Synthesis and Characterization of New Functionalized 1,2,3-Triazole-Based Acetaminophen Derivatives via Click Chemistry from Expired Commercial Acetaminophen Tablets
Abstract
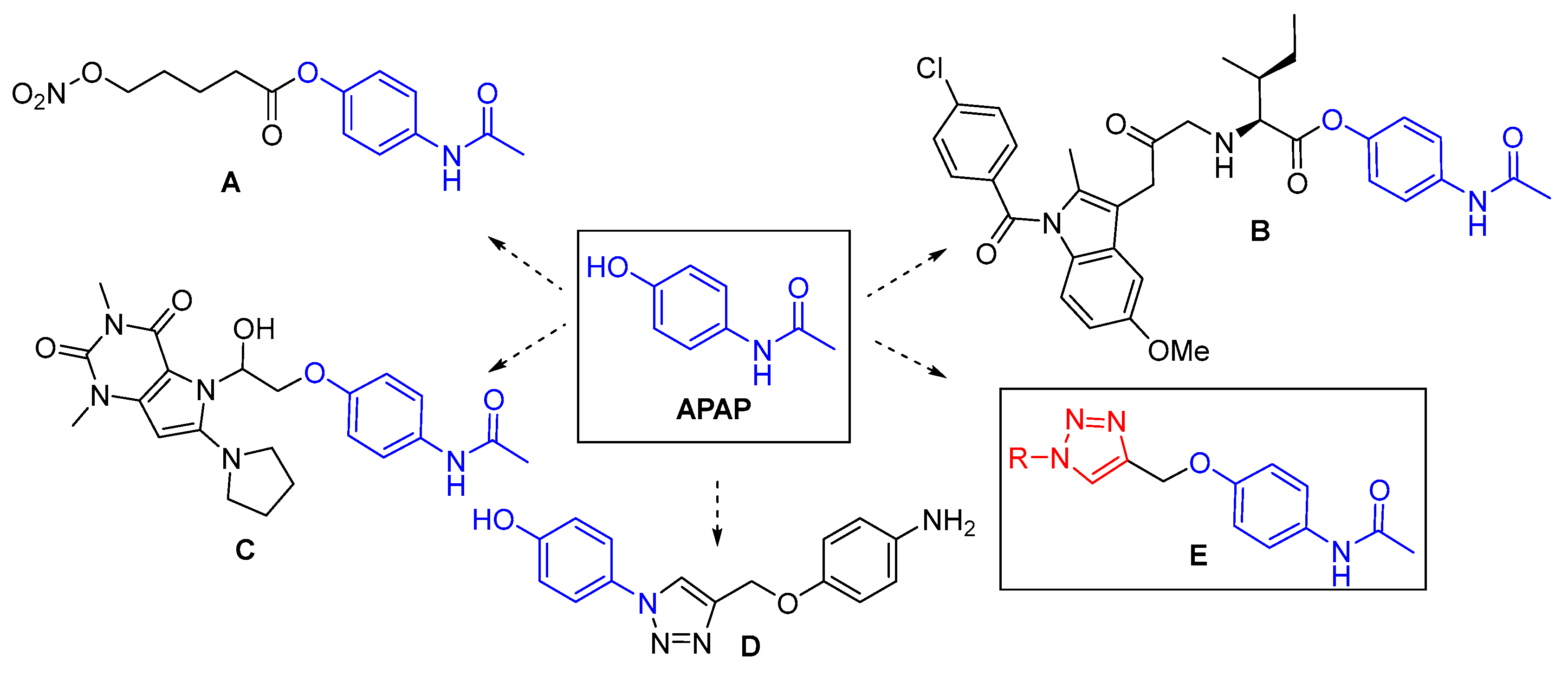
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
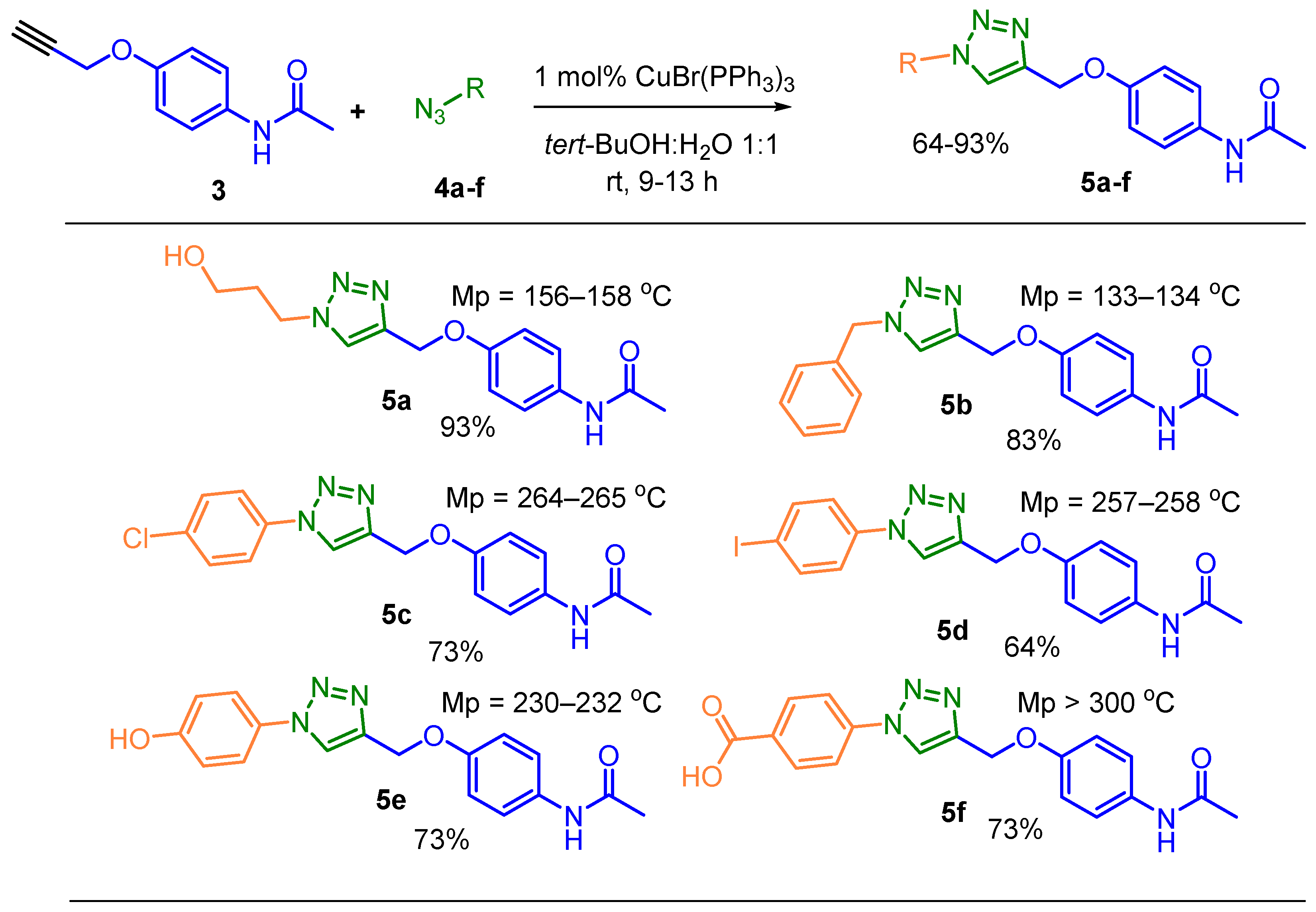
2.2. General Procedure for the Synthesis of 1,2,3-Triazole-Based APAP Derivatives 5a–f
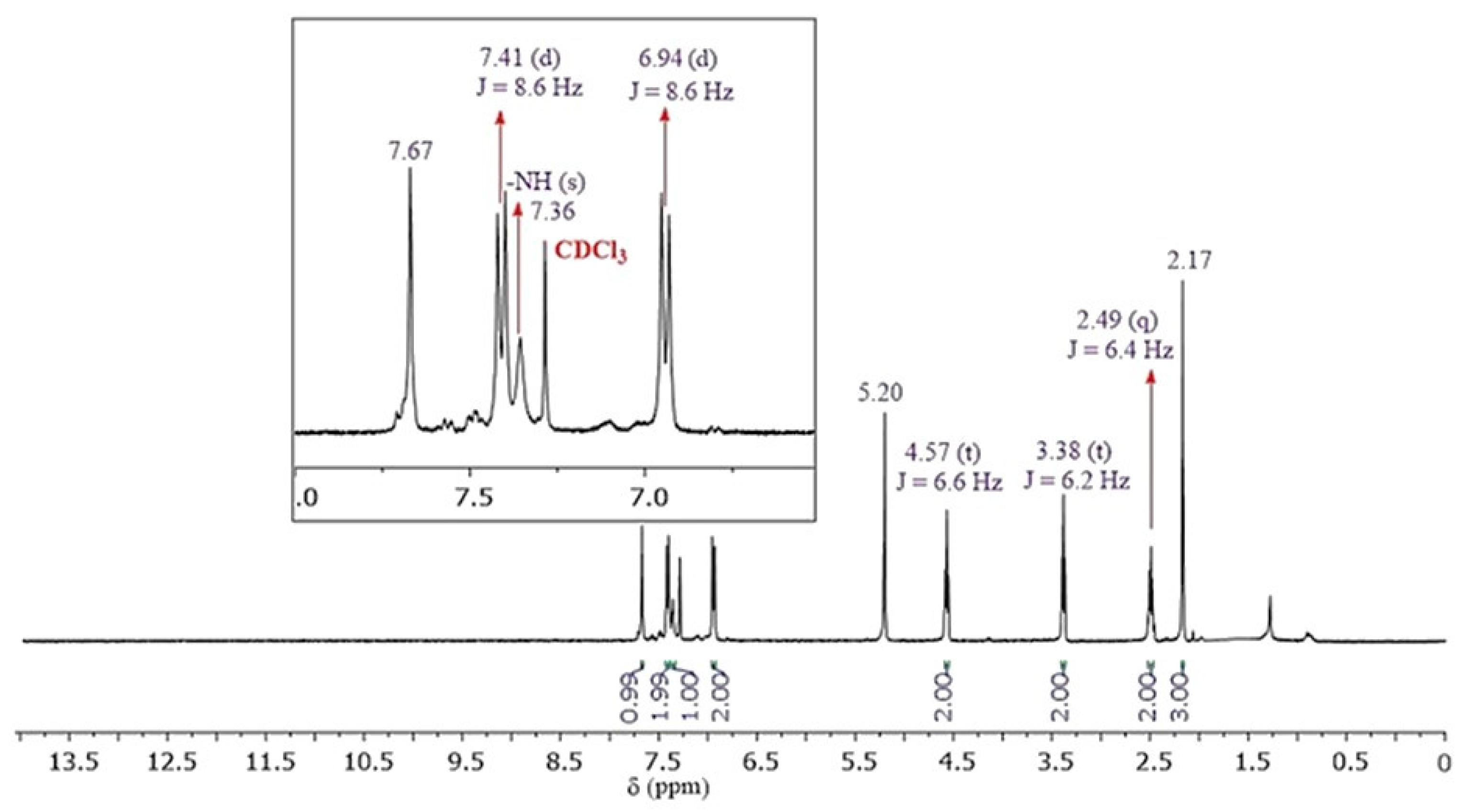
2.3. Spectroscopic Characterization
3. Results and Discussion
3.1. Chemistry
3.2. In Silico ADMET Prediction of Physicochemical Properties (Lipinski descriptors) of Prepared Triazole-APAP Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, B.J. Paracetamol (Acetaminophen): Mechanisms of action. Paediatr. Anaesth. 2008, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Klotz, U. Paracetamol (acetaminophen)—A popular and widely used nonopioid analgesic. Arzneimittelforschung 2012, 62, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Renner, B.; Tiegs, G. Acetaminophen/paracetamol: A history of errors, failures and false decisions. Eur. J. Pain 2015, 19, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A review of guideline recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication: Prescription Acetaminophen Products Are to Be Limited to 325 mg per Dosage Unit; A Boxed Warning Will Highlight the Potential for Severe Liver Failure. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit (accessed on 10 March 2023).
- Aminoshariae, A.; Khan, A. Acetaminophen: Old drug, new issues. J. End. 2015, 41, 588–593. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef]
- Hanel, A.M.; Lands, W.E. Modification of anti-inflammatory drug effectiveness by ambient lipid peroxides. Biochem. Pharmacol. 1982, 31, 3307–3311. [Google Scholar] [CrossRef]
- Suemaru, K.; Yoshikawa, M.; Aso, H.; Watanabe, M. TRPV1 mediates the anticonvulsant effects of acetaminophen in mice. Epilepsy Res. 2018, 145, 153–159. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic effect of acetaminophen: A review of known and novel mechanisms of action. Front. Pharmacol. 2020, 11, 580289. [Google Scholar] [CrossRef]
- Boutaud, O.; Aronoff, D.M.; Richardson, J.H.; Marnett, L.J.; Oates, J.A. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc. Natl. Acad. Sci. USA 2002, 99, 7130–7135. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, S.; Daiber, A.; Ghisla, S.; Cohen, R.A.; Bachschmid, M.M. Acetaminophen inhibits prostanoid synthesis by scavenging the PGHS-activator peroxynitrite. FASEB J. 2008, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef]
- Bessems, J.G.; Gaisser, H.D.; Te Koppele, J.M.; Van Bennekom, W.P.; Commandeur, J.N.; Vermeulen, N.P. 3,5-Disubstituted analogues of paracetamol. Synthesis, analgesic activity and cytotoxicity. Chem. Biol. Interact. 1995, 98, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Tavakolinejad-Kermani, E.; Saidi, K.; Islami, M.R. New synthesis of acetaminophen derivatives containing a phosphorus atom. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1879–1884. [Google Scholar] [CrossRef]
- Santos, C.; Mateus, M.L.; dos Santos, A.P.; Moreira, R.; de Oliveira, E.; Gomes, P. Cyclization-activated prodrugs. Synthesis, reactivity and toxicity of dipeptide esters of paracetamol. Bioorg. Med. Chem. Lett. 2005, 15, 1595–1598. [Google Scholar] [CrossRef]
- Jung, S.; Tsukuda, Y.; Kawashima, R.; Ishiki, T.; Matsumoto, A.; Nakaniwa, A.; Takagi, M.; Noguchi, T.; Imai, N. Convenient synthesis of acetaminophen analogues containing α-amino acids and fatty acids via their mixed carbonic carboxylic anhydrides in aqueous organic solvent. Tetrahedron Lett. 2013, 54, 5718–5720. [Google Scholar] [CrossRef]
- Tiwari, A.D.; Panda, S.S.; Girgis, A.S.; Sahu, S.; George, R.F.; Srour, A.M.; La Starza, B.; Hall, C.D.; Asiri, A.M.; Katritzky, A.R. Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers. Org. Biomol. Chem. 2014, 12, 7238–7249. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khalili, M.; Sadeghi, S.; Soleimani, N.; Nahri-Niknafs, B. Synthesis of New Acetaminophen Analogs and Their Ibuprofen Conjugates as Novel Analgesic Drugs. Pharm. Chem. J. 2016, 50, 369–376. [Google Scholar] [CrossRef]
- Vaccarino, A.L.; Paul, D.; Mukherjee, P.K.; de Turco, E.B.R.; Marcheselli, V.L.; Xu, L.; Trudell, M.L.; Minguez, J.M.; Matía, M.P.; Sunkel, C.; et al. Synthesis and in vivo evaluation of non-hepatotoxic acetaminophen analogs. Bioorg. Med. Chem. 2007, 15, 2206–2215. [Google Scholar] [CrossRef]
- Queiroz, L.M.; Rocha, J.R.; Leitão, A.; Montanari, C.A.; da Silva, A.B.; Sousa, P.J.; Borges, R.S. A Combined Study Using Ligand-Based Design, Synthesis, and Pharmacological Evaluation of Analogues of the Acetaminophen Ortho-Regioisomer with Potent Analgesic Activity. Chem. Biol. Drug Des. 2012, 80, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Alisi, M.A.; Brufani, M.; Cazzolla, N.; Ceccacci, F.; Dragone, P.; Felici, M.; Furlotti, G.; Garofalo, B.; La Bella, A.; Dragone, P.; et al. DPPH radical scavenging activity of paracetamol analogues. Tetrahedron 2012, 68, 10180–10187. [Google Scholar] [CrossRef]
- Reddy, Y.D.; Kumari, Y.B.; Dubey, P.K. Synthesis of a novel water soluble phthalimide derivative of acetaminophen as potential analgesic and antipyretic agent. Indian J. Chem. 2013, 52B, 691–693. [Google Scholar] [CrossRef]
- Raj, N.U.I. Synthesis, single crystal XRD and CT DNA/BSA binding studies of new paracetamol derivatives. J. Mol. Struct. 2020, 1208, 127911. [Google Scholar]
- Asghari, A.; Ameri, M.; Taghipour, S.; Ghaderi, O. Facile and clean electrochemical synthesis of new acetaminophen derivatives through electrochemical oxidation of acetaminophen in the presence of thiouracil derivatives. J. Sulphur Chem. 2017, 38, 163–172. [Google Scholar] [CrossRef]
- Profire, L.; Sunel, V.; Lupascu, D.; Baican, M.C.; Bibire, N.; Vasile, C. New theophylline derivatives with potential pharmacological activity. Farmacia 2010, 58, 170–176. [Google Scholar]
- Das, M.; Bhattacharjee, S.; Fronczek, F.R.; Bazan, N.G.; Trudell, M.L. Synthesis, hepatotoxic evaluation and antipyretic activity of nitrate ester analogs of the acetaminophen derivative SCP-1. Bioorg. Med. Chem. Lett. 2018, 28, 3798–3801. [Google Scholar] [CrossRef]
- Sahu, A.; Das, D.; Sahu, P.; Mishra, S.; Sakthivel, A.; Gajbhiye, A.; Agrawal, R. Bioisosteric replacement of amide group with 1,2,3-triazoles in acetaminophen addresses reactive oxygen species-mediated hepatotoxic insult in Wistar albino rats. Chem. Res. Toxicol. 2020, 33, 522–535. [Google Scholar] [CrossRef]
- Al-Swayeh, O.A.; Futter, L.E.; Clifford, R.H.; Moore, P.K. Nitroparacetamol exhibits anti-inflammatory and anti-nociceptive activity. Br. J. Pharmacol. 2000, 130, 1453–1456. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by design: A medicinal chemist’s perspective on multitargeting compounds. J. Med. Chem. 2018, 62, 420–444. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Ghasemi, J.B. Dual-acting of hybrid compounds-a new dawn in the discovery of multi-target drugs: Lead generation approaches. Curr. Top. Med. Chem. 2017, 17, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P. NCX-701. NicOx. Curr. Opin. Investig. 2004, 5, 755–759. [Google Scholar]
- Romero-Sandoval, E.A.; Curros-Criado, M.M.; Gaitan, G.; Molina, C.; Herrero, J.F. Nitroparacetamol (NCX-701) and Pain: First in a Series of Novel Analgesics. CNS Drug Rev. 2007, 13, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4-and 1,5-Disubstituted 1,2,3-Triazoles Good Pharmacophoric Groups? ChemMedChem 2014, 9, 2497–2508. [Google Scholar] [CrossRef]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Sahu, P.; Agrawal, R. A recent review on drug modification using 1,2,3-triazole. Curr. Chem. Biol. 2020, 14, 71–87. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A promising antitubercular agent. Chem. Biol. Drug. Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Song, M.X.; Deng, X.Q. Recent developments on triazole nucleus in anticonvulsant compounds: A review. J. Enzyme Inhib. Med. Chem. 2018, 33, 453–478. [Google Scholar] [CrossRef]
- Lal, K.; Yadav, P. Recent advancements in 1,4-disubstituted 1H-1,2,3-triazoles as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2018, 18, 21–37. [Google Scholar] [CrossRef]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, 2100158. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef]
- Nemallapudi, B.R.; Guda, D.R.; Ummadi, N.; Avula, B.; Zyryanov, G.V.; Reddy, C.S.; Gundala, S. New methods for synthesis of 1,2,3-triazoles: A review. Polycycl. Aromat. Compd. 2022, 42, 3874–3892. [Google Scholar] [CrossRef]
- Vala, D.P.; Vala, R.M.; Patel, H.M. Versatile Synthetic Platform for 1,2,3-Triazole Chemistry. ACS Omega 2022, 7, 36945–36987. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Huisgen, R. Cycloadditions—Definition, classification, and characterization. Angew. Chem. Int. Ed. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions(CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Pérez-Balderas, F.; Ortega-Munoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F.G.; Calvo-Asín, J.A.; Isac-García, J.; Santoyo-González, F. Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org. Lett. 2003, 5, 1951–1954. [Google Scholar] [CrossRef]
- Lal, S.; Diez-Gonzalez, S. [CuBr(PPh3)3] for azide− alkyne cycloaddition reactions under strict click conditions. J. Org. Chem. 2011, 76, 2367–2373. [Google Scholar] [CrossRef]
- Han, J. Barcoding drug information to recycle unwanted household pharmaceuticals: A review. Environ. Chem. Lett. 2022, 20, 2989–3003. [Google Scholar] [CrossRef] [PubMed]
- Pratama, D.E.; Hsieh, W.C.; Elmaamoun, A.; Lee, H.L.; Lee, T. Recovery of active pharmaceutical ingredients from unused solid dosage-form drugs. ACS Omega 2020, 5, 29147–29157. [Google Scholar] [CrossRef]
- Hsieh, D.S.; Lindrud, M.; Lu, X.; Zordan, C.; Tang, L.; Davies, M. A process for active pharmaceutical ingredient recovery from tablets using green engineering technology. Org. Process Res. Dev. 2017, 21, 1272–1285. [Google Scholar] [CrossRef]
- Messina, L.C.; de Espindola, C.S.; Omori, A.T. Direct One-Pot Synthesis of Propofol from Paracetamol Tablets. ACS Sustain. Chem. Eng. 2023, 11, 1638–1642. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas-Méndez, L.Y.; Zubkov, F.I. Recent Advances in Synthesis of Bioactive Quinoline-based 1,2,3-Triazoles via Cu-catalyzed Huisgen 1,3-Dipolar Cycloaddition (“Click reaction”). Mini-Rev. Org. Chem. 2016, 13, 488–503. [Google Scholar] [CrossRef]
- Luna-Parada, L.K.; Vargas-Méndez, L.Y.; Kouznetsov, V.V. Quinoline-Substituted 1,2,3-Triazole-Based Molecules, as Promising Conjugated Hybrids in Biomedical Research. Org. Med. Chem. 2018, 7, 555708. [Google Scholar]
- Luna-Parada, L.K.; Kouznetsov, V.V. 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl] methoxy}quinoline. Molbank 2019, 2019, M1038. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria-Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, Biological Evaluation and in silico Computational Studies of 7-Chloro-4-(1H-1,2,3-triazol-1-yl)quinoline Derivatives. Search for new controlling agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Calderón Lamus, D.; Puerto Galvis, C.E.; Kouznetsov, V.V. Cu(PPh3)3Br-catalyzed synthesis of new paracetamol-1,2,3-triazole molecular hybrids from expired commercial tablets and their in silico assessment to study their pharmacological properties. Med. Sci. Forum 2022, 14, 85. [Google Scholar]
- Pak, J.K.; Hesse, M. Synthesis of penta-N-protected homocaldopentamine and its selective acylation. J. Org. Chem. 1998, 63, 8200–8204. [Google Scholar] [CrossRef]
- Kutonova, K.V.; Trusova, M.E.; Postnikov, P.S.; Filimonov, V.D.; Parello, J. A simple and effective synthesis of aryl azides via arenediazonium tosylates. Synthesis 2013, 45, 2706–2710. [Google Scholar]
- Vong, L.B.; Nagasaki, Y. Nitric oxide nano-delivery systems for cancer therapeutics: Advances and challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.A.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.K.; Bhardwaj, T.R. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com (accessed on 15 January 2023).
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Organic Chemistry Portal. The OSIRIS Property Explorer Open-Source Program. Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 15 January 2023).


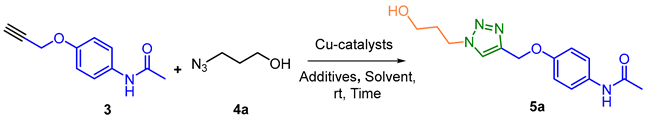
| Entry | Solvent | Catalyst | Additive: mol% NaAsC b | Load Catalyst (mol%) | Time (h) | Yield (%) c |
|---|---|---|---|---|---|---|
| 1 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 15 | 10 | 18 | 83 |
| 2 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 10 | 5 | 18 | 86 |
| 3 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 1 | 0.5 | 13 | 85 |
| 4 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 5 | 18 | 90 |
| 5 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 18 | 92 |
| 6 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 12 | 90 |
| 7 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 9 | 93 |
| 8 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 0.5 | 11 | 87 |
| 9 | CH3CN | [CuBr(PPh3)3] | - | 1 | 12 | 73 |
| 10 | CP | [CuBr(PPh3)3] | - | 1 | 18 | NR d |
| Comp. | MW b | cLogP c | HBA d | HBD e | ROTB f | TPSA g | n Violations |
|---|---|---|---|---|---|---|---|
| 5a | 290.32 | 0.60 | 7 | 2 | 7 | 89.28 | 0 |
| 5b | 322.37 | 2.56 | 6 | 1 | 6 | 69.05 | 0 |
| 5c | 342.79 | 2.92 | 6 | 1 | 5 | 69.05 | 0 |
| 5d | 434.24 | 3.32 | 6 | 1 | 5 | 69.05 | 0 |
| 5e | 324.34 | 1.76 | 7 | 2 | 5 | 89.28 | 0 |
| 5f | 352.35 | 2.15 | 8 | 2 | 6 | 106.35 | 0 |
| 6a | 353.22 | 1.97 | 6 | 1 | 7 | 69.05 | 0 |
| 7a | 335.32 | 1.86 | 10 | 1 | 9 | 124.11 | 0 |
| APAP | 151.16 | 0.68 | 3 | 2 | 1 | 49.33 | 0 |
| APAP-ONO2 | 296.28 | 2.17 | 8 | 1 | 9 | 110.46 | 0 |
| Comp. | Mut b | Tum c | Irr d | Rep e | Drug-Likeness | Drug-Score |
|---|---|---|---|---|---|---|
| 5a |  |  |  |  | −0.75 | 0.62 |
| 5b |  |  |  |  | 1.72 | 0.78 |
| 5c |  |  |  |  | 1.06 | 0.70 |
| 5d |  |  |  |  | 0.65 | 0.59 |
| 5e |  |  |  |  | 0.46 | 0.72 |
| 5f |  |  |  |  | −0.52 | 0.59 |
| 6a |  |  |  |  | −8.16 | 0.12 |
| 7a |  |  |  |  | −0.71 | 0.59 |
| APAP |  |  |  |  | 1.93 | 0.20 |
| APAP-ONO2 |  |  |  |  | −8.25 | 0.45 |
 = No risk,
= No risk,  = Moderated risk,
= Moderated risk,  = High risk; b Mutagenicity: Induction of permanent transmissible changes in the structure of the genetic material of cells or organisms; c Tumorigenicity: The process by which neoplastic cells grown in tissue culture form tumors; d Irritant: Stimulus from the compound that causes irritation; e Reproductive effect: Adverse effect of compounds that interferes with the reproductive organs of an organism, such as genitalia and gonads.
= High risk; b Mutagenicity: Induction of permanent transmissible changes in the structure of the genetic material of cells or organisms; c Tumorigenicity: The process by which neoplastic cells grown in tissue culture form tumors; d Irritant: Stimulus from the compound that causes irritation; e Reproductive effect: Adverse effect of compounds that interferes with the reproductive organs of an organism, such as genitalia and gonads.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouznetsov, V.V.; Calderón Lamus, D.; Puerto Galvis, C.E. Synthesis and Characterization of New Functionalized 1,2,3-Triazole-Based Acetaminophen Derivatives via Click Chemistry from Expired Commercial Acetaminophen Tablets. Reactions 2023, 4, 329-341. https://doi.org/10.3390/reactions4030020
Kouznetsov VV, Calderón Lamus D, Puerto Galvis CE. Synthesis and Characterization of New Functionalized 1,2,3-Triazole-Based Acetaminophen Derivatives via Click Chemistry from Expired Commercial Acetaminophen Tablets. Reactions. 2023; 4(3):329-341. https://doi.org/10.3390/reactions4030020
Chicago/Turabian StyleKouznetsov, Vladimir V., Daniela Calderón Lamus, and Carlos E. Puerto Galvis. 2023. "Synthesis and Characterization of New Functionalized 1,2,3-Triazole-Based Acetaminophen Derivatives via Click Chemistry from Expired Commercial Acetaminophen Tablets" Reactions 4, no. 3: 329-341. https://doi.org/10.3390/reactions4030020
APA StyleKouznetsov, V. V., Calderón Lamus, D., & Puerto Galvis, C. E. (2023). Synthesis and Characterization of New Functionalized 1,2,3-Triazole-Based Acetaminophen Derivatives via Click Chemistry from Expired Commercial Acetaminophen Tablets. Reactions, 4(3), 329-341. https://doi.org/10.3390/reactions4030020






