Abstract
Microbiome dysbiosis—an imbalance in gut microbial communities—has emerged as a critical factor in the pathogenesis of neurological disorders, particularly Alzheimer’s and Parkinson’s diseases. This review examines the role of gut microbiota in neurodegeneration, emphasizing how dysbiosis disrupts gut–brain communication through mechanisms such as impaired gut permeability, systemic inflammation, and neuroinflammation. The gastrointestinal and central nervous systems interact bidirectionally, with microbial metabolites like short-chain fatty acids playing a pivotal role in maintaining gut and brain health. Dysbiotic shifts in microbial composition can compromise the blood–brain barrier, enabling inflammatory molecules to alter brain biochemistry and potentially accelerate neurodegenerative processes. Additionally, this review explores therapeutic strategies—including probiotics, prebiotics, and dietary modifications—designed to restore microbial balance, reduce neuroinflammation, and slow disease progression. Further research is essential to refine microbiome-targeted therapies and fully elucidate their potential in managing neurodegenerative diseases.
1. Introduction
The human microbiota consists of diverse microorganisms that colonize and interact with the body. These interactions can be commensal, mutualistic, or pathogenic. The term microbiome refers to the collective genomic content of these microorganisms at specific body sites, including the skin, mucosa, gastrointestinal tract, respiratory tract, urogenital tract, and mammary glands [1]. Recent systematic reviews have highlighted differences in gut microbiome composition between individuals with various neurological diseases and healthy controls [2].
Since its discovery, the gut microbiota has been linked to multiple aspects of human health and disease, including diabetes, obesity, and neurodegenerative disorders [3]. An imbalance in the microbiome, known as dysbiosis, is implicated in the etiology of many diseases and may be managed through lifestyle adjustments, dietary modifications, and microbiome-targeted interventions [4]. Dysbiosis of the gut microbiota can disrupt molecular communication with nerve cells, potentially contributing to neurodegeneration and identifying new therapeutic targets for counteracting neuroinflammation [5]. Furthermore, large-scale analyses indicate that dysbiosis is associated with chronic gut inflammation, promotes a proinflammatory state, and compromises critical processes such as protein clearance and blood–brain barrier (BBB) integrity [6].
Dysbiosis of the gut microbiome imbalance of commensal bacterial communities in the gastrointestinal system facilitates neurodegenerative pathologies through derangement of the key gut–brain communicative interfaces. In general, dysbiosis is associated with the induction of widespread inflammation due to the enhancement of pro-inflammatory cytokines and the weakening of the gut and BBB, allowing the passage of noxious molecules to reach the brain and foster neuroinflammation [7].
This inflammatory response, therefore, triggers immune cells that can damage neurons and lead to the cognitive decline seen in neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s (PD). Moreover, dysbiotic gut microbes alter the production of neuroprotective metabolites like short-chain fatty acids (SCFAs) and neurotransmitters that impact brain health and mental well-being [8]. Dysbiosis also enhances the misfolding and aggregation of the hallmarks of these diseases, such as beta-amyloid and alpha-synuclein, which could migrate from the gut to the brain through neural pathways [9,10]. These mechanisms suggest a more complex relationship between gut health and the progression of neurodegenerative diseases, supporting the hypothesis that targeted microbiota interventions may offer therapeutic potential for managing or even preventing these conditions [11]. The gut microbiome, through such pathways, may thus influence brain functions; indeed, dysbiosis relates to disrupted gut–brain communication and the onset of neuroinflammation [12].
The aim of this review was to explore the role of gut microbiota in neurodegeneration, highlighting how dysbiosis disrupts gut–brain communication through various mechanisms, including impaired gut permeability, inflammation, and neuroinflammation. Furthermore, it discusses therapeutic strategies such as probiotics, prebiotics, and dietary modifications to restore microbial balance, mitigate neuroinflammation, and delay the progression of diseases like AD and PD.
Factors Contributing to Dysbiosis
Microbiome dysbiosis is increasingly identified in the major pathogenesis of neurological disorders such as AD and PD and modulates the gut–brain axis. Dysbiosis is defined as an imbalance in microbial composition, and depends on host-related factors such as genetic predisposition and health status, while environmental triggers include diet and exposure to xenobiotics [13]. Genetic background is known to be a critical element in the shaping of this relationship, with some gene products, such as the major histocompatibility complex (MHC) class I and II, influencing immune responses that may be associated with susceptibility to autoimmune and inflammatory diseases [14]. Whereby genes such as NOD2 and ATG16L1 act to modulate bacterial clearance, dysregulation induces bacterial dysbiosis and, correspondingly, host disease susceptibility [15]. Furthermore, gut microbe metabolic output may have direct effects on host gene expression, pointing toward an epigenetic interaction between microbiota and host extending to system health, including brain function [16]. Dysbiosis of the microbiome, through microbial metabolites and immune interactions, influences gene expression in AD and PD by altering DNA and histone structures while preserving the genetic code [17].
One key interaction highlighting this influence is the interplay between gut microbiota, mitochondria, and epigenetics. Mitochondria regulate gene expression both directly—through oxidative stress and calcium signaling—and indirectly, via microbial metabolites that influence mitochondrial function [18]. Therapeutically, manipulation of the gut–mitochondria axis using probiotics, prebiotics, and epigenetic drugs may be a beneficial step in various clinical approaches [19]. Health status can be a strong determinant of gut microbiome composition, since disturbances in immune homeostasis—infection or inflammation—can alter microbial communities. These become highly relevant in neurological contexts, given that the metabolites of the gut microbiome might have the potential to modulate immune responses across several tissues, including the brain [20,21]. The gut microbiome is quite important in neurological health and influences diseases such as AD and PD. The specific microbial shifts, such as an increase in Bacteroidetes and a reduction in Firmicutes induced by propionate, activate the FFAR3 receptor in the enteric nervous system, promoting neuroprotection [22]. Other butyrate-producing bacteria may be associated with lower rates of hospitalization and mortality from infections, suggesting the role of gut microbes in the immune system [23]. There are specific microbes that are associated with inflammatory cytokines that could subsequently influence risks for the development of the disease [24].
Diet is a key determinant of the composition, stability, and diversity of the gut microbiome, significantly influencing metabolic and intestinal health. Different dietary patterns, such as the Mediterranean diet, high-fiber diet, and Western diet, uniquely shape the gut microbiome, impacting the risk and prevention of diet-related diseases [25]. Given the individual variability of the gut microbiome, a movement toward personalized nutrition may be integral to optimizing health outcomes [26]. Gut flora plays an important role in the regulation of cognition and mood, enabling the brain’s potential to manage emotion and cognitive dysregulation. A combined strategy of dietary supplementation with exercise has also been proposed, which could potentially enhance gut health and cognitive function.
Exercise can alter the composition of gut bacteria, favoring homeostasis [27]. Different trials were systematically reviewed to understand the influence of exercise on gut microbiota, considering factors like type, frequency, intensity, and duration. It is likely that moderate to high-intensity exercises lasting from 30 to 90 min, at least three times a week for eight weeks or more, can change gut microbiota composition in both healthy and clinical populations [28]. Gut metabolites of endocannabinoids stimulate sensory neurons and increase levels of dopamine during exercise, a finding that could mean circulating signals derived from the gut may influence motivation to exercise [29].
Since the beginning of the 20th century, millions of people have been saved from bacterial infection by mass-produced and widely used antibiotics. However, there has been increasingly growing evidence to date that antibiotics accumulate in natural environments, particularly in water and soil [30,31]. The increasing knowledge of the gut microbiota’s important role in host health has thus drawn considerable attention to the persistent and significant impacts of antibiotic use on human gut microbiota [32]. Global antibiotic use disrupts the diversity and composition of the gut microbiome, whereby quinolone, metronidazole, and combined treatments commonly cause prolonged dysbiosis [30,31,32]. Antibiotics strongly interfere with the balance of gut microbiota but long-term implications for health, particularly in children, need to be determined [31,33]. The bacteriocidal effects of ampicillin, vancomycin, metronidazole, and neomycin were tested and resulted in profound changes within the intestinal microbiota: an overgrowth of Enterococcus and a reduction in Lactobacillus, which could well lead to the development of antibiotic-resistant strains, and thus may irreversibly alter the microbial flora, increasing disease vulnerability [34]. Furthermore, xenobiotics, such as environmental pollutants, are present in food, cosmetics, personal care products, and pharmaceuticals, which one can access quite easily and thus may be exposed to the gut microbiota. Exposure to such substances might cause an imbalance in the population composition of gut microbes, resulting in dysbiosis [35].
2. The Gut–Brain Axis
The gut–brain axis is a complex network with bidirectional communications in the central nervous system (CNS), autonomic nervous system (ANS), and enteric nervous system (ENS) through both neural and humoral signaling pathways. Microbial metabolites, including SCFAs, secondary bile acids, and tryptophan-derived products, further enhance gut–brain interactions. However, disruptions caused by pathogenic bacteria can lead to inflammation, impacting neuronal development and potentially triggering neurodegenerative processes [36].
Through this intricate network, the brain can detect gut-related threats and adjust physiological and immune responses accordingly, emphasizing the critical role of the microbiota in maintaining both gastrointestinal and brain health [37]. The gut–brain axis appears to be central to understanding neurodegenerative diseases such AD and PD [38]. Furthermore, dysbiosis may exacerbate neuroinflammation, accelerating neurodegeneration through chronic immune activation and protein aggregation in the brain [39].
2.1. Gut Barrier
The gut barrier is a composite of a highly distributed mucus layer and an epithelial layer with junctional proteins, among the most important interfaces between external and internal milieus. When barrier integrity is disrupted, permeability significantly increases and allows the translocation of commensal microbes, microbial products (including metabolites and virulence factors), and other luminal contents. This might impair the barrier functions, which leads to an inappropriate immune–inflammatory response, including inflammation, allergies, and autoimmune reactions driven by molecular mimicry and altered T-cell responses [10]. The cross-regulation by host–microbiota interactions means that barrier functions include the continuous sampling of the lumen by dendritic cells and activation of T cells, especially regulatory T cells (Tregs) [40]. These include certain commensal bacteria, such as Bacteroides fragilis, Bifidobacterium infantis, and species in Firmicutes, that foster the proliferation of Tregs like FOXP3+ and IL-10-producing Tregs. The importance of these Tregs pertains to suppressing pathological inflammation-misdirected effector T cells that enhance barrier function [41].
2.2. Mechanisms of Gut–Brain Communication
The microbiota–gut–brain axis comprises a network of neural connections, including the CNS, ANS, and ENS, and functions through neural and humoral signaling. Communication between the gut and brain involves continuous direct and indirect signals relayed via the immune system, neurological pathways, and systemic circulation [42]. The ENS interacts with the brain through afferent neural circuits composed of sensory nerves, which transmit modulatory signals that prompt gut reflexes. This enteric communication also modulates immune responses via receptors on immune cells. The brain’s perception of gut conditions is largely facilitated by the vagus nerve, composed of 80% visceral afferent fibers. Key neurotransmitters, such as serotonin, dopamine, and gamma aminobutyric acid (GABA), are essential in this signaling process, transmitting endogenous signals through both vagal and sympathetic pathways [43,44]. The brain detects microbial infections through early warning signals that activate the vagal sensory ganglia and the nucleus tractus solitarii [45]. In response to afferent signals, the CNS communicates with the gut through pathways crucial for regulating motility, mucus secretion, barrier integrity, and visceral sensitivity [5]. Beyond traditional neural pathways, the microbiota–gut–brain axis uses humoral signaling molecules and hormonal components. Disruptions in this network may lead to impaired gastrointestinal and brain function [46]. Signals traveling between the gut and brain can indicate harmful stimuli, such as intestinal distension, bacterial endotoxins, or pro-inflammatory cytokines, prompting the brain to alter gut physiology or immune responses, including cytokine secretion [47].
3. The Role of the Microbiome in Neuroinflammation
The microbiome has emerged as a critical regulator of neuroinflammation. Indeed, various studies have shown that an imbalanced microbiome leads to disruption of the gut barrier, allowing microbial metabolites, toxins, and pro-inflammatory cytokines to leak into the systemic circulation and reach the brain [48]. This process amplifies neuroinflammation and has been implicated in neurodegenerative diseases such as AD, multiple sclerosis (MS), and PD [49,50].
In dysbiosis, increased pro-inflammatory cytokines in AD mainly contribute to amyloid plaque formation and accelerate cognitive decline [51]. SCFAs, normally produced by a balanced microbiome, may modulate the function of the BBB, immune cells, and neuroinflammatory processes; low levels of SCFAs have been associated with higher brain inflammation [5,52]. Other studies suggest that microbial composition alteration is linked to many neuropsychiatric diseases. For example, Blautia and Gammaproteobacteria were associated with increased neuroinflammatory markers in major depressive disorder and schizophrenia [53]. These studies indicate that a predisposition toward neurodegenerative disease may exist via microbial profiles interacting with the immune system and CNS due to brain inflammation. The search for the restoration of microbial balance as a means of supporting the management of neuroinflammatory diseases has involved probiotics, prebiotics, and dietary modifications. For instance, high-fiber and -polyphenol diets enhance the production of SCFAs that protects against gut permeability and thus blunt the disruption of the BBB [54]. Probiotic and prebiotic interventions can help restore balance, prevent gut dysbiosis to some extent, and promote long-term health. However, permanent prevention of dysbiosis requires a holistic approach, including diet modification, stress management, and lifestyle adjustments. Similarly, microbial profiling for neurodegeneration predisposition is promising but requires further research to refine predictive accuracy and clinical application. It was demonstrated that microglia in adult germ-free mice, devoid of microbial signaling, manifested altered densities and morphologies [55]. Furthermore, the research on essential hypertension showed that microglial dysfunction and neuroinflammation impact gut microbial populations. In the same research, inhibition of microglial activation caused an alteration in phylum Proteobacteria, which further indicates that the activity of microglia shapes the composition of gut microbiota. Thus, the interplay among microglia and gut microbiota contributes to immune homeostasis, which sometimes might be disrupted, leading to neuroinflammation [40,55].
4. Neurotransmitters and Immune Signaling in the Gut–Brain Axis
Abnormalities in the gut–brain axis communication often indicate a disruption in the coordinated network of neurotransmitters across the endocrine, intestinal, and brain systems (Figure 1) [56,57,58]. Gut microbes modulate several functions in the body and influence behavior through chemical interactions with the nervous system both directly and indirectly [2]. Some microbes can synthesize neuroactive compounds directly, while others stimulate the host’s production of metabolites and neurotransmitters involved in gut–brain signaling [59]. The communications between the gut and CNS are mediated by three major pathways: direct neuronal pathways, endocrine signaling mediators, and interaction with the immune system. These systems together provide a highly integrated molecular communication network that connects systemic imbalances with neurodegenerative processes, affecting insulin regulation, fat metabolism, oxidative markers, and immune signaling [60,61].
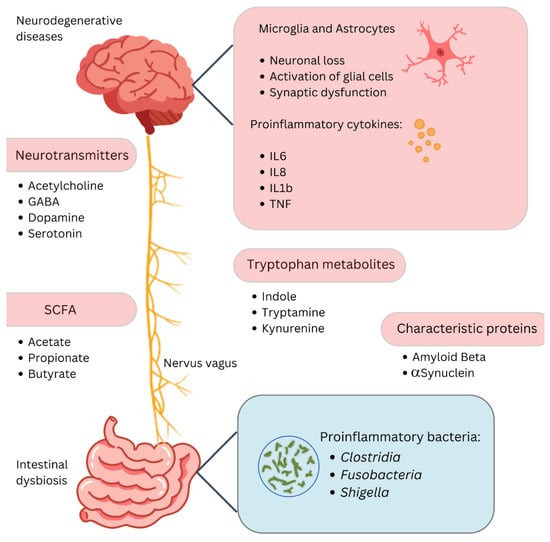
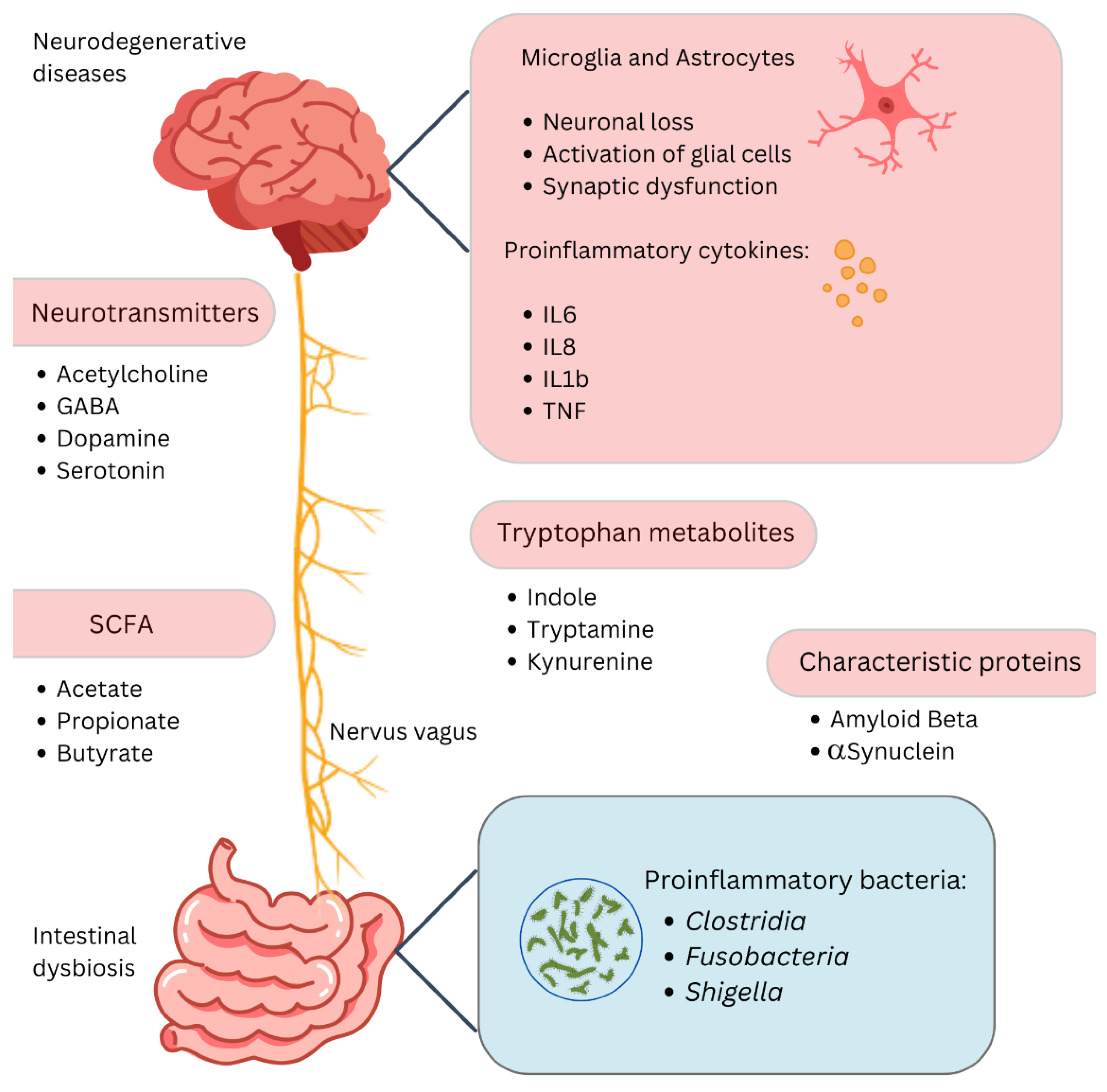
Figure 1.
The gut–brain axis and neurodegenerative diseases. This diagram highlights the link between gut dysbiosis and neurodegenerative diseases via the gut–brain axis. Changes in gut microbiota affect neurotransmitters like acetylcholine, GABA, dopamine, and serotonin, and produce short-chain fatty acids such as acetate, propionate, and butyrate, which modulate inflammation. Proinflammatory cytokines (IL6, IL8, IL1b, TNF) from activated microglia and astrocytes contribute to neuronal loss and synaptic dysfunction. Tryptophan metabolites (indole, tryptamine, kynurenine) and proinflammatory bacteria (Clostridia, Fusobacteria, Shigella) further drive inflammation. Proteins like amyloid beta and α-synuclein, linked to neurodegenerative diseases, are influenced by these interactions through pathways like the nervus vagus, connecting the gut and brain.
The microbiota is also indispensable in the development and function of the innate immune cells of the brain since microbial metabolic signals—SCFAs, for example—are required for the structural and functional maturation of these cells in germ-free mice [62,63]. Certain neurotransmitters produced by gut microbes include dopamine, serotonin, norepinephrine, and GABA, each of which impacts central nervous system functions in specific ways. For example, Bifidobacterium infantis increases plasma tryptophan levels, thereby affecting serotonin pathways in the brain, while GABA is synthesized by Lactobacillus and Bifidobacterium species, and serotonin is synthesized by Streptococcus, Escherichia, and Enterococcus species [64].
SCFAs are microbial metabolites produced through the fermentation of dietary fibers by gut bacteria, primarily acetate, propionate, and butyrate. These molecules play a critical role in maintaining the integrity of the gut barrier, modulating immune responses, and supporting neuroprotective functions in addition to direct impact on the immune system, epigenetics, and central nervous system plasticity to influence brain functions controlling metabolic and energy balance [65,66,67,68].
Moreover, gut microbiota can indirectly communicate with the nervous system via endocrine pathways, directly affecting appetite regulation due to the release of hormones such as GLP-1 from enteroendocrine cells [69]. Gut bacteria, including Escherichia, Bacteroides, and Bifidobacterium, can produce neurotransmitters GABA and serotonin and affect activity in the CNS in animal models [70]. SCFAs, indole derivatives, and tyramine are metabolic products, while other signaling molecules like lipopolysaccharides affect immune cell migration and neuroinflammation within the brain and, therefore, might influence CNS regulation and the development of neuroinflammatory disorders (Figure 2). Indeed, Harms et al. and Morais et al. illustrate this idea [2,71]. Double-stranded RNA and lipoproteins are other MAMPs that could be involved in mediating this gut–brain axis [72,73]. In germ-free animal studies, there are reduced hippocampal serotonin and tryptophan levels, indicating that microbial activity may be influencing neurotransmitter regulation within the CNS [74]. However, the extent to which these microbial metabolites influence CNS function remains unclear, as the transport mechanisms responsible for delivering these compounds to the brain have not yet been fully elucidated [75].
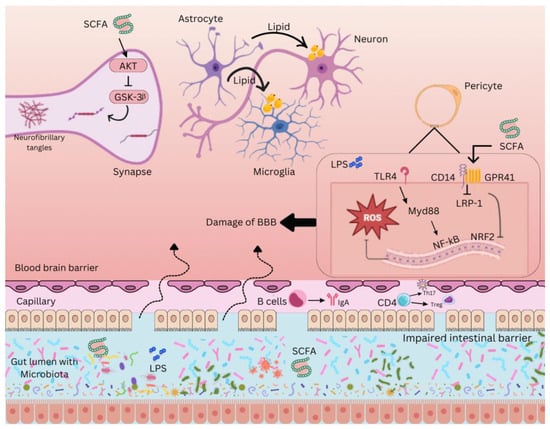
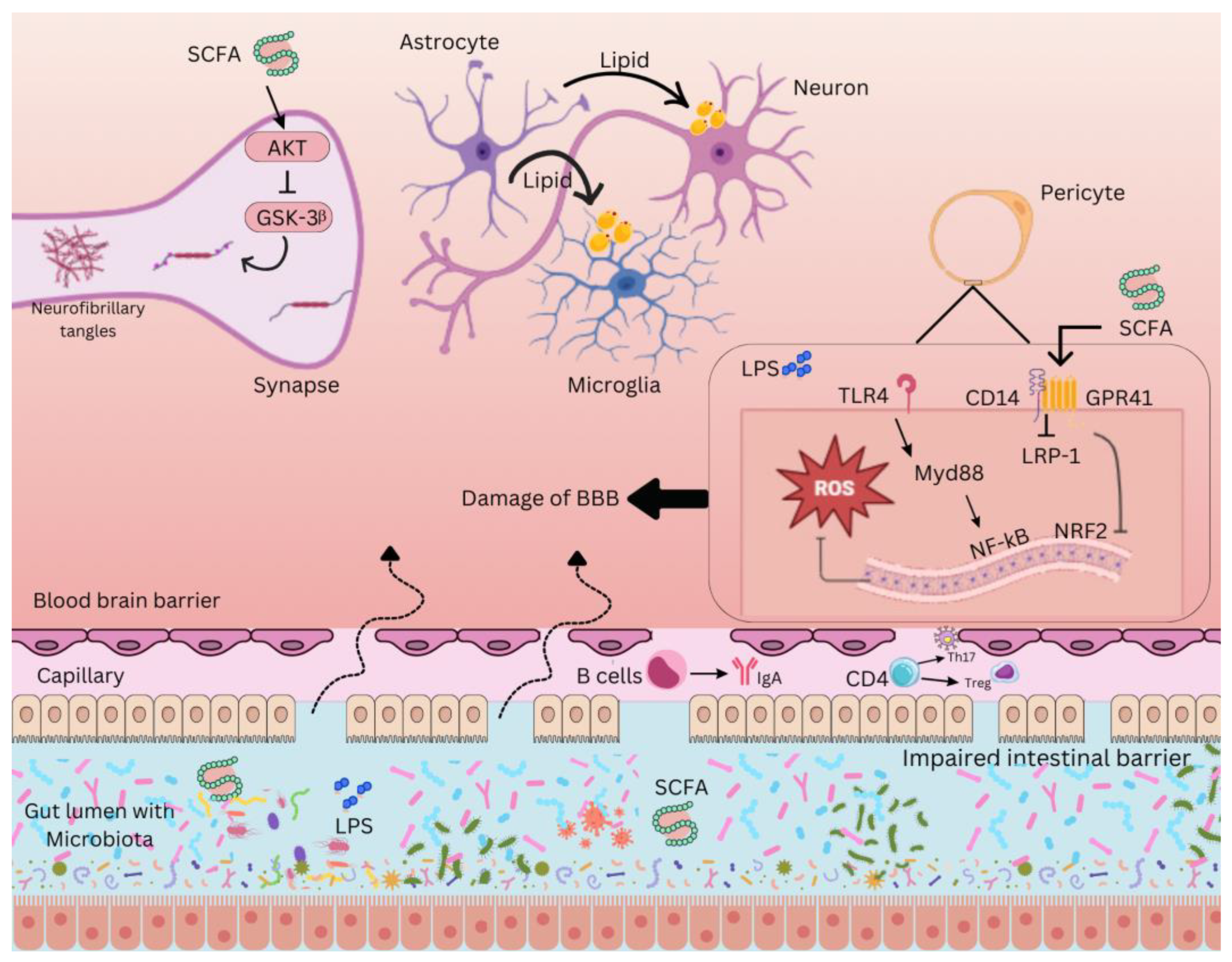
Figure 2.
Gut-brain axis interaction in neurodegeneration. The complex interaction between the gut microbiota and the CNS through various molecular pathways. Short-chain fatty acids (SCFAs), produced by gut bacteria through the fermentation of dietary fibers, play a crucial role in modulating neuroinflammation. SCFAs inhibit glycogen synthase kinase 3 beta (GSK-3β) via the protein kinase B (AKT) pathway, preventing the formation of neurofibrillary tangles, a hallmark of Alzheimer’s pathology. Damage to the blood-brain barrier (BBB), often facilitated by lipopolysaccharides (LPS) from gram-negative bacteria, leads to the infiltration of these endotoxins into the CNS, triggering an inflammatory response. Toll-like receptor 4 (TLR4) on immune cells recognizes LPS, activating an inflammatory cascade involving reactive oxygen species (ROS) and nuclear factor kappa B (NF-κB), a transcription factor that promotes the production of pro-inflammatory cytokines. MyD88, an adaptor protein, facilitates TLR4 signaling, while low-density lipoprotein receptor-related protein 1 (LRP-1) helps transport SCFAs across the BBB and modulate inflammation. Additionally, nuclear factor erythroid 2–related factor 2 (NRF2) regulates antioxidant responses, counteracting oxidative stress. G-protein coupled receptor 41 (GPR41) also modulates SCFA signaling, maintaining BBB integrity. The immune system’s role is further represented by immunoglobulin A (IgA), an antibody protecting the intestinal barrier from harmful microbes, showcasing the gut microbiota’s influence on neuroinflammatory processes.
5. Alzheimer’s Disease
AD is a neurodegenerative disease related to a decline in cognitive abilities, such as memory loss and changed behavior. It is the most common form of dementia in the elderly, and the incidence rises with age (Table 1). While it usually occurs after age 65, early-onset AD may be affecting people as young as 30 [76]. The primary symptom of the disease is short-term memory loss, but the long-term memory is still intact. In the further course of the disease, impaired judgment and problem-solving, language difficulties, and neuropsychiatric symptoms, such as apathy and agitation, emerge [77]. In the later stages, motor dysfunction, olfactory impairment, and disrupted sleep lead to total dependency on caregivers [78]. One of the risk factors for AD is physical inactivity, which heightens the risk for the development of the condition; midlife obesity is another major influence or factor that leads to the development of this fatal illness, just like low educational attainment and depression. It is further influenced by smoking and diabetes, while midlife hypertension and hearing loss add to these risks [79]. The amyloid hypothesis posits that the deposition of amyloid-β is related to the synaptic dysfunction and neurodegeneration of AD [80]. Aβ peptides are generated from a transmembrane amyloid precursor protein (APP), essential for neuronal development, synaptic function, and biological functions, including neural growth, signaling, and intracellular transport [10]. APP undergoes cleavage through two pathways, the non-amyloidogenic pathway, in which α-secretase cleaves APP to produce soluble APPα, and the amyloidogenic pathway, where β- and γ-secretases cleave APP to produce the toxic Aβ peptides [81]. Thus, microglia are the brain’s primary immune cells that infiltrate the brain early in development and maintain homeostasis as we age. The resident macrophages of the CNS, microglia, represent a phagocytic white blood cell that eliminates pathogens, cellular debris, and Aβ peptides. In theory, the acute neuroinflammatory response mediated by Aβ peptides is self-limiting and neuroprotective. With age, however, microglia become less effective at phagocytosing the neurotoxic Aβ plaques that characterize AD. Instead, accumulation of Aβ plaques activates microglia, promoting a chronic neuroinflammatory response [82].

Table 1.
Alzheimer’s disease (AD) characteristics.
The apolipoprotein E ε4 allele encoding ApoE4 is the strongest genetic risk factor identified to date for late-onset AD. The underlying mechanisms include the involvement of amyloid-β and tau pathologies with ApoE4 in cognitive impairment, but it remains unclear how the genotype influences AD development [83]. ApoE4 promotes mitochondrial dysfunction and neurotoxicity via increasing levels of reactive oxygen species (ROS) and mitochondrial dynamics. Moreover, ApoE4 translocates into the nucleus, where it regulates genes involved in aging, inflammation, and apoptosis. These may also be related to the pathogenesis of AD [84]. Recent advancements in AD diagnosis have come up with several biomarkers and imaging techniques, which include CSF biomarkers like the Aβ42/Aβ40 ratio and phosphorylated tau, and blood tests that detect amyloid-beta levels, thereby aiding in early detection even before symptoms appear [85]. These biomarkers correspond to the revised diagnostic criteria, focusing on advanced biological markers to stage the disease [86]. Imaging of the brain further amplifies diagnostic precision by disclosing structural and functional changes associated with the advancement of AD [58]. Current treatments of AD are symptom-oriented, for instance, with medications such as donepezil and memantine, but dietary and other non-pharmaceutical interventions have been attracting interest because they might favorably affect cognitive decline and reduce the rate of disease progression—for example, supplementation with omega-3 fatty acids and training in cognition [87,88].
5.1. Microbiome Alterations in Alzheimer’s Disease
Emerging evidence points to the involvement of the gut microbiome in AD. Indeed, the new criteria for AD have classified biomarkers into the broad categories termed “ATNIVS”, which expand the earlier concept focused on amyloid-beta A to include tau (T), neurodegradation (N), inflammation (I), vascular health (V), and synaptic dysfunction (S). The findings present changes in the gut microbiome signature over AD stages, its relationship with biomarker categories, and potential microbiome-based interventions, thus indicating research gaps and further directions [89]. Recent research began to focus on the gut–brain axis for its potential role in AD [90]. Modulations in the gut microbiota have been related to both known biomarkers of AD: amyloid-beta and tau. These observations give hope that manipulation of the gut microbiome will provide new therapeutic opportunities [91].
Current research highlights how gut microbiota modulates the biosynthesis of methionine (Met) and acetylcholine (ACh). Met serves as a biosynthetic precursor to choline, cysteine, and various neuroactive biogenic amines, making it particularly relevant to ACh synthesis and its implications in neurodegeneration. Choline occupies a central role in multiple metabolic pathways, contributing to the biosynthesis of glycerophospholipids—major components of cell membranes—including very-low-density lipoprotein (VLDL), phosphatidylcholine, and sphingolipids. These lipids are synthesized in the liver, secreted into the bloodstream, and distributed to various tissues, where they play key roles in phospholipid metabolism, biliary function, lipid regulation, lipoprotein dynamics, and energy metabolism [92].
A reaction catalyzed by the enzyme betaine homocysteine methyltransferase, betaine, or the oxidized form of choline methylates homocysteine to initiate the biosynthetic pathway for Met. The by-product of this reaction, dimethylglycine, has antioxidant properties and facilitates lymphocyte proliferation. Vitamin B12 also acts as a cofactor that helps in metabolizing folate and synthesizing aromatic amino acids, which are precursors for neurotransmitter synthesis. Moreover, Met is a general precursor of S-adenosylmethionine (SAM), produced by methionine-adenosyltransferase (MAT), which donates a methyl group for the conversion of phosphatidylethanolamine to phosphatidylcholine and, subsequently, for choline biosynthesis and further conversion into the neurotransmitter ACh [93,94].
Since choline is the precursor to ACh, and since essential amino acids—phenylalanine, tryptophan, and histidine, but not tyrosine, which is non-essential—are precursors for the other neurotransmitter biogenic amines, additional support is provided for an important ENS–CNS relationship. Furthermore, Met represents a precursor in the biosynthesis of histamine, serotonin, epinephrine, norepinephrine, and dopamine neurotransmitters that are crucial in neural transmission and maintenance of locomotion, muscle tone, mood, attention, and behavior [95,96]. Of particular note, changes in the microbiota, from both exogenous and endogenous influences, may affect neurotransmitter metabolism and production that are vital to the encephalon and, generally, to the health of the host, including neurodegenerative changes [95,97,98].
In the 1970s, post-mortem studies of AD brains showed a profound reduction in the activity of the biochemical enzyme choline acetyltransferase (ChAT), responsible for synthesizing ACh, a neurotransmitter involved in both the central and the peripheral nervous systems. ACh neuromodulates learning, memory, attention, and sensation, as well as cognitive functions [99]. Disturbances in the levels of ACh contribute to neurodegenerative diseases, NDs, and depressive disorders, leading to the cholinergic hypothesis. According to this hypothesis, cholinergic function can be enhanced by AChE inhibitors or nicotinic and muscarinic receptor agonists that increase ACh release or availability, a strategy underlying most current AD treatments [100,101].
Evidence supporting the cholinergic hypothesis points to the fact that AChE is the major enzyme responsible for ACh degradation, suggesting that inhibition of AChE may increase ACh levels in the synaptic cleft and slow neurodegeneration. Current evidence also suggests that AChE may also participate in the inflammation of the brain, neuronal apoptosis, oxidative stress, pathological aggregation of Aβ oligomers, and tau tangle formation—all the hallmarks of AD pathogenesis [93,100]. The multifunctional involvement of AChE in NDs has prompted the development of AChE inhibitors; a number of these are commercially available. Although these drugs act symptomatically only and do not provide a cure for AD, the past three decades have seen serious attempts by medicinal chemists and the pharmaceutical industry to identify and optimize new AChE inhibitors. This has resulted in some promising bioactive compounds with better pharmacological profiles compared with approved AChE inhibitors; yet, progress toward slowing the progression of AD continues to be very slow [93,102]. A close linkage can then be drawn between Met metabolism by the gut microbiota and AD pathogenesis through the ENS since Met is the biosynthetic precursor of the choline precursor that is present in reduced concentrations in the brains of patients with AD. This ACh deficit in AD may also be related to dysbiosis, given that dysbiosis can reduce choline production and thus limit the availability of ACh [93,100].
The Role of Microbial Amyloids in Neurodegeneration
Accumulation of amyloid plaques is one of the hallmarks of AD, and interestingly, a considerable number of amyloids are produced by human gut flora. Among those, the most studied bacterial amyloid is Curli, which is produced by Escherichia coli [103]. Synthesis of these amyloid proteins supports bacterial adhesion, biofilm formation, and resistance to physical and immunological stress. Bacterial amyloids could potentially act as prion proteins, exerting molecular mimicry through cross-seeding wherein one amyloidogenic protein—curl, tau, Aβ, α-syn, or prion—results in another instance, host proteins with a different base structure, to take on a pathogenic β-sheet structure [10,104]. Extracellular amyloid formation has been described in the human microbiome for many species, including Streptococcus, Staphylococcus, Salmonella, Mycobacteria, Klebsiella, Citrobacter, and Bacillus species. Amyloids can function as PAMPs, triggering the innate immune response via TLR4, TLR1, TLR2, NFκB, iNOS, and pro-inflammatory microRNAs [105,106]. The inflammatory reaction to amyloid deposits within the brain may also be controlled by the microbiota. Therefore, the priming of the immune system due to exposure of the gut to bacterial amyloid proteins may exacerbate the immune response against endogenous neuronal amyloid formation within the brain [107].
5.2. Research in Alzheimer’s Disease
Microbiota was investigated in patients with AD by comparing a population with mild cognitive and amnestic impairments to healthy controls using the Kyoto Encyclopedia of Genes and Genomes functional pathways analysis. Findings described the changes in taxonomic and functional composition of gut microbiota, capable of influencing brain function per se, indicating the possible contribution of intestinal microbiota to the development of amyloid pathology. Preceding that, a study was conducted using fecal samples from transgenic mice expressing the human APP-presenilin-1 (PS1) gene, CONVR-APPPS1—an established animal mouse model, Drosophila models, and Zebrafish models, along with clinical data for AD. Such studies have presented microbiota composition differences compared to control groups [108].
Conventionally raised AD pathology models include the CONVR-APP-PS1 mouse model, Drosophila models, and Zebrafish models, along with clinical data [55,109]. Comparison of the gut microbiota between conventionally raised APPS1 mice and wild-type mice shows a significant increase in Bacteroidetes as the mice age (1, 3.5, and 8 months), followed by a sharp decrease in Cyanobacteria, Firmicutes, and Proteobacteria content [110]. Human studies exhibit similar trends; fecal microbiota analyses in patients with and without AD show decreased microbial diversity, decreased Firmicutes and Bifidobacterium, and elevated Bacteroidetes in patients with AD. Elevations in CSF biomarkers of AD pathology have also been associated with an increase in relative bacterial abundance [107,111].
A second experimental methodology utilizes GF mice or mice that do not possess a gut microbiome, in combination with the transgenic APPPS1 mice. APPS1-transgenic mice reared under germ-free conditions had significant decreases in Aβ pathology compared to conventionally reared APPS1-transgenic mice, with histopathological staining revealing lower cerebral Aβ burdens in GF mice stained with thioflavin T [110]. In addition, there is a report of increased amyloid precursor protein load in the gut and an altered Firmicutes/Bacteroidetes ratio in 5xFAD mice, a transgenic AD model that expresses human APP and PSEN1 genes with five different AD-linked mutations, namely APP KM670/671NL, APP I716V, APP V7171, PSEN1M146L, and PSEN1L286V. The study implied that alterations in gut microbiota may be somehow linked to Aβ pathology in the brain [112,113].
In human studies, a recent investigation among cognitively impaired subjects with brain amyloidosis identified higher levels of proinflammatory Escherichia/Shigella coupled with lower anti-inflammatory Eubacterium rectale to be associated with peripheral inflammation [114]. However, it should be emphasized that different bacteria exhibit different actions: anti-inflammatory (e.g., Bacillus fragilis, Bacteroides fragilis, Eubacterium) and pro-inflammatory (e.g., Shigella, Proteobacteria, Pseudomonas) [107].
6. Parkinson’s Disease
PD is a neurodegenerative disease characterized by bradykinesia, tremors, and rigidity; it is also preceded by symptoms such as loss of smell, sleep disturbances, mood changes, and gastrointestinal problems (Table 2). All these symptoms, occurring 5–10 years before the degeneration of the dopaminergic neurons, have a great impact on patients despite the effectiveness of levodopa (L-dopa) on motor symptoms [115,116]. The pathophysiology of PD is generally associated with the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta, leading to a reduction in brain dopamine, especially within the striatum. Additionally, cytoplasmic inclusions of insoluble α-synuclein polymers, known as Lewy bodies, form toxic aggregates that represent a neuropathological hallmark of PD [117,118].

Table 2.
Parkinson’s disease characteristics.
Loss of dopamine precipitates the classic motor symptoms of PD, consisting of tremors, bradykinesia, rigidity, and postural instability [118]. The neurodegenerative outcomes of PD are influenced by oxidative stress and toxic by-product accumulation from either ROS or dopamine quinones. Genetic mutations within SNCA, LRRK2, PINK1, Parkin, and DJ-1 disrupt key cellular processes, including mitochondrial function and protein clearance, enhancing neuronal vulnerability [119]. The identification of α-synuclein in PD underlines its aggregation, leading to neuronal death via mitochondrial dysfunction, disruption of lysosomes, and disturbances in calcium homeostasis. Early inflammation associated with α-synuclein aggregation points out the connection between inflammation and synaptic dysfunction [120]. According to McCann et al., abnormal α-synuclein accumulation begins in the gut and propagates to the brain along the vagus nerve in a prion-like manner [121,122]. Pathophysiological evidence supports this, as α-synuclein inclusions appear early in the ENS and the vagal nerves [123]. Consistent with Braak’s hypothesis, an epidemiological study on patients undergoing truncal or selective vagotomy found that those who underwent complete truncal vagotomy had a reduced risk of developing PD, highlighting the vagus nerve’s key role in PD pathogenesis [124,125].
Lewy bodies, a pathognomonic feature in PD, further enhance oxidative stress and protein aggregation, with environmental toxins and heavy metals accelerating the disease process [126]. Neuroinflammation is also intricately linked with the pathogenesis of PD, where activated microglia and pro-inflammatory cytokines such as IL-1B and TNF-α promote neurotoxicity [127]. Additionally, infiltrating T lymphocytes (both CD8+ and CD4+) in the affected brain areas enhance neurodegeneration. One of the major concepts derived from preclinical studies is the potential benefit of targeting neuroinflammatory pathways [128]. The evolving diagnosis of PD, based on bradykinesia, includes unilateral onset, rest tremor, and positive levodopa response as supportive features [129]. Such diagnosis criteria include the Queen Square Brain Bank and the Movement Disorder Society criteria that enable the conducting of proper diagnosis, which is so important both for clinical management and for research [130]. Dysbiosis has been linked with neurologic disorders, including PD. Emerging evidence shows that signals from the gut could enhance neuroinflammation and thereby affect the course of the disease [107]. These findings open new possibilities for innovative therapeutic strategies interfering with the gut–brain axis to dampen inflammation and oxidative stress, thus potentially modifying the disease course in patients with PD [131].
6.1. Microbiome Alterations in Parkinson’s Disease
Multiple studies indicate that the neurodegenerative cascade may indeed start in the gut, subsequently spreading to the brain through gut–brain axis mechanisms, with the gut microbiota implicated in this process [132]. Alterations in gut microbiota composition can disrupt the intestinal barrier, impacting permeability and affecting not only epithelial and immune cells but also brain biochemistry [5,133]. The microbiota composition and diversity in patients with PD differ significantly from those of healthy individuals [134]. Gastrointestinal dysfunction is common in PD and can precede motor symptoms by more than ten years, potentially playing a role in α-synuclein misfolding, as patients with PD are known to have α-synuclein aggregates in their colons [135,136].
In individuals with Parkinson’s symptoms, the diversity of gut microbes, particularly within certain phyla such as Firmicutes, Bacteroidetes, and Fusobacteria, was found to be altered compared to healthy controls [137]. The enrichment of specific pathogenic microbial phyla and a reduction in beneficial microbial communities play a central role in regulating brain behavior. In Parkinson’s patients, SCFAs, known for their anti-inflammatory properties and their role in preventing the development of a neuroinflammatory microenvironment, were found to be deficient in the gastrointestinal tract due to the reduced abundance of SCFA-producing microorganisms, such as Bacteroidetes and Firmicutes [138]. A decreased presence of bacteria like Clostridium tyrobutyricum, which produces significant amounts of butyrate, is implicated in the progression of PD [139,140].
In PD patients, lipopolysaccharides from the numerous Gram-negative bacteria in the gut were observed to cross the BBB by modulating tight junction proteins such as occludin and claudin [141]. These changes in tight junction protein expression facilitate the infiltration of immune cells into the brain [7]. Consequently, activated microglial cells increase the production of pro-inflammatory molecules, with neuroinflammation recognized as a key hallmark in the initiation of neurodegenerative disorders [142].
The administration of levodopa, a dopamine precursor, remains the most common and effective treatment for managing PD [143]. However, the widespread prevalence of Helicobacter pylori (H. pylori) infection has been extensively studied concerning PD, as it is associated with reduced absorption of L-DOPA, leading to greater motor impairments compared to uninfected Parkinson’s patients [144]. Consequently, eradicating H. pylori has been shown to significantly improve motor fluctuations by enhancing the absorption of levodopa [145]. While previous studies have highlighted the role of H. pylori in lowering L-DOPA bioavailability in dopaminergic neurons, the precise mechanisms remain unclear [7].
One potential mechanism is that H. pylori may utilize L-DOPA as a nutrient source, thereby rendering it unavailable for the host. This consumption could also enhance the bacterium’s virulence, further exacerbating the neurological symptoms of PD. Since L-DOPA is derived from the amino acid phenylalanine, which is crucial for bacterial growth and motility, it is hypothesized that H. pylori consumption of L-DOPA may upregulate the expression of motility-related genes (e.g., flagellin), thereby increasing bacterial motility and intensifying the severity of infection in PD [146].
Another plausible mechanism involves the secretion of microbial enzymes that convert L-DOPA into abnormal or inactive products. Such biotransformation could reduce L-DOPA’s therapeutic efficacy by making it unavailable for PD treatment [147]. Further investigation is needed to elucidate these pathways and develop strategies to mitigate their impact on PD management.
6.2. Research in Parkinson’s Disease
While gastrointestinal symptoms and gut microbiota alterations are well documented in Parkinson’s disease, the mechanisms linking the gut microbiome to PD pathogenesis have only recently been explained [148]. Studies have indeed shown an increased abundance of Lactobacillus and Bifidobacterium genera in patients with PD, while the levels of butyrate-producing bacteria—such as Faecalibacterium, Coprococcus, Blautia, and Roseburia, which are known for their anti-inflammatory properties—are decreased [149,150]. Cirstea et al. observed an increase in Akkermansia and Bifidobacterium, as well as a decrease in Lachnospiraceae and Faecalibacterium in fecal samples from patients with PD compared to healthy individuals [151]. Aho et al. found significant alterations in gut microbiota, including a reduction in Prevotella in faster-progressing patients with PD [152]. In a machine learning study by Pietrucci et al., microbiota data from patients with PD showed increased Enterobacteriaceae and decreased Lachnospiraceae, correlating with disease severity and motor impairment [153]. Petrov et al. reported lower alpha diversity in patients with PD, observing an increase in Catabacter, Lactobacillus, Christensenella, Oscillopsia, and Bifidobacterium, while detecting only 9 genera and 15 species of microorganisms in the PD group [154].
The decreased abundance of Prevotellaceae, coupled with increased Enterobacteriaceae in patients with PD, has important implications. Prevotellaceae typically support mucin production and the generation of SCFAs through dietary fiber fermentation, and their reduction can increase gut permeability, exacerbating exposure to bacterial endotoxins and triggering inflammation, while dietary fibers have been shown to influence microglial activity linked to PD. Sampson et al. demonstrated it in their study with α-synuclein (α-syn) overexpressing (ASO) mice, where a fiber-rich diet impacted microglial function in a PD-like model [155]. This prebiotic diet lessens α-synuclein aggregation in the substantia nigra and consequently diminishes motor impairments, and the increased permeability allowed bacterial toxins to induce excessive α-synuclein expression in the colon, promoting its misfolding [156,157]. Nevertheless, this special type of feeding encourages the growth of protective disease-associated macrophage subsets of microglia and reverses pathogenic microglial conditions. Hence, even when feeding ASO mice with a prebiotic diet, the positive benefits are eliminated when microglia suffer depletion with a CSF1R inhibitor, causing motor impairments [157].
Another study concentrates on the transgenic rat (TG) model under investigation of over-expressing ɑ-Syn. Results were alterations in gut microbiota, intestinal inflammation, dysfunctional metabolites in plasma and feces, and a ɑ-Syn aggregation that was concurrent with the aging process. Specified traits exhibited similarly in human patients with PD. By altering the microbiome with a short-term antibiotic treatment cocktail, a reduction in the metabolite production in their feces and plasma can be achieved, as well as in the ɑ-syn expression in TG rats’ forebrain. This model finds early changes in avoidance and novelty-seeking behavior along with notable shifts in bacterial variety and metabolites. Additionally, it shows ɑ-syn accumulation in the colon walls of the rats before the development of pathological changes in the brain. This indicates that knowledge of gut physiology and microbiome/metabolite dynamics may help provide further insight into PD [158].
In contrast, the increase in Enterobacteriaceae results in increased amounts of lipopolysaccharides, which are structural components of the outer membranes of these Gram-negative bacteria [156]. Together with the decreased abundance of Prevotellaceae, this may facilitate the translocation of LPS and other neurotoxins across the intestinal barrier into the bloodstream, reflected by increased blood levels of LPS-binding protein in patients with PD. This process enhances gut inflammation and promotes systemic inflammation, which may activate immune pathways such as TLR4 and NF-κB, leading to the release of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-2, all of which compromise the integrity of the blood–brain barrier and contribute to neuroinflammation [150]. Therefore, the relative increase in Enterobacteriaceae and the decrease in Prevotellaceae might have an important role in starting and maintaining neuroinflammatory processes within the central nervous system.
Moreover, emerging evidence suggests that PD may be crucially linked to the pathophysiology of inflammatory bowel disease (IBD). Numerous reviews and meta-analyses have demonstrated that individuals suffering from IBD face a 20–90% higher risk of developing PD and additionally present with a reduced number of Firmicutes yet an increased abundance of Enterobacteriaceae in their gut microbiota [159]. Specifically, a study showed that patients with ulcerative colitis are at higher risk than individuals with Chron’s disease. This observation was underlined by the finding of ɑ-synuclein accumulation in the submucosa of patients with PD and UC [160]. An intriguing study additionally found that IBD patients receiving anti-TNF therapy have a 78% lower risk of developing PD than those who are not, due to neuroinflammation being prevented through modification of the gut microbiota [161].
7. Therapeutic and Future Directions
The broad impact of intestinal microbiota on brain functions opens a large door to investigating the treatment options available for neurodegenerative diseases such as AD and PD (Figure 3). Indeed, it is not an easy task to find therapeutic targets that may prevent or delay the processes of neurodegenerative diseases. In this context, it is also essential to emphasize that studies have demonstrated that the gut microbiome differs between males and females, both in human populations and in preclinical animal models [74,79]. These differences can arise from a combination of genetic, hormonal, and environmental factors that influence microbial composition, immune responses, and metabolic pathways. Given that both AD and PD exhibit well-documented sex disparities in incidence, progression, and symptomatology, it is plausible that gut microbiome differences contribute to these variations, but there is need for further investigations to clarify whether microbiome-targeted interventions should be tailored by sex. Consensus exists that all current approaches focusing on the CNS and targets like AChE yielding currently available medications have not shown effectiveness in symptomatic alleviation or retardation of disease progression [98,162]. Emerging data propose that alterations in gut microbiota with probiotics, prebiotics, synbiotics, antibiotics, or changes in diet can be associated with significant neurological and psychiatric changes [163,164]. It was reported that probiotic supplementation could improve cognitive test performance and elevate substances that may decelerate AD progress in experimental models [165,166]. In general, probiotics are considered a safe alternative therapy for several conditions and are capable of having a positive impact on the host gut microbiome and thus improving host health [165,166,167].
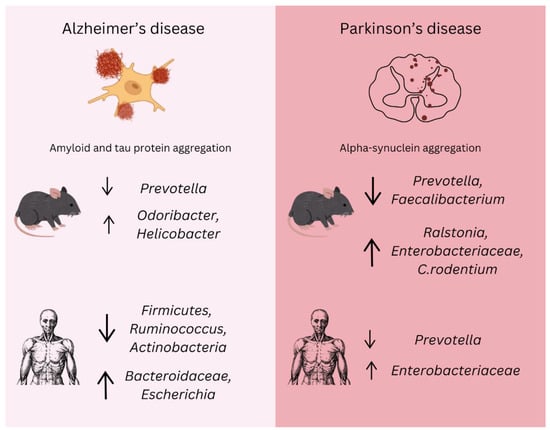
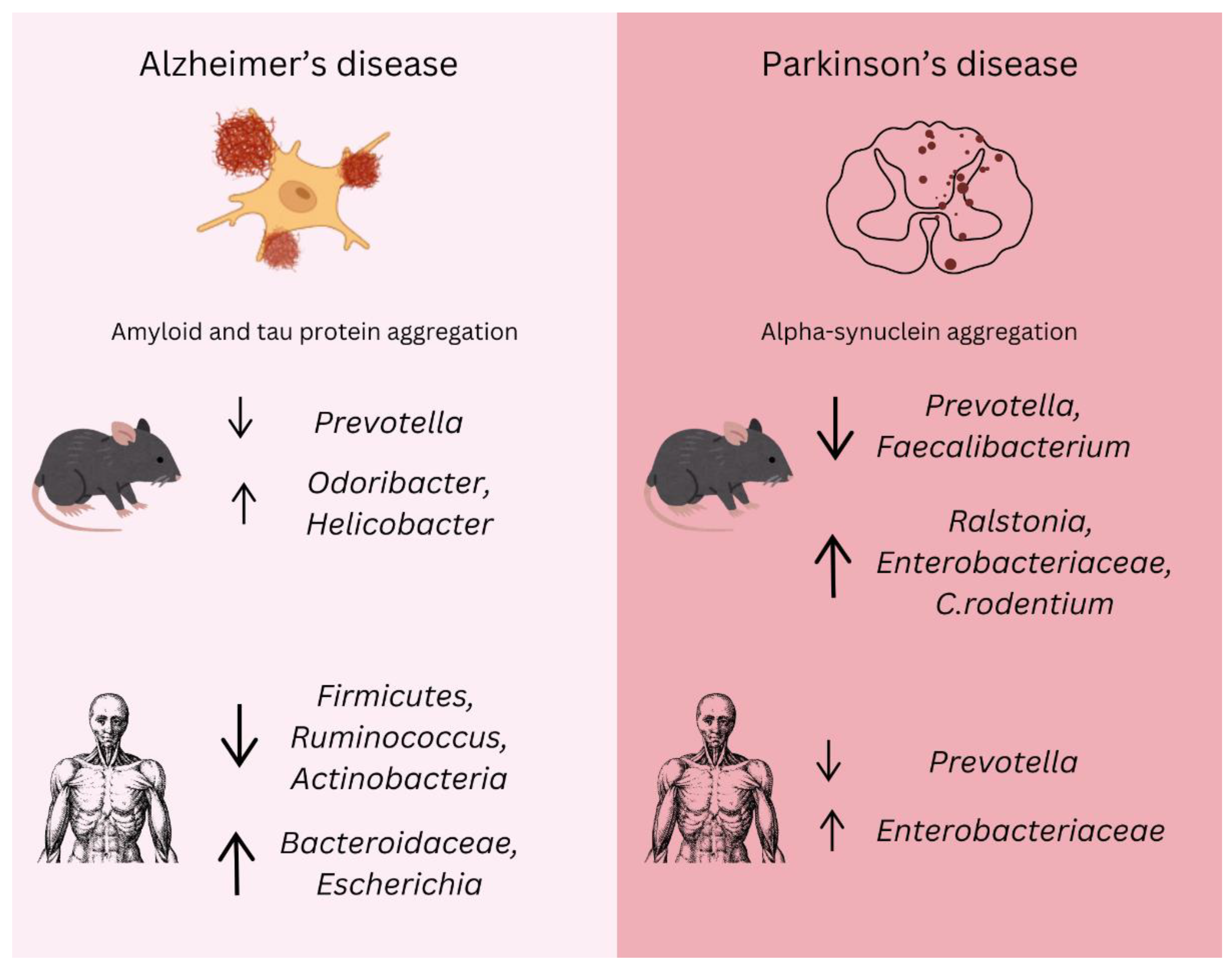
Figure 3.
Gut microbiota changes in Alzheimer’s and Parkinson’s disease. The alterations in gut microbiota composition associated with AD and PD in both animal models and human studies. In Alzheimer’s disease, amyloid and tau protein aggregation are major pathological features. Microbiota shifts include a reduction in beneficial bacteria such as Prevotella and an increase in Odoribacter and Helicobacter. Additionally, decreases in Firmicutes, Ruminococcus, and Actinobacteria are linked to neuroinflammation, while an increase in Bacteroidaceae and Escherichia correlates with the progression of amyloid pathology. In Parkinson’s disease, alpha-synuclein aggregation is a defining feature. Significant changes in the gut microbiota include a decrease in Prevotella and Faecalibacterium, while Ralstonia, Enterobacteriaceae, and Citrobacter rodentium show an increase, exacerbating inflammation and alpha-synuclein aggregation. A reduction in Prevotella is consistently observed in both animal models and human PD cases, while elevated Enterobacteriaceae levels correlate with motor symptoms. These microbial alterations suggest potential biomarkers and therapeutic targets, emphasizing the importance of the microbiota–gut–brain axis in the early diagnosis, progression, and management of neurodegenerative diseases.
Moreover, AD mouse models demonstrated that supplementation with Lactobacillus and Bifidobacterium restores GABA and glutamate levels in the frontal cortex and hippocampus, improving capabilities for memory and learning [95]. Bonfili et al. treated the AD model of transgenic 3xTgDA mice with the probiotic formulation SLAB51—a mixture of lactic acid and Bifidobacterium—and recorded significant inhibition of Aβ aggregation and a reduction in oxidative stress in the brain with the involvement of sirtuin 1 (SIRT1) [164].
Abdelhamid et al. studied the effects of oral supplementation with Bifidobacterium breve MCC1274 on cognitive performance and AD-like pathologies in mice. They demonstrated that probiotic treatment ameliorated memory deficits in AppNL–G–F mice and reduced hippocampal Aβ accumulation by increasing the α-disintegrin and metalloproteinase 10 (ADAM10) levels. This probiotic also induced the extracellular signal-regulated kinase (ERK)/hypoxia-inducible factor-α (HIF-1α) pathway, which was found to enhance the expression of ADAM10. Supplementation with Bifidobacterium breve MCC1274 also inhibited microglial activation and the mRNA expression of pro-inflammatory cytokines in the brain, and improved synaptic protein levels in the hippocampus [168].
All these supportive results confirm the beneficial effects of Bifidobacterium and Lactobacillus probiotics on memory, learning, inhibition of Aβ aggregation, and reduction in oxidative stress associated with AD. A recent clinical approach by Leblhuber et al. treated 20 AD outpatients (9 females, 11 males, aged 76.7 ± 9.6 years) who received routine lab tests and a mini-mental state examination (mean score: 18.5 ± 7.7). In four weeks with 18 patients, analyses before and after probiotic supplementation assessed biomarkers like immune activation, serum neopterin, L-tryptophan breakdown, intestinal inflammation, and microbiota composition in fecal samples. The post-treatment results showed a reduction in fecal zonulin and an increase in Faecalibacterium prausnitzii, while the serum kynurenine level was also elevated. These data reflected that multispecies probiotic supplementation in patients with AD affects gut bacterial composition along with serum L-tryptophan metabolism. In addition, it has also been observed that the correlation between the kynurenine/tryptophan and neopterin concentrations reflects the activation of macrophages and/or dendritic cells [169]. FMT of healthy donor fecal microbiota worldwide is considered a probable treatment for NDs. This approach was meant to be employed in the restoration of gut microbiota composition in treating these diseases, though the currently available evidence is not enough to confirm its efficacy in the progression of AD or PD [170,171,172].
Immune modulation, changes in endocrine pathways, and neurotrophic factors have been associated with probiotics due in part to the production of SCFAs [170,171,172]. SCFAs modulate immune pathways, inhibiting pro-inflammatory mediators and enhancing anti-inflammatory cytokines and interleukins. Through endocrine pathways, probiotics may stimulate the hypothalamic–pituitary–adrenal axis to stimulate adrenal cortisol release, the most potent anti-inflammatory hormone produced by the body. Probiotics stimulate neurotransmitter release, such as glutamate or serotonin through enterochromaffin cells. Together, these neurotransmitters and neuroactive metabolites may exert neuroprotection by preventing neuronal apoptosis [173,174].
8. Conclusions
Since the gut–brain axis has now become one of the most important areas for research in intestinal science, the development of relevant models has become extremely important in the presentation of mechanisms employed by gut and brain communication and maintaining gastrointestinal and neurological health via the gut–brain axis. Recent breakthroughs in microbiota research have significantly enhanced our understanding of the microbiota–gut–brain (MGB) axis, revealing how gut bacteria impact CNS health. This growing field offers promising new approaches for diagnosing, predicting, and treating NDs. Unlike other diseases, NDs often remain undiagnosed until significant, irreversible neuronal damage has occurred, as pathology can begin years before symptoms appear. Microbiota research could identify biomarkers capable of detecting NDs before clinical symptoms arise, enabling earlier intervention and potentially reducing long-term damage. Investigating the gut microbiota’s role in NDs also highlights the link between nutrition, microbiota, and disease. Emerging treatments, including novel antibiotics, prebiotics, probiotics, and microbiota transplants, offer exciting alternatives to conventional therapies. Managing the gut microbiota could transform both preventive and therapeutic approaches to NDs.
Dysbiosis, or an imbalance in gut microbiota, is associated with neuroinflammatory diseases such as AD and PD. It disrupts gut and blood–brain barrier integrity, enhances systemic inflammation, and alters the production of neuroactive compounds, exacerbating neurological decline. Restoring microbial balance by using probiotics, prebiotics, and dietary interventions, especially high-fiber and polyphenol-rich diets, has shown potential in enhancing SCFA production, reducing neuroinflammation, and slowing disease progression. Although these strategies are promising, much more research will be required to understand the molecular pathways and to develop personalized therapeutic approaches. Large-scale studies will be required to validate the efficacy of these interventions over a long period. Microfluidics and micromachining now allow for the making of dynamic cell culture systems and in vitro models recapitulating human organs. Nowadays, various results from in vitro models of the brain–gut axis are seen. These microarray systems represent powerful tools for predicting drug toxicity and modeling multiple disease states in the brain. They may continue to improve health care by increasing drug availability and reducing both unexpected drug side effects and the costs of drug development.
In conclusion, the evolving understanding of the MGB axis is reshaping research into NDs. The complex interactions between the gut microbiota, immune system, and CNS open new possibilities for early diagnosis, prognostic markers, and innovative therapies. This interdisciplinary approach is very promising for the future of neurological care. Continued research into these interactions holds great promise for advancing the treatment and prevention of NDs.
Author Contributions
Conceptualization, A.K., A.J., L.M.S.S. and L.B.; writing—original draft preparation, A.K., A.J. and L.M.S.S.; writing—review and editing, A.K., A.J., L.M.S.S. and L.B.; visualization, A.K.; supervision, L.B.; funding acquisition, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This work was written up as part of the scientific project “uniri-iskusni-biomed-23-88-3035: Influence of elective primary coronary intervention and coronary artery bypass grafting on the beating heart on endothelial dysfunction, oxidative stress, and inflammatory response in patients with ischemic heart disease”.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Many thanks to Marko Dolibasic for his hard work and help with the technical editing of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediat. Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health-Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Morgadinho, J.; Halverson, T. Approach to the Diagnosis and Management of Dysbiosis. Front. Nutr. 2024, 11, 1330903. [Google Scholar] [CrossRef] [PubMed]
- Intili, G.; Paladino, L.; Rappa, F.; Alberti, G.; Plicato, A.; Calabrò, F.; Fucarino, A.; Cappello, F.; Bucchieri, F.; Tomasello, G.; et al. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic Insights into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Verma, A.; Inslicht, S.S.; Bhargava, A. Gut-Brain Axis: Role of Microbiome, Metabolomics, Hormones, and Stress in Mental Health Disorders. Cells 2024, 13, 1436. [Google Scholar] [CrossRef]
- Mahbub, N.U.; Islam, M.M.; Hong, S.T.; Chung, H.J. Dysbiosis of the Gut Microbiota and Its Effect on α-Synuclein and Prion Protein Misfolding: Consequences for Neurodegeneration. Front. Cell. Infect. Microbiol. 2024, 14, 1348279. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Li, X.; Yu, J.T.; Wang, Y.J. Therapeutics for Neurodegenerative Diseases by Targeting the Gut Microbiome: From Bench to Bedside. Transl. Neurodegener. 2024, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Stolzer, I.; Scherer, E.; Süß, P.; Rothhammer, V.; Winner, B.; Neurath, M.F.; Günther, C. Impact of Microbiome-Brain Communication on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 14925. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Cohen, L.J.; Cho, J.H.; Gevers, D.; Chu, H. Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 2174–2189. [Google Scholar] [CrossRef]
- Muhammad, A.Y.; Amonov, M.; Baig, A.A.; Alvi, F.J. Gut Microbiome: An Intersection between Human Genome, Diet, and Epigenetics. Adv. Gut Microbiome Res. 2024, 2024, 6707728. [Google Scholar] [CrossRef]
- Boccuto, L.; Tack, J.; Ianiro, G.; Abenavoli, L.; Scarpellini, E. Human Genes Involved in the Interaction Between Host and Gut Microbiome: Regulation and Pathogenic Mechanisms. Genes 2023, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Epigenetic Regulation by Gut Microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.; Chen, X.; Wang, J.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Simão, V.A.; De Almeida Chuffa, L.G.; Ferder, L.; Inserra, F.; Manucha, W. Mitochondrial-Epigenetic Crosstalk as an Integrative Standpoint into Gut Microbiome Dysbiosis and Related Diseases. Biocell 2024, 48, 1429–1442. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of Gut Microbiota in Infectious and Inflammatory Diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation By-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut Microbiota-Derived Propionate Mediates the Neuroprotective Effect of Osteocalcin in a Mouse Model of Parkinson’s Disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Kullberg, R.F.J.; Wikki, I.; Haak, B.W.; Kauko, A.; Galenkamp, H.; Peters-Sengers, H.; Butler, J.M.; Havulinna, A.S.; Palmu, J.; McDonald, D.; et al. Association Between Butyrate-Producing Gut Bacteria and the Risk of Infectious Disease Hospitalisation: Results from Two Observational, Population-Based Microbiome Studies. Lancet Microbe 2024, 5, 100864. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; He, Z.; Zhuang, D.; Lin, F. The Influence of Gut Microbiota on Circulating Inflammatory Cytokines and Host: A Mendelian Randomization Study with Meta-Analysis. Life Sci. 2023, 332, 122105. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The Interplay Between Diet and the Gut Microbiome: Implications for Health and Disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The Role of Diet in Shaping Human Gut Microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Ge, X.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X. Regulation of the Gut Microbiota by Diet and Exercise: Improvements in Cognition and Emotion. Future Foods 2023, 8, 100256. [Google Scholar] [CrossRef]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The Effect of Exercise Prescription on the Human Gut Microbiota and Comparison Between Clinical and Apparently Healthy Populations: A Systematic Review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.; Litichevskiy, L.; Descamps, H.C.; et al. A Microbiome-Dependent Gut–Brain Pathway Regulates Motivation for Exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Tu, P.; Ru, H.; Lu, K. Studies of Xenobiotic-Induced Gut Microbiota Dysbiosis: From Correlation to Mechanisms. Gut Microbes 2021, 13, 1921912. [Google Scholar] [CrossRef] [PubMed]
- Nel Van Zyl, K.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of Antibiotics on the Human Microbiome: A Systematic Review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, S.; Huo, F.; Liu, H. Effects of Four Antibiotics on the Diversity of the Intestinal Microbiota. Microbiol. Spectrum 2022, 10, e0190421. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Ampatzoglou, A.; Aguilera-Gómez, M. Microbiota Analysis for Risk Assessment of Xenobiotics: Cumulative Xenobiotic Exposure and Impact on Human Gut Microbiota under One Health Approach. EFSA J. 2022, 20 (Suppl. S2), e200916. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The Communication Mechanism of the Gut-Brain Axis and Its Effect on Central Nervous System Diseases: A Systematic Review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Abdullah, N.; Defaye, M.; Altier, C. Neural Control of Gut Homeostasis. AJP Gastrointest. Liver Physiol. 2020, 319, G718–G732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Nguyen, M.; Palm, N.W. Gut Instincts in Neuroimmunity from the Eighteenth to Twenty-First Centuries. Semin. Immunopathol. 2022, 44, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Asha, N.; Sharma, K. Gut–Organ Axis: A Microbial Outreach and Networking. Lett. Appl. Microbiol. 2020, 72, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky Intestine and Impaired Microbiome in an Amyotrophic Lateral Sclerosis Mouse Model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-Brain Axis: A Cutting-Edge Approach to Target Neurological Disorders and Potential Synbiotic Application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Blakely, R.D. A Dialogue Between the Immune System and Brain, Spoken in the Language of Serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The Microbiota-Gut-Brain Axis: Pathways to Better Brain Health. Perspectives on What We Know, What We Need to Investigate and How to Put Knowledge into Practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
- Aatsinki, A.K.; Lahti, L.; Uusitupa, H.M.; Munukka, E.; Keskitalo, A.; Nolvi, S.; O’Mahony, S.; Pietilä, S.; Elo, L.L.; Eerola, E.; et al. Gut Microbiota Composition is Associated with Temperament Traits in Infants. Brain Behav. Immun. 2019, 80, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Gao, Y. Gut-Brain Axis and Neurodegeneration: Mechanisms and Therapeutic Potentials. Front. Neurosci. 2024, 18, 1481390. [Google Scholar] [CrossRef]
- Palacios, N.; Wilkinson, J.; Bjornevik, K.; Schwarzschild, M.A.; McIver, L.; Ascherio, A.; Huttenhower, C. Metagenomics of the Gut Microbiome in Parkinson’s Disease: Prodromal Changes. Ann. Neurol. 2023, 94, 486–501. [Google Scholar]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L.; et al. Gut Microbiome Composition May Be an Indicator of Preclinical Alzheimer’s Disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar]
- Zeng, H.; Huang, J.; Zhou, H.; Meilandt, W.J.; Dejanovic, B.; Zhou, Y.; Bohlen, C.J.; Lee, S.H.; Ren, J.; Liu, A.; et al. A Integrative In Situ Mapping of Single-Cell Transcriptional States and Tissue Histopathology in a Mouse Model of Alzheimer’s Disease. Nat. Neurosci. 2023, 26, 430–446. [Google Scholar] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations Between Gut Microbiota and Alzheimer’s Disease, Major Depressive Disorder, and Schizophrenia. J. Neuroinflamm. 2020, 17, 288. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal. Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.; Glass, C.K.; Mazmanian, S.K. Microbiome–Microglia Connections via the Gut–Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic Acid in Neurodegenerative Disorders—Unique Neuroprotection or Double-Edged Sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef]
- Al-Judaibi, A.A. Microbiota and Their Influence in the Human Body. J. Pure Appl. Microbiol. 2021, 15, 42–52. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction Between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar]
- Lyte, M. Microbial Endocrinology and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 3–24. [Google Scholar] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective Unconscious: How Gut Microbes Shape Human Behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar]
- Yang, W.; Cong, Y. Gut Microbiota-Derived Metabolites in the Regulation of Host Immune Responses and Immune-Related Inflammatory Diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Aresti Sanz, J.; El Aidy, S. Microbiota and Gut Neuropeptides: A Dual Action of Antimicrobial Activity and Neuroimmune Response. Psychopharmacology 2019, 236, 1597–1609. [Google Scholar]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The Gut Microbiota-Brain Axis in Neurological Disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef]
- Harms, A.S.; Thome, A.D.; Yan, Z.; Schonhoff, A.M.; Williams, G.P.; Li, X.; Liu, Y.; Qin, H.; Benveniste, E.N.; Standaert, D.G. Peripheral Monocyte Entry Is Required for Alpha-Synuclein-Induced Inflammation and Neurodegeneration in a Model of Parkinson’s Disease. Exp. Neurol. 2018, 300, 179–187. [Google Scholar] [PubMed]
- Schachtle, M.A.; Rosshart, S.P. The Microbiota-Gut-Brain Axis in Health and Disease and Its Implications for Translational Research. Front. Cell. Neurosci. 2021, 15, 698172. [Google Scholar]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis During Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [PubMed]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Marmol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota Modulate Sympathetic Neurons via a Gut-Brain Circuit. Nature 2020, 583, 441–446. [Google Scholar]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding Neuropsychiatric Symptoms in Alzheimer’s Disease: Challenges and Advances in Diagnosis and Treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated with Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584–591. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Chételat, G. Aβ-Independent Processes—Rethinking Preclinical AD. Nat. Rev. Neurol. 2013, 9, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Huang, H. Roles of APOE4 on the Pathogenesis in Alzheimer’s Disease and the Potential Therapeutic Approaches. Cell. Mol. Neurobiol. 2023, 43, 3115–3136. [Google Scholar] [CrossRef]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s Disease Pathogenesis—Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, C.; Cheng, M.; Geng, L.; Li, J.; Du, W.; Song, M.; Chen, N.; Yeleen, T.A.N.; Song, L.; et al. The Link Between Gut Microbiome and Alzheimer’s Disease: From the Perspective of New Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 5771–5788. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Li, J.; Ma, R.X.; Cheng, X.Y.; Ma, R.Y.; Zhou, T.Y.; Wu, Z.Q.; Yao, Y.; Li, J. Gut Microbiota Is an Impact Factor Based on the Brain-Gut Axis to Alzheimer’s Disease: A Systematic Review. Aging Dis. 2023, 14, 964–1678. [Google Scholar] [CrossRef]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The Gut Microbiome in Alzheimer’s Disease: What We Know and What Remains to Be Explored. Mol. Neurodegener. 2023, 18, 9. [Google Scholar] [CrossRef]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The Impact of Microbiota on the Gut-Brain Axis: Examining the Complex Interplay and Implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in Their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, I.H.R.; Abbasi, F.; Soomro, R.N.; Abd El-Hack, M.E.; Abdel-Latif, M.A.; Li, W.; Hao, R.; Sun, F.; Bodinga, B.M.; Hayat, K.; et al. Considering Choline as Methionine Precursor, Lipoproteins Transporter, Hepatic Promoter and Antioxidant Agent in Dairy Cows. AMB Express 2017, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Toledo, A.R.L.; Monroy, G.R.; Salazar, F.E.; Lee, J.Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut-Brain Axis as a Pathological and Therapeutic Target for Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef] [PubMed]
- Mazi, T.A.; Sarode, G.V.; Czlonkowska, A.; Litwin, T.; Kim, K.; Shibata, N.M.; Medici, V. Dysregulated Choline, Methionine, and Aromatic Amino Acid Metabolism in Patients with Wilson Disease: Exploratory Metabolomic Profiling and Implications for Hepatic and Neurologic Phenotypes. Int. J. Mol. Sci. 2019, 20, 5937. [Google Scholar] [CrossRef] [PubMed]
- Mohany, N.A.M.; Totti, A.; Naylor, K.R.; Janovjak, H. Microbial Methionine Transporters and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2021, 105, 3919–3929. [Google Scholar] [CrossRef]
- Rossor, M.N.; Garrett, N.J.; Johnson, A.L.; Mountjoy, C.Q.; Roth, M.; Iversen, L.L. A Post-Mortem Study of the Cholinergic and GABA Systems in Senile Dementia. Brain 1982, 105 Pt 2, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship Between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli Curli Operons in Directing Amyloid Fiber Formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Chapman, M.R. The Role of Microbial Amyloid in Neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Role of Toll-Like Receptor 4 in Alzheimer’s Disease. Front. Immunol. 2020, 11, 1588. [Google Scholar] [CrossRef]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Role of Gut Microbiota and Nutrients in Amyloid Formation and Pathogenesis of Alzheimer Disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Wu, X.; Cao, H.; Li, X.; Hou, Z.; Wang, B.; Liu, J.; Ji, X.; Zhang, P.; et al. A Comparison of the Composition and Functions of the Oral and Gut Microbiotas in Alzheimer’s Patients. Front. Cell. Infect. Microbiol. 2022, 12, 942460. [Google Scholar] [CrossRef]
- Wu, S.C.; Cao, Z.S.; Chang, K.M.; Juang, J.L. Intestinal Microbial Dysbiosis Aggravates the Progression of Alzheimer’s Disease in Drosophila. Nat. Commun. 2017, 8, 24. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; McCoy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthan, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Mezö, C.; Dokalis, N.; Mossad, O.; Staszewski, O.; Neuber, J.; Yilmaz, B.; Schnepf, D.; de Agüero, M.G.; Ganal-Vonarburg, S.C.; Macpherson, A.J.; et al. Different Effects of Constitutive and Induced Microbiota Modulation on Microglia in a Mouse Model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, L.; Jiang, B. The Role of Gut Microbiota in Aging and Aging Related Neurodegenerative Disorders: Insights from Drosophila Model. Life 2021, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Zafar, S.; Yaddanapudi, S.S. Parkinson Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Radad, K.; Moldzio, R.; Krewenka, C.; Kranner, B.; Rausch, W. Pathophysiology of Non-Motor Signs in Parkinson’s Disease: Some Recent Updating with Brief Presentation. Explor. Neuroprot. Ther. 2023, 3, 24–46. [Google Scholar] [CrossRef]
- Yang, X.; Qian, Y.; Xu, S.; Song, Y.; Xiao, Q. Longitudinal Analysis of Fecal Microbiome and Pathologic Processes in a Rotenone-Induced Mice Model of Parkinson’s Disease. Front. Aging Neurosci. 2018, 9, 441. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of Dopamine in the Pathophysiology of Parkinson’s Disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef]
- Cavallieri, F.; Cury, R.G.; Guimarães, T.; Fioravanti, V.; Grisanti, S.; Rossi, J.; Monfrini, E.; Zedde, M.; Di Fonzo, A.; Valzania, F.; et al. Recent Advances in the Treatment of Genetic Forms of Parkinson’s Disease: Hype or Hope? Cells 2023, 12, 764. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-Synuclein in Parkinson’s Disease and Other Synucleinopathies: From Overt Neurodegeneration Back to Early Synaptic Dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- McCann, H.; Cartwright, H.; Halliday, G.M. Neuropathology of α-Synuclein Propagation and Braak Hypothesis. Mov. Disord. 2016, 31, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Forloni, G. Alpha Synuclein: Neurodegeneration and Inflammation. Int. J. Mol. Sci. 2023, 24, 5914. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, F.; Pedersen, N.L.; Tillander, A.; Ludvigsson, J.F.; Ekbom, A.; Svenningsson, P.; Chen, H.; Wirdefeldt, K. Vagotomy and Parkinson Disease: A Swedish Register-Based Matched-Cohort Study. Neurology 2017, 88, 1996–2002. [Google Scholar] [CrossRef]
- Riederer, P.; Nagatsu, T.; Youdim, M.B.H.; Wulf, M.; Dijkstra, J.M.; Sian-Huelsmann, J. Lewy Bodies, Iron, Inflammation and Neuromelanin: Pathological Aspects Underlying Parkinson’s Disease. J. Neural Transm. 2023, 130, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, C.; Chang, K. Biomarker of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef]
- Grotemeyer, A.; McFleder, R.L.; Wu, J.; Wischhusen, J.; Ip, C.W. Neuroinflammation in Parkinson’s Disease—Putative Pathomechanisms and Targets for Disease-Modification. Front. Immunol. 2022, 13, 878771. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Munhoz, R.P.; Tumas, V.; Pedroso, J.L.; Silveira-Moriyama, L. The Clinical Diagnosis of Parkinson’s Disease. Arq. Neuropsiquiatr. 2024, 82, 1–10. [Google Scholar] [CrossRef]
- Caradonna, E.; Nemni, R.; Bifone, A.; Gandolfo, P.; Costantino, L.; Giordano, L.; Mormone, E.; Macula, A.; Cuomo, M.; Difruscolo, R.; et al. The Brain–Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review. J. Clin. Med. 2024, 13, 4130. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal Barrier Permeability: The Influence of Gut Microbiota, Nutrition, and Exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. npj Parkinsons Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Lekchand Dasriya, V.; Samtiya, M.; Dhewa, T.; Puniya, M.; Kumar, S.; Ranveer, S.; Chaudhary, V.; Vij, S.; Behare, P.; Singh, N.; et al. Etiology and Management of Alzheimer’s Disease: Potential Role of Gut Microbiota Modulation with Probiotics Supplementation. J. Food Biochem. 2022, 46, e14043. [Google Scholar] [CrossRef] [PubMed]
- Talman, L.; Safarpour, D. An Overview of Gastrointestinal Dysfunction in Parkinsonian Syndromes. Semin. Neurol. 2023, 43, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Zapała, B.; Stefura, T.; Wójcik-Pędziwiatr, M.; Kabut, R.; Bałajewicz-Nowak, M.; Milewicz, T.; Dudek, A.; Stój, A.; Rudzińska-Bar, M. Differences in the Composition of Gut Microbiota Between Patients with Parkinson’s Disease and Healthy Controls: A Cohort Study. J. Clin. Med. 2021, 10, 5698. [Google Scholar] [CrossRef]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, J.; Liu, X.; Zhong, Y.; Cai, X.; Long, L. Review: The Role of Intestinal Dysbiosis in Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 615075. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut Microbial Metabolites in Depression: Understanding the Biochemical Mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Van Ijzendoorn, S.C.D.; Derkinderen, P. The Intestinal Barrier in Parkinson’s Disease: Current State of Knowledge. J. Park. Dis. 2019, 9, S323–S329. [Google Scholar] [CrossRef]
- Gorlé, N.; Bauwens, E.; Haesebrouck, F.; Smet, A.; Vandenbroucke, R.E. Helicobacter and the Potential Role in Neurological Disorders: There Is More Than Helicobacter pylori. Front. Immunol. 2021, 11, 584165. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Medical Management of Parkinson’s Disease. Pharm. Ther. 2008, 33, 590–606. [Google Scholar]
- Nyholm, D.; Hellström, P.M. Effects of Helicobacter pylori on Levodopa Pharmacokinetics. J. Park. Dis. 2021, 11, 61–69. [Google Scholar] [CrossRef]
- Lolekha, P.; Sriphanom, T.; Vilaichone, R.-K. Helicobacter Pylori Eradication Improves Motor Fluctuations in Advanced Parkinson’s Disease Patients: A Prospective Cohort Study (HP-PD Trial). PLoS ONE 2021, 16, e0251042. [Google Scholar] [CrossRef]
- Abdollahi, H.; Tadjrobehkar, O. The Role of Different Sugars, Amino Acids and Few Other Substances in Chemotaxis Directed Motility of Helicobacter Pylori. Iran. J. Basic Med. Sci. 2012, 15, 787–794. [Google Scholar] [CrossRef]
- Rekdal, V.M.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and Inhibition of an Interspecies Gut Bacterial Pathway for Levodopa Metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef]
- Bi, M.; Feng, L.; He, J.; Liu, C.; Wang, Y.; Jiang, H.; Liu, S.J. Emerging Insights Between Gut Microbiome Dysbiosis and Parkinson’s Disease: Pathogenic and Clinical Relevance. Ageing Res. Rev. 2022, 82, 101759. [Google Scholar] [CrossRef]
- Parashar, A.; Udayabanu, M. Gut Microbiota: Implications in Parkinson’s Disease. Parkinsonism Relat. Disord. 2017, 38, 1–7. [Google Scholar] [PubMed]
- Kustrimovic, N.; Balkhi, S.; Bilato, G.; Mortara, L. Gut Microbiota and Immune System Dynamics in Parkinson’s and Alzheimer’s Diseases. Int. J. Mol. Sci. 2024, 25, 12164. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated with Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut Microbiota in Parkinson’s Disease: Temporal Stability and Relations to Disease Progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [PubMed]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A Gut Bacterial Amyloid Promotes α-Synuclein Aggregation and Motor Impairment in Mice. Elife 2020, 9, e53111. [Google Scholar] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased Intestinal Permeability Correlates with Sigmoid Mucosa Alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.; Boktor, J.C.; Cantu-Jungles, T.M.; Thron, T.; Zhang, M.; Bostick, J.W.; Khazaei, T.; Chilakala, S.; Morais, L.H.; et al. A Prebiotic Diet Modulates Microglial States and Motor Deficits in α-Synuclein Overexposing Mice. eLife 2022, 11, e81453. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Trautwein, C.; Romani, J.; Salker, M.S.; Neckel, P.H.; Fraccaroli, I.; Abeditashi, M.; Woerner, N.; Admard, J.; Dhariwal, A.; et al. Overexpression of Human Alpha-Synuclein Leads to Dysregulated Microbiome/Metabolites with Ageing in a Rat Model of Parkinson Disease. Mol. Neurodegener. 2023, 18, 44. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Guo, J. Parkinson’s Disease and Gut Microbiota: From Clinical to Mechanistic and Therapeutic Studies. Transl. Neurodegener. 2023, 12, 59. [Google Scholar] [CrossRef]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Chun, J.; Han, K.; Soh, H.; Kang, E.A.; Lee, H.J.; Im, J.P.; Kim, J.S. Patients with Inflammatory Bowel Disease Are at an Increased Risk of Parkinson’s Disease: A South Korean Nationwide Population-Based Study. J. Clin. Med. 2019, 8, 1191. [Google Scholar] [CrossRef]
- Guo, L.; Xu, J.; Du, Y.; Wu, W.; Nie, W.; Zhang, D.; Luo, Y.; Lu, H.; Lei, M.; Xiao, S.; et al. Effects of Gut Microbiota and Probiotics on Alzheimer’s Disease. Transl. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic Potential of Bifidobacterium breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Neta, F.I.; de Souza, F.E.S.; Batista, A.L.; Pinheiro, F.I.; Cobucci, R.N.; Guzen, F.P. Effects of Supplementation with Probiotics in Experimental Models of Alzheimer’s Disease: A Systematic Review of Animal Experiments. Curr. Alzheimer Res. 2022, 19, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Gong, C.; Cuccioloni, M.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation as Preventative and Therapeutic Approach in Alzheimer’s Disease. FEBS J. 2021, 288, 2836–2855. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.G.; Michikawa, M. Probiotic Bifidobacterium breve Prevents Memory Impairment Through the Reduction of Both Amyloid-β Production and Microglia Activation in APP Knock-In Mouse. J. Alzheimer’s Dis. 2022, 85, 1555–1571. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia—An Explorative Intervention Study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Debbarma, S. Fecal Microbiota Transplantation: A Microbiome Modulation Technique for Alzheimer’s Disease. Cureus 2021, 13, e16503. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-Like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Jiang, Z.D.; DuPont, A.W.; Utay, N.S. Abnormal Intestinal Microbiome in Medical Disorders and Potential Reversibility by Fecal Microbiota Transplantation. Dig. Dis. Sci. 2020, 65, 741–756. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered Microbiomes Distinguish Alzheimer’s Disease from Amnestic Mild Cognitive Impairment and Health in a Chinese Cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).