A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects
Abstract
:1. Introduction
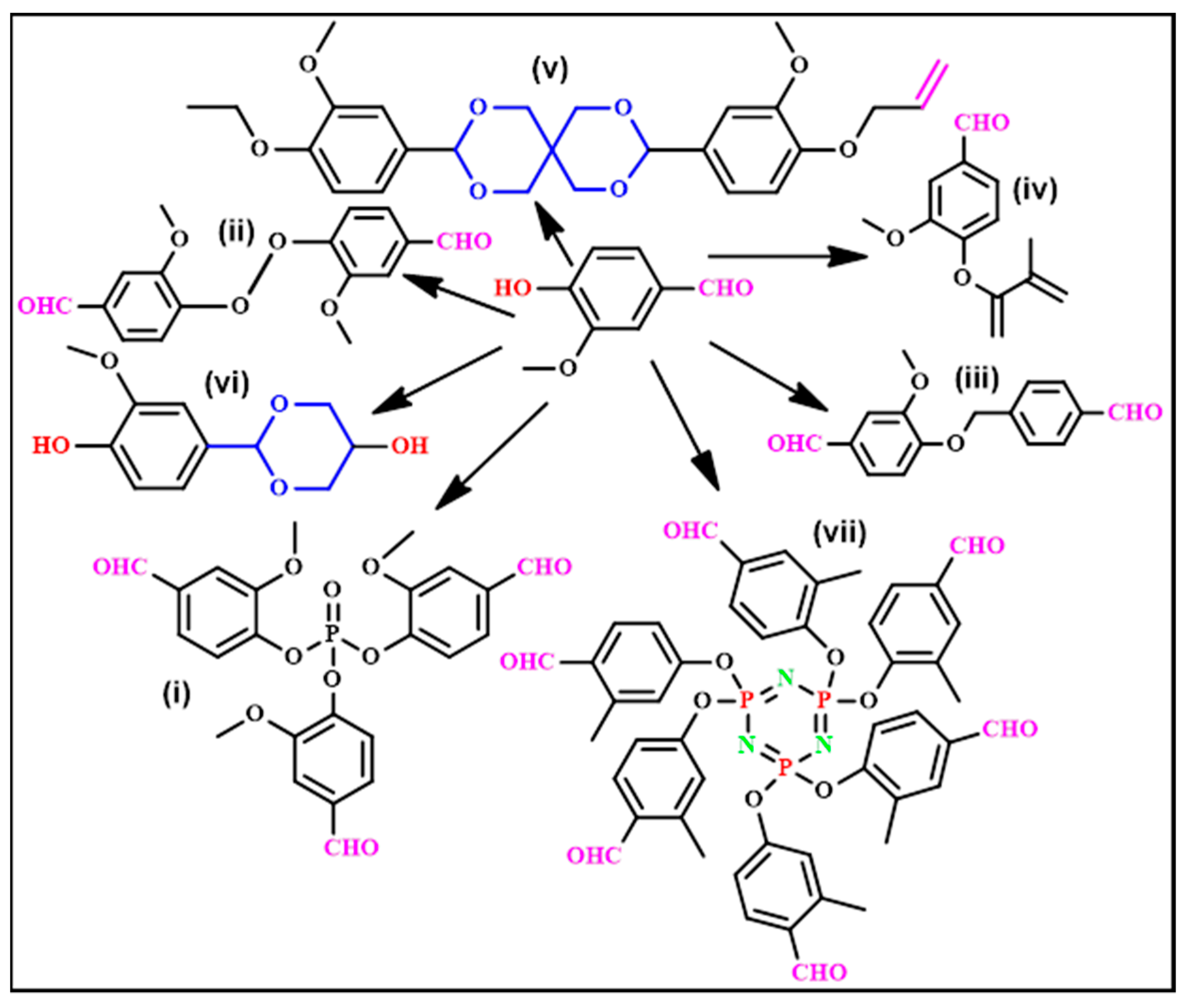
2. Vanillin-Modified Epoxy Monomers
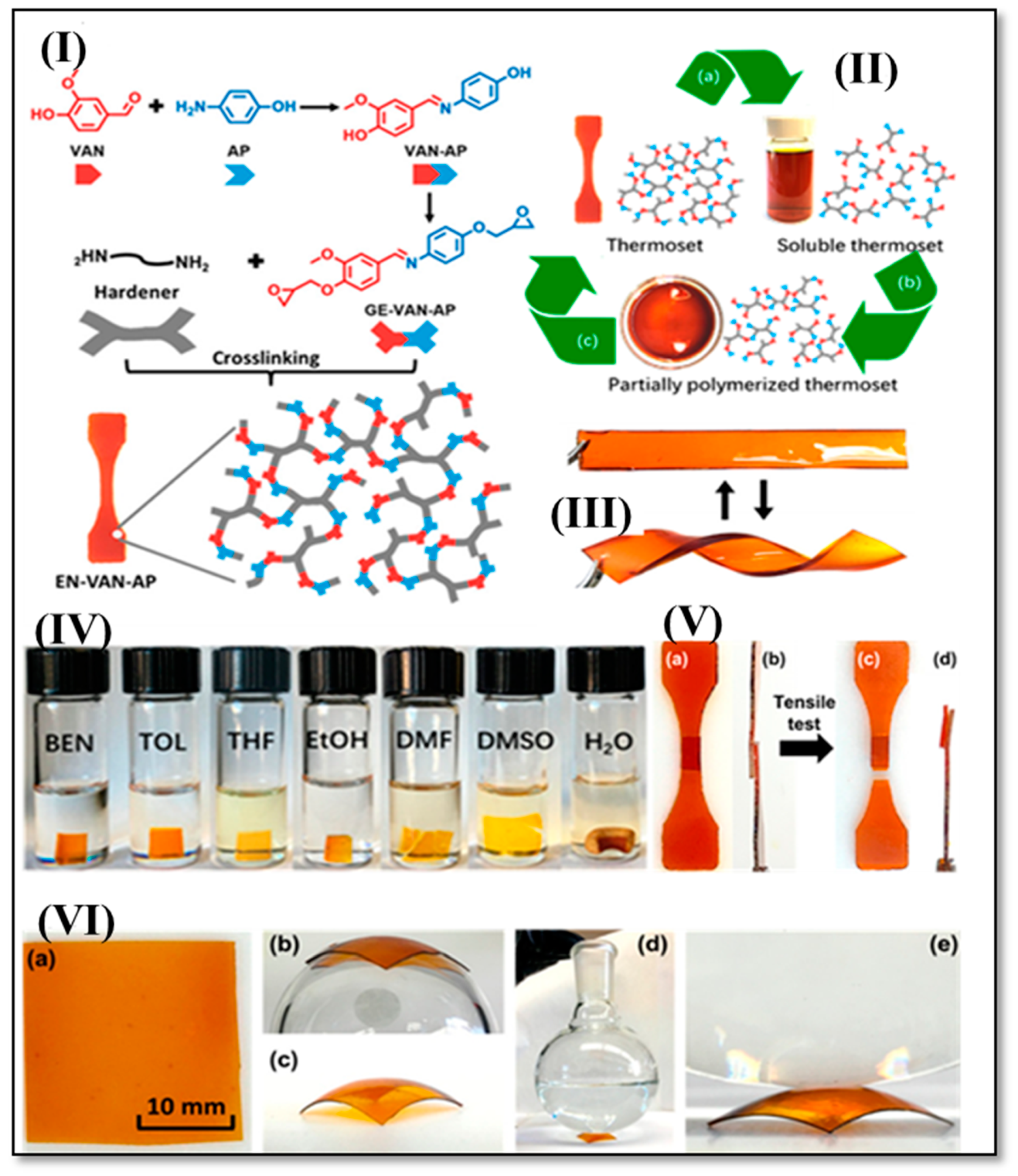
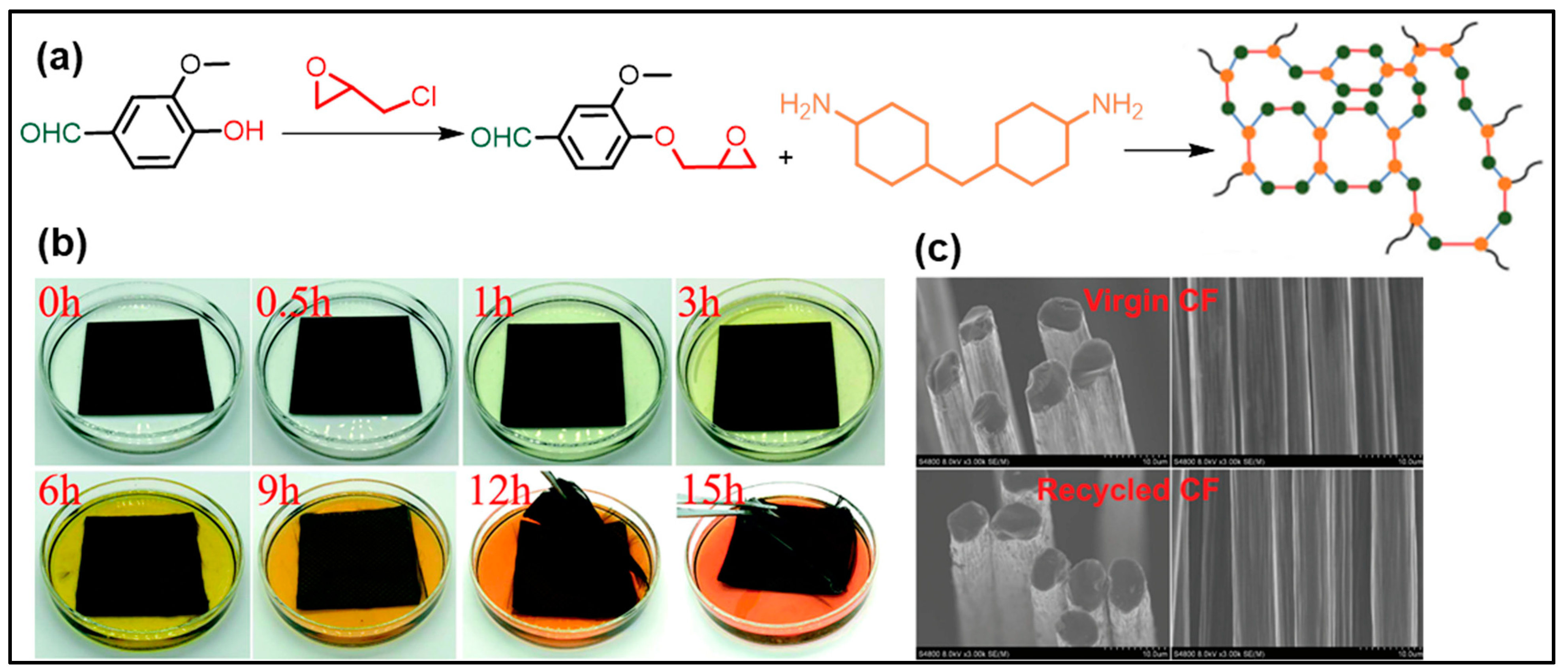
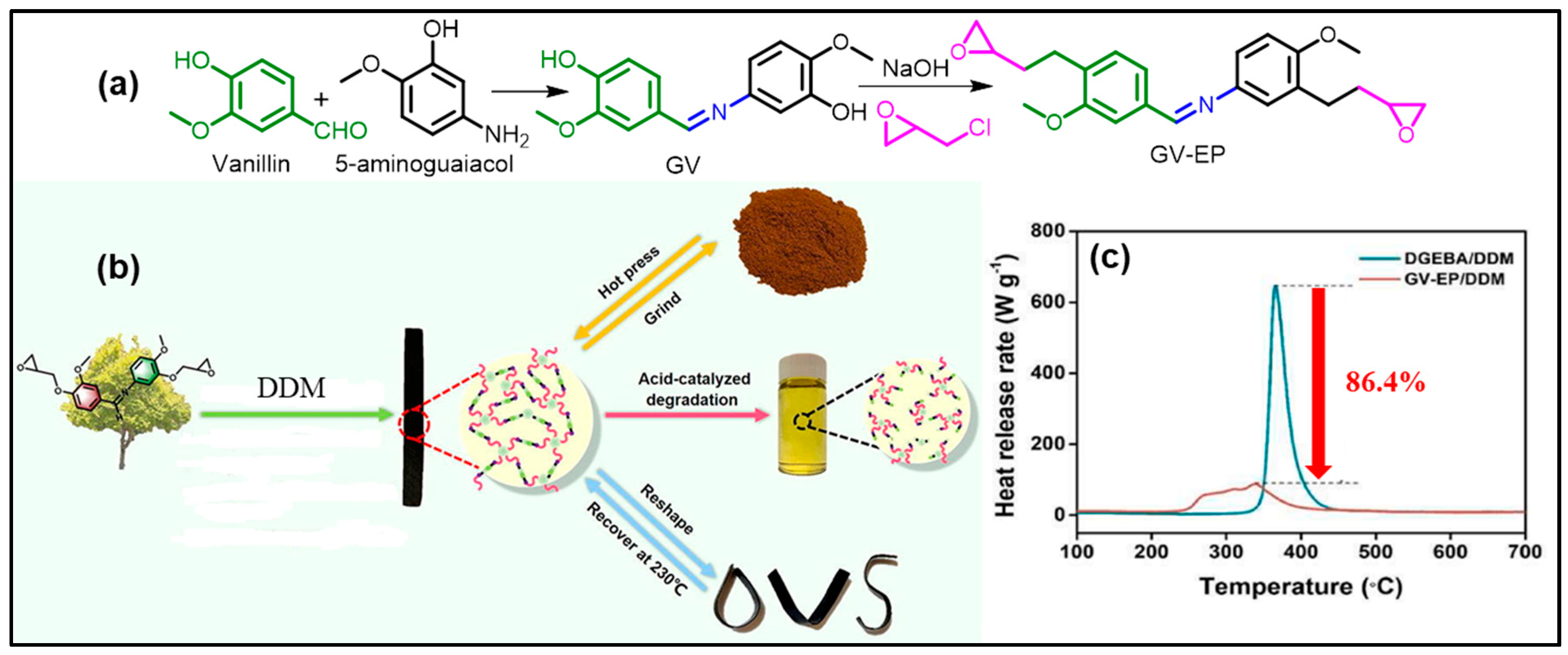
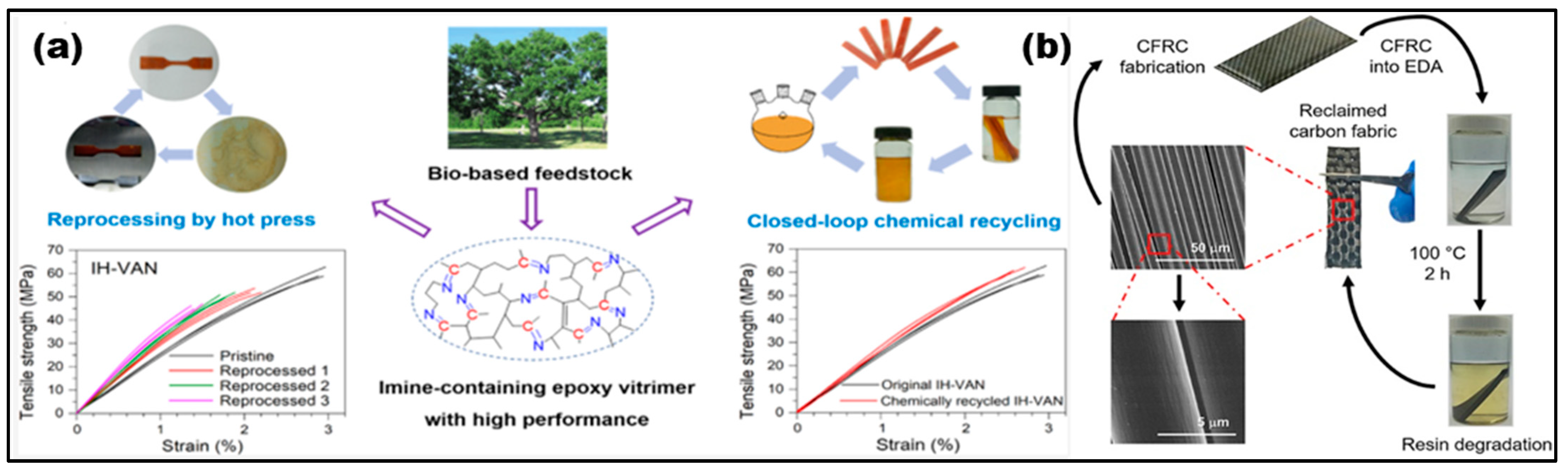
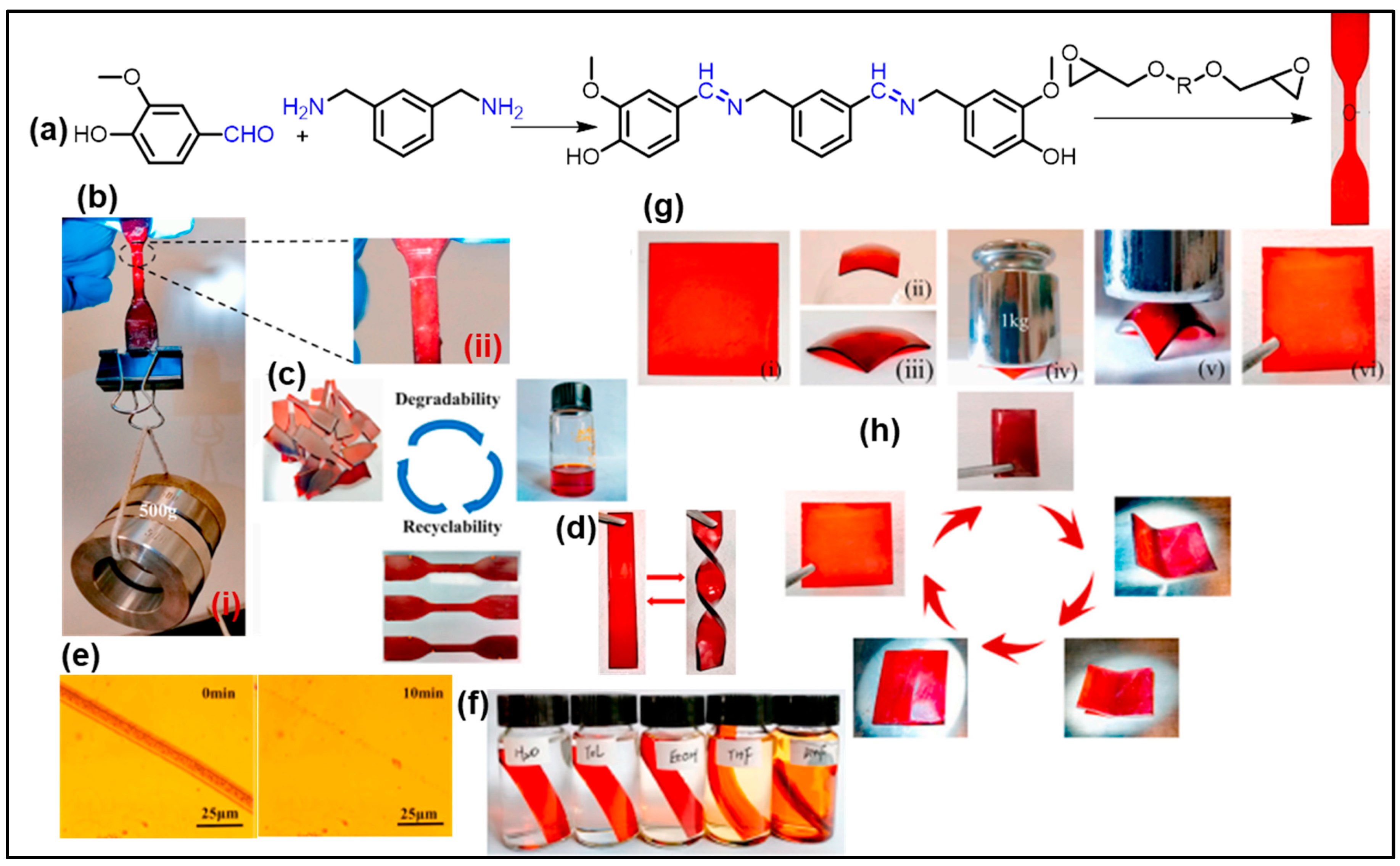
3. Vanillin-Modified Vitrimers
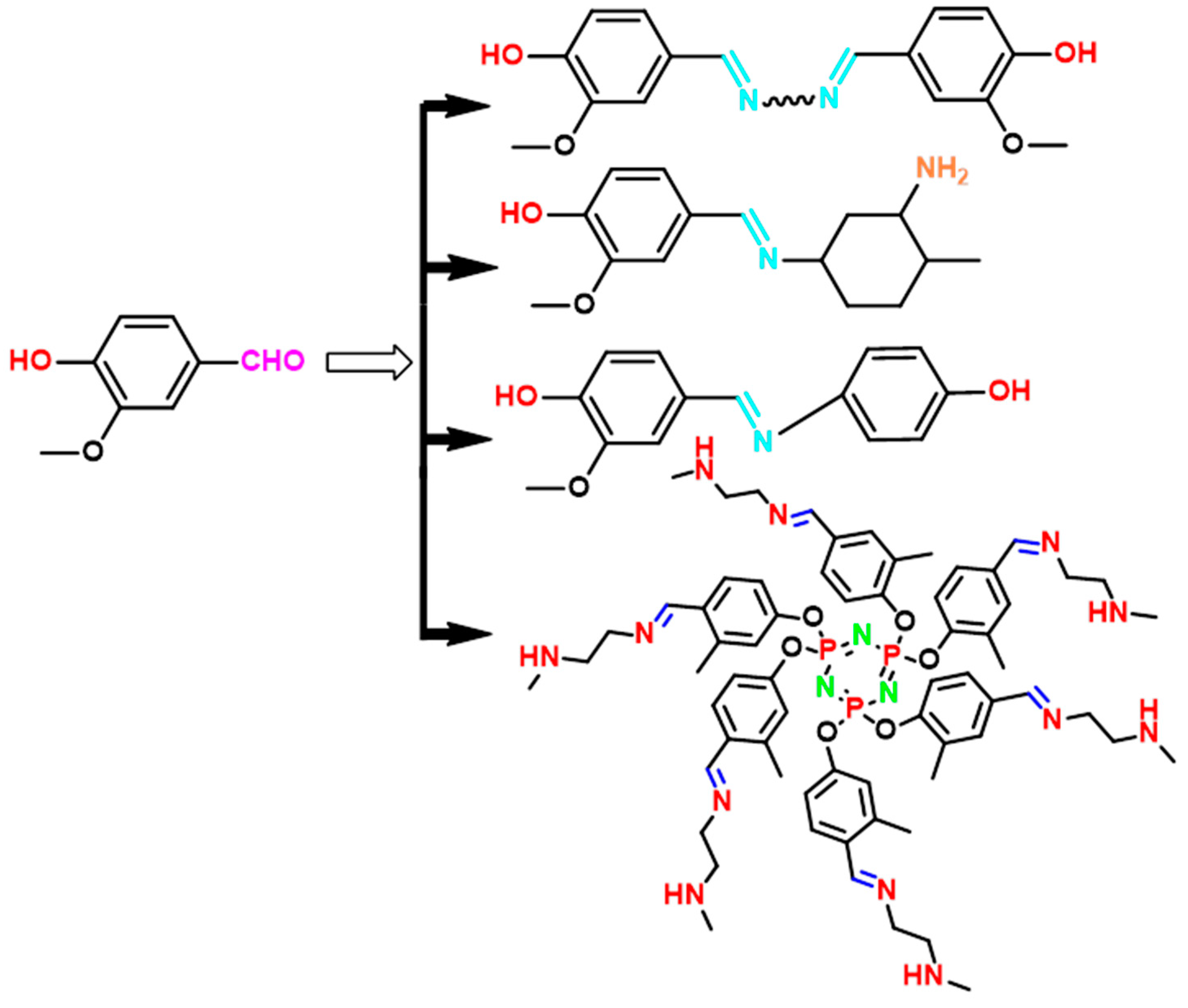
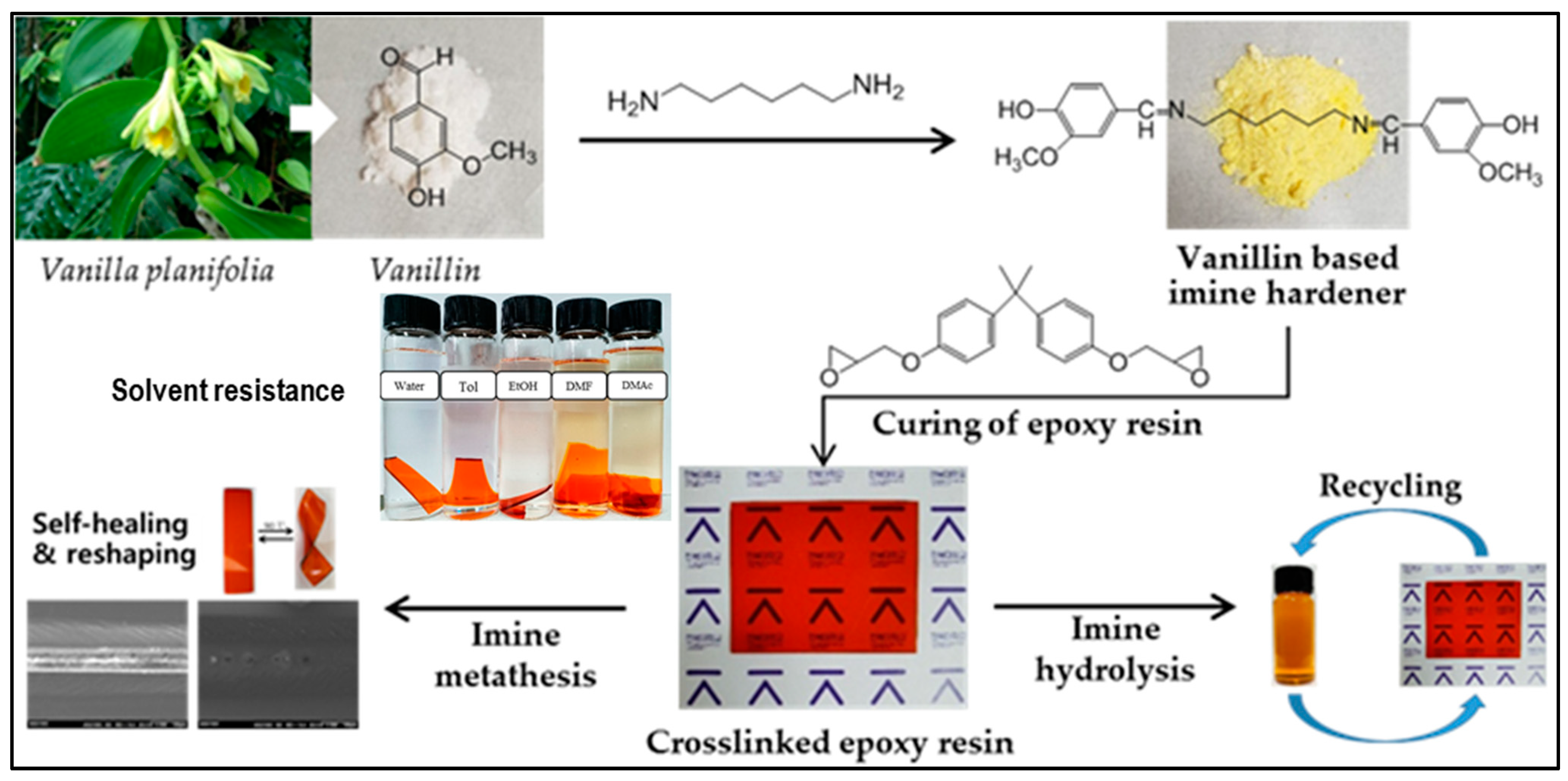
4. Vanillin-Modified Curing Agents
5. Fully Biobased Vitrimers
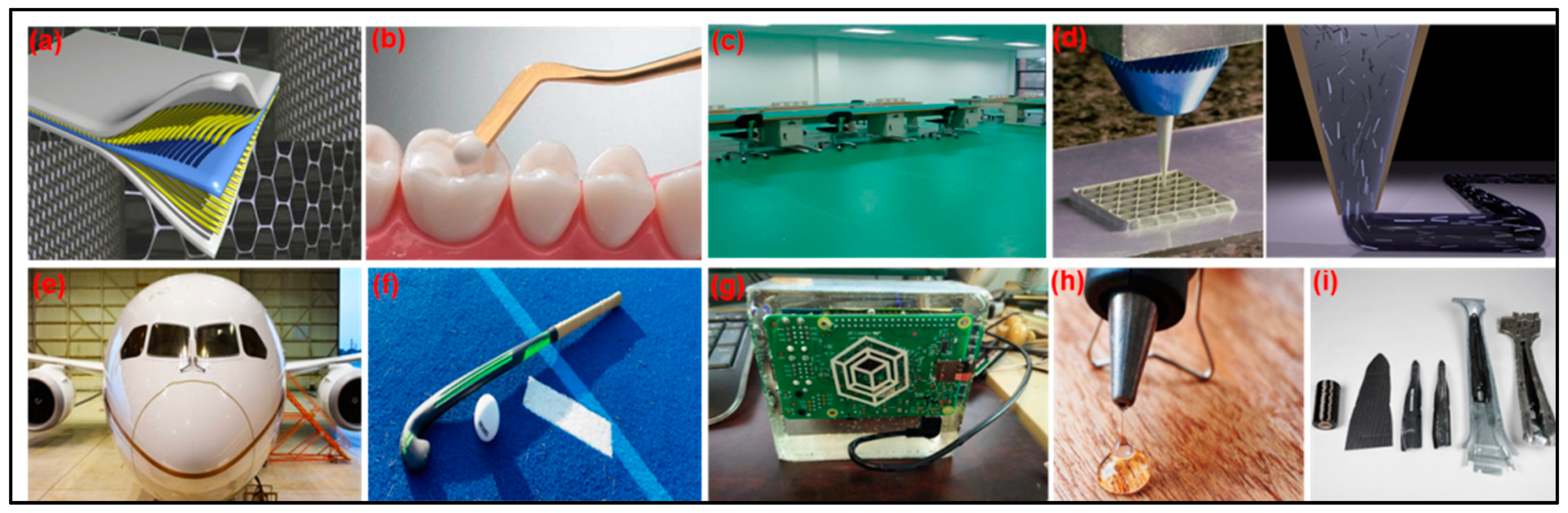
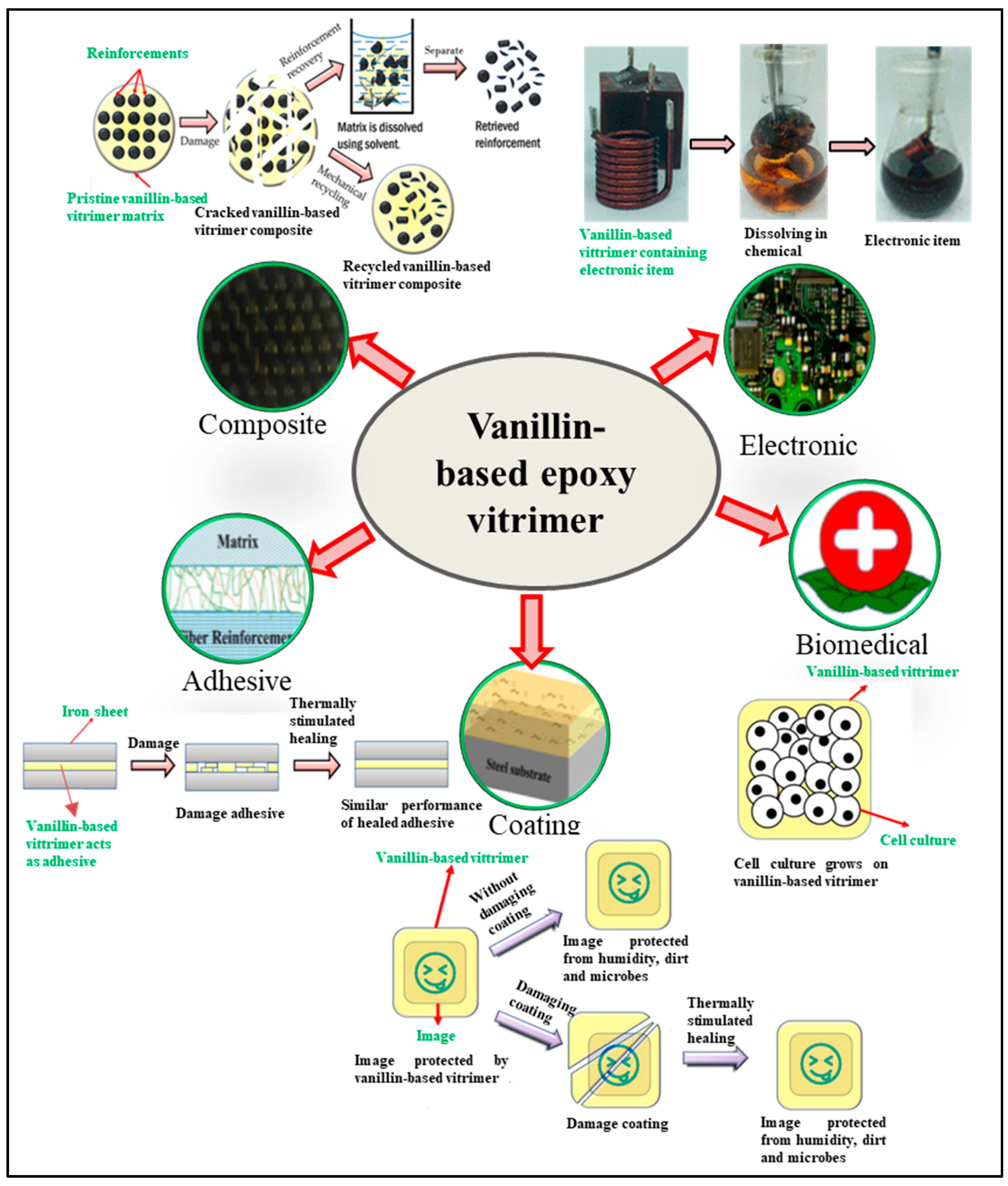
6. Potential Applications of Vanillin-Modified Epoxy Vitrimers
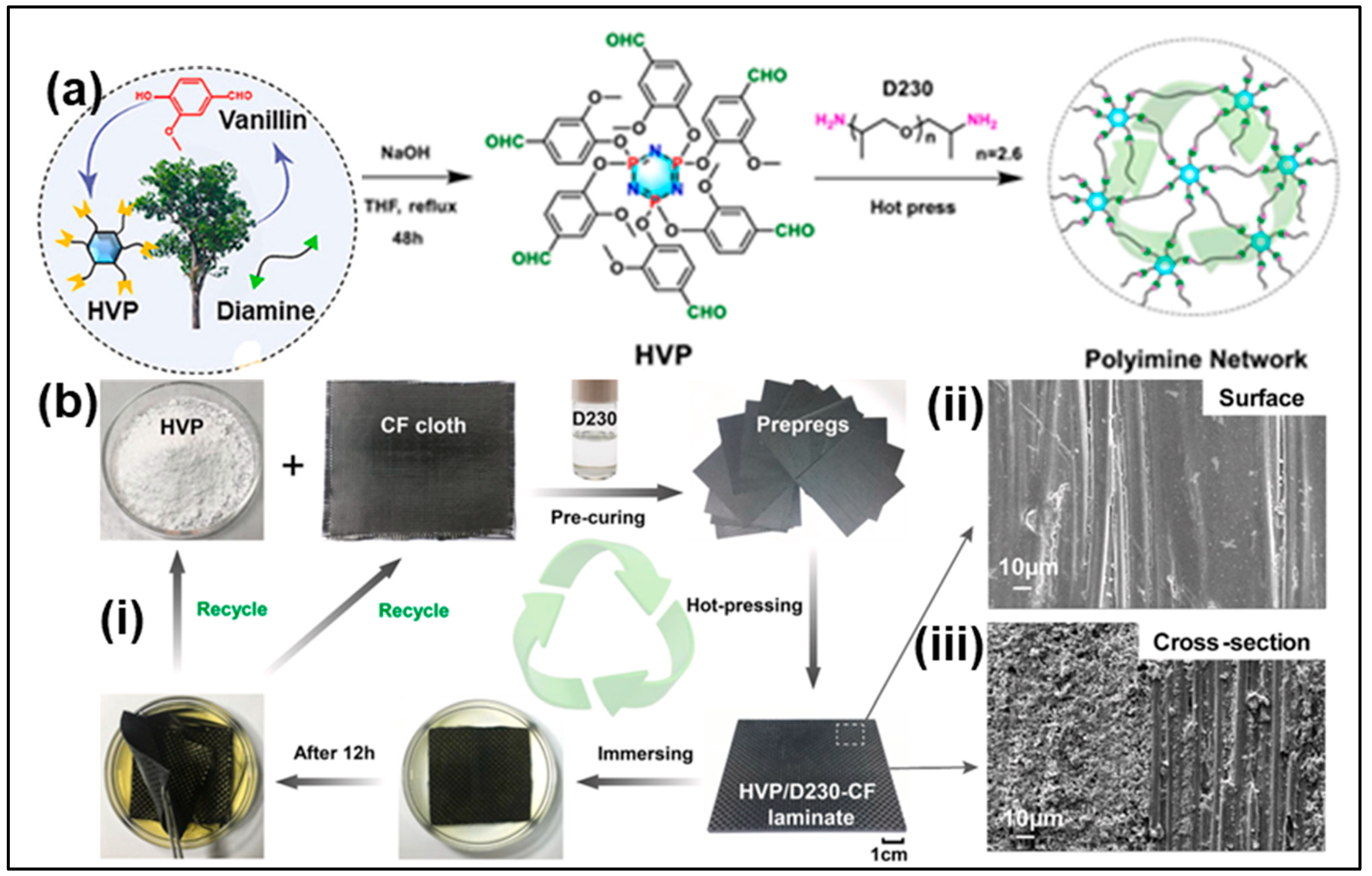
6.1. Green Matrix for Composites
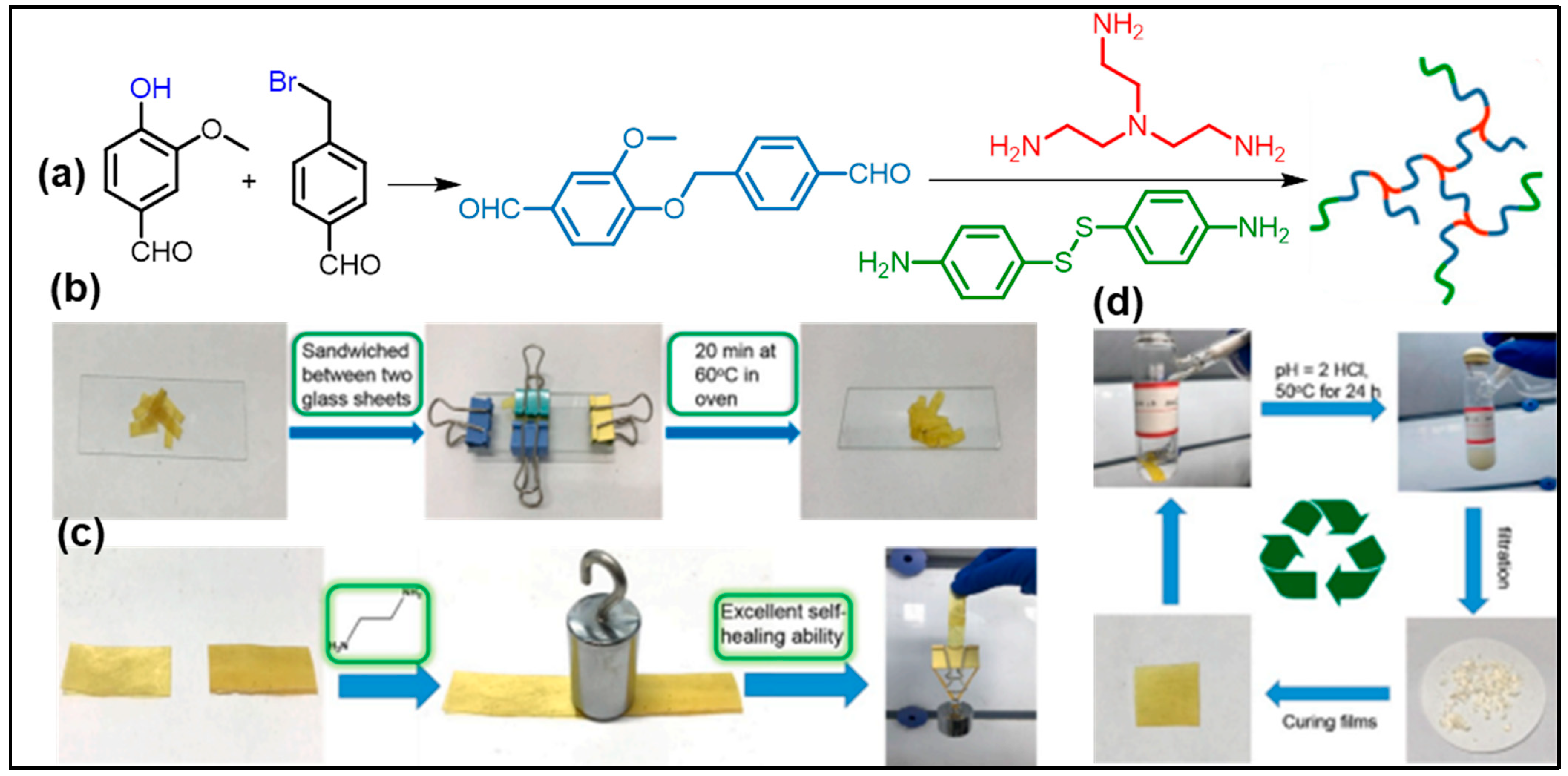
6.2. Curable and Regenerative Adhesives
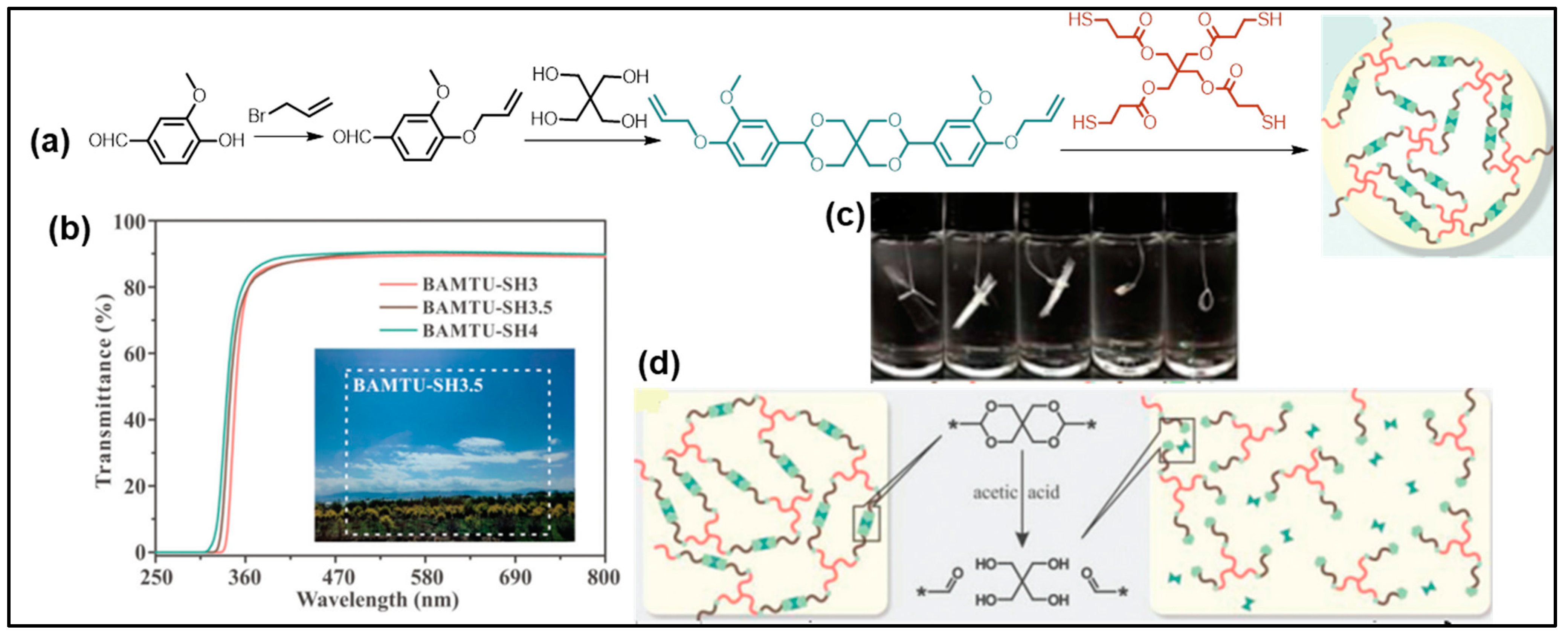
6.3. Curable Coating
6.4. Biomedical Applications
6.5. Electronic Devices
7. Difficulties and Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of reversible dynamic bonds containing intrinsically flame retardant biomass thermosets. Eur. Polym. J. 2022, 173, 111263. [Google Scholar] [CrossRef]
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review on intrinsically recyclable flame retardant thermosets enabled through covalent bonds. J. Appl. Polym. Sci. 2022, 139, e52493. [Google Scholar] [CrossRef]
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of intrinsically recyclable biobased epoxy thermosets enabled by dynamic chemical bonds. Polym.-Plast. Technol. Mater. 2022, 61, 1740–1782. [Google Scholar]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, E.; Feng, Z.; Liu, J.; Chen, B.; Liang, L. Degradable bio-based epoxy vitrimers based on imine chemistry and their application in recyclable carbon fiber composites. J. Mater. Sci. 2021, 56, 15733–15751. [Google Scholar] [CrossRef]
- Rashid, M.A.; Zhu, S.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W. A Quercetin-Derived Polybasic Acid Hardener for Reprocessable and Degradable Epoxy Resins Based on Transesterification. ACS Appl. Polym. Mater. 2022, 4, 5708–5716. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liang, L.; Lu, M.; Song, X.; Liu, H.; Chen, G. Water-resistant bio-based vitrimers based on dynamic imine bonds: Self-healability, remodelability and ecofriendly recyclability. Polymer 2020, 210, 123030. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Zhou, Z.; Su, X.; Liu, J.; Liu, R. Synthesis of Vanillin-Based Polyimine Vitrimers with Excellent Reprocessability, Fast Chemical Degradability, and Adhesion. ACS Appl. Polym. Mater. 2020, 2, 5716–5725. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, J.; Qu, D.; Wang, H.; Chai, C.; Feng, L. Thermo-adjusted self-healing epoxy resins based on Diels–Alder dynamic chemical reaction. Polym. Eng. Sci. 2021, 61, 2257–2266. [Google Scholar] [CrossRef]
- Lucherelli, M.A.; Duval, A.; Avérous, L. Biobased vitrimers: Towards sustainable and adaptable performing polymer materials. Prog. Polym. Sci. 2022, 127, 101515. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Liguori, A.; Hakkarainen, M. Designed from Biobased Materials for Recycling: Imine-Based Covalent Adaptable Networks. Macromol. Rapid Commun. 2022, 43, 2100816. [Google Scholar] [CrossRef]
- Lorero, I.; Rodríguez, A.; Campo, M.; Prolongo, S. Thermally remendable, weldable, and recyclable epoxy network crosslinked with reversible Diels-alder bonds. Polymer 2022, 259, 125334. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Yaseen, M.; Huang, K. A review on the self-healing ability of epoxy polymers. J. Appl. Polym. Sci. 2021, 138, 50260. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Thamizh Selvan, R.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Recycling technology of epoxy glass fiber and epoxy carbon fiber composites used in aerospace vehicles. J. Compos. Mater. 2021, 55, 3281–3292. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, Z.; Feng, Z.; Xue, B.; Xiong, C.; Duan, C.; Ni, Y. New Kind of Lignin/Polyhydroxyurethane Composite: Green Synthesis, Smart Properties, Promising Applications, and Good Reprocessability and Recyclability. ACS Appl. Mater. Interfaces 2021, 13, 28938–28948. [Google Scholar] [CrossRef]
- Yao, J.; Yang, C.; Zhu, C.; Hou, B. Preparation Process of Epoxy Resin Microcapsules for Self—healing Coatings. Prog. Org. Coat. 2019, 132, 440–444. [Google Scholar]
- Liu, Y.; Wang, B.; Ma, S.; Yu, T.; Xu, X.; Li, Q.; Wang, S.; Han, Y.; Yu, Z.; Zhu, J. Catalyst-free malleable, degradable, bio-based epoxy thermosets and its application in recyclable carbon fiber composites. Compos. Part B Eng. 2021, 211, 108654. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Liang, X.; Yin, N.; Liang, S.; Yang, R.; Liu, S.; Lu, Y.; Jiang, L.; Zhou, Q.; Jiang, G.; Faiola, F. Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length. Food Chem. Toxicol. 2020, 135, 111015. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Qiu, W.; Zhan, H.; Hu, J.; Zhang, T.; Xu, H.; Wong, M.; Xu, B.; Zheng, C. The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: A critical review of recent progress. Ecotoxicol. Environ. Saf. 2019, 173, 192–202. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Yin, K.; Liu, L.; Gu, H. Green Paradox or Forced Emission Reduction—The Dual Effects of Environmental Regulation on Carbon Emissions. Int. J. Environ. Res. Public Health 2022, 19, 11058. [Google Scholar] [CrossRef]
- Moreira, V.B.; Alemán, C.; Rintjema, J.; Bravo, F.; Kleij, A.W.; Armelin, E. A Biosourced Epoxy Resin for Adhesive Thermoset Applications. ChemSusChem 2022, 15, e202102624. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Zhu, S.; Jiang, Q.; Wei, Y.; Liu, W. Developing Easy Processable, Recyclable, and Self-Healable Biobased Epoxy Resin through Dynamic Covalent Imine Bonds. ACS Appl. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Meng, X.; Pu, Y.; Ragauskas, A.J. Recent Advances in the Application of Functionalized Lignin in Value-Added Polymeric Materials. Polymers 2020, 12, 2277. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Pinto, P.C.R.; Loureiro, J.M.; Rodrigues, A.E. Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Fache, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Savonnet, E.; Grau, E.; Grelier, S.; Defoort, B.; Cramail, H. Divanillin-Based Epoxy Precursors as DGEBA Substitutes for Biobased Epoxy Thermosets. ACS Sustain. Chem. Eng. 2018, 6, 11008–11017. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Yu, Q.; Du, Y.; Goncalves, R.B.; Francis, L.F.; Reineke, T.M. Vanillin-based degradable epoxy vitrimers: Reprocessability and mechanical properties study. Eur. Polym. J. 2019, 117, 55–63. [Google Scholar] [CrossRef]
- Liu, T.; Peng, J.; Liu, J.; Hao, X.; Guo, C.; Ou, R.; Liu, Z.; Wang, Q. Fully recyclable, flame-retardant and high-performance carbon fiber composites based on vanillin-terminated cyclophosphazene polyimine thermosets. Compos. Part B Eng. 2021, 224, 109188. [Google Scholar] [CrossRef]
- Li, J.; Weng, Z.; Cao, Q.; Qi, Y.; Lu, B.; Zhang, S.; Wang, J.; Jian, X. Synthesis of an aromatic amine derived from biomass and its use as a feedstock for versatile epoxy thermoset. Chem. Eng. J. 2022, 433, 134512. [Google Scholar] [CrossRef]
- Stanzione Iii, J.F.; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-based resin for use in composite applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Mai, V.D.; Shin, S.-R.; Lee, D.-S.; Kang, I. Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers 2019, 11, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Renewable Polymers Prepared from Vanillin and Its Derivatives. Macromol. Chem. Phys. 2015, 216, 1816–1822. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A Self-Healable High Glass Transition Temperature Bioepoxy Material Based on Vitrimer Chemistry. Macromolecules 2018, 51, 5577–5585. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wu, J.; Yang, J.; Wang, B.; Wang, S.; Li, Q.; Feng, J.; You, S.; Zhu, J. High-performance, command-degradable, antibacterial Schiff base epoxy thermosets: Synthesis and properties. J. Mater. Chem. A 2019, 7, 15420–15431. [Google Scholar] [CrossRef]
- Ma, S.; Wei, J.; Jia, Z.; Yu, T.; Yuan, W.; Li, Q.; Wang, S.; You, S.; Liu, R.; Zhu, J. Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger. J. Mater. Chem. A 2019, 7, 1233–1243. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Yuan, W.; Ma, S.; Wang, S.; Li, Q.; Wang, B.; Xu, X.; Huang, K.; Chen, J.; You, S.; Zhu, J. Synthesis of fully bio-based diepoxy monomer with dicyclo diacetal for high-performance, readily degradable thermosets. Eur. Polym. J. 2019, 117, 200–207. [Google Scholar] [CrossRef]
- Wang, B.; Ma, S.; Li, Q.; Zhang, H.; Liu, J.; Wang, R.; Chen, Z.; Xu, X.; Wang, X.; Lu, N.; et al. Facile synthesis of “digestible”, rigid-and-flexible, bio-based building block for high-performance degradable thermosetting plastics. Green Chem. 2020, 22, 1275–1290. [Google Scholar] [CrossRef]
- Su, X.; Zhou, Z.; Liu, J.; Luo, J.; Liu, R. A recyclable vanillin-based epoxy resin with high-performance that can compete with DGEBA. Eur. Polym. J. 2020, 140, 110053. [Google Scholar] [CrossRef]
- Mo, R.; Song, L.; Hu, J.; Sheng, X.; Zhang, X. An acid-degradable biobased epoxy-imine adaptable network polymer for the fabrication of responsive structural color film. Polym. Chem. 2020, 11, 974–981. [Google Scholar] [CrossRef]
- Yang, Y.; Boom, R.; Irion, B.; Heerden, D.J.V.; Kuiper, P.; Wit, H.D. Recycling of composite materials. Chem. Eng. Process. Process Intensif. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Saccani, A.; Manzi, S.; Lancellotti, I.; Lipparini, L. Composites obtained by recycling carbon fibre/epoxy composite wastes in building materials. Constr. Build. Mater. 2019, 204, 296–302. [Google Scholar] [CrossRef]
- Fang, Z.; Nikafshar, S.; Hegg, E.L.; Nejad, M. Biobased Divanillin As a Precursor for Formulating Biobased Epoxy Resin. ACS Sustain. Chem. Eng. 2020, 8, 9095–9103. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Yuan, W.; Wang, B.; Zhu, J. Robust, Fire-Safe, Monomer-Recovery, Highly Malleable Thermosets from Renewable Bioresources. Macromolecules 2018, 51, 8001–8012. [Google Scholar] [CrossRef]
- Geng, H.; Wang, Y.; Yu, Q.; Gu, S.; Zhou, Y.; Xu, W.; Zhang, X.; Ye, D.-Z. Vanillin-Based Polyschiff Vitrimers: Reprocessability and Chemical Recyclability. ACS Sustain. Chem. Eng. 2018, 6, 15463–15470. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhou, L.; Wang, L.; Majeed, K.; Zhang, B.; Zhou, F.; Zhang, Q. Preparation of environmentally friendly bio-based vitrimers from vanillin derivatives by introducing two types of dynamic covalent C N and S–S bonds. Polymer 2020, 197, 122483. [Google Scholar] [CrossRef]
- Xu, Y.; Odelius, K.; Hakkarainen, M. Photocurable, Thermally Reprocessable, and Chemically Recyclable Vanillin-Based Imine Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 17272–17279. [Google Scholar] [CrossRef]
- Wang, B.; Ma, S.; Xu, X.; Li, Q.; Yu, T.; Wang, S.; Yan, S.; Liu, Y.; Zhu, J. High-Performance, Biobased, Degradable Polyurethane Thermoset and Its Application in Readily Recyclable Carbon Fiber Composites. ACS Sustain. Chem. Eng. 2020, 8, 11162–11170. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Zhang, L.; Chen, Z.; Qin, R.; Liu, Y.; Jiang, X.; Ye, D.; Liu, Z. Recyclable Carbon Fiber Reinforced Vanillin-Based Polyimine Vitrimers: Degradation and Mechanical Properties Study. Macromol. Mater. Eng. 2022, 307, 2100893. [Google Scholar] [CrossRef]
- Cortés-Guzmán, K.P.; Parikh, A.R.; Sparacin, M.L.; Remy, A.K.; Adegoke, L.; Chitrakar, C.; Ecker, M.; Voit, W.E.; Smaldone, R.A. Recyclable, Biobased Photoresins for 3D Printing Through Dynamic Imine Exchange. ACS Sustain. Chem. Eng. 2022, 10, 13091–13099. [Google Scholar] [CrossRef]
- Ge, M.; Miao, J.T.; Zhang, K.; Wu, Y.; Zheng, L.; Wu, L. Building biobased, degradable, flexible polymer networks from vanillin via thiol–ene “click” photopolymerization. Polym. Chem. 2021, 12, 564–571. [Google Scholar] [CrossRef]
- Yang, X.; Ke, Y.; Chen, Q.; Shen, L.; Xue, J.; Quirino, R.L.; Yan, Z.; Luo, Y.; Zhang, C. Efficient transformation of renewable vanillin into reprocessable, acid-degradable and flame retardant polyimide vitrimers. J. Clean. Prod. 2022, 333, 130043. [Google Scholar] [CrossRef]
- Zamani, P.; Zabihi, O.; Ahmadi, M.; Mahmoodi, R.; Kannangara, T.; Joseph, P.; Naebe, M. Biobased Carbon Fiber Composites with Enhanced Flame Retardancy: A Cradle-to-Cradle Approach. ACS Sustain. Chem. Eng. 2022, 10, 1059–1069. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Iii, J.F.S. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Y.; Liu, G.-L.; Li, Y.-D.; Weng, Y.; Zeng, J.-B. Biobased High-Performance Epoxy Vitrimer with UV Shielding for Recyclable Carbon Fiber Reinforced Composites. ACS Sustain. Chem. Eng. 2021, 9, 4638–4647. [Google Scholar] [CrossRef]
- Peng, J.; Xie, S.; Liu, T.; Wang, D.; Ou, R.; Guo, C.; Wang, Q.; Liu, Z. High-performance epoxy vitrimer with superior self-healing, shape-memory, flame retardancy, and antibacterial properties based on multifunctional curing agent. Compos. Part B Eng. 2022, 242, 110109. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y.; Zhang, L.; Jiang, Q.; Liu, W. An imine-containing epoxy vitrimer with versatile recyclability and its application in fully recyclable carbon fiber reinforced composites. Compos. Sci. Technol. 2020, 199, 108314. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zeng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Nabipour, H.; Niu, H.; Wang, X.; Batool, S.; Hu, Y. Fully bio-based epoxy resin derived from vanillin with flame retardancy and degradability. React. Funct. Polym. 2021, 168, 105034. [Google Scholar] [CrossRef]
- Hong, K.; Sun, Q.; Zhang, X.; Fan, L.; Wu, T.; Du, J.; Zhu, Y. Fully Bio-Based High-Performance Thermosets with Closed-Loop Recyclability. ACS Sustain. Chem. Eng. 2022, 10, 1036–1046. [Google Scholar] [CrossRef]
- Turani, M.; Baggio, A.; Casalegno, V.; Salvo, M.; Sangermano, M. An Epoxy Adhesive Crosslinked through Radical-Induced Cationic Frontal Polymerization. Macromol. Mater. Eng. 2021, 306, 2100495. [Google Scholar] [CrossRef]
- Quan, D.; Deegan, B.; Alderliesten, R.; Dransfeld, C.; Murphy, N.; Ivankovic, A.; Benedictus, R. The influence of interlayer/epoxy adhesion on the mode-I and mode-II fracture response of carbon fibre/epoxy composites interleaved with thermoplastic veils. Mater. Des. 2020, 192, 108781. [Google Scholar] [CrossRef]
- Putnam-Neeb, A.A.; Kaiser, J.M.; Hubbard, A.M.; Street, D.P.; Dickerson, M.B.; Nepal, D.; Baldwin, L.A. Self-healing and polymer welding of soft and stiff epoxy thermosets via silanolates. Adv. Compos. Hybrid Mater. 2022, 5, 3068–3080. [Google Scholar] [CrossRef]
- Mulcahy, K.R.; Kilpatrick, A.F.R.; Harper, G.D.J.; Walton, A.; Abbott, A.P. Debondable adhesives and their use in recycling. Green Chem. 2022, 24, 36–61. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Catechol-Mediated Glycidylation toward Epoxy Vitrimers/Polymers with Tunable Properties. Macromolecules 2019, 52, 3646–3654. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Zhang, H.; Bi, L.; Jiang, J.; Chen, Y. Reprocessable, Self-Adhesive, and Recyclable Carbon Fiber-Reinforced Composites Using a Catalyst-Free Self-Healing Bio-Based Vitrimer Matrix. ACS Sustain. Chem. Eng. 2021, 9, 16281–16290. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-free self-healing bio-based vitrimer for a recyclable, reprocessable, and self-adhered carbon fiber reinforced composite. Chem. Eng. J. 2022, 429, 132518. [Google Scholar] [CrossRef]
- Tang, J.; Wan, L.; Zhou, Y.; Pan, H.; Huang, F. Strong and efficient self-healing adhesives based on dynamic quaternization cross-links. J. Mater. Chem. A 2017, 5, 21169–21177. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Liang, G.; Gu, A. Developing Reversible Self-Healing and Malleable Epoxy Resins with High Performance and Fast Recycling through Building Cross-Linked Network with New Disulfide-Containing Hardener. Ind. Eng. Chem. Res. 2018, 57, 12397–12406. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Mikkelsen, A.; Zhao, B.; Zhang, J. Hempseed Oil-Based Covalent Adaptable Epoxy-Amine Network and Its Potential Use for Room-Temperature Curable Coatings. ACS Sustain. Chem. Eng. 2020, 8, 14964–14974. [Google Scholar] [CrossRef]
- Kadam, A.; Pawar, M.; Yemul, O.; Thamke, V.; Kodam, K. Biodegradable biobased epoxy resin from karanja oil. Polymer 2015, 72, 82–92. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Khatri, M.; Hussain, N.; Khatri, Z.; Yamamoto, T.; Kim, S.H.; Kobayashi, S.; Kim, I.S. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1,4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1,4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofibers and their blended composition. Mater. Today Commun. 2021, 26, 102113. [Google Scholar]
- Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, eaaq0508. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Sun, Y.-C.; Wang, J.; Qi, H.J.; Wang, T.; Naguib, H.E. Flexible, Reconfigurable, and Self-Healing TPU/Vitrimer Polymer Blend with Copolymerization Triggered by Bond Exchange Reaction. ACS Appl. Mater. Interfaces 2020, 12, 8740–8750. [Google Scholar] [CrossRef]
- Jia, H.; Gu, S.-Y. Remote and efficient infrared induced self-healable stretchable substrate for wearable electronics. Eur. Polym. J. 2020, 126, 109542. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Y.; Bao, Z.; Yan, X. Skin-Inspired Electronics Enabled by Supramolecular Polymeric Materials. CCS Chem. 2019, 1, 431–447. [Google Scholar] [CrossRef]




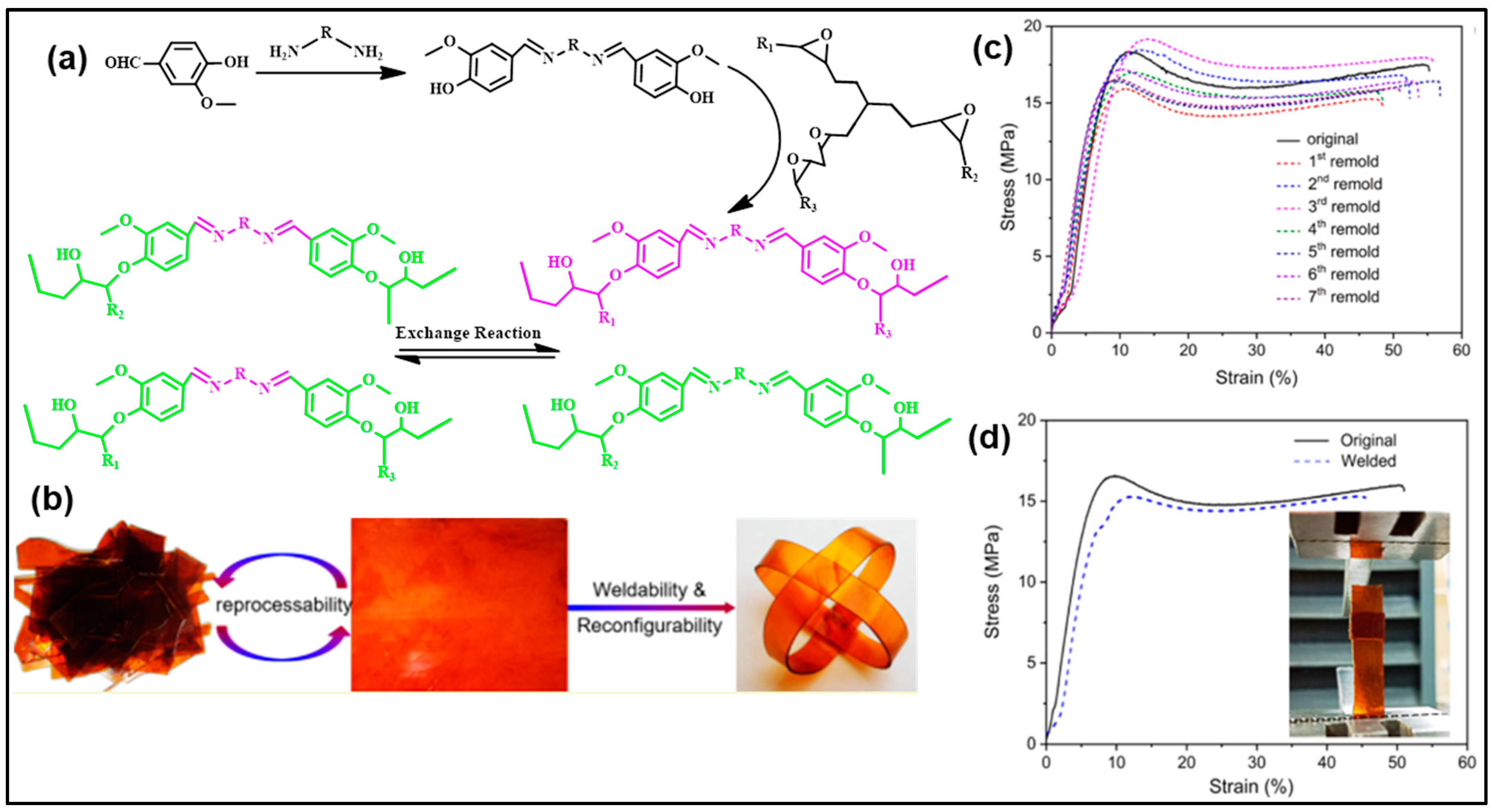
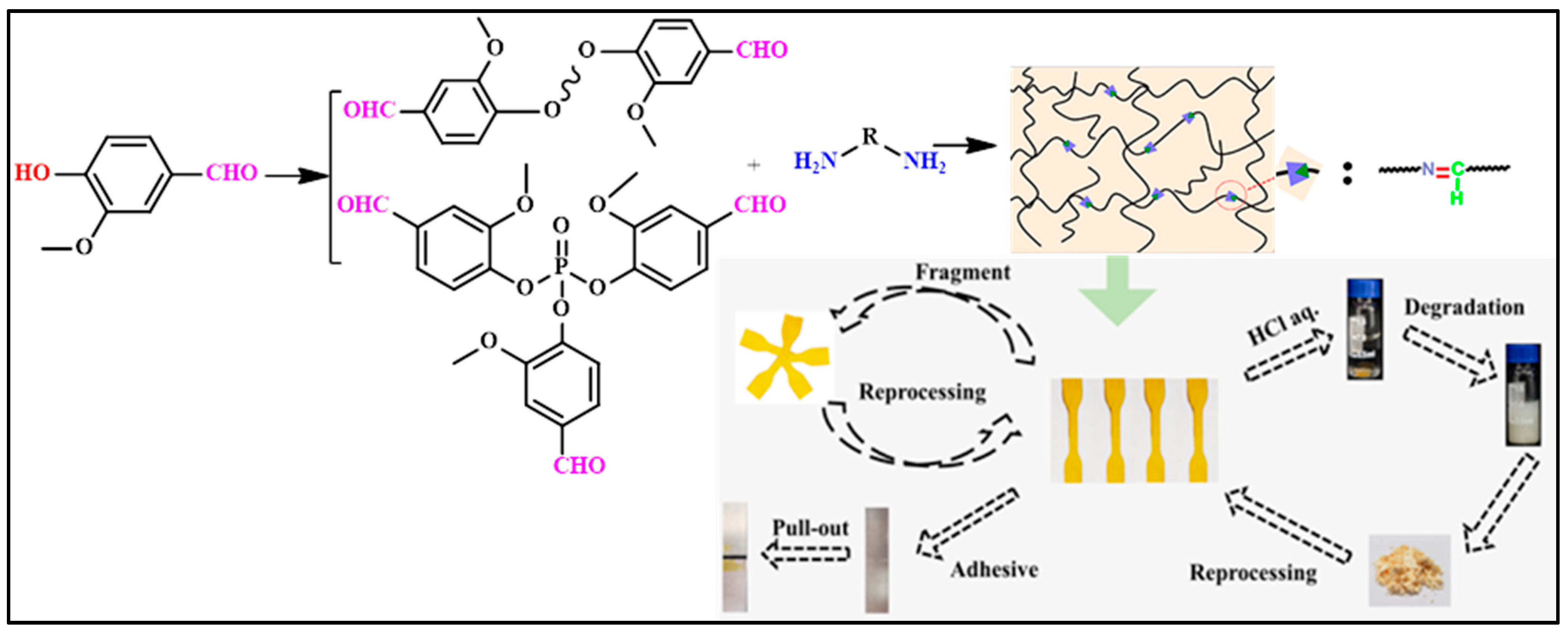
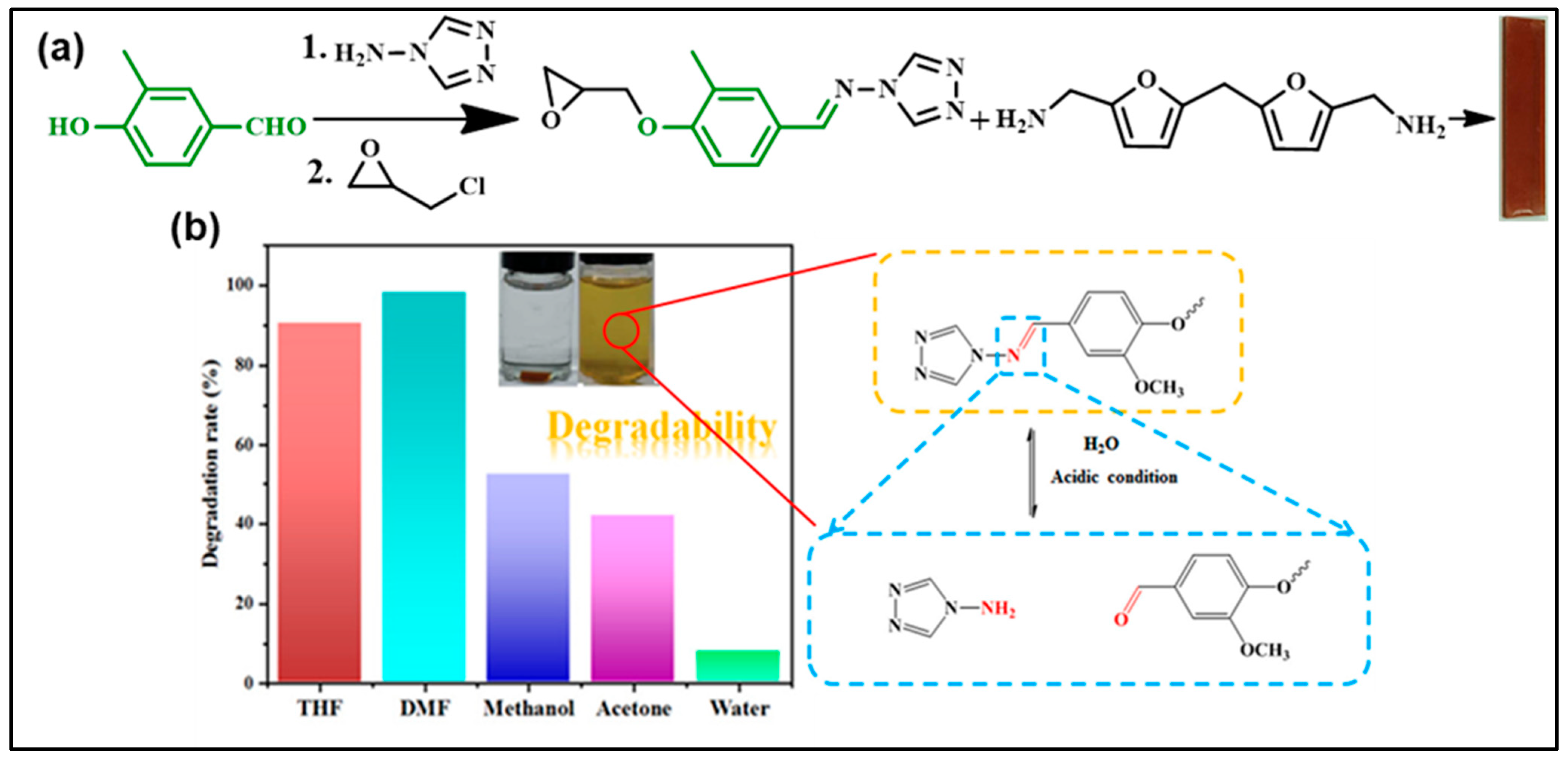
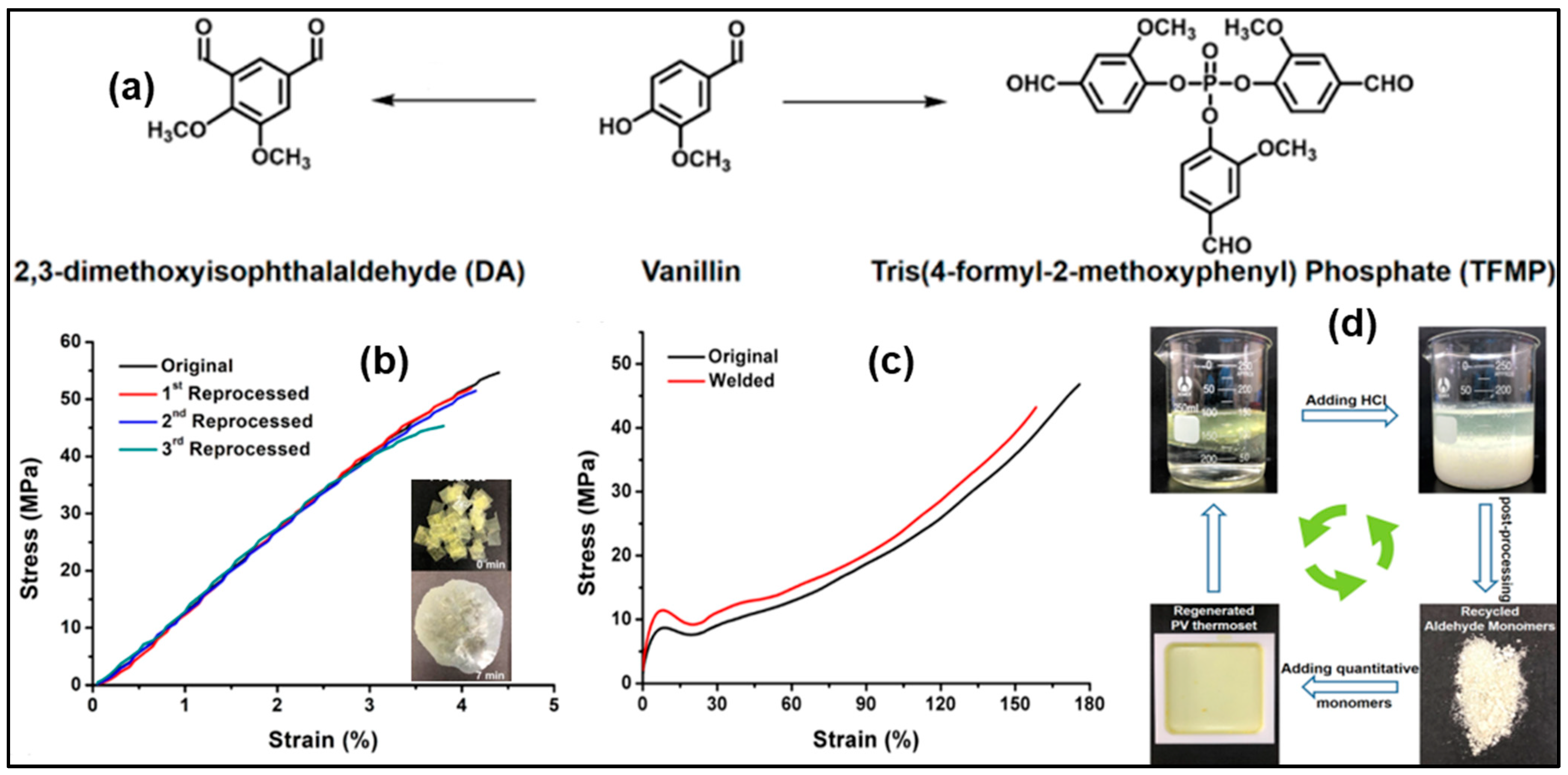
| Composition | Pristine Products | Recycling Conditions | Recycled Products | Ref. | |||
|---|---|---|---|---|---|---|---|
| Strength (MPa) | Tg (°C) | Thermal | Chemical | Strength (MPa) | Tg (°C) | ||
| TEP + MHHPA | 69 | 187 | 220 °C, 10 m | - | - | - | [56] |
| GE-VAN-AP + Jeffamine | 46 | 71 | 100 °C, 60 s | 65 °C, 30 m, acid | 41 | 72 | [49] |
| VBE + DDM | 93 | 181 | - | 50 °C, 12 h, acid | - | - | [57] |
| Spiro-acetal epoxy + IPDA | 87 | 169 | - | 50 °C, 9 m, acid | - | - | [58] |
| MB + PACM | 81 | 172 | 180 °C, 2 m | r.t., 4 h, acid | 81 | 175 | [59] |
| Van-EP + IPDA | 65 | 109 | 130 °C, 5 m | 70 °C, 24 h, acid | 66 | - | [50] |
| DGEVE + DDS | 71 | 184 | 50 °C, 40 m, acid | - | - | [60] | |
| DGHMDO + DDM | 105 | 164 | - | 50 °C, 5.5 h, acid | - | - | [61] |
| DADE + D230 | 57 | 106 | 150 °C, 10 m | 50 °C, 24 h, acid | 47 | 97 | [62] |
| GV-EP + DDM | - | 220 | 230 °C, 2 h | 90 °C, 24 h, acid | - | 237 | [52] |
| Composition | Pristine Products | Recycling Conditions | Recycled Products | Ref. | |||
|---|---|---|---|---|---|---|---|
| Strength (MPa) | Tg (°C) | Thermal | Chemical | Strength (MPa) | Tg (°C) | ||
| TFMP + diamine | 69 | 178 | 180 °C, 10 m | r.t., 24 h, acid | 69 | - | [67] |
| DAV + diamine | 51 | 75 | 150 °C, 1 h | 50 °C, 24 h, acid | 52 | - | [68] |
| VC + triamine + 4-AFD | 17 | 43 | 60 °C, 20 m | 50 °C, 24 h, acid | 16 | - | [69] |
| MVL + amine | 22 | 75 | 200 °C, 10 m | r.t., 24 h, amine | 21 | - | [70] |
| HMDO + HDI trimer | 68 | 130 | - | 50 °C, 40 m, acid | - | - | [71] |
| HVP + D230 | 58 | 98 | 120 °C, 5 m | r.t., 2 h, acid | 56 | - | [51] |
| Composition | Pristine Products | Recycling Conditions | Recycled Products | Ref. | |||
|---|---|---|---|---|---|---|---|
| Strength (MPa) | Tg (°C) | Thermal | Chemical | Strength (MPa) | Tg (°C) | ||
| DGEBA + Van2HMDA | 85 | 78 | 110 °C, 15 m | 70 °C, 8 h, acid | 10 | 63 | [54] |
| DGEBA + IH-VAN | 60 | 120 | 170 °C, 30 m | 60 °C, 3 h, IPDA | 58 | 127 | [18] |
| DGEBA + Van-OH | 79 | 96 | 120 °C,12 h | 50 °C, 2 h, acid | 49 | - | [11] |
| Gte + VA | 62 | 70 | 140 °C, 10 m | 50 °C, 2 h, EDA | 63 | 70 | [78] |
| GDE + HVPA | 39 | 82 | 100 °C, 3 m | r.t., 45 m, acid | 35 | - | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.A.; Hasan, M.N.; Dayan, M.A.R.; Ibna Jamal, M.S.; Patoary, M.K. A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects. Reactions 2023, 4, 66-91. https://doi.org/10.3390/reactions4010003
Rashid MA, Hasan MN, Dayan MAR, Ibna Jamal MS, Patoary MK. A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects. Reactions. 2023; 4(1):66-91. https://doi.org/10.3390/reactions4010003
Chicago/Turabian StyleRashid, Muhammad Abdur, Md. Nabiul Hasan, Md. Anisur Rahman Dayan, Mohammad Salman Ibna Jamal, and Mohammed Kayes Patoary. 2023. "A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects" Reactions 4, no. 1: 66-91. https://doi.org/10.3390/reactions4010003
APA StyleRashid, M. A., Hasan, M. N., Dayan, M. A. R., Ibna Jamal, M. S., & Patoary, M. K. (2023). A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects. Reactions, 4(1), 66-91. https://doi.org/10.3390/reactions4010003








