Hemetsberger–Knittel and Ketcham Synthesis of Heteropentalenes with Two (1:1), Three (1:2)/(2:1) and Four (2:2) Heteroatoms
Abstract
1. Introduction
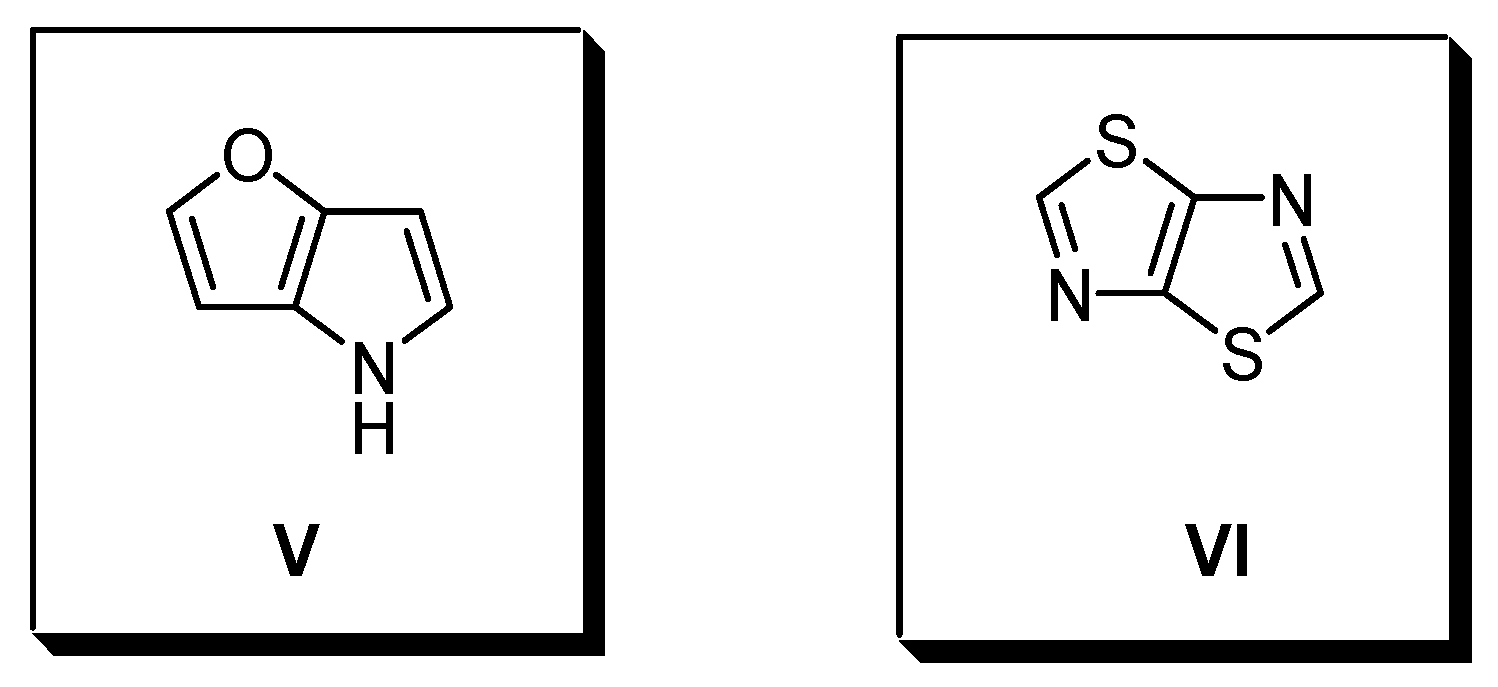
2. Furo-, Thieno- and Selenopheno [3,2-b]pyrroles
2.1. Hemetsberger–Knittel Synthesis of Furo [3,2-b]pyrroles and Related Compounds
2.2. Behaviour of the Hemetsberger–Knittel Procedure
2.3. Mechanism of the Hemetsberger–Knittel Synthesis
2.4. Application of the Hemetsberger–Knittel Synthesis towards a Variety of [3,2-b]HPs
2.5. Structural Modifications to [3,2-b]HPs through Subsequent Treatment
2.6. Application Potential of Seleno-, Thieno- and Pyrrolo [3,2-b]pyrroles as HP Related to Furo[3,2-b]pyrroles
3. Thiazolo [5,4-d]thiazoles
3.1. Ketcham’s Cyclocondensation Reaction
3.2. Mechanism of the Ketcham Reaction
3.3. Synthesis of Asymmetrical Thiazolo [5,4-d]thiazoles by Ketcham’s Reaction
3.4. Cyclopolymerisations Following Ketcham’s Reaction Protocol
- The polycondensation reaction of the Ketcham-type of dithiooxamide with triethylamine and carbazole-based aldehydes was published by Dabuliene at al. in 2022 [98]. GPC analysis showed the average molecular weights of triphenylamine-based compounds (62) (Figure 11b) between 2980 and 3080, while in the case of carbazole containing derivatives (63) it was from 1640 to 3290. The published GPC results indicated that the molecules contained approximately three to seven repeating units.
- Zhu et al. (2014) [99] demonstrated the preparation of a porous cross-linked polymer 64 (Figure 11c) containing TzTz and phenyl units. Similar phenyl-based monomers with three carbaldehyde groups, such as tris(4-formylphenyl)-benzene and tetra(4- formylphenyl)-benzene, can be also condensed with dithiooxamide to give a cross-linked copolymer with a porous structure [100].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stecko, S.; Gryko, T.G. Multifunctional Heteropentalenes: From Synthesis to Optoelectronic applications. J. Am. Chem. Soc. 2022, 2, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.J.; Rosenberg, M. The pentalenyl dianion. J. Am. Chem. Soc. 1962, 84, 865–866. [Google Scholar] [CrossRef]
- Alkorta, I.; Blanco, F.; Elguero, J. Heteropentalenes aromaticity. J. Mol. Struct. 2008, 851, 75–83. [Google Scholar] [CrossRef]
- Ramsden, C.A. Mesomeric betaine derivatives of heteropentalenes. Tetrahedron 1977, 33, 3193–3202. [Google Scholar] [CrossRef]
- Molander, G.A.; Shaumann, E.; Thomas, E.J.; Aitken, R.A.; Ameduri, B.; Braverman, S.; Brønsded Nielsen, M.; Cherkinsky, M.; Heydt, H.; Meehan, A.; et al. Product class 21: Five-five fused heteroarenes with one heteroatom in each ring. In Science of Synthesis: Knowledge Updates 2014/3, 1st ed.; Barnet, A., Nielsen Brønsded, M., Drabowicz, J., Joule, J.A., Schaumann, E., Weinreb, S.M., Eds.; Thieme: Delaware City, DE, USA, 2014; Volume 3. [Google Scholar]
- Kruotšíková, A. Bicyclic 5-5 Systems: Two heteroatoms. In Comprehensive Heterocyclic Chemistry II, 1st ed.; Katrizky, A.R., Reeds, C., Scriven, E.F.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 7, pp. 1–47. [Google Scholar]
- Katrizky, A.R.; Ramsden, C.; Scrive, E.; Taylor, R.; Neuville, L.; Zhu, J. Bicyclic 5-5 Systems with one Bridgehead (Ring Junction) Nitrogen Atoms: Four Extra Heteroatoms 2:2. In Comprehensive Heterocyclic Chemistry II, 1st ed.; Katrizky, A.R., Ramsden, C., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 11, pp. 1–13718. [Google Scholar]
- Panova, Y.S.; Khristolyubova, A.V.; Sushev, V.V.; Zolotareva, N.V.; Baranov, E.V.; Fukin, G.K.; Kornev, A.N. Rearrangements and reductive cleavage of 3a,6a-diaza-1,4-diphosphapentalenes. New J. Chem. 2021, 45, 18491–18496. [Google Scholar] [CrossRef]
- Kashida, J.; Shoji, Y.; Ikabata, Y.; Taka, H.; Sakai, H.; Hasobe, T.; Nakai, H.; Fukushima, T. An Air-and Water-stable B4N4-Heteropentalene serving as a Host Material for Phosphorescent OLED. Angew. Chem. Int. Ed. 2021, 60, 23812–23818. [Google Scholar] [CrossRef]
- Su, B.; Kostenko, A.; Yao, S.; Driess, M. Isolable Dibenzo[a,e]disilylpentalene with a Dichotomic reactivity toward CO2. J. Am. Chem. Soc. 2020, 142, 16935–16941. [Google Scholar] [CrossRef]
- Stanforh, S.P. Product class 21: Five-five-Fused Hetarenes with One Heteroatom in Each ring. In Science of Synthesis: Knowledge Updates 2014/3, 1st ed.; Barnet, A., Nielsen Brønsded, M., Drabowicz, J., Joule, J.A., Schaumann, E., Weinreb, S.M., Eds.; Thieme: Delaware City, DE, USA, 2014; Volume 3. [Google Scholar]
- Katrizky, A.R.; Rachwal, S. Synthesis of Heterocycles Mediated by Benzotriazole. 2. Bicyclic Systems. Chem. Rev. 2011, 111, 7063–7120. [Google Scholar] [CrossRef]
- Zlatoidský, P.; Martinelli, E.; Svensson, E.; Pruvost, A. A Facile Access to Novel (5+5)Annellated Heterocycles: Synthesis of a Furopyrrole, an Imidazoimidazole and a Pyrroloimidazole. Synthesis 2019, 51, 3491–3498. [Google Scholar] [CrossRef]
- Musicki, B. Synthesis and 1,3-Dipolar Cycloaddition Reactions of Novel Heteropentalene Mesomeris Betaines, Pyrrolo[1,2-c]Imidazole Mesomeric Betaines. J. Org. Chem. 1990, 55, 910–918. [Google Scholar] [CrossRef]
- Zemanová, I.; Gašparová, R.; Boháč, A.; Maliar, T.; Kraic, F.; Addová, G. Synthesis and antibacterial activity of furo[3,2-b]pyrrole derivatives. ARKIVOC 2017, v, 204–215. [Google Scholar] [CrossRef]
- Tokárová, Z.; Eckstein-Andicsová, A.; Balogh, R.; Tokár, K. Survey of the Ketcham reaction for series of furan-substituted thiazolo[5,4-d]thiazoles. Tetrahedron 2021, 89, 132155. [Google Scholar] [CrossRef]
- Milkiewicz, L.K.; Parks, J.D.; Lu, T. Synthesis of a novel series of tetrasubstituted furan[3,2-b]pyrrols. Tetrahedron Lett. 2003, 44, 4257–4260. [Google Scholar] [CrossRef]
- Gajdoš, P.; Pavlíková, S.; Bureš, F.; Krutošíková, A. 2-[3-(Trifluoromethyl)phenyl]furo[3,2-b]pyrroles: Synthesis and reactions. Cent. Eur. J. Chem. 2005, 3, 311–332. [Google Scholar] [CrossRef]
- Ilyin, A.P.; Dmitrieva, I.G.; Kustova, V.A.; Manaev, A.V.; Ivachtchenko, A.V. Synthesis of Heterocyclic Compounds Possessing the 4H-Thieno[3,2-b]Pyrrole Moiety. J. Comput. Chem. 2007, 91, 96–106. [Google Scholar]
- Kawashima, Y.; Amanuma, F.; Sato, M.; Okuyama, S.; Nakashima, Y.; Sota, K.; Moriguchi, I. Structure-activity studies of 4,6-disubstituted 2-(morpholinocarbonyl)furo[3,2-b]indole derivatives with analgesic and antiinflammatory activities. J. Med. Chem. 1986, 29, 2284–2290. [Google Scholar] [CrossRef]
- Garzan, A.; Willby, M.J.; Green, K.D.; Tsodikov, O.V.; Posey, J.E.; Garneau-Tsodikova, S. Discovery and Optimization of Two Eis Inhibitor Families as Kanamycin Adjuvants against Drug-Resistant M. tuberculosis. ACS Med. Chem. Lett. 2016, 7, 1219–1221. [Google Scholar] [CrossRef]
- Sparey, T.; Abeywickrema, P.; Almond, S.; Brandon, N.; Byrne, N.; Campbell, A.; Hutson, P.H.; Jacobson, M.; Jones, B.; Munshi, S.; et al. The discovery of fused pyrrole carboxylic acids as novel, potent d-amino acid oxidase (DAO) inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3386–3391. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.; McAtee, J.; Hemscheidt, T.K. Highly Brominated Antimicrobial Metabolites from a Marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Umezawa, K.; Matsui, A.; Nakamura, Y.; Citterio, D.; Suzuki, K. Bright, color-tunable fluorescent dyes in the VIS/NIR region: Establishment of new „Tailor-Made“ multicolor fluorophores based on borondipyrromethene. Chem. Eur. J. 2009, 15, 1096–1103. [Google Scholar] [CrossRef]
- Jiang, K.J.; Boxer, M.B.; Vander Heiden, M.G.; Shen, M.; Skoumbourdis, A.P.; Southall, N.; Veith, H.; Leister, W.; Austin, C.P.; Park, H.W.; et al. Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of tumor cell specific M2 isoform of pyruvate kinase. Bioorg. Med. Chem. Lett. 2010, 20, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Sindac, J.A.; Yestrepsky, B.D.; Barraza, S.J.; Bolduc, K.L.; Blakely, P.K.; Keep, R.F.; Irani, D.N.; Miller, D.J.; Larsen, S.D. Novel Inhibitors of Neurotropic Alphavirus Replication That Improve Host Survival in a Mouse Model of Acute Viral Encephalitis. J. Med. Chem. 2012, 55, 3535–3545. [Google Scholar] [CrossRef]
- Jones, C.; Boudinet, D.; Xia, Y.; Denti, M.; Das, A.; Facchetti, A.; Driver, T.G. Synthesis and Properties of Semiconducting Bispyrrolothiophenes for Organic Field-Effect Transistors. Chem. Eur. J. 2014, 20, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Hemetsberger, H.; Knittel, D. Syntheses and Thermolyzen von α-Azidoacrylestern. Monatsh. Chem. 1972, 103, 194–204. [Google Scholar] [CrossRef]
- Bingul, M.; Kumar, N.; StCBlack, D. The Hemetsberger reaction: A new approach to the synthesis of novel dihydroindoloindole systems. Arkivoc 2020, vii, 16–26. [Google Scholar] [CrossRef]
- Bingul, M.; Arndt, G.M.; Marshall, G.M.; Cheung, B.B.; Kumar, N.; Black, D.S. Synthesis, characterization and biological evaluation of novel dihydropyranoindoles improving the anticancer effects of HDAC inhibitors. Molecules 2020, 25, 1377. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Jones, G.B. Extending the versatility of the Hemetsberger–Knittel indole synthesis through microwave and flow chemistry. Bioorg. Med. Chem. Lett. 2013, 23, 1740–1742. [Google Scholar] [CrossRef]
- Roy, P.J.; Dufresne, C.; Lachance, N.; Leclerc, J.-P.; Boisvert, M.; Wang, Z.; Leblanc, Y. The Hemetsberger-Knittel Synthesis of Substituted 5-, 6-, and 7-Azaindoles. Synthesis 2005, 16, 2751–2757. [Google Scholar] [CrossRef]
- Bobošík, V.; Krutošíková, A. Synthesis of N-phenylsulfonyl protected furo[3,2-b]pyrroles. Collect. Czech. Chem. Commun. 1994, 59, 499–502. [Google Scholar] [CrossRef]
- Eras, J.; Galvez, C.; Garcia, F. Reactivity of thienopyrroles. Synthesis of isomeric nitro and bromothienopyrroles. J. Heterocycl. Chem. 1984, 21, 215–217. [Google Scholar] [CrossRef]
- Welch, M.; Phillips, R.S. Improved Syntheses of [3,2-b]- and [2,3-b]-fused Selenolo- and Thienopyrroles, and of Furo[3,2-b]pyrrole. Heterocycl. Commun. 1999, 5, 305–310. [Google Scholar] [CrossRef]
- Heaner, W.L., IV; Gelbaum, C.S.; Gelbaum, L.; Pollet, P.; Richman, K.W.; DuBay, W.; Butler, J.D.; Wells, G.; Liotta, C.L. Indoles via Knoevenagel–Hemetsberger reaction sequence. RSC Adv. 2013, 3, 13232–13242. [Google Scholar] [CrossRef]
- Jacobs, L.; Kock, C.; Taylor, D.; Pelly, S.C.; Blackie MA, L. Synthesis of five libraries of 6,5-fused heterocycles to establish the importance of the heterocyclic core for antiplasmodial activity. Bioorg. Med. Chem. 2018, 26, 5730–5741. [Google Scholar] [CrossRef]
- Roy, P.; Boisvert, M.; Leblanc, Y. Preparation of substituted 5-azaindoles: Methyl 4-chloro-1H-pyrrolo[3,2-c]pyridine-2-carboxylate. Org. Synth. 2007, 84, 262–271. [Google Scholar]
- O’Brien, A.G.; Lévesque, F.; Seeberger, P.H. Continuous flow thermolysis of azidoacrylates for the synthesis of heterocycles and pharmaceutical intermediates. Chem. Commun. 2011, 47, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Hemetsberger, H.; Knittel, D.; Weidmann, D. Thermolyzen von α-Azidoacrylestern: Synthese von Indole-derivaten. Monatsh. Chem. 1970, 101, 161–165. [Google Scholar] [CrossRef]
- Soth, S.; Farnier, M.; Paumier, C. Recherches en série hétérocyclique. XXIX. Sur des voies dàccès à des thiéno, sélénolo, furo et pyrrolopyrroles. Can. J. Chem. 1978, 56, 1429–1434. [Google Scholar] [CrossRef]
- Kralovičová, E.; Krutošíková, A.; Kováč, J.; Dandárová, M. Electrophilic substitution reactions of furo[3,2-b]pyrrole derivatives. Collect. Czech. Chem. Commun. 1986, 51, 106–111. [Google Scholar] [CrossRef]
- Krutošíková, A.; Kováč, J.; Dandárová, M.; Leško, J.; Ferík, S. Synthesis and reactions of furo[3,2-b]pyrrole derivatives. Collect. Czech. Chem. Commun. 1981, 45, 2564–2572. [Google Scholar] [CrossRef]
- Krutošíková, A.; Dandárová, M.; Chýlová, J.; Végh, D. Condensed O-, N-heterocycles by the Transformations of Azidoacrylates. Monatsh. Chem. 1992, 123, 807–815. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Rainbolt, E.A.; Sista, P.; Stefan, M.C. Synthesis and Polymerization of Fused-Ring Thienodipyrrole Monomers. Macromol. Chem. Phys. 2012, 213, 425–430. [Google Scholar] [CrossRef]
- Shefer, N.; Rozen, S. Synthesis of Oxidized Thienopyrroles using HOF.CH3CN. J. Org. Chem. 2011, 76, 4611–4616. [Google Scholar] [CrossRef]
- Shafiee, A.; Mazloumi, A.; Cohen, V.I. Selenium heterocycles. XXVIII. Synthesis of pyrrolo[3,2-d]selenazole and pyrrolo[3,2-d]thiazole. Two novel heterocycles. J. Heterocycl. Chem. 1979, 16, 1563–1566. [Google Scholar] [CrossRef]
- Athmani, S.; Farhat, M.F.; Iddon, B. Azoles. Part 9. Synthesis of Derivatives of Thieno[2,3-d]thiazole, 4H-Pyrrolo-[2,3-d]thiazole, 2H- Pyrazolo[3,4-d]thiazole and lsoxazolo [3,4-d]thiazole from Thiazolidine-2,4-dione. J. Chem. Soc Perkin Trans. 1992, 1, 973–977. [Google Scholar] [CrossRef]
- Shaffie, A.; Hadizadeh, F. Synthesis of sbstituted pyrrolo[3,2-d]imidazoles. J. Heterocycl. Chem. 1997, 34, 549–550. [Google Scholar] [CrossRef]
- Shafiee, A.; Mojarrad, J.S.; Jalili, M.A.; Adhami, H.R.; Hadizadeh, F. Syntheses of substituted pyrrolo[2,3-d]imidazole-5-carboxylates and substitued pyrrolo[3,2-d]imidazole-5-carboxylates. J. Heterocycl. Chem. 2002, 39, 367–373. [Google Scholar] [CrossRef]
- Sartori, L.; Mercurio, C.; Amigoni, F.; Cappa, A.; Fagá, G.; Fattori, R.; Legnaghi, E.; Ciossani, G.; Mattevi, A.; Meroni, G.; et al. Thieno[3,2-b]pyrrole-5-carboxamides as New Reversible Inhibitors of Histone Lysine Demethylase KDM1A/LSD1. Part 1: High-Throughput Screening and Preliminary Exploration. J. Med. Chem. 2017, 60, 1673–1692. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.-C.; Kam, Y.-W.; Merits, A.; Ng, L.F.P.; Chai, C.L.L. Trisubstituted thieno[3,2-b]pyrrole 5-carboxamides as potent inhibitors of alphaviruses. J. Med. Chem. 2015, 58, 9196–9213. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Peng, W.; Peltier, D.C.; Larsen, M.J.; Kirchhoff, P.D.; Larsen, S.D.; Neubig, R.R.; Miller, D.J. Identification of thieno[3,2-b]pyrrole derivatives as novel small molecule inhibitors of neurotropic alphaviruses. J. Infect. Dis. 2009, 199, 950–957. [Google Scholar] [CrossRef]
- Welch, M.; Phillips, R.S. Enzymatic syntheses of 6-(4H-selenolo[3,2-b]pyrrolyl)-L-alanine, 4-(6H-selenolo[3,2-b]pyrrolyl)-L-alanine, and 6-(4H-furo[3,2-b]pyrrolyl)-L-alanine. Bioorg. Med. Chem. Lett. 1999, 9, 637–640. [Google Scholar] [CrossRef]
- Bae, J.-H.; Alefelder, S.; Kaiser, J.T.; Friedrich, R.; Moroder, L.; Hubert, R.; Budisa, N. Incorporation of β-selenolo[3,2-b]pyrrolyl-alanine into proteins. J. Mol. Biol. 2001, 309, 925–936. [Google Scholar] [CrossRef]
- Blair, J.P.; Maruna-Lewicka, D.; Kanthasamy, A.; Lucaites, V.L.; Nelson, D.L.; Nichols, D.E. Thieno[3,2-b]pyrrole and thieno[2,3-b]pyrrole bioisosteric analogues of the hallucinogen and serotonin agonist N,N-dimethyltraptamine. J. Med. Chem. 1999, 42, 1106–1111. [Google Scholar] [CrossRef]
- Gamage, P.L.; Gedara, C.M.U.; Ma, Z.; Bhadran, A.; Gunawardhana, R.; Biewer, H.C.; Stefan, M.C. Incorporation of selenopheno[3,2-b]pyrrole into benzothiadiazole-based small molecules for organic field effect tranzistors. ACS Appl. Electron. Mater. 2021, 3, 5335–5344. [Google Scholar] [CrossRef]
- Gedara, C.M.U.; Ma, Z.; Talukder, M.M.; Gunawardkana, R.; Biewer, M.C.; Stefan, M.C. Siloxane side-chain-modified diketopyrrolopyrrole and thienopyrrole containing small molecules for organic field effect tranzistors. ACS Appl. Electron. Mater. 2022, 4, 5340–5350. [Google Scholar] [CrossRef]
- Domínguez, R.; Montcada, N.F.; de la Cruz, P.; Palomarez, E.; Langa, F. Pyrrolo[3,2-b]pyrrole as the central core of the electron donor for the solution-processed organic solar cells. Chem. Plus. Chem. 2017, 82, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ko, S.-J.; Shin, H.; Jin, Y.; Kim, I.; Kim, J.Y.; Suh, H. Pyrrolo[3,2-b]pyrrole small molecules as donor materials for OPVs. Sol. Energy Sol. Cells 2013, 112, 120–126. [Google Scholar] [CrossRef]
- Tasior, M.; Kowalczyk, P.; Przybyl, M.; Cichy, M.; Janasik, P.; Bousquel, M.H.E.; Lapkowski, M.; Rammo, M.; Rebane, A.; Jacquemin, D.; et al. Going beyond borders: Pyrrolo[3,2-b]pyrroles with deep red emission. Chem. Sci. 2021, 12, 15935–15946. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.N.; Kolesar, J.M.; Hall, S.R.; Saleh, N.A.; Jones, D.S.; Walter, M.G. Thiazolothiazole Fluorophores Exhibiting Strong Fluorescence and Viologen-Like Reversible Electrochromism. J. Am. Chem. Soc. 2017, 139, 8467–8473. [Google Scholar] [CrossRef]
- Johnson, J.R.; Ketcham, R. Thiazolothiazoles. I. The Reaction of Aromatic Aldehydes with Dithiooxamide. J. Am. Chem. Soc. 1960, 82, 2719–2724. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.; Inoue, Y.; Yamashita, Y. Synthesis, physical properties, and field-effect transistors of novel thiophene/thiazolothiazole co-oligomers. Science 2004, 14, 1787–1790. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.; Fujiwara, E.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. Characterization and Field-Effect Transistor Performance of Heterocyclic Oligomers Containing a Thiazolothiazole Unit. Chem. Lett. 2004, 33, 1170–1171. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.-I.; Tada, H.; Inoue, Y.; Tokio, S.; Yamashita, Y. High performance n-type organic field-effect transistors based on pi-electronic systems with trifluoromethylphenyl groups. J. Am. Chem. Soc. 2005, 127, 5336–5337. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Zhang, R.; Sauvé, G.; Smiligies, D.-F.; Kowalewski, T.; McCullough, R.D. High-Lamellar Ordering and Amorphous-Like π-Network in Short-Chain Thiazolothiazole−Thiophene Copolymers Lead to High Mobilities. J. Am. Chem. Soc. 2009, 131, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-H.; Jang, S.-Y.; Kang, M.; Lee, S.; Kim, Y.-A.; Heo, Y.-J.; Lee, M.-H.; Kim, D.-Y. A systematic study on molecular planarity and D–A conformation in thiazolothiazole- and thienylenevinylene-based copolymers for organic field-effect transistors. J. Mater. Chem. C 2017, 5, 10126–10132. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.-A.; Hung, W.-Y.; Tang, W.-i.-F.; Hsu, Y.-H.; Chen, C.h.-L.; Meng, F.-Y.; Chou, P.-T. Control of the Reversibility of Excited-State Intramolecular Proton Transfer (ESIPT) Reaction: Host-Polarity Tuning White Organic Light Emitting Diode on a New Thiazolo[5,4-d]thiazole ESIPT System. Chem. Mater. 2016, 28, 8815–8824. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kang, M.; Chun, J.; Lee, J.; Kim, J.; Kim, J.; Kim, Y.; Kim, S.-J.; Lee, C.; Yoon, J. A thiazolothiazole based Cu2+ selective colorimetric and fluorescent sensor via unique radical formation. Chem. Commun. 2013, 49, 176–178. [Google Scholar] [CrossRef]
- Luo, J.; Hu, B.; Debruler, C.; Liu, T.L. A π-Conjugation Extended Viologen as a Two-Electron Storage Anolyte for Total Organic Aqueous Redox Flow Batteries. Angew. Chem. Int. Ed. 2018, 57, 231–235. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, S.; Wang, J.; Xu, Y.; Hou, J. Recent advances in non-fullerene organic solar cells: From lab to fab. Chem. Commun. 2020, 56, 14337–14352. [Google Scholar] [CrossRef]
- Dessi, A.; Calamante, M.; Mordini, A.; Peruzzini, M.; Sinicropi, A.; Basosi, R.; de Biani, F.F.; Taddei, M.; Colonna, D.; di Carlo, A.; et al. Organic dyes with intense light absorption especially suitable for application in thin-layer dye-sensitized solar cells. Chem. Commun. 2014, 50, 13952–13955. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Min, J.; Zhang, S.; Zhang, J.; Zhang, M.; Li, Y. Alkyl chain engineering on a dithieno[3,2-b:2′,3′-d]silole-alt-dithienylthiazolo[5,4-d]thiazole copolymer toward high performance bulk heterojunction solar cells. Chem. Commun. 2011, 47, 9474–9476. [Google Scholar] [CrossRef] [PubMed]
- Nazim, M.; Ameen, S.; Akhtar, M.S.; Nazeeruddin, M.K.; Shin, H.S. Tuning electronic structures of thiazolo[5,4-d]thiazole-based hole-transporting materials for efficient perovskite solar cells. Sol. Energy Mat. Sol. Cells 2018, 180, 334–342. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.; Fang, J.; Guo, X.; Zhu, L.; Guo, B.; Wang, Y.; Zhang, G.; Arunagiri, L.; Liu, F.; et al. Random terpolymer based on thiophene-thiazolothiazole unit enabling efficient non-fullerene organic solar cells. Nat. Commun. 2020, 11, 4612. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Earmme, T.; Subramaniyan, S.; Jenekhe, S.A. Side chain engineering of n-type conjugated polymer enhances photocurrent and efficiency of all-polymer solar cells. Chem. Commun. 2014, 50, 10801–10804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, D.; Xiao, H.; Song, J.; Qu, L.; Wang, L.; Zhou, X.; Xu, Z.-X.; Xiang, H. Syntheses and photophysical properties of axially chiral thiazolothiazoles: Multi-stimuli-responsive fluorescence and circularly polarized luminescence. Dye. Pigment. 2022, 197, 109906–109916. [Google Scholar] [CrossRef]
- Sayresmith, N.A.; Saminathan, A.; Sailer, J.K.; Patberg, S.M.; Sandor, K.; Krishan, Y.; Walter, M.G. Photostable Voltage-Sensitive Dyes Based on Simple, Solvatofluorochromic, Asymmetric Thiazolothiazoles. J. Am. Chem. Soc. 2019, 141, 18780–18790. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, T.; Liu, Y.A.; Hu, W.; Yang, H.; Zhang, Y.-B.; Wen, K. Thiazolo[5,4-d]thiazole-based donor-acceptor covalent organic framework for sunlight-driven hydrogen evolution. Angew. Chem. Int. Ed. 2021, 61, 1869–1874. [Google Scholar] [CrossRef]
- Ephraim, J. Ueber die Einwirkung von Aldehyden auf Thioamide I. Eur. J. Inorg. Chem. 1891, 24, 1026–1031. [Google Scholar] [CrossRef]
- Tokárová, Z.; Biathová, A. Synthesis and structure-physicochemical properties relationship of thiophene substituted bis(5,4-d)thiazoles. Nova Biotech. Chim. 2018, 17, 193–200. [Google Scholar] [CrossRef]
- Osaka, I.; Sauvé, G.; Zhang, R.; Kowalewski, T.; McCullough, R.D. Novel thiophene-thiazolothiazole copolymers for organic field-effect transistors. Adv. Mater. 2007, 19, 4160–4165. [Google Scholar] [CrossRef]
- Papernaya, L.K.; Shatrova, A.A.; Sterkhova, I.V.; Levkovskaya, G.G.; Rozentsveig, I.B. Microwave-Assisted Synthesis of 2,5-Diarylthiazolo[5,4-d]thiazoles from benzaldehydes and dithioxamide. Russ. J. Org. Chem. 2015, 51, 389–393. [Google Scholar] [CrossRef]
- Nazim, M.; Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shik, H.; Shin, H.S. Novel liquid crystalline oligomer with thiazolothiazole-acceptor for efficient BHJ small molecule organic solar cells. Synth. Met. 2014, 187, 178–184. [Google Scholar] [CrossRef]
- Schneider, J.A.; Black, H.; Lin, H.P.; Perepichka, D.F. Polymorphism in new thienothiophene-thiazolothiazole organic semiconductors. ChemPhysChem 2015, 16, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kang, M.; Choi, H.; Jung, Y.; Swamy, K.M.K.; Kim, S.; Kim, D.; Kim, J.; Lee, C.; Yoon, J. Organic radical-induced Cu2+ selective sensing based on thiazolothiazole derivatives. Sens. Actuators B Chem. 2014, 192, 691–696. [Google Scholar] [CrossRef]
- Ziessel, R.; Nano, A.; Heyer, E.; Bura, T.; Retailleau, P. Rational design of new thiazolo-thiazole dyes as input energy units in molecular dyads. Chem.—A Eur. J. 2013, 19, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Sui, Y.; Lei, Y.; Jin, Y.; Wu, Y. Imidazole-thiazolo[5,4-d]thiazoles as corrosion inhibitors for mild steel in acidic media: Experimental and theoretical investigation. J. Mater. Sci. 2022, 35, 16904–16922. [Google Scholar] [CrossRef]
- Bevk, D.; Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. Thiazolo[5,4-d]thiazoles-promising building blocks in the synthesis of semiconductors for plastic electronics. RSC Adv. 2013, 3, 11418–11431. [Google Scholar] [CrossRef]
- Reginato, G.; Mordini, A.; Zani, L.; Calamante, M.; Dessì, A. Photoactive Compounds Based on the Thiazolo[5,4-d]thiazole Core and Their Application in Organic and Hybrid Photovoltaics. Eur. J. Org. Chem. 2016, 2016, 233–251. [Google Scholar] [CrossRef]
- Biswal, B.P.; Becker, D.; Chandrasekhar, N.; Seenath, J.S.; Paasch, S.; Machill, S.; Hennersdorf, F.; Brunner, E.; Weigand, H.; Berger, R.; et al. Exploration of Thiazolo[5,4-d]thiazole Linkages in Conjugated Porous Organic Polymers for Chemoselective Molecular Sieving. Chem. Eur. J. 2018, 24, 10868–10875. [Google Scholar] [CrossRef]
- Kumar, V.; Sony, S.; Kaur, N.; Mobin, S.M.; Kaur, P.; Singh, K. Thiazolothiazole based donor-π-acceptor fluorophore: Protonation/deprotonation triggered molecular switch, sensing and bio-imaging applications. Anal. Chim. Acta 2022, 1206, 339776. [Google Scholar] [CrossRef]
- Flores, E.; Muñoz-Osses, M.; Torrent, C.; Vásquez-Martínez, Y.; Gómez, A.; Martin, M.C.; Vega, A.; Martí, A.A.; Godoy, F.; Mascayano, C. Design, Synthesis and Biological Evaluation of Ferrocenyl Thiazole and Thiazolo[5,4- d]thiazole Catechols as Inhibitors of 5-hLOX and as Antibacterials against Staphylococcus aureus. Structural Relationship and Computational Studies. Organometallics 2020, 39, 2672–2681. [Google Scholar] [CrossRef]
- Sathiyan, G.; Sakthivel, P.; Thangamuthu, R.; Thangamuthu, R.; Sakthivel, P. Synthesis of carbazole-based copolymers containing carbazole-thiazolo[5,4-d]thiazole groups with different dopants and their fluorescence and electrical conductivity applications. RSC Adv. 2016, 6, 69196–69205. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Wang, Y.; Yang, Y.W. Pillararene-enriched linear conjugated polymer materials with thiazolo[5,4-d]thiazole linkages for photocatalysis. Chem. Commun. 2021, 57, 6546–6549. [Google Scholar] [CrossRef] [PubMed]
- Dabuliene, A.; Dainyte, A.; Andruleviciene, V.; Lygaitis, R.; Punniyakoti, S.J.; Tomkeviciene, A.; Velasco, D.; Obushak, M.; Grazulevicius, J.V. Low-molar-mass and oligomeric derivatives of carbazole and triphenylamine containing thiazolo[5,4-d]thiazole moieties. Polym. Bull. 2023, 80, 1477–1493. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Jin, T.; Wang, J.; Mahurin, S.M.; Mei, W.; Xiong, Y.; Hu, J.; Feng, X.; Liu, H.; et al. Thiazolothiazole-linked porous organic polymers. Chem. Commun. 2014, 50, 15055–15058. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, L.; Wang, N.; Zhu, X.; Jin, S.; Tan, B. Layered Thiazolo[5,4-d] Thiazole-Linked Conjugated Microporous Polymers with Heteroatom Adoption for Efficient Photocatalysis Application. ACS Appl. Mater. Interfaces 2019, 11, 15861–15868. [Google Scholar]
- Biswal, B.P.; Vignolo-González, H.A.; Banerjee, T.; Grunenberg, L.; Savasci, G.; Gottschling, K.; Nuss, J.; Ochsenfeld, C.; Lotsch, B.V. Sustained Solar H2 Evolution from a Thiazolo[5,4-d]thiazole-Bridged Covalent Organic Framework and Nickel-Thiolate Cluster in Water. J. Am. Chem. Soc. 2019, 141, 11082–11092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Dong, X.; Zhang, F.; Lang, X. Triazine-based two dimensional porous materials for visible light-mediated oxidation of sulfides to sulfoxides with O2. J. Colloid Interface Sci. 2022, 616, 846–857. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Pan, Q.; Ding, N.; Yang, C.; Zhang, Z.; Jia, C.; Li, Z.; Liu, J.; Zhao, Y. Construction of Thiazolo[5,4-d]thiazole-based Two-Dimensional Network for Efficient Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 46483–46489. [Google Scholar] [CrossRef]
- Sami, M.M.; Mekhemer, I.M.A.; Mohamed, M.G.; Elsayed, M.H.; Lin, K.; Chen, Y.; Wu, T.; Chou, H. Conjugated microporous polymers incorporating Thiazolo[5,4-d]thiazole moieties for Sunlight-Driven hydrogen production from water. Chem. Eng. J. 2022, 446, 137158. [Google Scholar]
- Samal, M.; Valligatla, S.; Saad, N.A.; Rao, M.V.; Rao, D.N.; Sahu, R.; Biswal, B.P. A thiazolo[5,4-d] thiazole-bridged porphyrin organic framework as a promising nonlinear optical material. Chem. Commun. 2019, 55, 11025–11028. [Google Scholar] [CrossRef] [PubMed]


























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokárová, Z.; Gašparová, R.; Kabaňová, N.; Gašparová, M.; Balogh, R. Hemetsberger–Knittel and Ketcham Synthesis of Heteropentalenes with Two (1:1), Three (1:2)/(2:1) and Four (2:2) Heteroatoms. Reactions 2023, 4, 254-273. https://doi.org/10.3390/reactions4020015
Tokárová Z, Gašparová R, Kabaňová N, Gašparová M, Balogh R. Hemetsberger–Knittel and Ketcham Synthesis of Heteropentalenes with Two (1:1), Three (1:2)/(2:1) and Four (2:2) Heteroatoms. Reactions. 2023; 4(2):254-273. https://doi.org/10.3390/reactions4020015
Chicago/Turabian StyleTokárová, Zita, Renáta Gašparová, Natália Kabaňová, Marcela Gašparová, and Róbert Balogh. 2023. "Hemetsberger–Knittel and Ketcham Synthesis of Heteropentalenes with Two (1:1), Three (1:2)/(2:1) and Four (2:2) Heteroatoms" Reactions 4, no. 2: 254-273. https://doi.org/10.3390/reactions4020015
APA StyleTokárová, Z., Gašparová, R., Kabaňová, N., Gašparová, M., & Balogh, R. (2023). Hemetsberger–Knittel and Ketcham Synthesis of Heteropentalenes with Two (1:1), Three (1:2)/(2:1) and Four (2:2) Heteroatoms. Reactions, 4(2), 254-273. https://doi.org/10.3390/reactions4020015




