Abstract
(1) Background: Lower gastrointestinal bleeding (LGIB) accounts for 20% of all gastrointestinal bleeds. LGBI originates in the colon, rectum, and anus, mainly in patients who are receiving antiaggregant or anticoagulant treatment. The major causes are diverticular disease, colitis, hemorrhoids, and angiodysplasia. The literature studies underline that Direct Oral Anticoagulants (DOACs) are effective in reducing the risk of thromboembolic events but are associated with a higher risk of lower gastrointestinal bleeding (LGIB), particularly lower hemorrhoid bleeding. (2) Methods: The aim of our review is to revise the risk of hemorrhoid bleeding, pathophysiology, and management in patients taking DOACs in light of the most modern evidence. (3) Conclusions: central to the management of hemorrhoid bleeding in patients receiving DOAC therapy is the consideration of a tailored approach that respects the delicate equilibrium between the need for thromboembolic prophylaxis and the potential for bleeding complications. Cessation of anticoagulation, if clinically feasible, constitutes a fundamental cornerstone in the control of hemorrhage. This pause in therapy aims to mitigate the exacerbation of bleeding risk while offering a window for the implementation of local measures to manage hemorrhoid bleeding.
1. Introduction
In recent years, the medical landscape has witnessed a paradigm shift in the management of thromboembolic events with the emergence of Direct Oral Anticoagulants (DOACs). These agents, including both direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) and a direct thrombin inhibitor (dabigatran), have swiftly gained recognition as indispensable therapeutic options for patients with atrial fibrillation, deep vein thrombosis, and pulmonary embolism. The allure of DOACs stems from their favorable pharmacokinetic profiles, predictable anticoagulant responses, and reduced interactions with dietary factors compared to traditional vitamin K antagonists like warfarin. These attributes have translated into enhanced patient adherence and a reduction in the need for stringent monitoring, revolutionizing the landscape of anticoagulation therapy [1,2].
However, as with any medical advancement, the introduction of DOACs has necessitated a meticulous evaluation of their benefits against potential drawbacks. Foremost among these concerns is the risk of bleeding, a phenomenon intricately linked to the inherent anticoagulant properties of these agents. While DOACs indisputably demonstrate efficacy in lowering the risk of thromboembolic events, the perturbation of the coagulation cascade places patients at an increased vulnerability to bleeding events. This concern is underscored by the recognition that bleeding complications, when they arise, can impact patient outcomes, quality of life, and healthcare resources [1,2,3].
One facet of bleeding that warrants focused consideration is gastrointestinal (GI) bleeding, of which hemorrhoid bleeding constitutes a significant subset. Hemorrhoids, vascular structures situated within the anal canal, possess a unique susceptibility to bleeding due to their rich vascular supply and location. The interaction between DOAC therapy and hemorrhoid bleeding carries clinical implications that extend beyond the realm of anticoagulation, involving the delicate balance between hemostasis and vascular integrity. Consequently, a comprehensive understanding of the risk factors, pathophysiology, and management strategies associated with hemorrhoid bleeding in the context of DOAC administration is imperative.
In light of this background, this review aims to provide a comprehensive exploration of the intricate relationship between DOACs and hemorrhoid bleeding. By delving into the multifaceted interplay of pharmacology, vascular biology, and clinical management, we seek to enhance our understanding of the challenges posed by hemorrhoid bleeding in patients undergoing DOAC therapy. In doing so, we hope to facilitate informed clinical decision making, improve patient care, and contribute to the ongoing refinement of anticoagulation strategies in an era dominated by the remarkable therapeutic advances of DOACs.
2. Results
2.1. Risk of Hemorrhoid Bleeding with DOACs
The increased risk of GI bleeding with DOACs is caused primarily by three processes. The topical anticoagulant impact comes first, followed by mucosal healing inhibition, and ultimately by direct caustic action.
All DOACs are P glycoprotein (P-gp) substrates, a protein that governs both their absorption in the intestine and their distribution in the body. As a result, active DOAC medication concentrations may be observed in the lower gastrointestinal tract, in contrast to the absorption and excretion paths of VKAs and antiplatelet drugs. Moreover, in dabigatran etexilate, the tartaric acid can cause direct caustic injury [4,5].
DOACs have fewer drug–drug interactions than warfarin, although drugs that inhibit P-gp and/or CYP3A4 may increase the plasma concentration of the DOAC, which could result in increased bleeding risk.
The dosing of DOACs is another point to take into account for the risk of GI bleeding. For example, if we compare two Xa inhibitors such as rivaroxaban and apixaban, both administered in active form and with similar bioavailability, we would observe a different risk of GIB; in particular, the once-daily dosing of rivaroxaban may be related to the higher peak level of than twice-daily dosing of apixaban [2].
The risk of hemorrhoid bleeding with DOACs is dependent on the dose and duration of therapy, as well as patient factors such as age, comorbidities, and concomitant medications [6].
Several studies have examined the risk of hemorrhoid bleeding in patients taking DOACs [5,6,7].
A meta-analysis of randomized controlled trials (RCTs) comparing DOACs to placebo or no treatment found that DOACs were associated with an increased risk of GI bleeding, including hemorrhoid bleeding, compared to placebo or no treatment (odds ratio [OR] 1.46, 95% confidence interval [CI] 1.14–1.87) [3]. The risk of GI bleeding was highest in patients taking dabigatran (OR 1.92, 95% CI 1.45–2.53) and rivaroxaban (OR 1.46, 95% CI 1.02–2.08), compared to placebo or no treatment [8]. However, there was no significant difference in the risk of GI bleeding between patients taking apixaban and placebo or no treatment (OR 1.13, 95% CI 0.80–1.60) [9].
Another study found that patients taking DOACs had a higher incidence of hemorrhoid bleeding compared to those taking warfarin, a traditional oral anticoagulant [10]. The study included 15,189 patients with atrial fibrillation and found that the incidence of hemorrhoid bleeding was 1.1% per year in patients taking DOACs, compared to 0.6% per year in patients taking warfarin (hazard ratio [HR] 1.97, 95% CI 1.55–2.49) [11]. The risk of hemorrhoid bleeding was highest in patients taking dabigatran (HR 2.51, 95% CI 1.73–3.64) and rivaroxaban (HR 2.17, 95% CI 1.47–3.22), compared to warfarin [11].
A retrospective study [12] performed on 658 patients who take DOAC for non-valvular atrial fibrillation showed, in a 4-year time interval, that the most common causes of lower gastrointestinal bleeding were telangiectasia and hemorrhoid, which occurred relatively early after starting DOAC and were not clinically serious. In a multivariate analysis, the clinical factors relating to LGIB were the concomitant use of NSAIDs and dual antiplatelet.
In an interesting paper, researchers evaluated the different characteristics of GI bleeding in patients taking oral anticoagulants (VKA or DOACs) or antiplatelet drugs. The authors showed that hemorrhoid bleeding was considerably more common in patients who took DOAC compared to those who took VKA or an antiplatelet drug (33 vs. 10 vs. 8.7%, respectively) [13].
2.2. Pharmacokinetic Difference among DOACs
DOACs have different pharmacokinetic profiles, which can impact their clinical use. Dabigatran has a poor oral bioavailability (~6.5%); requires acidic environment for absorption and is hence formulated with tartaric acid; and the time to peak concentration is 1–2 h after ingestion with low plasma protein binding (~35%).
It is not metabolized by CYP450 enzymes and has an elimination half-life between 12 and 17 h. The excretion is primarily renal (80% unchanged in urine).
Rivaroxaban has a high oral bioavailability (~80–100% with food); is taken with meals to improve absorption with a time to peak concentration between 2 and 4 h after ingestion; and has moderate plasma protein binding (~92–95%).
It is metabolized by CYP3A4, CYP2J2, and other CYP-independent mechanisms with an elimination half-life of 5–9 h (younger patients), 11–13 h (elderly). Approximately 66% was eliminated via renal (36% unchanged) and fecal/biliary routes.
Apixaban has a good oral bioavailability (~50%), with a time to peak concentration of 3–4 h after ingestion. It has high plasma protein binding (~87%), and it is metabolized mainly by CYP3A4. The elimination half-life is between 8 and 15 h, with excretion occurring at approximately 27% through kidney, while the rest occurs via fecal/biliary routes.
Edoxaban has a good oral bioavailability (~62%), with a time to peak concentration of around 1–2 h after ingestion, with low plasma protein binding (~55%). It shows a minimal metabolism via CYP3A4, and it is primarily metabolized by hydrolysis and conjugation. The elimination half-life is 10–14 h. Approximately 50% is excreted by renal, while the rest is excreted via fecal/biliary routes.
The following summarizes the key differences among DOACs: 1. Bioavailability: Dabigatran has the lowest, while rivaroxaban is almost fully absorbed with food; 2. Peak concentration time: Similar across DOACs, but slightly longer for apixaban; 3. Plasma protein binding: Highest for apixaban and rivaroxaban, lowest for dabigatran and edoxaban; 4. Metabolism: Rivaroxaban and apixaban are heavily dependent on CYP3A4, while dabigatran is not metabolized by CYP450 enzymes; 5. Half-life: Longest for dabigatran and apixaban, shortest for rivaroxaban in younger patients; 6. Excretion: Dabigatran is mostly renal, while the others have a more balanced renal and fecal/biliary excretion.
These differences can influence the choice of DOAC depending on the patient’s renal function, risk of gastrointestinal issues, and potential for drug–drug interactions [14,15,16,17,18].
DOACs can interact with various drugs, which may affect their efficacy and safety. A detailed overview is presented below (Table 1) of significant drug interactions for the commonly used DOACs: dabigatran, rivaroxaban, apixaban, and edoxaban [18,19].

Table 1.
Pharmacologic interactions between DOACs and other medications.
2.3. Pathophysiology of Hemorrhoids
Hemorrhoids, intricate vascular structures nestled within the anal canal, fulfill a pivotal role in the maintenance of bowel continence and overall anorectal function. Comprising a complex amalgamation of arteriovenous channels, resilient connective tissue, and contractile smooth muscle elements, hemorrhoids are indispensable components of the intricate anatomy of the anal region [20]. The categorization of hemorrhoids into internal or external variants hinges upon their specific positioning relative to the dentate line, a demarcation of functional significance.
Internal hemorrhoids, strategically situated proximal to the dentate line, are enshrouded by a protective layer of mucosa. This arrangement safeguards these vascular entities from the potential friction and mechanical stress associated with stool passage. In contrast, external hemorrhoids take up residence distal to the dentate line, encompassed by the more robust protective covering of skin. This positioning exposes them to the dynamic interactions of the external environment, potentially influencing their susceptibility to inflammation and related sequelae [21].
For practical purposes, internal hemorrhoids are further classified based on their appearance and degree of prolapse, according to Goligher’s classification: First-degree hemorrhoids (Grade I): the anal cushions bleed but do not prolapse; Second-degree hemorrhoids (Grade II): the anal cushions prolapse through the anus during straining but spontaneously reduce; Third-degree hemorrhoids (Grade III): the anal cushions prolapse through the anus during straining or exertion and require manual reduction into the anal canal; Fourth-degree hemorrhoids (Grade IV): the prolapse remains external at all times and is irreducible [22].
Within the intricate factors’ interplay contributing to hemorrhoidal health, the specter of inflammation and engorgement looms. Inflammatory processes can precipitate hemorrhoidal swelling, ultimately culminating in the distressing phenomenon of hemorrhoid bleeding.
Central to the pathophysiology lies the notion of increased venous pressure, a key protagonist in the hemorrhoidal bleeding risk. Straining during bowel movements, prolonged periods of sitting, and the physiological demands of pregnancy can all contribute to elevate venous pressure within the delicate vascular networks of the anal canal. This increase in pressure sets the stage for venous stasis, where the typically efficient venous return from these vascular beds becomes compromised. Venous stasis, in turn, can be attributed to the relaxation of the smooth muscle tonicity in the walls of the hemorrhoidal veins. This intricate balance between muscle tone and vascular dynamics can be perturbed by a myriad of factors, ranging from lifestyle choices to underlying medical conditions [23,24,25]. Particularly regarding increased venous pressure, it is worth mentioning that contrary to previous teachings, the incidence of hemorrhoid disease in patients with liver cirrhosis complicated by portal hypertension is similar to that in the general population. However, rectal varices, which result from porto-systemic communication via the hemorrhoidal veins, are common in patients with portal hypertension. Despite this, bleeding from rectal varices is rare, contributing to less than 1% of massive bleeding cases in portal hypertension. When such bleeding occurs, it is typically treated with portal decompression. For this subset of hepatopathic patients and concomitant atrial fibrillation with an indication for anticoagulation, the American Gastroenterological Association (AGA) suggests using anticoagulation over no anticoagulation. However, particularly for those patients with more advanced cirrhosis (Child-Turcotte-Pugh class C) and/or low CHA2DS2-VASC scores, when clinical conditions impose higher value on avoiding the bleeding risk on anticoagulation and lower value on the stroke reduction, no anticoagulation could reasonably be chosen [26].
Trauma forms yet another thread in the tapestry of hemorrhoidal pathophysiology. Trauma, in this context, signifies mechanical insults that can befall these delicate structures. Constipation, characterized by the arduous passage of hardened stool, and its converse—diarrhea—can both impose mechanical stresses on hemorrhoids. Furthermore, the strain of repeated anal intercourse can contribute to the trauma equation, exacerbating the vulnerability of these vascular entities to inflammation and, consequently, hemorrhoid bleeding [23].
Intriguingly, the nexus between hemorrhoid bleeding and the use of DOACS has emerged as an area of interest. DOACs, heralded for their targeted interference with key components of the coagulation cascade—be it thrombin or factor Xa—bear the paradoxical duality of offering anticoagulation benefits while simultaneously introducing the risk of bleeding. This enhanced risk of hemorrhoid bleeding in individuals on DOAC therapy finds its roots in the very mechanism that renders these agents efficacious. The inhibition of specific clotting factors inherently disrupts the hemostatic equilibrium, engendering a scenario in which the propensity for bleeding, including hemorrhoid bleeding, is amplified in this subset of patients [6].
In summation, the intricate orchestration of vascular dynamics, inflammation, and coagulation underscores the enigmatic realm of hemorrhoidal pathophysiology. The interaction between these facets is pivotal in comprehending the development of hemorrhoid bleeding, an entity further complicated by the administration of DOACs.
2.4. Management of Hemorrhoid Bleeding in Patients Taking DOACs
The management of hemorrhoid bleeding in patients taking DOACs (Figure 1) is similar to that in patients taking traditional oral anticoagulants, such as warfarin. The most important difference between the two classes is that DOACs have a fast onset of action and a short half-life. Both properties simplify treatment initiation, as well as the interruption of oral anticoagulation if needed.
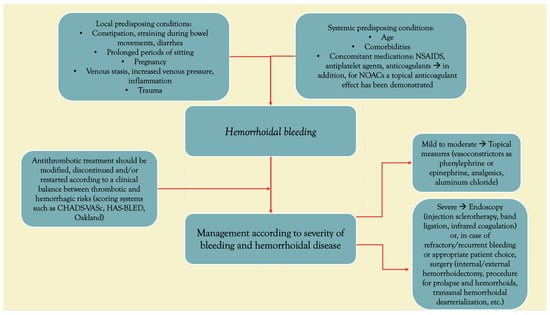
Figure 1.
Hemorrhoidal bleeding: schematic illustration of risk factors and management.
The goals of management include controlling bleeding, preventing recurrence, and minimizing the risk of thromboembolic events [7].
Initial management of hemorrhoid bleeding in patients taking DOACs should include cessation of the anticoagulant therapy, if possible. The decision to discontinue anticoagulation should be based on the severity of the bleeding and the patient’s risk of thromboembolic events. Diseases of the cardiovascular system, including those of a thromboembolic nature, constitute a serious public health problem because they are one of the leading causes of morbidity and mortality [27]. Therefore, in patients with severe bleeding and a high risk of thromboembolic events, anticoagulation may need to be continued or replaced with a different anticoagulant, such as unfractionated heparin or low-molecular-weight heparin (LMWH) [28,29].
In patients with mild to moderate bleeding, local measures may be sufficient to control the bleeding. These measures include the use of topical vasoconstrictors, such as phenylephrine or epinephrine, and topical or oral analgesics. Topical astringents, such as aluminum chloride, may also be used to help control bleeding.
In patients with severe bleeding, endoscopic treatment may be necessary to control the bleeding. Endoscopic treatment options include injection sclerotherapy, rubber band ligation, or infrared coagulation. These procedures can be performed safely in patients taking DOACs with appropriate monitoring and reversal agents available if needed [13,25,30,31,32].
The decision to restart anticoagulation after an episode of hemorrhoid bleeding should be based on the severity of the bleeding and the patient’s risk of thromboembolic events [33]. In patients with severe bleeding or a high risk of thromboembolic events, anticoagulation may need to be delayed or restarted at a lower dose. In patients with mild to moderate bleeding, anticoagulation can be restarted after the bleeding has resolved. For instance, due to the increasing thromboembolic risk over time, for patients who were under vitamin-K antagonist, it is advisable to restart warfarin as soon as possible from day 7 onward after its interruption. For patients with a high thrombotic risk, cardiology societies recommend resuming anticoagulation, rapidly titrating from prophylactic doses to therapeutic doses of low-molecular-weight heparin within 48–72 h [28,34].
The use of reversal agents may be necessary in patients taking DOACs who experience severe hemorrhoid bleeding or require urgent surgery [35]. Reversal agents for DOACs include idarucizumab for dabigatran, andexanet alfa for factor Xa inhibitors, and ciraparantag for all DOACs [36,37]. These agents can rapidly reverse the anticoagulant effect of DOACs, allowing for prompt control of bleeding or the safe performance of surgery.
A digital rectal examination (DRE) is a valuable part of a complete physical examination, but it is not routinely performed before starting DOAC specifically to check for hemorrhoids. However, DRE is indicated in patients who present with anal or peri-anal pain, rectal bleeding, anorectal mass, pruritus, incontinence, constipation, or weight loss. If disease in the anal canal is suspected, anoscopy can be used for further examination. DOACs are primarily prescribed for their anticoagulant effects, and DRE is not a standard prerequisite for their initiation. If a patient has specific symptoms or risk factors related to hemorrhoids, a DRE may be considered, but it is not universally required before starting DOACs [38].
Calculating the HAS-BLED score is important for assessing the risk of bleeding in patients with atrial fibrillation who take DOACs, including those with comorbid conditions such as hemorrhoids. The HAS-BLED score helps guide clinicians in balancing the risks and benefits of anticoagulation therapy. According to the results, we can distinguish low risk (0–1): generally safe to initiate anticoagulation with appropriate monitoring; moderate risk (2–3): caution is warranted; closer monitoring is needed; and high risk (≥4): high risk of bleeding; alternative strategies or more intensive monitoring may be required [39].
For a patient with hemorrhoids, the decision to anticoagulate should be carefully weighed against the bleeding risk. Minor hemorrhoidal bleeding typically does not contraindicate anticoagulation but requires monitoring and potentially addressing hemorrhoidal symptoms to reduce bleeding risk.
Diosmin exhibits multiple mechanism of action, in particular the improvement of venous tone, the increased lymphatic drainage, the protection of capillary bed microcirculation, the inhibition of inflammatory reactions, and finally the reduction in capillary permeability. Diosmin exerts its phlebotonic effect by prolonging the vasoconstrictor effect of norepinephrine on the vein wall, enhances venous tone, and thus reduces distensibility and stasis [40].
In a placebo-controlled trial, authors reported the efficacy of micronized diosmin in reducing the duration and/or intensity of individual symptoms of grade 1 or 2 acute internal hemorrhoids compared to placebo. In all, the efficacy of diosmin is well established in attenuating and treating the symptoms of hemorrhoids [41].
3. Discussion
The advent of DOACs has undoubtedly reshaped the landscape of anticoagulation therapy, offering a compelling alternative to traditional agents for the prevention of thromboembolic events. These agents, characterized by their targeted interference with pivotal components of the coagulation cascade, have heralded a new era of anticoagulant pharmacotherapy. However, this remarkable progress is not devoid of considerations, particularly when it comes to the critical balance between their therapeutic benefits and the associated risk of bleeding, hemorrhoid bleeding being no exception [42,43].
The comprehensive assessment of the interplay between DOACs and hemorrhoid bleeding is a complex task that draws upon the amalgamation of pharmacology, patient characteristics, and clinical context. A salient facet in this matter is the realization that the risk of hemorrhoid bleeding in the realm of DOAC administration is not uniformly distributed. Rather, it is shaped by a constellation of factors, including the dose and duration of therapy and the intricate interplay of patient variables such as age, comorbidities, and concomitant medications, which collectively define the individual’s hemorrhagic susceptibility.
Central to the management of hemorrhoid bleeding in patients receiving DOAC therapy is the consideration of a tailored approach that respects the delicate equilibrium between the need for thromboembolic prophylaxis and the potential for bleeding complications. Cessation of anticoagulation, if clinically feasible, constitutes a fundamental cornerstone in the control of hemorrhage. This pause in therapy aims to mitigate the exacerbation of bleeding risk while offering a window for the implementation of local measures designed to curtail hemorrhoid bleeding. These local measures, encompassing an array of conservative interventions, such as topical agents, dietary adjustments, and meticulous hygiene practices, aim to address mild to moderate bleeding episodes, striking a balance between bleeding control and anticoagulation continuity.
However, the spectrum of hemorrhoidal bleeding is not always amenable to conservative interventions alone. In cases where the bleeding assumes a more severe form, endoscopic interventions may emerge as imperative tools in the clinician’s arsenal. The judicious deployment of endoscopic treatments seeks to staunch hemorrhagic torrents, restore hemostasis, and ultimately, avert the repercussions of unchecked bleeding.
The decision to recommence anticoagulation following hemorrhoid bleeding is an intricate deliberation that hinges on the fine equilibrium between the urgency of thromboembolic prevention and the lingering specter of bleeding complications. The severity of the hemorrhage, the patient’s inherent thromboembolic risk, and their overall clinical stability collectively steer the decision-making process, guiding the optimal timing for anticoagulation resumption.
In conclusion, the trajectory of DOACs and their intertwining with hemorrhoid bleeding weaves a narrative replete with therapeutic potential and clinical challenges. The clinician’s compass in this journey lies in understanding the multifaceted determinants of hemorrhoidal bleeding within the context of DOAC therapy. By embracing a patient-centric approach that synergizes evidence-based interventions with individualized care, we navigate the realm in which thromboembolic risk and bleeding vulnerability coalesce, striving for optimal patient outcomes in an era defined by the dynamic interplay of anticoagulation innovation and clinical pragmatism.
4. Materials and Methods
In this narrative review, we analyzed studies published in PubMed® (8600 Rockville Pike Bethesda, MD 20894, USA), UpToDate® (34 Washington Street, Wellesley, MA 02481, USA), Web of Science® (1500 Spring Garden Philadelphia, PA 19130, USA), and Cochrane® (The Atrium Southern Gate, Chichester, West Sussex, PO19 8AQ, UK) about the risk of hemorrhoidal bleeding in patients under DOACs and its management.
Author Contributions
Conceptualization, V.O. and C.P.; methodology, A.S. and M.B.; validation, V.O., C.P. and A.M.; resources, A.S. and C.P.; data curation, C.P.; writing—original draft preparation, M.C., A.S., C.P. and A.M.; writing—review and editing, V.O., M.B. and L.L.M.; supervision, V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Altiok, E.; Marx, N. Oral Anticoagulation. Dtsch. Arztebl. Int. 2018, 115, 776–783. [Google Scholar] [CrossRef]
- Cheung, K.S.; Leung, W.K. Gastrointestinal bleeding in patients on novel oral anticoagulants: Risk, prevention and management. World J. Gastroenterol. 2017, 23, 1954–1963. [Google Scholar] [CrossRef]
- Xu, Y.; Siegal, D.M. Anticoagulant-associated gastrointestinal bleeding: Framework for decisions about whether, when and how to resume anticoagulants. J. Thromb. Haemost. 2021, 19, 2383–2393. [Google Scholar] [CrossRef]
- Grymonprez, M.; Vanspranghe, K.; Capiau, A.; Boussery, K.; Steurbaut, S.; Lahousse, L. Impact of P-glycoprotein and/or CYP3A4-interacting drugs on effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 3039–3051. [Google Scholar] [CrossRef]
- Saviano, A.; Brigida, M.; Petruzziello, C.; Candelli, M.; Gabrielli, M.; Ojetti, V. Gastrointestinal Bleeding Due to NOACs Use: Exploring the Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 13955. [Google Scholar] [CrossRef]
- Benamouzig, R.; Guenoun, M.; Deutsch, D.; Fauchier, L. Review Article: Gastrointestinal Bleeding Risk with Direct Oral Anticoagulants. Cardiovasc. Drugs Ther. 2022, 36, 973–989. [Google Scholar] [CrossRef]
- Drezdzon, M.K.; Peterson, K.J. Evaluation and Management of Lower GI Bleeding. Dis. Colon. Rectum. 2022, 65, 785–788. [Google Scholar] [CrossRef]
- Elwood, P.C.; Morgan, G.; Galante, J.; Chia, J.W.; Dolwani, S.; Graziano, J.M.; Kelson, M.; Lanas, A.; Longley, M.; Phillips, C.J.; et al. Systematic Review and Meta-Analysis of Randomised Trials to Ascertain Fatal Gastrointestinal Bleeding Events Attributable to Preventive Low-Dose Aspirin: No Evidence of Increased Risk. PLoS ONE 2016, 11, e0166166. [Google Scholar] [CrossRef]
- Caldeira, D.; Barra, M.; Ferreira, A.; Rocha, A.; Augusto, A.; Pinto, F.J.; Costa, J.; Ferreira, J.J. Systematic review with meta-analysis: The risk of major gastrointestinal bleeding with non-vitamin K antagonist oral anticoagulants. Aliment. Pharmacol. Ther. 2015, 42, 1239–1249. [Google Scholar] [CrossRef]
- Chen, J.; Bi, G.; Wu, F.; Qin, X. Direct oral anticoagulants versus standard anticoagulation in children treated for acute venous thromboembolism. Pediatr. Res. 2022, 93, 1491–1498. [Google Scholar] [CrossRef]
- Osumi, S.; Abe, K.; Arizumi, T.; Watari, Y.; Kozuma, K.; Aiso, M.; Asaoka, Y.; Kodashima, S.; Yamamoto, T.; Tanaka, A. Low prevalence of colonic mucosal injury and bleeding in patients taking direct oral anticoagulants for non-valvular atrial fibrillation. Int. J. Clin. Pharmacol. Ther. 2021, 59, 662–667. [Google Scholar] [CrossRef]
- Maruyama, K.; Yamamoto, T.; Aoyagi, H.; Isono, A.; Abe, K.; Kodashima, S.; Kita, H.; Watari, Y.; Kozuma, K. Difference between the Upper and the Lower Gastrointestinal Bleeding in Patients Taking Nonvitamin K Oral Anticoagulants. Biomed. Res. Int. 2018, 2018, 7123607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pannach, S.; Goetze, J.; Marten, S.; Schreier, T.; Tittl, L.; Beyer-Westendorf, J. Management and outcome of gastrointestinal bleeding in patients taking oral anticoagulants or antiplatelet drugs. J. Gastroenterol. 2017, 52, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 216210, Dabigatran. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dabigatran (accessed on 24 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9875401, Rivaroxaban. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rivaroxaban (accessed on 24 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10280735, Edoxaban. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Edoxaban (accessed on 24 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10182969, Apixaban. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Apixaban (accessed on 24 June 2024).
- Hindley, B.; Lip, G.Y.H.; McCloskey, A.P.; Penson, P.E. Pharmacokinetics and pharmacody-namics of direct oral anticoagulants. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 911–923. [Google Scholar] [CrossRef]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.H.; Miletello, M.; et al. Drug Interactions Affecting Oral Anticoagulant Use. Circ. Arrhythm. Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef]
- Ray-Offor, E.; Amadi, S. Hemorrhoid disease: Predilection sites, pattern of presentation, and treatment. Ann. Afr. Med. 2019, 18, 12–16. [Google Scholar] [CrossRef]
- Sun, Z.; Migaly, J. Review of Hemorrhoid Disease: Presentation and Management. Clin. Colon. Rectal Surg. 2016, 29, 22–29. [Google Scholar] [CrossRef]
- Clinical Practice Committee; American Gastroenterological Association. American Gastroenterological Association medical position statement: Diagnosis and treatment of hemorrhoids. Gastroenterology 2004, 126, 1461–1462. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Hemorrhoids: From basic pathophysiology to clinical management. World J. Gastroenterol. 2012, 18, 2009–2017. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Sheikh, P.; Bandolon, R.; Ren, D.L.; Roslani, A.C.; Schaible, K.; Freitag, A.; Martin, M.; Yaltirik, P.; Godeberge, P. Recurrence Rates and Pharmacological Treatment for Hemorrhoid Disease: A Systematic Review. Adv. Ther. 2023, 40, 117–132. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.S.; Davitkov, P.; Ko, C.W.; Rajasekhar, A.; Su, G.L.; Sultan, S.; Allen, A.M.; Falck-Ytter, Y. AGA Clinical Practice Guideline on the Management of Coagulation Disorders in Patients with Cirrhosis. Gastroenterology 2021, 161, 1615–1627.e1. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K. Cardiac Troponin Serum Concentration Measurement Is Useful Not Only in the Diagnosis of Acute Cardiovascular Events. J. Pers. Med. 2024, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, K.; Gkolfakis, P.; Gralnek, I.M.; Oakland, K.; Manes, G.; Radaelli, F.; Awadie, H.; Camus Duboc, M.; Christodoulou, D.; Fedorov, E.; et al. Correction: Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, C10, Erratum in Endoscopy 2021, 53, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Sakurai, T.; Moriyasu, S.; Shimbo, T.; Okubo, H.; Watanabe, K.; Yokoi, C.; Yanase, M.; Akiyama, J.; Uemura, N. Impact of INR monitoring, reversal agent use, heparin bridging, and anticoagulant interruption on rebleeding and thromboembolism in acute gastrointestinal bleeding. PLoS ONE 2017, 12, e0183423. [Google Scholar] [CrossRef] [PubMed]
- Kodilinye, S.M.; Kalloo, A.N. Endoscopic approaches to the management of hemorrhoids. Curr. Opin. Gastroenterol. 2023, 39, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Schleinstein, H.P.; Averbach, M.; Averbach, P.; Correa, P.A.F.P.; Popoutchi, P.; Rossini, L.G.B. Endoscopic Band Ligation For The Treatment of Hemorrhoidal Disease. Arq. Gastroenterol. 2019, 56, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, L.J.; Xiang, J.; He, Z.; Peng, Z.Y.; Huang, G.M.; Ji, G.Z.; Zhang, F.M. Cap-assisted endoscopic sclerotherapy for hemorrhoids: Methods, feasibility and efficacy. World J. Gastrointest. Endosc. 2015, 7, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Milling, T.J.; Refaai, M.A.; Sengupta, N. Anticoagulant Reversal in Gastrointestinal Bleeding: Review of Treatment Guidelines. Dig. Dis. Sci. 2021, 66, 3698–3714. [Google Scholar] [CrossRef]
- Veitch, A.M.; Vanbiervliet, G.; Gershlick, A.H.; Boustiere, C.; Baglin, T.P.; Smith, L.A.; Radaelli, F.; Knight, E.; Gralnek, I.M.; Hassan, C.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy 2016, 48, c1, Erratum in Endoscopy 2016, 48, 385–402. [Google Scholar] [CrossRef]
- Thomas, S.; Makris, M. The reversal of anticoagulation in clinical practice. Clin. Med. 2018, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeefi, M.; Badreldin, H.A.; Alsuwayyid, F.; Alqahtani, T.; Alshaya, O.; Al Yami, M.S.; Bin Saleh, K.; Al Harbi, S.A.; Alshaya, A.I. Practical Guide for Anticoagulant and Antiplatelet Reversal in Clinical Practice. Pharmacy 2023, 11, 34. [Google Scholar] [CrossRef]
- Sengupta, N.; Feuerstein, J.D.; Jairath, V.; Shergill, A.K.; Strate, L.L.; Wong, R.J.; Wan, D. Management of Patients with Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline. Am. J. Gastroenterol. 2023, 118, 208–231. [Google Scholar] [CrossRef]
- Rajab, T.K.; Bordeianou, L.G.; von Keudell, A.; Rajab, H.; Zhou, H. Digital Rectal Examination and Anoscopy. N. Engl. J. Med. 2018, 378, e30. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Frison, L.; Halperin, J.L.; Lane, D.A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J. Am. Coll. Cardiol. 2011, 57, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bo-gucka-Kocka, A. Effect of Diosmin Administration in Patients with Chronic Venous Dis-orders on Selected Factors Affecting Angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, P.; Lohsiriwat, V.; Shelygin, Y. Micronized Purified Flavonoid Fraction in Hem-orrhoid Disease: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 2792–2812. [Google Scholar] [CrossRef] [PubMed]
- Maemoto, R.; Tsujinaka, S.; Miyakura, Y.; Machida, E.; Fukui, T.; Kakizawa, N.; Tamaki, S.; Ishikawa, H.; Rikiyama, T. Effect of Antithrombotic Therapy on Secondary Bleeding After Proctological Surgery. Cureus 2021, 13, e14983. [Google Scholar] [CrossRef]
- Pigot, F.; Juguet, F.; Bouchard, D.; Castinel, A. Do we have to stop anticoagulant and platelet-inhibitor treatments during proctological surgery? Colorectal Dis. 2012, 14, 1516–1520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).