Synthesis of 2-Benzylidene-3-Pyrrolines and Their Synthetic Transformation
Abstract
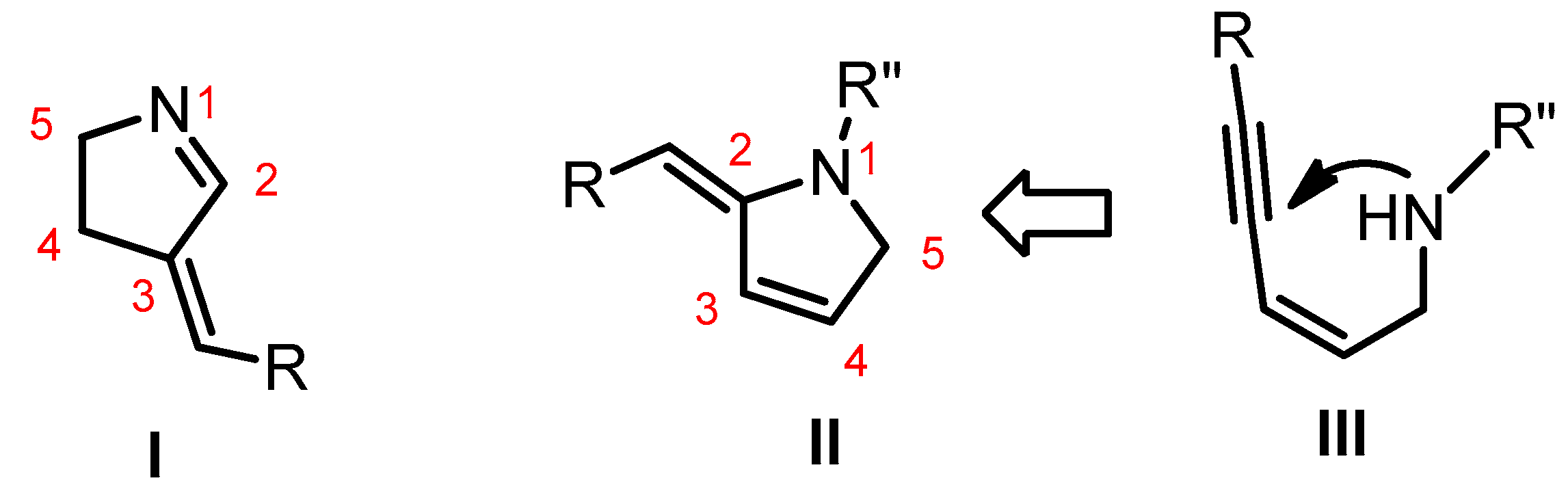
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis
2.3. Spectroscopic Characterization
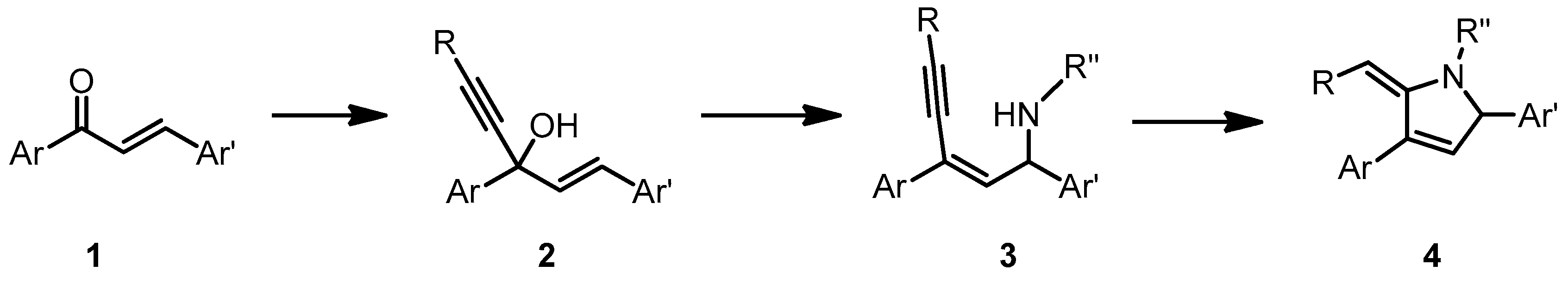
3. Results and Discussion
3.1. Synthetic Design
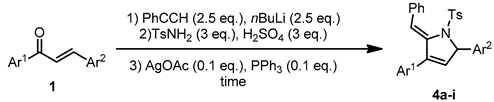
3.2. Optimization of Each Step
3.3. Reaction Scope
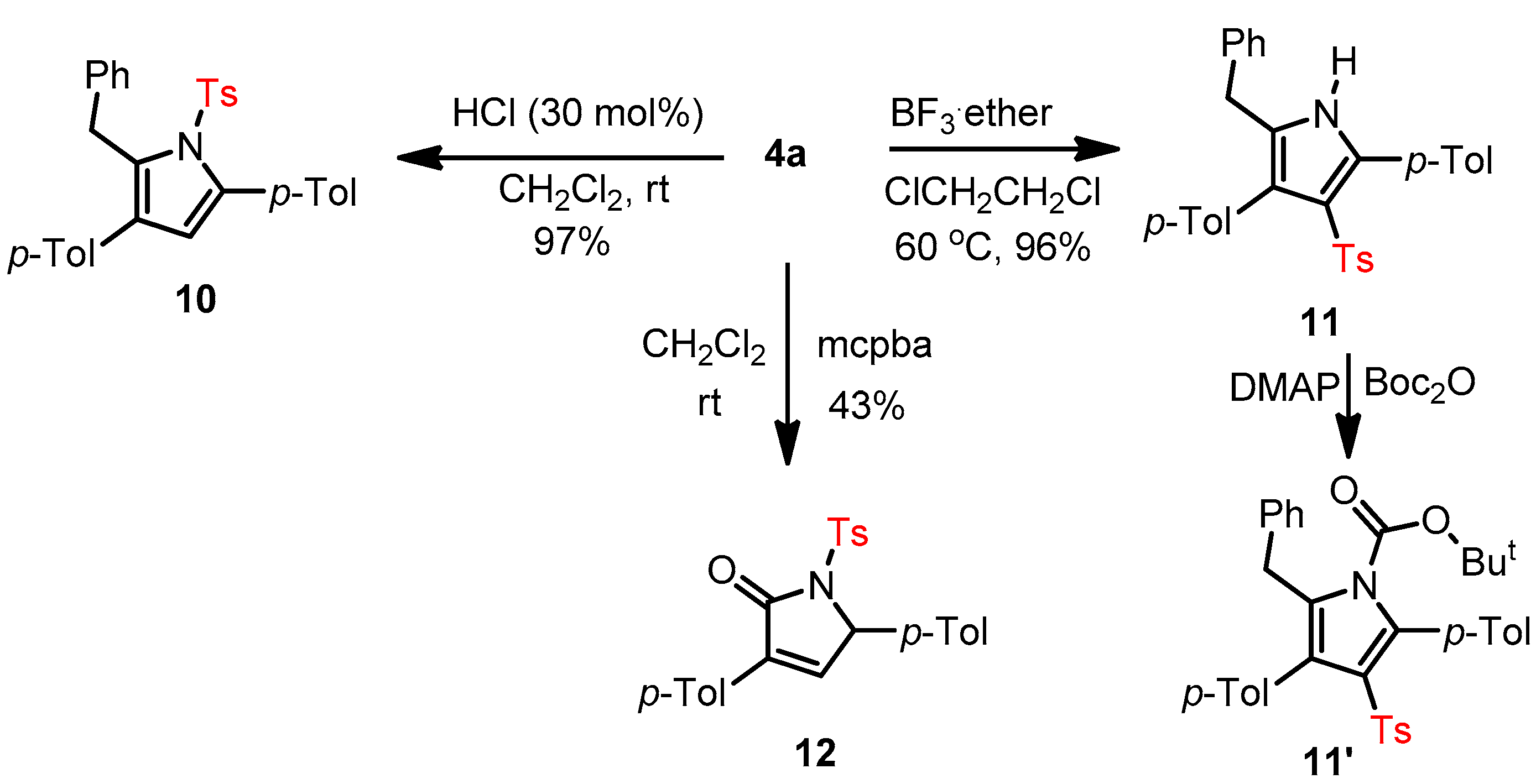
3.4. Further Synthetic Transformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khajuria, R.; Dham, S.; Kapoor, K.K. Active methylenes in the synthesis of a pyrrole motif: An imperative structural unit of pharmaceuticals, natural products and optoelectronic materials. RSC Adv. 2016, 6, 37039–37066. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Bauer, I.; Knoelker, H.-J. Synthesis of pyrrole and carbazole alkaloids. Top. Curr. Chem. 2012, 309, 203–254. [Google Scholar] [PubMed]
- de Laszlo, S.E.; Hacker, C.; Li, B.; Kim, D.; MacCoss, M.; Mantlo, N.; Pivnichny, J.V.; Colwell, L.; Koch, G.E.; Cascieri, M.A.; et al. Potent, orally absorbed glucagon receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 641–646. [Google Scholar] [CrossRef]
- Zubia, A.; Ropero, S.; Otaegui, D.; Ballestar, E.; Fraga, M.F.; Boix-Chornet, M.; Berdasco, M.; Martinez, A.; Coll-Mulet, L.; Gil, J.; et al. Identification of (1H)-pyrroles as histone deacetylase inhibitors with antitumoral activity. Oncogene 2009, 28, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Fraley, M.E.; Garbaccio, R.M.; Arrington, K.L.; Hoffman, W.F.; Tasber, E.S.; Coleman, P.J.; Buser, C.A.; Walsh, E.S.; Hamilton, K.; Fernandes, C.; et al. Kinesin spindle protein (KSP) inhibitors. Part 2: The design, synthesis, and characterization of 2,4-diaryl-2,5-dihydropyrrole inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett. 2006, 16, 1775–1779. [Google Scholar] [CrossRef]
- Cox, C.D.; Coleman, P.J.; Breslin, M.J.; Whitman, D.B.; Garbaccio, R.M.; Fraley, M.E.; Buser, C.A.; Walsh, E.S.; Hamilton, K.; Schaber, M.D.; et al. Kinesin Spindle Protein (KSP) Inhibitors. 9. Discovery of (2S)-4-(2,5-Difluorophenyl)-N-[(3R,4S)-3-fluoro-1-methylpiperidin-4-yl]-2-(hydroxymethyl)-N-methyl-2-phenyl-2,5-dihydro-1H-pyrrole-1-carboxamide (MK-0731) for the Treatment of Taxane-Refractory Cancer. J. Med. Chem. 2008, 51, 4239–4252. [Google Scholar] [PubMed]
- Castellano, S.; Fiji, H.D.G.; Kinderman, S.S.; Watanabe, M.; de Leon, P.; Tamanoi, F.; Kwon, O. Small Molecule Inhibitors of Protein Geranylgeranyltransferase Type I. J. Am. Chem. Soc. 2007, 129, 5843–5845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazizov, A.S.; Smolobochkin, A.V.; Burilov, A.R.; Pudovik, M.A. 3-Ylidene-1-pyrrolines: Synthesis, reactions and perspectives. Tetrahedron Lett. 2020, 61, 152371. [Google Scholar] [CrossRef]
- Atkinson, J.H.; Atkinson, R.S.; Johnson, A.W. The structure and reactions of some pyrrolin-2-ones. J. Chem. Soc. 1964, 5999–6009. [Google Scholar] [CrossRef]
- Atkinson, J.H.; Johnson, A.W. Some reactions of 2-(2-pyrrolyl)-1-pyrrolines. J. Chem. Soc. 1965, 2614–2621. [Google Scholar] [CrossRef]
- Zahedi, E.; Mozaffari, M.; Shahsavar, F.; Shiroudi, A.; Deleuze, M.S. Understanding the kinetics and mechanism of thermal cheletropic elimination of N2 from (2,5-dihydro-1H-pyrrol-1-ium-1-ylidene) amide using RRKM and ELF theories. Res. Chem. Intermed. 2017, 43, 1575–1590. [Google Scholar] [CrossRef] [Green Version]
- Li, M.B.; Grape, E.S.; Bäckvall, J.E. Palladium-Catalyzed Stereospecific Oxidative Cascade Reaction of Allenes for the Construction of Pyrrole Rings: Control of Reactivity and Selectivity. ACS Catal. 2019, 9, 5184–5190. [Google Scholar] [CrossRef]
- Yu, Q.; Li, X.; Wang, X.; Liu, J. Regioselective Synthesis of 2,5-Disubstituted Pyrroles via Stepwise Iododesilylation and Coupling Reactions. Aust. J. Chem. 2018, 71, 95–101. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, P.; Zhang, H.; Zhong, L.; Wang, Z.-X.; Huang, X. Gold-Catalyzed Atom-Economic Synthesis of Sulfone-Containing Pyrrolo[2,1-a]isoquinolines from Diynamides: Evidence for Consecutive Sulfonyl Migration. ACS Catal. 2019, 9, 2610–2617. [Google Scholar] [CrossRef]



 | |||||
| Entry | Catalyst (eq.) | Solvent 2 | Temp. | Time (h) | Yield of 4a 2 |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 (0.1) | DCM | rt | 24 | 0% |
| 2 | PdCl2 (0.1)/tBuOK (1.0) | toluene | 50 °C | 24 | 0% |
| 3 | AgOAc (0.1)/PPh3 (0.1) | DCM/MeOH | rt | 24 | 23% |
| 4 | AgOAc (0.1) | DCE/MeOH | 60 °C | 2 | 26% |
| 5 | AgOAc (0.1)/PPh3 (0.1) | DCE/MeOH | 60 °C | 2 | 65% |
| 6 | AgOAc (0.1)/PPh3 (0.1) | DCE/MeOH | 60 °C | 3 | 100% (86%) 3 |
| 7 | AgOAc (0.1)/P(oTol)3 (0.1) | DCE/MeOH | 60 °C | 2 | 57% |
| 8 | AgOAc (0.1)/P(OPh)3 (0.1) | DCE/MeOH | 60 °C | 2 | 0% |
| 9 | AgOAc (0.1)/dppe (0.05) | DCE/MeOH | 60 °C | 2 | 44% |
 | |||
| Entry | Substituents | Time | Product (Yield) |
|---|---|---|---|
| 1 | Ar1 = p-MeC6H4-; Ar2 = p-MeC6H4- | 4 h | 4a (86%) |
| 2 | Ar1 = p-MeOC6H4-; Ar2 = p-MeC6H4- | 25 h | 4b (42%) |
| 3 | Ar1 = p-FC6H4-; Ar2 = p-MeC6H4- | 22 h | 4c (81%) |
| 4 | Ar1 = p-ClC6H4-; Ar2 = p-MeC6H4- | 22 h | 4d (70%) |
| 5 | Ar1 = p-BrC6H4-; Ar2 = p-MeC6H4- | 22 h | 4e (55%) |
| 6 | Ar1 = p-MeC6H4-; Ar2 = p-FC6H4- | 46 h | 4f (71%) |
| 7 | Ar1 = p-MeC6H4-; Ar2 = p-ClC6H4- | 66 h | 4g (61%) |
| 8 | Ar1 = o-MeC6H4-; Ar2 = p-MeC6H4- | 4 h | 4h (72%) |
| 9 | Ar1 = p-MeC6H4-; Ar2 = 1-naphthyl | 22 h | 4i (64%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-T.; Liu, Y.-H.; Liu, S.-T. Synthesis of 2-Benzylidene-3-Pyrrolines and Their Synthetic Transformation. Reactions 2020, 1, 47-53. https://doi.org/10.3390/reactions1020005
Hsu M-T, Liu Y-H, Liu S-T. Synthesis of 2-Benzylidene-3-Pyrrolines and Their Synthetic Transformation. Reactions. 2020; 1(2):47-53. https://doi.org/10.3390/reactions1020005
Chicago/Turabian StyleHsu, Ming-Tsung, Yi-Hung Liu, and Shiuh-Tzung Liu. 2020. "Synthesis of 2-Benzylidene-3-Pyrrolines and Their Synthetic Transformation" Reactions 1, no. 2: 47-53. https://doi.org/10.3390/reactions1020005
APA StyleHsu, M.-T., Liu, Y.-H., & Liu, S.-T. (2020). Synthesis of 2-Benzylidene-3-Pyrrolines and Their Synthetic Transformation. Reactions, 1(2), 47-53. https://doi.org/10.3390/reactions1020005






