Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains
Abstract
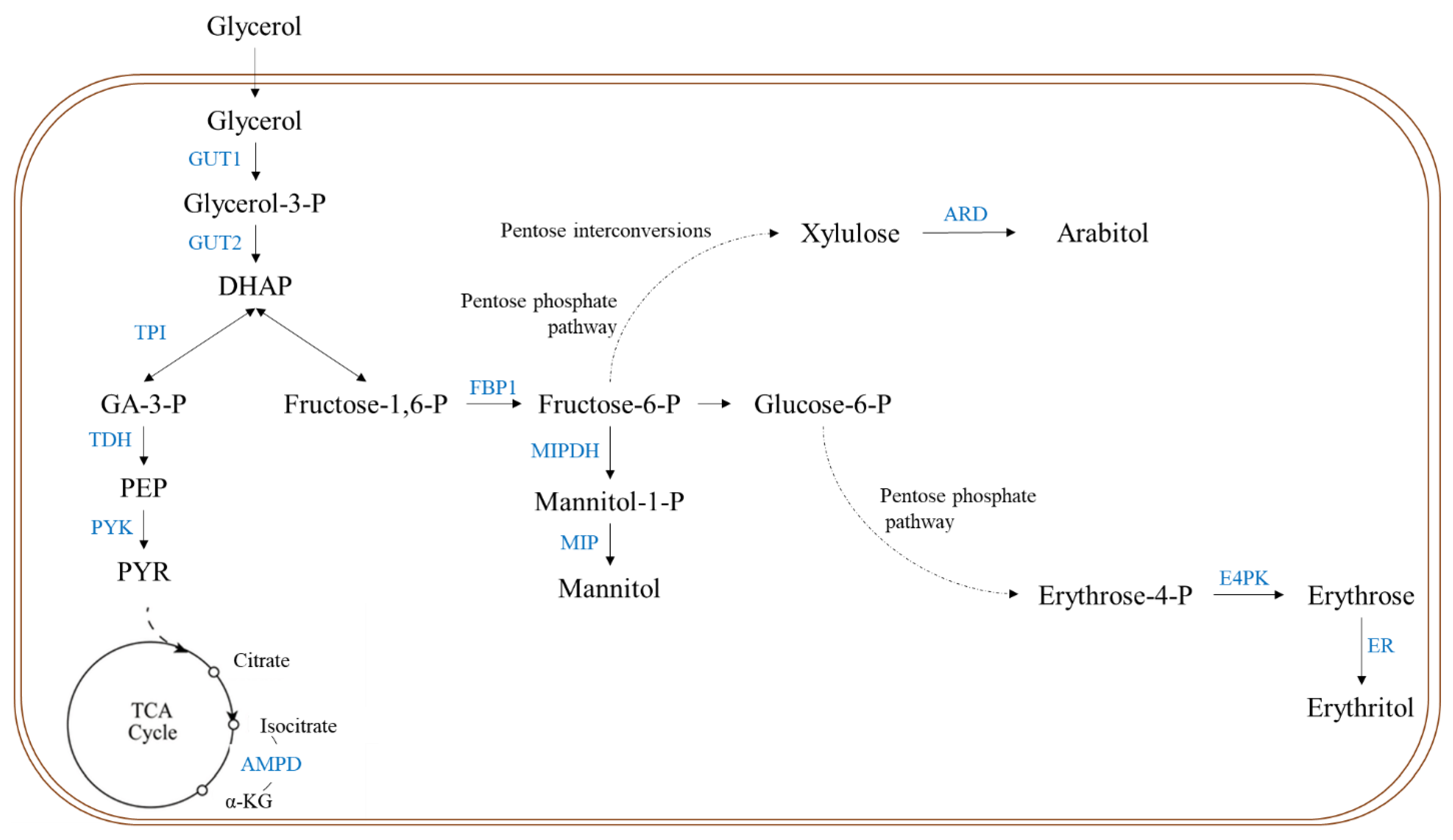
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Raw Materials
2.3. Fermentation Media
2.4. Culture Conditions
2.5. Analyses
2.6. Nomenclature
3. Results
3.1. Trials of Yarrowia lipolytica Strains on Crude Glycerol at Different Initial pH Values
3.2. Effect of Glycerol Purity and Initial Concentration on Polyols Production by Yarrowia lipolytica FMCC Y-74
3.3. Fed-Batch Fermentation for Mannitol Production by Yarrowia lipolytica FMCC Y-74
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Dheskali, E.; Papapostolou, H.; de Castro, A.M.; Freire, D.M.G.; Nychas, G.J.E.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess development for 2,3-butanediol production from crude glycerol and conceptual process design for aqueous conversion into methyl ethyl ketone. ACS Sustain. Chem. Eng. 2021, 9, 8692–8705. [Google Scholar] [CrossRef]
- World Energy Resources 2016 by the World Energy Council. Archived from the Executive Summary World Energy Resources. 2016. Available online: https://www.worldenergy.org/assets/images/imported/2016/10/World-Energy-Resources-Full-report-2016.10.03.pdf (accessed on 24 July 2021).
- Fickers, P.; Cheng, H.; Lin, C.S.K. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K.; De Bruyn, M.; Clark, J.H.; Koutinas, A.A. Sunflower-based biorefinery: Poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour. Technol. 2014, 172, 121–130. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Yang, X.; Lin, C.S.K. Green and sustainable succinic acid production from crude glycerol by engineered Yarrowia lipolytica via agricultural residue based in situ fibrous bed bioreactor. Bioresour. Technol. 2018, 249, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Metsoviti, M.; Paraskevaidi, K.; Koutinas, A.; Zeng, A.-P.; Papanikolaou, S. Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process. Biochem. 2012, 47, 1872–1882. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.; KArendt, E.; Coffey, A. A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Kordowska-Wiater, M. Production of arabitol by yeasts: Current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef]
- Expert Market Research. Global Mannitol Market Outlook. Available online: https://www.expertmarketresearch.com/reports/mannitol-market (accessed on 30 August 2021).
- Ortiz, M.E.; Bleckwedel, J.; Raya, R.R.; Mozzi, F. Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2013, 97, 4713–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.B.; Zhan, X.B.; Zheng, Z.Y.; Wu, J.R.; Gao, M.J.; Lin, C.C. A novel osmotic pressure control fed-batch fermentation strategy for improvement of erythritol production by Yarrowia lipolytica from glycerol. Bioresour. Technol. 2014, 151, 120–127. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Kim, S. Controlling substrate concentration in fed-batch Candida magnoliae culture increases mannitol production. Biotechnol. Prog. 2003, 19, 768–775. [Google Scholar] [CrossRef]
- Khan, A.; Bhide, A.; Gadre, R. Mannitol production from glycerol by resting cells of Candida magnoliae. Bioresour. Technol. 2009, 100, 4911–4913. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Suzuki, T. Microbial production of D-mannitol and D-fructose from glycerol. Biotechnol. Bioeng. 1970, 12, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Filippousi, R.; Antoniou, D.; Tryfinopoulou, P.; Nisiotou, A.A.; Nychas, G.; Koutinas, A.A.; Papanikolaou, S. Isolation, identification and screening of yeasts towards their ability to assimilate biodiesel-derived crude glycerol: Microbial production of polyols, endopolysaccharides and lipid. J. Appl. Microbiol. 2019, 127, 1080–1100. [Google Scholar] [CrossRef]
- Sarantou, S.; Stoforos, N.G.; Kalantzi, O.; Papanikolaou, S. Biotechnological valorization of biodiesel-derived glycerol: Trials with the non-conventional yeasts Yarrowia lipolytica and Rhodosporidium sp. Carbon Resour. Convers. 2021, 4, 61–75. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nicaud, J.-M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- André, A.; Chatzifragkou, A.; Diamantopoulou, P.; Sarris, D.; Philippoussis, A.; Galiotou-Panayotou, M.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel-derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Stoforos, N.G.; Xenopoulos, E.; Sarris, D.; Psarianos, D.; Philippoussis, A.; Papanikolaou, S. Lipid production by Cryptococcus curvatus growing on commercial xylose and subsequent valorization of fermentation waste-waters for the production of edible and medicinal mushrooms. Biochem. Eng. J. 2021, 162, 107706. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid production by yeasts growing on commercial xylose in submerged cultures with process water being partially replaced by olive mill wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Onishi, H.; Suzuki, T. Production of D-mannitol and glycerol by yeasts. Appl. Microbiol. 1968, 16, 1847–1852. [Google Scholar] [CrossRef]
- Blomberg, A.; Adler, L. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 1992, 33, 145–212. [Google Scholar] [CrossRef]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, J.; Habe, H.; Morita, T.; Fukuoka, T.; Imura, T.; Iwabuchi, H.; Uemura, S.; Tamura, T.; Kitamoto, D. Production of mannitol from raw glycerol by Candida azyma. J. Biosci. Bioeng. 2014, 117, 725–729. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk, A.M.; Furgała, J.; Rakicka, M.; Rymowicz, W. Enhanced production of erythritol by Yarrowia lipolytica on glycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2014, 41, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka-Pustułka, M.; Rywińska, A.; Rymowicz, W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper host for efficient erythritol biosynthesis from glycerol. Process. Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Carly, F.; Vandermies, M.; Telek, S.; Steels, S.; Thomas, S.; Nicaud, J.-M.; Fickers, P. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab. Eng. 2017, 42, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Rakicka-Pustułka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef] [PubMed]


| Time (h) | X (g L−1) | Glycons (g L−1) | Mannitol (g L−1) | YM/Gly (g g−1) | YL/X (g g−1) | YIPS/X (g g−1) | ||
|---|---|---|---|---|---|---|---|---|
| pH = 3.0 ± 0.3 | a | 120 | 10.38 | 37.70 | 3.51 | 0.09 | 0.08 | 0.17 |
| b, c | 48 | 6.62 | 36.33 | 19.64 | 0.54 | 0.11 | 0.20 | |
| d | 72 | 7.26 | 37.70 | 17.33 | 0.46 | 0.09 | 0.21 | |
| pH = 4.0 ± 0.3 | a | 144 | 11.13 | 37.70 | 2.97 | 0.08 | 0.06 | 0.13 |
| b | 48 | 6.69 | 34.15 | 14.89 | 0.44 | 0.09 | 0.19 | |
| c | 96 | 9.00 | 37.70 | 14.20 | 0.38 | 0.09 | 0.17 | |
| d | 72 | 9.83 | 37.70 | 11.46 | 0.30 | 0.11 | 0.29 | |
| pH = 5.0 ± 0.3 | a | 120 | 11.86 | 37.70 | 8.45 | 0.22 | 0.07 | 0.19 |
| b | 96 | 9.55 | 37.05 | 13.41 | 0.36 | 0.09 | 0.35 | |
| c, d | 72 | 11.70 | 34.33 | 8.45 | 0.25 | 0.10 | 0.37 | |
| pH = 6.0 ± 0.3 | a | 120 | 12.99 | 37.70 | 4.52 | 0.20 | 0.06 | 0.22 |
| b | 96 | 10.48 | 37.70 | 10.35 | 0.28 | 0.09 | 0.31 | |
| c, d | 72 | 10.26 | 37.70 | 9.38 | 0.25 | 0.11 | 0.42 | |
| pH = 7.0 ± 0.3 | a | 120 | 13.40 | 37.70 | 4.32 | 0.12 | 0.08 | 0.18 |
| b, c, d | 96 | 5.20 | 37.70 | 6.67 | 0.18 | 0.10 | 0.35 | |
| Time (h) | X (g L−1) | Glycons (g L−1) | Mannitol (g L−1) | YM/Gly (g g−1) | YL/X (g g−1) | YIPS/X (g g−1) | ||
|---|---|---|---|---|---|---|---|---|
| pH = 3.0 ± 0.3 | a, c, d | 48 | 10.60 | 32.75 | 10.13 | 0.31 | 0.12 | 0.23 |
| b | 96 | 7.08 | 36.14 | 10.52 | 0.29 | 0.08 | 0.13 | |
| pH = 4.0 ± 0.3 | a | 144 | 11.25 | 36.14 | 6.32 | 0.18 | 0.06 | 0.13 |
| b | 96 | 9.13 | 36.14 | 16.11 | 0.45 | 0.09 | 0.15 | |
| c | 72 | 10.00 | 36.14 | 12.54 | 0.35 | 0.11 | 0.20 | |
| d | 48 | 10.80 | 34.54 | 9.63 | 0.28 | 0.09 | 0.30 | |
| pH = 5.0 ± 0.3 | a | 144 | 10.43 | 36.14 | 9.63 | 0.27 | 0.06 | 0.20 |
| b | 96 | 9.13 | 36.14 | 13.31 | 0.37 | 0.09 | 0.19 | |
| c | 72 | 9.57 | 36.14 | 12.08 | 0.33 | 0.10 | 0.21 | |
| d | 48 | 8.00 | 34.99 | 11.53 | 0.33 | 0.08 | 0.31 | |
| pH = 6.0 ± 0.3 | a, c | 72 | 11.20 | 36.14 | 12.23 | 0.35 | 0.11 | 0.25 |
| b | 96 | 10.45 | 36.14 | 12.26 | 0.34 | 0.09 | 0.17 | |
| d | 48 | 7.40 | 30.73 | 12.19 | 0.40 | 0.09 | 0.34 | |
| pH = 7.0 ± 0.3 | a, c | 72 | 11.60 | 36.14 | 8.32 | 0.23 | 0.12 | 0.29 |
| b | 48 | 5.00 | 30.27 | 8.74 | 0.29 | 0.09 | 0.29 | |
| d | 96 | 10.87 | 36.14 | 8.07 | 0.22 | 0.11 | 0.34 | |
| Time (h) | X (g L−1) | Glycons (g L−1) | Mannitol (g L−1) | Erythritol (g L−1) | Arabitol (g L−1) | ||
|---|---|---|---|---|---|---|---|
| Crude glycerol (88%) | a, b | 144 | 10.90 | 36.06 | 15.20 | 1.00 | 3.42 |
| Crude glycerol (74%) | a, b | 171 | 11.94 | 38.52 | 13.68 | 2.41 | 5.78 |
| Pure glycerol | a, b | 144 | 11.20 | 34.82 | 15.50 | 1.00 | 3.23 |
| Gly0 (g L−1) | Time (h) | X (g L−1) | Glycons (g L−1) | Mannitol (g L−1) | Erythritol (g L−1) | Arabitol (g L−1) | Polyols (g L−1) | YPolyols/Gly (g g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| ≈60 | 96 | a | 7.58 | 38.62 | 6.13 | 4.02 | 3.21 | 13.36 | 0.35 |
| 144 | c, d | 4.12 | 45.66 | 11.58 | 5.90 | 5.53 | 23.01 | 0.50 | |
| 264 | b | 6.01 | 55.67 | 14.34 | 3.79 | 3.63 | 21.76 | 0.39 | |
| ≈80 | 96 | a | 7.00 | 37.83 | 9.17 | 6.49 | 4.11 | 19.77 | 0.52 |
| 168 | c | 3.78 | 65.62 | 15.62 | 15.77 | 7.19 | 38.58 | 0.59 | |
| 216 | d | 3.05 | 73.36 | 16.45 | 13.07 | 9.73 | 39.25 | 0.54 | |
| 240 | b | 2.54 | 76.18 | 19.14 | 14.59 | 6.91 | 40.64 | 0.53 | |
| ≈120 | 96 | a | 6.89 | 40.15 | 4.82 | 3.68 | 2.63 | 11.13 | 0.28 |
| 312 | b, c, d | 4.85 | 104.27 | 21.74 | 24.59 | 10.31 | 56.64 | 0.54 |
| Strain | Erythritol (g L−1) | Mannitol (g L−1) | Arabitol (g L−1) | Polyols (g L−1) | YPol/Gly (g g−1) | Cultivation Type | Reference |
|---|---|---|---|---|---|---|---|
| Wratislavia 1.31 † | 132.0 | 23.0 | - | 155.0 | 0.52 | Fed-batch reactor | Rymowicz et al. [36] |
| Wratislavia K1 † | 170.0 | 12.0 | - | 182.0 | 0.60 | Fed-batch reactor | Rymowicz et al. [36] |
| A-15 & | 71.0 | 8.0 | 1.8 | 80.8 | 0.50 | Batch reactor | Tomaszewska et al. [13] |
| A UV’1 † | 63.0 | 8.8 | 9.2 | 81.0 | 0.50 | Batch reactor | Tomaszewska et al. [13] |
| Wratislavia K1 † | 80.0 | 2.6 | 0.3 | 82.9 | 0.51 | Batch reactor | Tomaszewska et al. [13] |
| Wratislavia K1 † | 135.5 | 3.9 | 0.1 | 139.5 | 0.58 | Repeated-batch reactor | Mirończuk et al. [37] |
| Wratislavia 1.31 † | 26.2 | 16.8 | 3.7 | 46.7 | 0.36 | Batch reactor | Tomaszewska et al. [25] |
| Wratislavia K1 † | 40.7 | 15.1 | 2.9 | 58.7 | 0.40 | Batch reactor | Tomaszewska et al. [25] |
| MK1 † | 79.5 | 2.7 | 0.4 | 82.6 | 0.55 | Batch reactor | Mirończuk et al. [38] |
| MK1 † | 177.3 | 2.2 | - | 179.5 | 0.67 | Repeated-batch reactor | Mirończuk et al. [38] |
| FCY 218 † | 80.6 | n.i. | n.i. | 80.6 | 0.53 | Batch reactor | Carly et al. [39] |
| HA 1251 &¶ | ≈4 | ≈32 | ≈5 | ≈41 | n.i. | Batch reactor | Egermeier et al. [34] |
| ACA YC 5030 &¶ | 35.5 | 32.1 | - | 67.6 | 0.49 | Batch flasks | Papanikolaou et al. [8] |
| AIB & | 56.7 | 12.6 | 6.0 | 75.3 | 0.49 | Fed-batch reactor | Rakicka et al. [40] |
| ACA-DC 5033 &¶ | 25.9 | 17.5 | 4.2 | 47.6 | 0.58 | Batch flasks | Sarantou et al. [20] |
| FMCC Y-74 | 2.41 | 36.84 | 3.02 | 42.27 | 0.59 | Fed-batch flasks | Present study |
| FMCC Y-74 | 24.59 | 21.74 | 10.31 | 56.64 | 0.54 | Batch flasks | Present study |
| FMCC Y-74 | 14.59 | 19.14 | 6.91 | 40.64 | 0.53 | Batch flasks | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vastaroucha, E.-S.; Maina, S.; Michou, S.; Kalantzi, O.; Pateraki, C.; Koutinas, A.A.; Papanikolaou, S. Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains. Reactions 2021, 2, 499-513. https://doi.org/10.3390/reactions2040032
Vastaroucha E-S, Maina S, Michou S, Kalantzi O, Pateraki C, Koutinas AA, Papanikolaou S. Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains. Reactions. 2021; 2(4):499-513. https://doi.org/10.3390/reactions2040032
Chicago/Turabian StyleVastaroucha, Eleni-Stavroula, Sofia Maina, Savvoula Michou, Ourania Kalantzi, Chrysanthi Pateraki, Apostolis A. Koutinas, and Seraphim Papanikolaou. 2021. "Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains" Reactions 2, no. 4: 499-513. https://doi.org/10.3390/reactions2040032
APA StyleVastaroucha, E.-S., Maina, S., Michou, S., Kalantzi, O., Pateraki, C., Koutinas, A. A., & Papanikolaou, S. (2021). Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains. Reactions, 2(4), 499-513. https://doi.org/10.3390/reactions2040032







