Insights Based on Gut–Liver Axis: Nutritional Target for Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement. Int. J. Mol. Sci. 2020, 21, 3066. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo, B.A.; Vendemiale, G. Hepatic Mitochondria-Gut Microbiota Interactions in Metabolism-Associated Fatty Liver Disease. Metabolites 2023, 13, 322. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Howard, A.G.; Meyer, K.A.; Tsilimigras, M.C.B.; Avery, C.L.; Sha, W.; Sun, S.; Zhang, J.; Su, C.; et al. Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults. Nutrients 2020, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Q.; An, Y.X.; Yu, C.G.; Ke, J.; Zhao, D.; Yu, K. The Association Between Fecal Short-Chain Fatty Acids, Gut Microbiota, and Visceral Fat in Monozygotic Twin Pairs. Diabetes, Metab. Syndr. Obesity Targets Ther. 2022, 15, 359–368. [Google Scholar] [CrossRef]
- Miranda, V.P.N.; Dos Santos Amorim, P.R.; Bastos, R.R.; de Faria, E.R.; de Castro Moreira, M.E.; do Carmo Castro Franceschini, S.; do Carmo Gouveia Peluzio, M.; de Luces Fortes Ferreira, C.L.; Priore, S.E. Abundance of Gut Microbiota, Concentration of Short-Chain Fatty Acids, and Inflammatory Markers Associated with Elevated Body Fat, Overweight, and Obesity in Female Adolescents. Mediat. Inflamm. 2019, 2019, 7346863. [Google Scholar] [CrossRef]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Zeng, K.Y.; Bao, W.Y.; Wang, Y.H.; Liao, M.; Yang, J.; Huang, J.Y.; Lu, Q. Non-invasive evaluation of liver steatosis with imaging modalities: New techniques and applications. World J. Gastroenterol. 2023, 29, 2534–2550. [Google Scholar] [CrossRef]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef]
- Reshetova, M.; Zolnikova, O.; Ivashkin, V.T.; Ivashkin, K.; Appolonova, S.A.; Lapina, T.L. Gut Microbiota and its Metabolites in Pathogenesis of NAFLD. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Zolnikova, O.; Reshetova, M.S.; Ivanova, M.N.; Ivashkin, V.T. Metabolomic profiles as a new understanding of disease processes. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 46–52. [Google Scholar] [CrossRef]
- La Cuesta-Zuluaga, D.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef]
- Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Diet-Related Changes of Short-Chain Fatty Acids in Blood and Feces in Obesity and Metabolic Syndrome. Biology 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef]
- Iván, J.; Major, E.; Sipos, A.; Kovács, K.; Horváth, D.; Tamás, I.; Bay, P.; Dombrádi, V.; Lontay, B.; Smolková, K.; et al. The short–chain fatty acid propionate inhibits adipogenic differentiation of human chorion–derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef]
- Aguilar, E.C.; da Silva, J.F.; Navia-Pelaez, J.M.; Leonel, A.J.; Lopes, L.G.; Menezes-Garcia, Z.; Ferreira, A.V.M.; Capettini, L.d.S.A.; Teixeira, L.G.; Lemos, V.S.; et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR–gamma in obese Apo E knockout mice. Nutrition 2018, 47, 75–82. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Albillos, A.; Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Campisano, S.; Colla, A.L.; Echarte, S.M.; Chisari, A.N. Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases. Nutr. Res. Rev. 2019, 32, 128–145. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, F.; Strappe, P.; Liu, W.; Zheng, J.; Zhou, Z.; Zhang, Y. Microbiota fermentation characteristics of acylated starches and the regulation mechanism of short-chain fatty acids on hepatic steatosis. Food Funct 2021, 12, 8659–8668. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef]
- Associazione Italiana per lo Studio del Fegato (AISF); Società Italiana di Diabetologia (SID); Società Italiana dell’Obesità (SIO); Members of the guidelines panel; Coordinator; AISF Members; SID Members; SIO Members; Metodologists. Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Dig. Liver Dis. 2022, 54, 170–182. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Maevskaya, M.V.; Zharkova, M.S.; Kotovskaya, Y.V.; Tkacheva, O.N.; Troshina, E.A.; Shestakova, M.V.; Maev, I.V.; Breder, V.V.; Gheivandova, N.I.; et al. Clinical Practice Guidelines of the Russian Scientific Liver Society, Russian Gastroenterological Association, Russian Association of Endocrinologists, Russian Association of Gerontologists and Geriatricians and National Society for Preventive Cardiology on Diagnosis and Treatment of Non-Alcoholic Liver Disease. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 104–140. [Google Scholar] [CrossRef]
- Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites 2023, 13, 859. [Google Scholar] [CrossRef]
- Sdwd. Available online: https://patents.google.com/patent/RU2220755C1/ru (accessed on 1 January 2021).
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; Cardoso, A.C.; et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: An individual patient data meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.D.; Hooker, J.; Tu, X.; Bettencourt, R.; et al. Magnetic Resonance vs Transient Elastography Analysis of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).