Abstract
Introduction: Chronic hepatitis B (CHB) is a significant public health issue worldwide, especially in the Middle East region. Around 8% to 20% of patients with CHB develop cirrhosis, which may progress to hepatocellular carcinoma. The significant morbidity and mortality associated with CHB denote the importance of high-quality treatment. Methods: We searched the PubMed, Medline, and Cochrane databases from inception to January 2024 to identify relevant studies. Search terms were generated using established treatment guidelines for CHB. We also manually searched the bibliographies of relevant literature to obtain additional papers. Results: In this narrative review, we evaluated the seven currently licensed antiviral therapies for chronic Hepatitis B treatment, including nucleos(t)ide analogs (NAs) and pegylated interferon-alpha (PEG-IFNα). NAs can be divided into two categories: high barrier to resistance and low barrier to resistance. Tenofovir disoproxil fumarate, tenofovir alafenamide, and entecavir are NAs with a high barrier to resistance. Telbivudine has shown promise in providing high efficacy with low viral resistance rates; however, it is not recommended because of insufficient evidence and lack of cost-effectiveness. Lamivudine and adefovir dipivoxil, despite being efficacious, have a low barrier to resistance, the primary reason they are no longer recommended. PEG-IFNα has high efficacy and can be completed in 48 weeks. It is not associated with resistance; however, it has been reported to have several systemic adverse effects. Conclusions: Current first-line NA treatments in the Middle East include entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide. These drugs are favored over other NAs because of their low rates of resistance. PEG-IFNα has superiority over NAs in inducing a more durable antiviral response and having a finite treatment duration. The main drawback of PEG-IFNα is an unfavorable safety profile.
1. Introduction
The World Health Organisation (WHO) reports that Hepatitis B virus (HBV) infection remains a significant public health issue worldwide. The WHO reports that 254 million people were living with chronic hepatitis B infection in 2022, with 1.2 million new infections each year [1]. The yearly risk of hepatocellular carcinoma (HCC) in patients with cirrhosis is 2–5%, and there is an estimated 5-year incidence of 8–20% if CHB is untreated [2]. HCC remains the main concern for CHB patients, highlighting the importance of reducing the risk through treatment. While Western countries report that the prevalence of HBV infection is <1% of the total population, Middle Eastern countries have a comparatively high prevalence of hepatitis B, which is reported to be 2–8% [3,4].
A combination of public health strategies, new antiviral medications, and vaccination programs is employed to control and eradicate HBV infection in the Middle East [5]. The introduction of antiviral medications is a major step towards achieving the WHO goal of eradicating viral hepatitis by 2030 [6]. However, access to hepatitis B treatment is inconsistent across various countries and regions in the Middle East. While certain nations have robust healthcare systems and offer affordable access to antiviral medications, others may encounter difficulties in providing consistent treatment and care [7].
Hepatitis B virus (HBV) belongs to the Hepadnaviridae family of small, enveloped, primarily hepatotropic DNA viruses. HBV integrates itself into the human host genome. It is transmitted parenterally via contaminated blood and bodily fluids [8]. Patients unable to clear the virus can develop Chronic Hepatitis B (CHB) [8]. The lifecycle of HBV, as depicted in Figure 1, offers many targets for antiviral therapies.
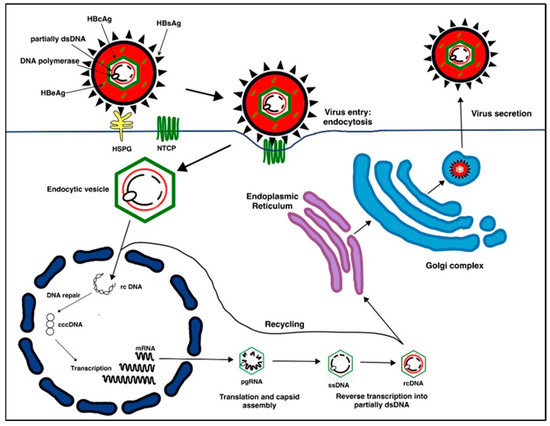
Figure 1.
The Lifecycle of Hepatitis B Virus (HBV): HBV binds to HSPG (Heparan Sulfate Proteoglycan) and NTCP (sodium taurocholate co-transporting polypeptide) cell surface receptors and is endocytosed into the hepatocyte cell. The genomic DNA is released into the semipermeable nucleus, and relaxed, circular, partially double-stranded DNA (rcDNA) is repaired to form covalently closed circular DNA (cccDNA). Viral RNAs are produced from cccDNA. The mRNA strands (pregenomic, precode, Hex, PreS1, and PreS2/S RNA) are reverse transcribed and translated back to viral DNA. DNA nucleocapsids are recycled back into the nucleus or enveloped and secreted from hepatocytes as new HBV virions.
The assessment of chronic HBV infection takes the presence of HBsAg (Hepatitis B surface antigen), HBeAg (Hepatitis B e-antigen), HBV DNA levels] HBeAg, HBV DNA levels, alanine aminotransferase (ALT) values and eventually the presence or absence of liver inflammation. The serological diagnosis of HBV is assessed by measurement of the markers outlined in Table 1.

Table 1.
A table showing the presence/absence of serological markers [HBsAg (Hepatitis B surface antigen), HBeAg (Hepatitis B e-antigen), and HBV DNA] associated with HBeAg positive/negative chronic hepatitis B.
Anti-viral treatment is indicated in patients with elevated alanine aminotransferase (ALT) with evidence of necroinflammation or fibrosis on biopsy and elevated HBV DNA (>2000 IU/mL if HBeAg positive or >20,000 IU/mL if HBeAg negative). Patients with both cirrhosis and detectable HBV DNA should be treated regardless of ALT levels [2,9].
Response to antivirals is measured by virologic (serum HBV DNA suppression), biochemical (ALT normalization), and serological (loss of HBsAg with or without seroconversion) endpoints [2]. An optimal endpoint for antivirals is HBsAg loss, as it is associated with clinical remission and is considered a functional cure for CHB [2].
There are two main treatment options for CHB patients: nucleos(t)ide analogs (NA) and interferon (IFN) therapy. Both therapies are available in the Middle East region but have variable access between the countries. Our objective is to review and appraise evidence on the efficacy and safety of the currently licensed therapies for CHB.
2. Methods
We conducted a search of the literature in the PubMed, Medline, and Cochrane databases for articles. Bibliographies of the retrieved studies were searched for other relevant studies. We also reviewed the reference articles that had been cited in the European Association for the Study of the Liver (EASL) guidelines. Key reference terms included in the search were chronic hepatitis B, tenofovir disoproxil fumarate, tenofovir alafenamide, entecavir, lamivudine, telbivudine, adefovir dipivoxil, and pegylated interferon-alpha.
2.1. Nucleos(t)ide Analogues (NAs)
NAs target the HBV DNA polymerase and hence suppress the replicational activity of the virus. Licensed NAs include tenofovir, entecavir, lamivudine, telbivudine and adefovir dipivoxil.
2.2. Tenofovir (TFV)
TFV is available as tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). These drugs demonstrate similar efficacy with HBeAg-positive and HBeAg-negative patients. In ongoing international multicentre studies of CHB, 93% of HBeAg-negative participants receiving TDF achieved HBV DNA <29 IU/mL compared with 94% of participants receiving TAF after 48 weeks (n = 426) [10]. In HBeAg-positive participants, 64% of those receiving TAF and 67% of participants receiving TDF achieved HBV DNA <29 IU/mL (n = 873) [11]. The difference between TDF and TAF did not differ statistically [10,11].
The metabolism of both drugs causes different tenofovir distribution, affecting drug safety profiles. TDF is converted to tenofovir in the plasma, resulting in high circulating levels due to low permeability [12,13]. However, TAF remains stable within the plasma and is only converted to tenofovir intracellularly [14]. As TAF results in higher intracellular tenofovir, it can be prescribed at a lower dose, resulting in reduced systemic tenofovir exposure compared with TDF. The recommended dose of TAF is 25 mg/day compared with 245 mg/day TDF [2]. Consequently, TDF is associated with higher systemic tenofovir exposure, resulting in greater adverse effects (AEs) on bones and renal function than TAF.
TAF has improved renal and bone profiles compared with TDF. After 48 weeks of treatment, patients on TAF had a lower median decrease in estimated glomerular filtration rate compared with TDF (−1.8 mL/min [IQR −7.8 to 6.0] vs. −4.8 mL/min [–12.0 to 3.0], p = 0.004) [10]. Additionally, 10% of patients receiving TAF treatment had a >3% decrease in hip bone mineral density (BMD) at week 48 compared with 33% of those receiving TDF (p < 0.0001) [10]. Similarly, Chan et al. determined a 1.62% statistically significant difference in BMD between participants receiving TDF and TAF [11]. This is further supported by smaller studies demonstrating improved BMD at the hip in patients that switched from TDF to TAF, with a mean change of 0.66% [SD 2.08], compared with those that stayed on TDF, −0.51% [SD (Standard Deviation) 1.91], (p < 0.0001) [15].
The extent of renal impairment with TDF varies across studies. Pan et al. reported no patients (n = 90) with >0.5 mg/dL increase in serum creatinine or creatinine clearance <50 mL/min [16]. However, Marcellin et al. (n = 585) reported thirteen patients with ≥0.5 mg/dL increase in serum creatinine and seven with creatinine clearance <50 mL/min [17]. Furthermore, 5.1% of participants experienced renal impairment after 10 years, within the previously reported range of 2–7% [17]. A small sample size with Pan et al. and a shorter duration of study could have resulted in confounding and lack of detection. Renal function and serum phosphate testing are recommended before commencing treatment and then every 4 weeks following [2].
In studies so far, negligible resistance has been detected except in the circumstance of non-compliance, such as in the Australian real-world trial that experienced three episodes (n = 92) [18]. A high barrier to resistance allows this to be a favorable therapeutic option as it results in a predictably high and long-term efficacy.
2.3. Entecavir
Entecavir, a guanosine analog, is the most widely used antiviral alongside tenofovir in CHB patients. A dose of 0.5 mg is recommended in treatment-naive patients; however, the dose should be increased to 1 mg in patients with evidence of decompensated liver disease [2].
The ENUMERATE study investigated the efficacy of entecavir on NA-treatment naïve patients (n = 658) [19]. Notable levels of seroconversion (8.8%), ALT normalization, and HBV DNA suppression were recorded after 1 year. It was also recognized that HBeAg-negative patients, when compared with HBeAg-positive patients, had a notably higher rate of ALT normalization (72.8% vs. 65.9%) and HBV DNA suppression (81.9% vs. 34.6%). This study primarily focuses on Asian patients, and as several studies have found regional differences in response to HBV infection and treatments for CHB, the global applicability of this trial may be limited [20,21]. Significant discrepancies are also seen in age and race between the two groups, resulting in confounding. However, an ongoing phase 3 trial comparing young CHB patients (<18 years) treated with entecavir with a placebo group also demonstrates a significant difference in ALT normalization (67.5% vs. 23.3%; p < 0.0001) and HBV DNA suppression (49.2% vs. 3.3%; p < 0.0001) [22].
Long-term entecavir treatment has been associated with a lower incidence of HCC in CHB patients compared with a control group after 5 years (3.7% vs. 13.7%; p < 0.001). There was adjustment for other causes of HCC; however, notably, a key risk factor of family history was not included [23]. Similarly, the C-TEAM study (n = 1818) also identified a significantly higher risk of developing HCC in untreated patients compared with those receiving entecavir treatment (p < 0.0001), as well as an increased risk of variceal bleeds and liver-related mortality in patients on 4-year entecavir therapy compared with the control group (p = 0.324, p = 0.0003) [24].
Entecavir resistance has proved to be minimal in treatment-naïve patients, where rates of 0.8% in adults and 0.6% in patients <18 have been noted [22,24]. A study monitoring entecavir resistance for extended treatment in naive patients identified a cumulative entecavir resistance incidence of 1.2 after 5 years, while lamivudine-resistant patients receiving entecavir treatment recorded 51% genotypic resistance [25].
Across these studies, common AEs experienced fatigue, headaches, and abdominal discomfort. The ENUMERATE (n = 658) study recorded eight patients discontinuing entecavir treatment because of AEs, whereby two patients suffered from lactic acidosis [19]. However, evidence suggests that lactic acidosis occurrence is only associated with MELD (Model for End-stage Liver Disease) scores >20 [26]. EASL recommends cessation of entecavir treatment if liver function deteriorates due to the risk of lactic acidosis [2].
2.4. Lamivudine
Lamivudine, a cytidine analog, was the first NA to be approved for the treatment of CHB. The recommended dose of lamivudine for adults is 100 mg orally daily [2]. It is usually well-tolerated, with rare and mild side effects.
A large, randomized controlled trial (RCT) investigated the efficacy of lamivudine vs. placebo in HBeAg-positive CHB patients (n = 358) [27]. In this 52-week study, lamivudine treatment resulted in a 98% rate of HBV DNA suppression, compared with 54% in the placebo group (p < 0.001). In addition, 72% of the patients receiving lamivudine had sustained ALT normalization compared with 24% in the placebo group (p < 0.001). By performing liver biopsies in all patients at the end of the treatment period, this study was able to show that lamivudine treatment is associated with a significant improvement in necro-inflammatory activity when compared with placebo (56% vs. 25% respectively, p < 0.001). This landmark study was pivotal in demonstrating the clinical and virologic efficacy of lamivudine. This study is limited by its short follow-up period and by recruitment of Chinese patients only. However, several subsequent studies have further validated the efficacy of lamivudine and evaluated the safety profile of lamivudine as comparable to placebo [28,29,30,31].
Lamivudine has the highest viral resistance rates among currently licensed therapies for CHB; the principal reason lamivudine monotherapy is no longer recommended by international guidelines as a first-line therapeutic option for CBV [2]. The cause of HBV resistance to lamivudine is because of amino acid substitutions in the methionine residue of the conserved tyrosine (Y), methionine (M), aspartate (D), aspartate (D) motif (YMDD) of RNA-dependent DNA polymerase [31,32]. The incidence of the YMDD mutants increases with the duration of therapy, ranging from 14–32% after 1 year of monotherapy and 53–76% after 3 years [33]. Although most YMDD mutants appear early after initiation of therapy, Koukoulioti et al. showed that even in patients who have had a long-term response to lamivudine for over 10 years, there remains a risk of developing resistance [34].
The clinical response to lamivudine can be attenuated after the development of YMDD mutants. Liu et al. investigated the viral load in CHB patients with YMDD mutants (n = 25) and showed that HBV DNA level and HBeAg were significantly higher in the patients with YMDD mutants compared with the negative controls [HBV DNA: 5.77 log10 IU/mL vs. 2.63 log10 IU/mL (p < 0.001), HBeAg: 1.23 IU/mL vs. 0.1 IU/mL (p < 0.05)] [35]. Notably, there was heterogeneity in the different CHB genotypes. However, the findings are validated by existing evidence; virologic breakthroughs can be observed within 2–3 months after the emergence of the YMDD mutant [36].
2.5. Telbivudine
Telbivudine, a synthetic thymidine analog, has been critically evaluated in the pivotal phase 3 GLOBE trial (n = 1367). It is currently not recommended as CHB treatment by EASL guidelines [2]. Telbivudine patients had a significantly superior therapeutic response to lamivudine over a two-year period in HBeAg-positive (63% vs. 48%; p < 0.001) and HBeAg-negative (78% vs. 66%; p = 0.007) patients. Additionally, telbivudine’s resistance profile was shown to be preferable to lamivudine in HBeAg-positive (25.1% vs. 39.5%; p < 0.001) and HBeAg-negative (10.8% vs. 25.9%; p < 0.001) patients. This is supported by a Chinese phase 3 RCT (n = 332), which yielded parallel results. Telbivudine patients had a significantly greater reduction in HBV DNA and a significant difference in the percentage of patients with undetectable HBV DNA (61.9% vs. 38.5%; p < 0.0001). The percentage of telbivudine patients with viral resistance was half that of lamivudine, though this was not found to be significantly different [37].
Furthermore, GLOBE patients who enrolled in a follow-up trial (n = 502) and continued taking telbivudine for 4 years continued with a high rate of viral suppression and resistance rates of 10% [38,39]. Applicability of the GLOBE trial to the wider patient population is limited by population demographics. Ethnic subgroups are highly imbalanced; over 50% of participants were Chinese, and only 1% were Caucasian. As the second RCT was also conducted in China, the evidence for telbivudine’s therapeutic superiority is only applicable to ethnicities that make up the largest sub-groups [40].
The frequency of common AEs, such as fatigue, was comparable in telbivudine and alternative Nas [40,41]. However, serious telbivudine-associated AEs, including nervous system damage, cardiac arrhythmias, and myopathies due to creatinine kinase elevation, have been reported in up to 5% of patients [40,41].
Patients receiving telbivudine had a superior therapeutic response as measured by the reduction in HBV DNA and drug resistance. However, telbivudine monotherapy is not currently recommended [2]. This is due to existing evidence for efficacy being drawn from a singular RCT (GLOBE), insufficient data for cost-effectiveness, and insignificant clinical differences between the drugs.
2.6. Adefovir Dipivoxil (ADV)
ADV, a prodrug of the NA adefovir, is also not recommended as CHB treatment by EASL guidelines [2]. ADV has demonstrated efficacy in HBeAg-positive and HBeAg-negative patients in two double-blinded RCT trials. Marcellin et al. randomized HBeAg-positive patients (n = 515) with 10 mg ADV, 30 mg ADV, or placebo for 48 weeks [20]. Significantly more patients who received 10 mg or 30 mg ADV compared with placebo had a reduced serum HBV DNA (21% vs. 39% vs. 0%, p < 0.001), normalization of ALT levels (48% vs. 55% vs. 16%, p < 0.001) and seroconversion (12% vs. 14% vs. 6%, p < 0.05) [20]. ADV has shown clinical benefit; however, as in the aforementioned trials, over 50% of this study cohort was composed of Asian patients, limiting its global applicability.
Hadziyannis et al. randomized patients with 10 mg ADV or placebo in HBeAg-negative patients (n = 185) for 48 weeks [42]. In those receiving ADV compared with placebo, more patients had a significant reduction in serum HBV DNA (51% vs. 0%, p < 0.01) and normalization of ALT levels (72% vs. 29%, p < 0.01) [42]. This trial included >80% of males; studies have reported gender influences in response to treatment; therefore, the efficacy in females may differ [42,43].
The overall safety of ADV was similar to that of the placebo in both trials with respect to headache, abdominal pain, and nausea [42,44]. However, long-term ADV is associated with AEs such as renal dysfunction in 12% of patients (95% CI 0.08–0.16) and a significant rise in serum creatinine (>0.5 mg/dL) from patients’ baseline in those receiving ADV for 5 years [45].
Resistance is rare before 48 weeks of treatment; however, treatment for 5 years is associated with mutations leading to resistance in 20–29% of patients [20,45]. This is the primary reason for ADV not being recommended and its replacement by antivirals with higher barriers to resistance [2].
2.7. Interferons (IFN)
IFN is thought to target multiple facets of the HBV life cycle to generate an effective antiviral response. Epigenetic modifications have recently been shown to have a role in enhancing this response to clear HBV-infected cells [46]. Pegylated interferon-alpha (PEG-IFNα) is given as a subcutaneous injection, and the standard treatment duration is 48 weeks [2].
PEG-IFNα is structurally different from the traditional standard-IFN by the presence of a polyethylene glycol attachment. It has overtaken standard-IFNα as a standard of care because of its superior efficacy. This was demonstrated in a multicentre study by Cooksley et al., where a significantly higher combined response (HBeAg loss, HBV DNA suppression, and ALT normalization) was observed in PEG-IFNα compared with standard-IFNα (p = 0.036) [47]. By adding a polyethylene glycol attachment, this increases the half-life of interferon in the blood. The efficacy of PEG-IFNα therapy was further validated in a multinational study (n = 814), where CHB patients were randomly assigned to receive either PEG-IFNα monotherapy or in combination with lamivudine or lamivudine monotherapy for a treatment duration of 48 weeks [48]. By following up with patients 24 weeks after the treatment duration, this study successfully evaluated the sustained HBeAg seroconversion. A higher rate of sustained HBeAg seroconversion was noted in patients being treated with PEG-IFNα therapy monotherapy and combination therapy compared with lamivudine monotherapy [32% vs. 19% (p < 0.001) and 27% vs. 19% (p = 0.02), respectively].
PEG-IFNα therapy has several advantages over NA therapy. PEG-IFNα has a more finite treatment duration compared with NAs. In an RCT (n = 544) of HBeAg-positive CBV, HBeAg seroconversion was seen in 36% of patients taking PEG-IFNα (180 μg) for 48 weeks [49]. Moreover, a significant benefit of PEG-IFNα therapy is that there is no risk of drug resistance, unlike NAs, where there is a significant limitation. Furthermore, HBsAg clearance can reach 40% in patients receiving PEG-IFNα, which is much higher than NA monotherapy. The antiviral response is also much more durable in PEG-IFNα therapy [50].
The safety profile of PEG-IFNα therapy is a major limitation; a retrospective study (n = 11,241) of 73 different centers showed that IFN therapy can have several systemic adverse effects that can cause morbidity [51]. Initial influenza-like illness is common, and other frequently reported symptoms include fatigue, anorexia, and weight loss. Patients may experience emotional lability and depression, which may be associated with suicidal ideations. Bone marrow suppression and thyroid hormone disturbances have also been reported [52]. Furthermore, there is a substantial risk of other serious complications, such as serious infections and hepatic failure; it is therefore contraindicated in patients with decompensated cirrhosis [53,54]. The unfavorable safety profile limits the number of suitable candidates for therapy, and patients often require dose reductions or early cessation of treatment.
2.8. Combination Therapies
There are now several licensed therapies available for CHB treatment. The utility of combining these therapies has been actively investigated. Notably, this approach has shown improved outcomes in patients with HIV and chronic hepatitis C infection [55,56].
There is limited evidence that combining NAs in treatment-naive patients has greater antiviral efficacy than monotherapy. Lok et al. compared the efficacy of entecavir to entecavir with TDF in HBeAg-positive and HBeAg–negative CHB patients (n = 379) [57]. In this RCT, the primary endpoint (HBV DNA < 50 IU/mL) was comparable between entecavir and entecavir-TDF (76% vs. 83% respectively, p = 0.088), additionally the rate of seroconversion was also similar in both groups. Peterson et al. showed that the same combination can be safe and effective in causing a rapid suppression of HBV DNA in advanced liver disease. In patients with advanced liver disease who have a poor response to therapy, there is an elevated risk of developing HCC; this specific combination may, therefore, be considered in these patients [58]. Other combinations, such as lamivudine-adefovir and lamivudine–telbivudine, have shown no superior virologic or biochemical response compared with monotherapy of any of these drugs [59,60].
Combination therapy of NAs with PEG-IFNα has shown promise as a viable treatment option, but its use remains controversial. Several treatment strategies for combination therapy can be considered, including de novo combination therapy, switch therapy, or add-on therapy.
Marcellin et al. compared de novo combination therapy of TDF and PEG-IFNα compared with monotherapy of TDF or PEG-IFNα and showed that the combination therapy induced a higher rate of HBsAg clearance [61]. Although the de novo combination was shown to be effective, these findings were not uniform across different genotypes. However, a meta-analysis evaluated the combination of NAs with IFN and included studies assessing de novo combination therapy (n = 33), add-on therapy (n = 15), and switch therapy (n = 12) [62]. The probability of HBsAg clearance was found to be highest with de novo combination therapy (Relative Risk 15.59, 95% CI: 3.22–75.49) compared with NA monotherapy.
There remains insufficient robust evidence proving that combination therapies achieve treatment targets to a significantly greater extent than currently available monotherapies; hence, there is difficulty justifying their use as a standard of care. This is further reflected by the EASL guidelines, which do not currently recommend the routine use of combination therapies in clinical practice [2].
2.9. Current Landscape of Antiviral Therapies in the Middle East
In 2016, the World Health Organisation outlined an ambitious but achievable target to eliminate viral hepatitis, setting a target of a 90% reduction in new infections and a 65% reduction in viral hepatitis-related mortality by 2030. However, there remain challenges in the Middle East that need to be addressed, such as lack of disease awareness and knowledge, lack of proper screening, underdiagnosis, social stigma, lack of an established referral system, and treatment costs [5]. Authorities should work to improve access to affordable and effective antiviral therapies to achieve elimination goals [63].
Furthermore, it is imperative to implement public health initiatives that prioritize education, screening, and awareness in order to combat hepatitis B in the Middle East. Public health organizations can alleviate the burden of hepatitis B in the region by promoting widespread testing and increasing awareness of the significance of early detection and treatment.
Treatment of CHB in the Middle East has been transformed with the advent of NAs and PEG-IFNα. Long-term follow-up studies have indicated that NAs and PEG-IFNα decrease the risk of cirrhosis, cirrhosis decompensation, and HCC [64,65]. The choice of initial NA therapy in most CHB patients includes entecavir, TDF, and TAF [2]. These regimens are considered first-line options because of their high antiviral potency, favorable safety profile, and high genetic barrier to resistance compared with other licensed therapies. Advantages of PEG-IFNα therapy include a finite treatment duration and a more durable antiviral response compared with NAs; it is a first-line treatment in patients with mild to moderate disease. PEG-IFNα is contraindicated in decompensated cirrhosis, whereas NAs can be used safely [2].
The choice of licensed therapy is, therefore, based on the patient’s preference and clinical assessment of the predicted likelihood of response. The overall rate of HBsAg clearance is low during NA treatment, and virological relapse is common when NAs are discontinued [66]. NAs must, therefore, be administered indefinitely, which increases the risk of resistance and can be unfavorable from an economic perspective [67,68]. Moreover, PEG-IFNα must be administered as subcutaneous injections and is associated with numerous systemic AEs.
The significant role of chronic inflammation as a driver of hepatocellular carcinoma (HCC) in HBV patients has been demonstrated in recent studies demonstrating the important role of CD4+ CD25+ regulatory T cells in chronic inflammation leading to HCC development [69]. The risk of HCC has reduced since the introduction of these therapies; however, CHB remains a leading cause of HCC worldwide. The proper treatment of HBV has a significant impact on the incidence of HBV complications such as cirrhosis and, most of all, hepatocellular carcinoma in Western countries [70]. The observed improvement in overall incidence and survival of HCC is due to better screening programs, HBV vaccination, surveillance, and treatment [70]. Furthermore, the impact of alcohol intake is an independent predictor of cirrhosis and death in subjects with chronic HBV [71]. This factor must be considered when planning an effective national program for HBV management.
With the availability of a highly effective cure for chronic hepatitis C, there is now an active investigation to identify novel therapeutic targets that can overcome the limitations of currently licensed drugs and achieve a safe, clinical cure. Advances in technology and understanding of HBV have led to the development of novel agents. These novel drugs can target multiple aspects of the HBV lifecycle and will hopefully provide a path to HBV eradication.
Many patients with hepatitis C virus (HCV) have also been exposed to hepatitis B virus (HBV). The two viruses interact, and in most cases, HCV suppresses HBV. Treatment of HBV/HCV coinfected patients can represent a challenge. American Association for this Study of Liver Diseases (AASLD) recommends starting people with HBV/HCV coinfection who meet the criteria for treatment of active HBV infection [72] on therapy at the same time or before starting direct-acting antiviral (DAA) for HCV treatment. When HCV is treated with direct antiviral agents, this suppressive effect is removed, HBV replication may increase, and a flare in liver enzymes with liver injury occurs. All patients with chronic HCV should, therefore, be checked for serologic HBV. Patients with hepatitis B surface antigen are at the highest risk for reactivation, and these patients should be monitored closely and receive prophylactic treatment of HBV if there is any serological signal of reactivation during HCV treatment.
3. Conclusions
In summary, the Middle East’s hepatitis B epidemic necessitates a multifaceted strategy that includes vaccination, access to antiviral medications, and comprehensive public health interventions. Healthcare systems in the Middle East can make substantial progress in the fight against hepatitis B and the enhancement of the general health of the population by prioritizing these endeavors. We have evaluated the current key literature determining the efficacy, viral resistance, and safety profile of the currently licensed therapies for CBV in the Middle East. NAs and PEG-IFNα have become the mainstay of management and have significantly improved outcomes in CHB. However, major limitations exist with these therapies, and further advancements are needed to identify a potential cure for HBV.
Author Contributions
Conceptualization, L.A.; Writing—original draft preparation, H.B., N.D., K.K., M.M.K. and R.H.P.; writing—review and editing, all authors; visualization, L.A.; supervision, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Hepatitis B. World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 18 July 2024).
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Akyıldız, M.; Ahıskalı, E.; Zeybel, M.; Yurdaydın, C. Regional Epidemiology, Burden, and Management of Hepatitis B Virus in the Middle East. Clin. Liver Dis. 2020, 14, 212–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco, E.; Bagnato, B.; Marino, M.G.; Meleleo, C.; Serino, L.; Zaratti, L. Hepatitis B: Epidemiology and prevention in developing countries. World J. Hepatol. 2012, 4, 74–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanai, F.; Alkhatry, M.; Alzanbagi, A.; Kumar, S. Hepatitis B virus infection in Saudi Arabia and the UAE: Public health challenges and their remedial measures. J. Infect. Public Health 2023, 16, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Brierley, R. Elimination of viral hepatitis by 2030: Ambitious, but achievable. Lancet Gastroenterol. Hepatol. 2019, 4, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaye, A.; Tariq, Z.; Alrubaiy, L. Prevalence of hepatitis B seromarkers and hepatitis C antibodies in blood donors in Basra, Iraq. BMJ Open Gastroenterol. 2016, 3, e000067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwon, S.Y.; Lee, C.H. Epidemiology and prevention of hepatitis B virus infection. Korean J. Hepatol. 2011, 17, 87–95. [Google Scholar] [CrossRef]
- Vlachogiannakos, J.; Papatheodoridis, G.V. Hepatitis B: Who and when to treat? Liver Int. 2018, 38, 71–78. [Google Scholar] [CrossRef]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.Y.; Chuang, W.; Stepanova, T.; Hui, A.-J.; Lim, Y.-S.; Mehta, R.; Janssen, H.L.A.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Fung, S.; Seto, W.K.; Chuang, W.; Chen, C.; Kim, H.J.; Hui, A.J.; Janssen, H.L.A.; Chowdhury, A.; Tsang, T.Y.O.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 185. [Google Scholar] [CrossRef]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Cusato, J.; Marinaro, L.; Trentini, L.; Alcantarini, C.; Mussa, M.; Simiele, M.; D’Avolio, A.; Di Perri, G.; Bonora, S. Clinical pharmacology of tenofovir clearance: A pharmacokinetic/pharmacogenetic study on plasma and urines. Pharmacogenomics J. 2015, 16, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; He, G.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective Intracellular Activation of a Novel Prodrug of the Human Immunodeficiency Virus Reverse Transcriptase Inhibitor Tenofovir Leads to Preferential Distribution and Accumulation in Lymphatic Tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Arribas, J.R.; Andrade-Villanueva, J.; DiPerri, G.; Van Lunzen, J.; Koenig, E.; Elion, R.; Cavassini, M.; Madruga, J.V.; Brunetta, J.; et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: A randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet. Infect. Dis. 2016, 16, 43–52. [Google Scholar] [CrossRef]
- Pan, C.Q.; Trinh, H.; Yao, A.; Bae, H.; Lou, L.; Chan, S. Efficacy and Safety of Tenofovir Disoproxil Fumarate in Asian-Americans with Chronic Hepatitis B in Community Settings. PLoS ONE 2014, 9, e89789. [Google Scholar] [CrossRef]
- Marcellin, P.; Wong, D.K.; Sievert, W.W.; Buggisch, P.P.; Petersen, J.; Flisiak, R.; Manns, M.; Kaita, K.; Krastev, Z.; Lee, S.S.; et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019, 39, 1868. [Google Scholar] [CrossRef]
- Lovett, G.C.; Nguyen, T.; Iser, D.M.; Holmes, J.A.; Chen, R.; Demediuk, B.; Shaw, G.; Bell, S.J.; Desmond, P.V.; Thompson, A.J. Efficacy and safety of tenofovir in chronic hepatitis B: Australian real world experience. World J. Hepatol. 2017, 9, 48. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.M.; Lim, J.K.; Pan, C.Q.; Nguyen, M.H.; Ray Kim, W.; Mannalithara, A.; Trinh, H.; Chu, D.; Tran, T.; et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US—The ENUMERATE study. Aliment. Pharmacol. Ther. 2016, 43, 134–144. [Google Scholar] [CrossRef]
- Marcellin, P.; Chang, T.; Lim, S.G.L.; Sievert, W.; Tong, M.; Arterburn, S.; Borroto-Esoda, K.; Frederick, D.; Rousseau, F. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen–positive chronic hepatitis B. Hepatology 2008, 48, 750–758. [Google Scholar] [CrossRef]
- Zou, X.J.; Jiang, X.Q.; Tian, D.Y. Clinical features and risk factors of creatine kinase elevations and myopathy associated with telbivudine. J. Viral Hepat. 2011, 18, 892–896. [Google Scholar] [CrossRef]
- Jonas, M.M.; Chang, M.; Sokal, E.; Schwarz, K.B.; Kelly, D.; Kim, K.M.; Ling, S.C.; Rosenthal, P.; Oraseanu, D.; Reynolds, L.; et al. Randomized, Controlled Trial of Entecavir Versus Placebo in Children With Hepatitis B Envelope Antigen-Positive Chronic Hepatitis B. Hepatology 2016, 63, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Su, T.H.; Hu, T.H.; Chen, C.Y.; Huang, Y.H.; Chuang, W.L.; Lin, C.C.; Wang, C.C.; Su, W.W.; Chen, M.Y.; Peng, C.Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Tenney, D.J.; Rose, R.E.; Baldick, C.J.; Pokornowski, K.A.; Eggers, B.J.; Fang, J.; Wichroski, M.J.; Xu, D.; Yang, J.; Wilber, R.B.; et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009, 49, 1503–1514. [Google Scholar] [CrossRef]
- Lange, C.M.; Bojunga, J.; Hofmann, W.P.; Wunder, K.; Mihm, U.; Zeuzem, S.; Sarrazin, C. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 2009, 50, 2001–2006. [Google Scholar] [CrossRef]
- Lai, C.-L.; Chien, R.-N.; Leung, N.W.; Chang, T.-T.; Guan, R.; Tai, D.-I.; Ng, K.-Y.; Wu, P.-C.; Dent, J.C.; Barber, J.; et al. A One-Year Trial of Lamivudine for Chronic Hepatitis B. N. Engl. J. Med. 1998, 339, 61–68. [Google Scholar] [CrossRef]
- Lok, A.S.F.; Lai, C.; Leung, N.; Yao, G.; Cui, Z.; Schiff, E.R.; Dienstag, J.L.; Heathcote, E.; Little, N.R.; Griffiths, D.A.; et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003, 125, 1714–1722. [Google Scholar] [CrossRef]
- Liaw, Y.; Sung, J.J.Y.; Chow, W.C.; Farrell, G.; Lee, C.; Yuen, H.; Tanwandee, T.; Tao, Q.-M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef]
- Srivastava, A. Clinical trial of lamivudine in children with chronic hepatitis B. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2002, 21, 169. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Leung, N.W.Y.; Chang, T.T.; Guan, R.; Tai, D.I.; Ng, K.Y.; Chien, R.; Dent, J.; Roman, L.; Edmundson, S.; et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology 2000, 119, 172–180. [Google Scholar] [CrossRef]
- Dienstag, J.L.; Schiff, E.R.; Wright, T.L.; Perrillo, R.P.; Hann, H.-W.L.; Goodman, Z.; Crowther, L.; Condreay, L.D.; Woessner, M.; Rubin, M.; et al. Lamivudine as Initial Treatment for Chronic Hepatitis B in the United States. N. Engl. J. Med. 2008, 341, 1256–1263. [Google Scholar] [CrossRef]
- Lai, C.; Dienstag, J.; Schiff, E.; Leung, N.W.Y.; Atkins, M.; Hunt, C.; Brown, N.; Woessner, M.; Boehme, R.; Condreay, L. Prevalence and Clinical Correlates of YMDD Variants during Lamivudine Therapy for Patients with Chronic Hepatitis B. Clin. Infect. Dis. 2003, 36, 687–696. [Google Scholar] [CrossRef]
- Koukoulioti, E.; Brodzinski, A.; Mihm, U.; Sarrazin, C.; Jung, M.C.; Schott, E.; Fülöp, B.; Schlosser, B.; Berg, T.; van Bömmel, F. Risk factors for resistance development against lamivudine during long-term treatment of chronic hepatitis B virus infections. Eur. J. Gastroenterol. Hepatol. 2019, 31, 845–852. [Google Scholar] [CrossRef]
- Liu, H.; Wan, Z.; She, L.; Zhu, Y.; Cai, Z.; Wu, B.; Zhuang, Q.; Ke, P.; Wu, X.; Li, Z.; et al. Inflammation Pharmacological Reaction and YMDD Mutational Patterns in Lamivudine Therapeutics Hepatitis B Virus. Front. Pharmacol. 2021, 12, 648170. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Z.; Ma, S.; Zeng, G.; Zhou, Z.; Luo, K.; Hou, J. Clinical and virological characteristics of lamivudine resistance in chronic hepatitis B patients: A single center experience. J. Med. Virol. 2005, 75, 391–398. [Google Scholar] [CrossRef]
- Hou, J.; Yin, Y.; Xu, D.; Tan, D.; Niu, J.; Zhou, X.; Wang, Y.; Zhu, L.; He, Y.; Ren, H.; et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology 2008, 47, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thongsawat, S.; Gane, E.J.; Liaw, Y.-; Jia, J.; Hou, J.; Chan, H.L.Y.; Papatheodoridis, G.; Wan, M.; Niu, J.; et al. Efficacy and safety of continuous 4-year telbivudine treatment in patients with chronic hepatitis B. J. Viral Hepat. 2013, 20, e37–e46. [Google Scholar] [CrossRef]
- Gane, E.J.; Wang, Y.; Liaw, Y.F.; Hou, J.; Thongsawat, S.; Wan, M.; Moon, Y.M.; Jia, J.; Chao, Y.C.; Niu, J.; et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011, 31, 676–684. [Google Scholar] [CrossRef]
- Liaw, Y.; Gane, E.; Leung, N.; Zeuzem, S.; Wang, Y.; Lai, C.L.; Heathcote, E.J.; Manns, M.; Bzowej, N.; Niu, J.; et al. 2-Year GLOBE Trial Results: Telbivudine Is Superior to Lamivudine in Patients with Chronic Hepatitis B. Gastroenterology 2009, 136, 486–495. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.; Seto, W.; Yuen, M. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J. Antimicrob. Chemother. 2011, 66, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Wulfsohn, M.S.; et al. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Negative Chronic Hepatitis B. N. Engl. J. Med. 2009, 348, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and therapeutic treatments for viral diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2012, 34, 1050. [Google Scholar] [CrossRef]
- Marcellin, P.; Chang, T.; Lim, S.G.; Tong, M.J.; Sievert, W.; Shiffman, M.L.; Jeffers, L.; Goodman, Z.; Wulfsohn, M.S.; Xiong, S.; et al. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Positive Chronic Hepatitis B. N. Engl. J. Med. 2003, 348, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Ma, J.; et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006, 131, 1743–1751. [Google Scholar] [CrossRef]
- Gailhouste, L.; Sudoh, M.; Qin, X.; Watashi, K.; Wakita, T.; Ochiya, T.; Matsuura, T.; Kojima, S.; Furutani, Y. Epigenetic reprogramming promotes the antiviral action of IFNα in HBV-infected cells. Cell Death Discov. 2021, 7, 130. [Google Scholar] [CrossRef]
- Cooksley, W.G.E.; Piratvisuth, T.; Lee, S.D.; Mahachai, V.; Chao, Y.C.; Tanwandee, T.; Chutaputti, A.; Chang, W.Y.; Zahm, F.E.; Pluck, N. Peginterferon alpha-2a (40 kDa): An advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J. Viral Hepat. 2003, 10, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.K.K.; Piratvisuth, T.; Luo, K.X.; Marcellin, P.; Thongsawat, S.; Cooksley, G.; Gane, E.; Fried, M.W.; Chow, W.C.; Paik, S.W.; et al. Peginterferon Alfa-2a, Lamivudine, and the Combination for HBeAg-Positive Chronic Hepatitis B. N. Engl. J. Med. 2005, 352, 2682–2695. [Google Scholar] [CrossRef]
- Liaw, Y.-F.; Jia, J.-D.; Chan, H.; Han, K.; Tanwandee, T.; Chuang, W.; Tan, D.; Chen, X.; Gane, E.; Piratvisuth, T.; et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 2011, 54, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Su, C.; Wang, Y.; Lee, K.; Huo, T.; Lin, H.; Huang, Y. Predictors of Response to Pegylated Interferon in Chronic Hepatitis B: A Real-World Hospital-Based Analysis; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Fattovich, G.; Giustina, G.; Favarato, S.; Ruol, A. A survey of adverse events in 11 241 patients with chronic viral hepatitis treated with alfa interferon. J. Hepatol. 1996, 24, 38–47. [Google Scholar] [CrossRef]
- Kozielewicz, D.; Zaleśna, A.; Dybowska, D. Can pegylated interferon α 2a cause development of thyroid disorders in patients with chronic hepatitis B? Expert Opin. Drug Saf. 2014, 13, 1009–1014. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Di Bisceglie, A.M.; Waggoner, J.G.; Park, Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology 1993, 104, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Perrillo, R.; Tamburro, C.; Regenstein, F.; Balart, L.; Bodenheimer, H.; Silva, M.; Schiff, E.; Bodicky, C.; Miller, B.; Denham, C.; et al. Low-Dose, Titratable Interferon Alfa in Decompensated Liver Disease Caused by Chronic Infection with Hepatitis B Virus; Elsevier BV: Amsterdam, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Jordan, R.; Gold, L.; Cummins, C.; Hyde, C. Systematic review and meta-analysis of evidence for increasing numbers of drugs in antiretroviral combination therapy. BMJ 2002, 324, 757–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Gonçales, F.L.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D.; et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef]
- Lok, A.S.; Trinh, H.; Carosi, G.; Akarca, U.S.; Gadano, A.; Habersetzer, F.; Sievert, W.; Wong, D.; Lovegren, M.; Cohen, D.; et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 2012, 143, 619–628.e1. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Su, J.; Jen, C.; You, S.; Lu, S.; Huang, G.; Iloeje, U.; for the REVEAL-HBV Study Group. Risk of Hepatocellular Carcinoma Across a Biological Gradient of Serum Hepatitis B Virus DNA Level. JAMA J. Am. Med. Assoc. 2006, 295, 65–73. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Lai, J.Y.; Zeuzem, S.; Chow, W.C.; Heathcote, E.J.; Perrillo, R.P.; Brosgart, C.L.; Woessner, M.A.; Scott, S.A.; Gray, D.F.; et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J. Hepatol. 2008, 48, 728–735. [Google Scholar] [CrossRef]
- Lai, C.L.; Leung, N.; Teo, E.K.; Tong, M.; Wong, F.; Hann, H.W.; Han, S.; Poynard, T.; Myers, M.; Chao, G.; et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005, 129, 528–536. [Google Scholar] [CrossRef]
- Marcellin, P.; Ahn, S.H.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkashab, M.; Chuang, W.-L.; Lim, S.-G.; Tabak, F.; Mehta, R.; et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 2016, 150, 134–144.e10. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Zhang, W.; Cheng, Y.; He, Q.; Wang, F. Effect of combination treatment based on interferon and nucleos(t)ide analogues on functional cure of chronic hepatitis B: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184, Erratum in Lancet Gastroenterol. Hepatol. 2019, 4, e4. https://doi.org/10.1016/S2468-1253(19)30099-8. PMID: 30647010. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Sypsa, V.; Dalekos, G.; Yurdaydin, C.; van Boemmel, F.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; et al. Eight-year survival in chronic hepatitis B patients under long-term entecavir or tenofovir therapy is similar to the general population. J. Hepatol. 2018, 68, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Mak, C.W.; Lee, S.K.; Ip, Z.M.; Lam, A.T.; Iu, H.W.; Leung, J.M.; Lai, J.W.; Lo, A.O.; et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013, 58, 1537. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.; Wong, D.K.; Fung, J.; Huang, F.; Lai, C.; Yuen, M. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology 2013, 58, 923. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, G.; Li, Y.; Li, G.; Liang, Y.; Zhou, F.; Zhou, S.; Yang, Y.; Jia, W.; Gao, Y.; et al. The trend of direct medical costs and associated factors in patients with chronic hepatitis B in Guangzhou, China: An eight-year retrospective cohort study. BMC Med. Inform. Decis. Mak. 2021, 21, 1–71. [Google Scholar] [CrossRef]
- Allard, N.L.; Maclachlan, J.H.; Dev, A.; Dwyer, J.; Srivatsa, G.; Spelman, T.; Thompson, A.J.; Cowie, B.C. Adherence in chronic hepatitis B: Associations between medication possession ratio and adverse viral outcomes. BMC Gastroenterol. 2020, 20, 140. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bucci, L.; Garuti, F.; Lenzi, B.; Pecorelli, A.; Farinati, F.; Giannini, E.G.; Granito, A.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; et al. The evolutionary scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2017, 37, 259–270. [Google Scholar] [CrossRef]
- Bedogni, G.; Miglioli, L.; Masutti, F.; Ferri, S.; Castiglione, A.; Lenzi, M.; Crocè, L.S.; Granito, A.; Tiribelli, C.; Bellentani, S. Natural course of chronic HCV and HBV infection and role of alcohol in the general population: The Dionysos Study. Am. J. Gastroenterol. 2008, 103, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Aronsohn, A.; Price, J.; Lo Re, V. AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2023, ciad319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).