Abstract
At the time of colorectal cancer (CRC) diagnosis, approximately 25% of patients present with liver metastases, and 70% develop them during follow-up. This is the primary cause of therapeutic failure and most associated deaths, making it imperative to understand the molecular mechanisms involved in this process and the biological components involved. In the process of anaerobic glycolysis occurring in these cells, to maintain cellular homeostasis, excess lactate is removed via monocarboxylate transporters (MCTs). This study aimed to characterize monocarboxylate transporter 4 (MCT4), human glucose transporter protein isoform 1(GLUT1), cluster of differentiation 147 (CD147), and the acidic cell surface adhesion protein (CD44) in various cellular and histological compartments of liver metastases from CRC in 45 patients diagnosed with metastatic CRC. The characterization revealed significant correlations between the compartmentalization of these markers and the patients’ clinicopathological data. The findings for MCT4, GLUT1, CD147, and CD44 obtained in this study are very promising in relation to considering these markers as therapeutic targets in further investigations.
1. Introduction
Colorectal cancer (CRC) is one of the cancers associated with the highest morbidity and mortality worldwide, being the third most frequent type of cancer in men and the second in women [].
At the time of primary tumor resection, up to 25% of patients with CRC present metastases, and the 5-year survival rate is only around 11%. The presence of metastases, the high recurrence rate of the disease, and the presence of locally advanced tumors results in a poor prognosis [,,].
Since, in cancer, the metastasis of neoplastic cells leads to a worse prognosis and is the main cause of therapeutic failure and most associated deaths, it is imperative to understand the molecular mechanisms that facilitate this process and the biological components involved. For metastasis to occur, neoplastic cells require a greater energy supply to ensure rapid cell division, which is achieved through metabolic adaptation mechanisms []. This reprogramming of cellular metabolism, also known as the Warburg effect, is currently recognized as one of the hallmarks of cancer and is thought to be closely related to the high rate of uncontrolled proliferation of neoplastic cells []. In metastasis, several metabolic markers are involved, notably the isoform 1 of the glucose transporter, GLUT1, due to the increased need for glucose uptake for anaerobic glycolysis [,,]. Monocarboxylate transporters (MCTs) [,,], which remove lactate and thus maintain intracellular pH homeostasis, and CD44, among others, are also involved [].
To explore the existence of metabolic compartmentalization in CRC hepatic metastases and better understand their metabolic programming, this study aimed to analyze the expression of the transporters GLUT1 and MCT4 and the glycoproteins CD44 and CD147 in various cellular and histological compartments of CRC hepatic metastases through immunohistochemical characterization, and to determine possible associations between their expression and clinicopathological data on tumor and patient survival.
2. Results
2.1. Sample Characterization
The sample used in this study comprises 45 patients selected according to the established inclusion criteria during the period of 1 January 2003 to 31 December 2010.
An analysis of sex distribution revealed that 77.8% (n = 35) of the patients were male, while 22.2% (n = 10) were female.
The average age of the patients was 65 ± 12 years, with males averaging 67 ± 11 years and females averaging 58 ± 15 years.
The age group with the highest proportion of cases, 20.0% (n = 9), was between 75 and 79 years.
Regarding the anatomical location of the primary tumor in the analyzed patients, the most frequent locations were the rectum in 40% of the cases (n = 18) and the sigmoid colon in 33.3% (n = 15), as can be observed in Table 1.

Table 1.
Primary tumor localization.
In male patients, the most frequent tumor location was the rectum, occurring in 42.9% (n = 15) of the cases. In female patients, the most frequent location was the sigmoid colon, with 50% (n = 5) of the cases.
In the evaluation of tumor staging, it was found that the majority of cases, 62.5% (n = 25), were in stage IV, as can be observed in Table 2.

Table 2.
Primary tumor staging.
After resection of the primary tumor, venous involvement was found in 62.5% of the patients (n = 20) and lymphatic involvement in 69.7% (n = 23). Surgical margins showed lesions in 6.1% of the patients (n = 2).
Regarding liver metastases, 46.7% of the patients (n = 21) had synchronous metastases and 53.3% had metachronous metastases (n = 24). Metachronous metastases appeared on average 24 ± 22 months after the primary tumor, with a minimum appearance time of 1 month and a maximum of 107 months.
Among the hepatic segments, segment V was the most frequently affected, occurring in 51.3% of the cases (n = 20), followed by segment VI, in 48.7% (n = 19). Information on the anatomical location of hepatic metastases was not available for six cases, as can be observed in Table 3

Table 3.
Anatomical location of liver metastases.
CEA values were also evaluated at the time of diagnosis of CRC and hepatic metastases. At the time of primary tumor diagnosis, 71.4% of the patients (n = 20) had CEA values between 2.5 and 200 ng/mL and 10.7% (n = 3) had values above 200 ng/mL. At the time of hepatic metastasis diagnosis, 67.5% of the cases (n = 27) had values between 2.5 and 200 ng/mL, and 10% of the patients (n = 4) had values above 200 ng/mL.
During the follow-up evaluation of these patients, 18 developed new metastases, namely hepatic (n = 12), pulmonary (n = 8), bone (n = 2), brain (n = 1), and cutaneous (n = 1). Adjuvant therapy was evaluated, and all patients underwent some form of therapy at some stage of their treatment. Thus, 81.8% of the patients (n = 36) underwent chemotherapy alone and 18.2% (n = 8) underwent chemotherapy and radiotherapy.
Out of the 45 patients evaluated, data could not be obtained for 3 (6.7%); 1 (2.2%) had survived; and 41 (91.1%) had died before the study was conducted.
2.2. Evaluation of Immunohistochemical Expression of MCT4, GLUT1, CD147, and CD44 in Histological and Cellular Compartments of Hepatic Metastases
The representative immunohistochemical expression of MCT4, GLUT1, CD147, and CD44 in cells and stroma of CRC hepatic metastasis tissue can be observed in Figure 1.
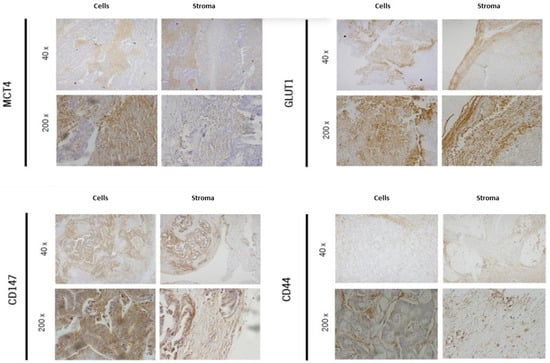
Figure 1.
Representative immunohistochemical expression of MCT4, GLUT1, CD147, and CD44 in cells and stroma of CRC hepatic metastasis tissue.
The frequencies of marker expression in the histological compartments are summarized in Table 4.

Table 4.
Relative frequency of marker expression in different histological compartments.
Regarding the immunoreaction in neoplastic cells, GLUT1 was the marker with the highest expression, with positivity criteria in 100% of the cases (n = 43). The other markers also showed high expression frequencies in cells, with MCT4 being the one with the lowest expression, at 92.7% (n = 38).
In the stroma, there was a greater disparity in expression among the different markers. MCT4 was the marker with the highest expression in this compartment, with a positive reaction in 33.3% (n = 14) of the cases, and GLUT1 had the lowest expression, with a positive reaction in only 9.3% (n = 4) of the cases.
The studied markers were not significantly expressed in the vessels, with no expression being found for MCT4 and CD147, and expression frequencies of only 2.4% and 2.3% being found for CD44 and GLUT1, respectively.
The frequencies of marker expression in the cellular compartments are summarized in Table 5.

Table 5.
Relative frequency of marker expression in different cellular compartments.
Regarding the distribution of marker expression by cellular compartment, it was found that in most cases, both CD44 and CD147 and GLUT1 exhibited primary immunoreaction in the membrane, with GLUT1 being the marker with the highest expression in this compartment. In the evaluation of MCT4, 52.4% (n = 22) of the cases expressed this marker in the cytoplasm.
2.3. Determination of Possible Correlations between the Expression of MCT4, GLUT1, CD147, and CD44 and Clinicopathological Data
The association between MCT4, GLUT1, CD147, and CD44 immunoexpression in histological compartments of CRC hepatic metastases and clinicopathological parameters can be observed, respectively, in Table 6, Table 7, Table 8 and Table 9.

Table 6.
Association between MCT4 immunoexpression in histological compartments of CRC hepatic metastases and clinicopathological parameters.

Table 7.
Association between GLUT1 immunoexpression in histological and cellular compartments of CRC hepatic metastases and clinicopathological parameters.

Table 8.
Association between CD44 immunoexpression in histological and cellular compartments of CRC hepatic metastases and clinicopathological parameters.

Table 9.
Association between CD147 immunoexpression in histological and cellular compartments of CRC hepatic metastases and clinicopathological parameters.
In the evaluation of possible correlations with clinicopathological data of the primary tumor, a significant association was observed between the expression of GLUT1 in the membrane/cytoplasm and the value of CEA at diagnosis (p = 0.014) (Table 7).
No significant correlations were observed in the analysis of the remaining variables under study.
Kaplan–Meier survival curves were also analyzed for the different markers and compartments. However, no differences in survival were found in patients whose hepatic metastases showed expression of MCT4, CD44, CD147, and GLUT1, neither in the histological compartments nor in the cellular compartments under study (LogRank p > 0.05), as can be observed in Table 10.

Table 10.
LogRank p-values obtained through Kaplan–Meier curves.
3. Materials and Methods
The study population consisted of patients from the General Surgery Service of Braga Hospital (SCG-HB) diagnosed with CRC liver metastases, spanning the period from 1 January 2003 to 31 December 2010. The inclusion criteria required patients to have a confirmed diagnosis of CRC with liver metastases and to have undergone either a biopsy or resection of their liver metastases. The exclusion criteria included patients with metastatic CRC who had not undergone any diagnostic or therapeutic intervention on their liver metastases, as well as cases where it was not possible to retrieve the liver metastasis tissue block.
The study’s target population was selected based on the SCG-HB database, and clinical information was gathered from patient medical records and histopathological results of surgical specimens. Data collection and usage were approved by the HB Ethics Committee in June 2011. Immunoexpression analysis was performed, and a database was created to include sociodemographic information (gender and age), pathological details of the primary tumor (location, staging, histological grade, and lymphovascular invasion (LVI)), CEA levels (serum levels) at primary tumor and liver metastasis diagnosis, the presence of extrahepatic metastases, adjuvant therapy, and patient mortality.
The immunohistochemistry technique was performed based on the peroxidase–biotin-avidin complex principle (Ultravision Detection System, Anti-polyvalent HRP [Lab Vision Corp]) using primary antibodies specific for MCT4 (Santa Cruz Biotech; SC-50329), GLUT1 (AB15309-500), and CD147 (ZYMED; 18-7344) at a dilution of 1:500, and for CD44 (Serotec; MCA2726) at a dilution of 1:1000. Tissue sections were cut, deparaffinized, and hydrated using an AutoStainer. Antigen retrieval was performed in a 10 mM citrate solution (pH 6.0) for MCT4, GLUT1, and CD44, and in a 1 mM EDTA solution (pH 8.0) for CD147, both heated to 98 °C for 20 min. After cooling for 20 min, sections were washed twice in phosphate-buffered saline (PBS), inactivated for endogenous peroxidases with 3% hydrogen peroxide in methanol for 10 min, and then blocked with UV-Block (LabVision) for 10 min.
Sections were then incubated with the respective primary antibodies for 2 h at room temperature, followed by incubation with secondary antibodies (LabVision) and streptavidin peroxidase for 10 min each, with PBS washes between each step. Finally, sections were incubated with 3,3′-diaminobenzidine (DAB) for 10 min, washed in distilled water for 2 min, counterstained with hematoxylin using an AutoStainer, and mounted. The expression of GLUT1, MCT4, and the membrane glycoproteins CD44 and CD147 was evaluated semi-quantitatively based on the depth and intensity of their immunoreactions in histological compartments (cells, stroma, and blood vessels) and cellular compartments (membrane and cytoplasm). Two independent observers assessed the immunoreaction depth and intensity, with discordant results discussed until consensus was achieved. Immunoreaction depth was scored as zero for no reaction, one for less than 5% reactivity, two for 5–50% reactivity, and three for more than 50% reactivity. Staining intensity was scored as zero for no reaction, one for weak staining, two for intermediate staining, and three for strong staining. The final immunoreaction score was the sum of these parameters, categorized as negative (zero to one) or positive (two to six).
Data were stored and analyzed using SPSS. A simple descriptive analysis was performed on all variables, determining total case numbers and relative frequencies for valid cases. All correlations were tested for statistical significance using Pearson’s Chi-square test where applicable and Fisher’s Exact Test for smaller sample sizes, with confidence values (p-values) less than 0.05 being considered significant. Additionally, a survival analysis was conducted using Kaplan–Meier curves to evaluate patient outcomes.
4. Discussion
The roles of MCT4, GLUT1, CD147, and CD44 in the metabolism of neoplastic cells have been investigated previously with the aim of understanding the molecular interactions underlying the metastasis process [,,,].
Martins et al. demonstrated that MCT4, CD147, and GLUT1 are overexpressed in the plasma membrane of CRC cells, as well as in hepatic metastases, compared to non-neoplastic tissue []. However, conclusive studies on metabolic compartmentalization in colorectal cancer metastases, which would better elucidate the metabolic functioning differences between primary tumors and metastases, are still lacking.
This study aimed to describe the immunoexpression of the markers MCT4, GLUT1, CD147, and CD44 in various histological and cellular compartments of colorectal cancer hepatic metastases and to establish possible associations between this expression and the clinicopathological data of the studied patients. Additionally, a survival analysis was conducted to find a potential association between the expression of the studied markers and patient survival, which did not demonstrate significant associations.
In this study, the expression of MCT4, GLUT1, CD44, and CD147 was analyzed in both histological compartments (cells, stroma, and vessels) and cellular compartments (membrane and cytoplasm), as these regions have distinct metabolic functions that could influence the dynamics of the metastasis process.
The significant expression of MCT4, GLUT1, CD44, and CD147 found at the cellular level is consistent with the literature [,,,]. The expression of MCT4, CD44, and CD147 in the stroma was higher compared to GLUT1 expression, with MCT4 being the most expressed marker in this compartment, which is also consistent with data described in other studies [].
Weak expression of CD44 was found in the vessels of the studied tissue, which can possibly be explained by its role in tumor angiogenesis []. GLUT1 also showed weak expression in the vessels, which aligns with studies on this marker demonstrating that this family of markers acts at the membrane level []. Indeed, in the study of GLUT1 expression in the compartments of the neoplastic cells, membrane expression was observed in the vast majority of cases (90.7%), contrary to its cytoplasmic expression. CD44 also showed significant membrane expression, which is expected given the transmembrane nature of this glycoprotein family []. CD44 is known to be important in many cancer cases. A recent study has suggested that CD44 may be a promising target for intervening with the metastatic spreading of liver cancer. CD44 upregulates the expression of integrin β2 and promotes the transendothelial migration of liver cancer cells, which may facilitate metastatic spreading [].
In the cytoplasm of the observed cells, MCT4 was the most expressed marker, a finding that has also been demonstrated in recent studies, particularly in other cancer types, such as prostate cancer [].
Regarding the correlation analysis between the expression of MCT4, CD44, CD147, and GLUT1 and the clinicopathological data of the studied patients, a significant correlation was found only between GLUT1 membrane expression and the CEA value of the primary tumor at diagnosis. This significant GLUT1 membrane expression in tumor cells aligns with other studies, demonstrating the importance of this transporter in the metabolic adaptation of these cells, particularly in increasing metabolism and energy utilization dependent on increased glucose uptake []. In oncology, this process of high glucose uptake and glycolysis followed by lactic acid fermentation is called the Warburg effect. There are recent studies that have evaluated the expression of proteins associated with the Warburg effect (LDHA, GLUT1, MCT4, PKM2, p53, PTEN) in relation to survival in colorectal cancer. Offermans et al. demonstrated that patients with high protein expression had the poorest overall survival, independent of known prognostic factors []. The Warburg effect is thought to increase the malignant potential of tumor cells and may contribute to therapy resistance [,].
The absence of correlations between the expression of the other markers and the clinicopathological data could be due to the small sample size of this study. Although associations between increased GLUT1 expression and clinicopathological data, such as invasion depth and histological differentiation, have been demonstrated in the literature [], this is the first study to expose an association between GLUT1 membrane expression in hepatic metastases of colorectal cancer and the CEA values of the primary tumor at diagnosis.
This study has a limitation regarding the amplitude of the tissue research due to the limited size of the samples, which prevented a more accurate analysis of the cytological compartments of the tumor cells and the surrounding tissues.
5. Conclusions
This is likely the first study conducted on colorectal cancer hepatic metastases that assesses the immunohistochemical expression of MCT4 and GLUT1 transporters and the membrane glycoproteins CD147 and CD44 in various histological and cellular compartments. Our findings reveal significant correlations between the expression of these markers and various clinicopathological parameters, underscoring their potential roles in the progression and metastasis of colorectal cancer. Notably, the high expression levels of MCT4 and GLUT1 support the hypothesis that anaerobic glycolysis and lactate transport are critical metabolic adaptations in metastatic cancer cells, facilitating their survival and proliferation in the liver microenvironment. Similarly, the elevated levels of CD147 and CD44 suggest that these glycoproteins may contribute to the metastatic potential and aggressiveness of colorectal cancer cells.
These results highlight the importance of targeting metabolic pathways and cell adhesion mechanisms in the development of novel therapeutic strategies for colorectal cancer with liver metastases. By focusing on the molecular underpinnings of metastasis, treatments can be tailored to disrupt the specific processes that enable cancer cells to colonize and thrive in distant organs. Future research should aim to further elucidate the functional roles of MCT4, GLUT1, CD147, and CD44 in metastatic progression and explore the therapeutic efficacy of inhibitors targeting these molecules.
The results regarding the expression of these markers in neoplastic cells support the data mentioned in the literature in describing an increase in their immunohistochemical expression at the tumor level, thus suggesting their role in tumor progression and the metastasis process. However, the lack of studies on metabolic compartmentalization in various cancer types makes it difficult to compare the obtained results with those found in the literature. Therefore, additional studies with larger sample sizes and analyses of the expression of the studied markers in different metabolic compartments would complement this work and contribute to a better understanding of the metabolic adaptation phenomenon in the tumor metastasis process.
Author Contributions
Conceptualization—A.L.-F. and S.F.M.; design—N.C., A.L.-F. and S.F.M.; supervision—A.L.-F. and S.F.M.; materials collection—A.P., M.C., N.M., C.B. and C.V.; data collection—N.C., M.F., A.P., M.C., N.M., C.B. and C.V.; analysis and interpretation—N.C., A.L.-F. and S.F.M.; literature search—N.C. and M.F.; writing—N.C. and M.F.; critical review—A.L.-F. and S.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted under compliance with the Declaration of Helsinki and was approved by the Ethics Committee of Hospital de Braga, HB 32_2013, and the Ethics Committee of the Life and Health Science Research Institute, SECVS_064/2013.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef]
- Valderrama-Trevino, A.I.; Barrera-Mera, B.; Ceballos-Villalva, J.C.; Montalvo-Jave, E.E. Hepatic Metastasis from Colorectal Cancer. Euroasian J. Hepatogastroenterol. 2017, 7, 166–175. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Qian, C.N.; Mei, Y.; Zhang, J. Cancer metastasis: Issues and challenges. Chin. J. Cancer 2017, 36, 38. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Lei, T.; et al. The prognostic value of GLUT1 in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, I.; Bang, H.; Kim, H.C.; Lee, Z.W.; Lee, W.Y.; Yun, S.H.; Lee, J.; Lee, S.J.; Park, Y.S.; et al. MCT4 Expression Is a Potential Therapeutic Target in Colorectal Cancer with Peritoneal Carcinomatosis. Mol. Cancer Ther. 2018, 17, 838–848. [Google Scholar] [CrossRef]
- Xin, X.; Zeng, X.; Gu, H.; Li, M.; Tan, H.; Jin, Z.; Hua, T.; Shi, R.; Wang, H. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32804. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Gotanda, Y.; Akagi, Y.; Kawahara, A.; Kinugasa, T.; Kage, M.; Shirouzu, K. Expression of monocarboxylate transporter (MCT)-4 in colorectal cancer and its role: MCT4 contributes to the growth of colorectal cancer with vascular endothelial growth factor. Anticancer Res. 2013, 33, 2941–2947. [Google Scholar]
- Koch, A.; Lang, S.A.; Wild, P.J.; Gantner, S.; Mahli, A.; Spanier, G.; Berneburg, M.; Müller, M.; Bosserhoff, A.K.; Hellerbrand, C. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget 2015, 6, 32748–32760. [Google Scholar] [CrossRef]
- Martins, S.F.; Martins, S.F.; Amorim, R.; Viana-Pereira, M.; Pinheiro, C.; Costa, R.F.A.; Silva, P.; Couto, C.; Alves, S.; Fernandes, S.; et al. Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer 2016, 16, 535. [Google Scholar] [CrossRef]
- Wang, J.; Ye, C.; Chen, C.; Xiong, H.; Xie, B.; Zhou, J.; Chen, Y.; Zheng, S.; Wang, L. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 16875–16886. [Google Scholar] [CrossRef]
- Granchi, C.; Fortunato, S.; Minutolo, F. Anticancer agents interacting with membrane glucose transporters. Medchemcomm 2016, 7, 1716–1729. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Gerardo-Ramírez, M.; Giam, V.; Becker, D.; Groth, M.; Hartmann, N.; Morrison, H.; May-Simera, H.; Radsak, M.; Jarquardt, J.; Galle, P.; et al. Deletion of Cd44 Inhibits Metastasis Formation of Liver Cancer in Nf2-Mutant Mice. Cells 2023, 12, 1257. [Google Scholar] [CrossRef]
- Pertega-Gomes, N.; Vizcaíno, J.R.; Miranda-Gonçalves, V.; Pinheiro, C.; Silva, J.; Pereira, P.; Monteiro, P.; Henrique, R.M.; Lopes, C.; Baltazar, F. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer 2011, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Offermans, K.; Jenniskens, J.C.; Simons, C.C.; Samarska, I.; Fazzi, G.; Smits, K.; Schouten, L.; Weijenberg, M.; Grabsch, H.; Piet A van den Brandt. Expression of proteins associated with the Warburg-effect and survival in colorectal cancer. J. Pathol. Clin. Res. 2022, 8, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Maeda, T.; Suzuki, A.; Baba, Y. Cancer metabolism: New insights into classic characteristics. Jpn. Dent. Sci. Rev. 2018, 54, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Schuurbiers, O.C.; Kaanders, J.H.; van der Heijden, H.F.; Dekhuijzen, R.P.; Oyen, W.J.; Bussink, J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 761–767. [Google Scholar] [CrossRef]
- Sakashita, M.; Aoyama, N.; Minami, R.; Maekawa, S.; Kuroda, K.; Shirasaka, D.; Ichihara, T.; Kuroda, Y.; Maeda, S.; Kasuga, M. Glut1 expression in T1 and T2 stage colorectal carcinomas: Its relationship to clinicopathological features. Eur. J. Cancer 2001, 37, 204–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).