The Formation of Small Amounts of Cyclopropane during Pulsed Pyrolysis of C4–C5 Acyclic Alkanes in the Adiabatic Compression Reactor
Abstract
:1. Introduction
2. Materials and Methods
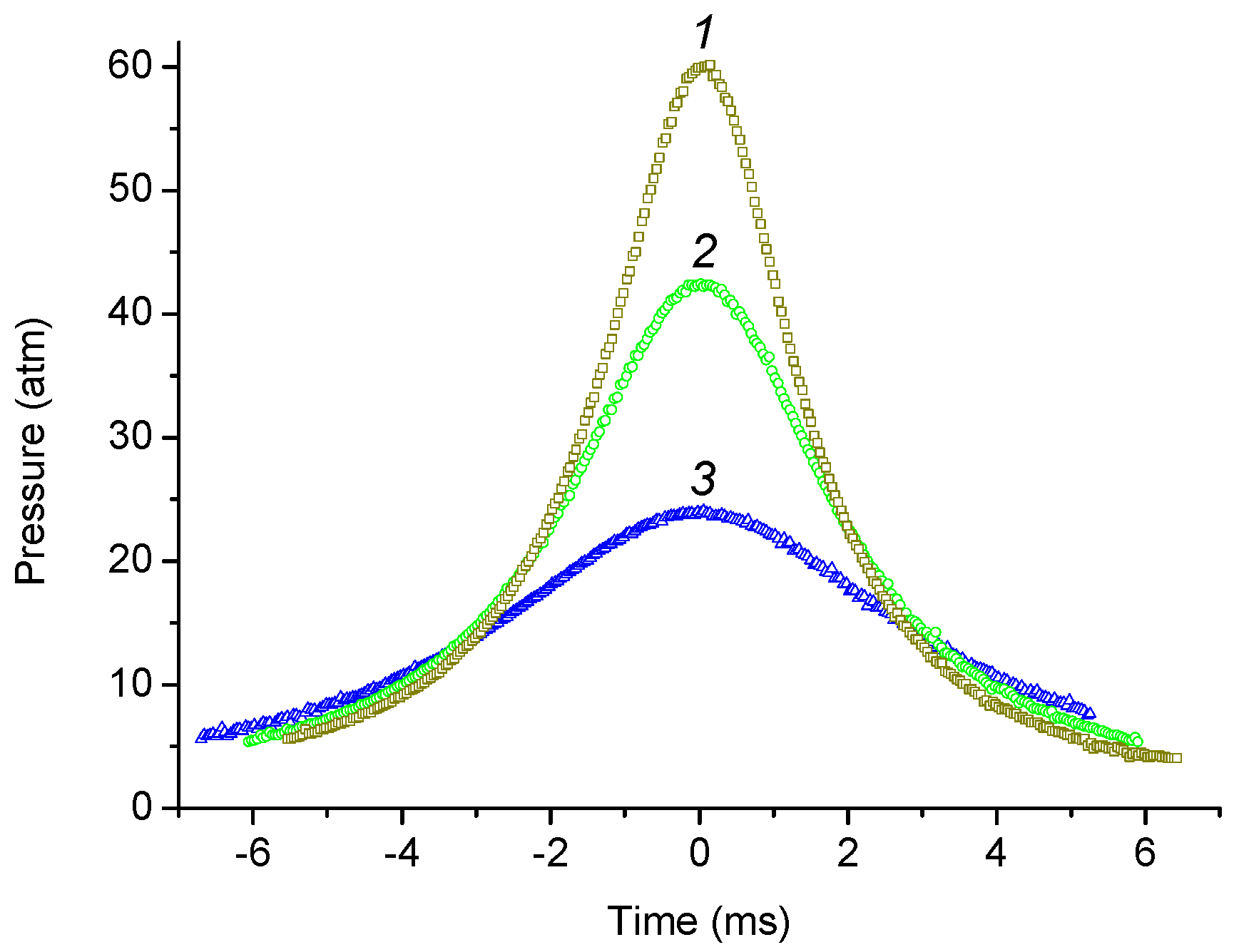
2.1. Adiabatic Compression of Gases with Free Flying Piston
2.2. Experimental Setup
2.3. Materials
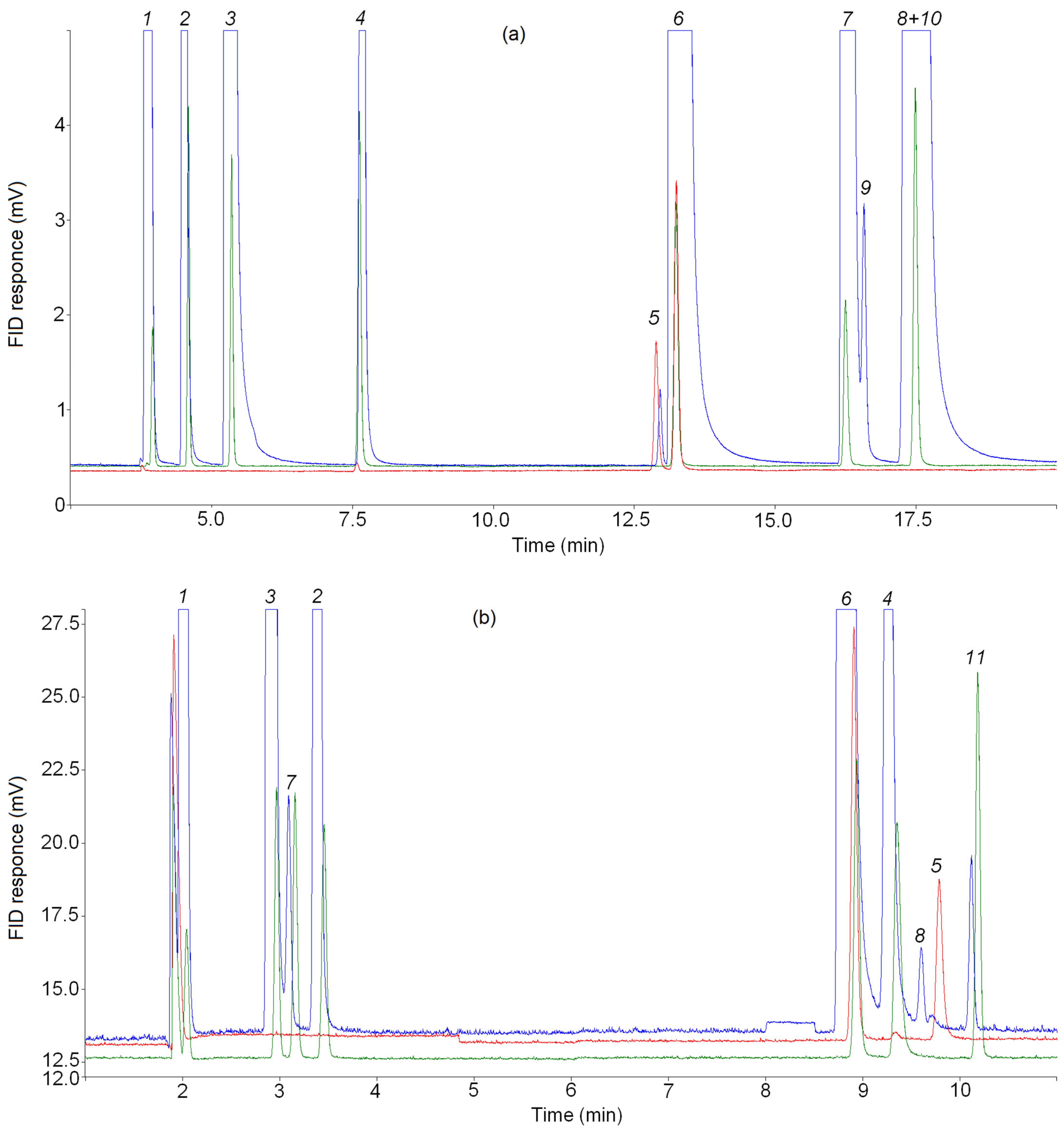
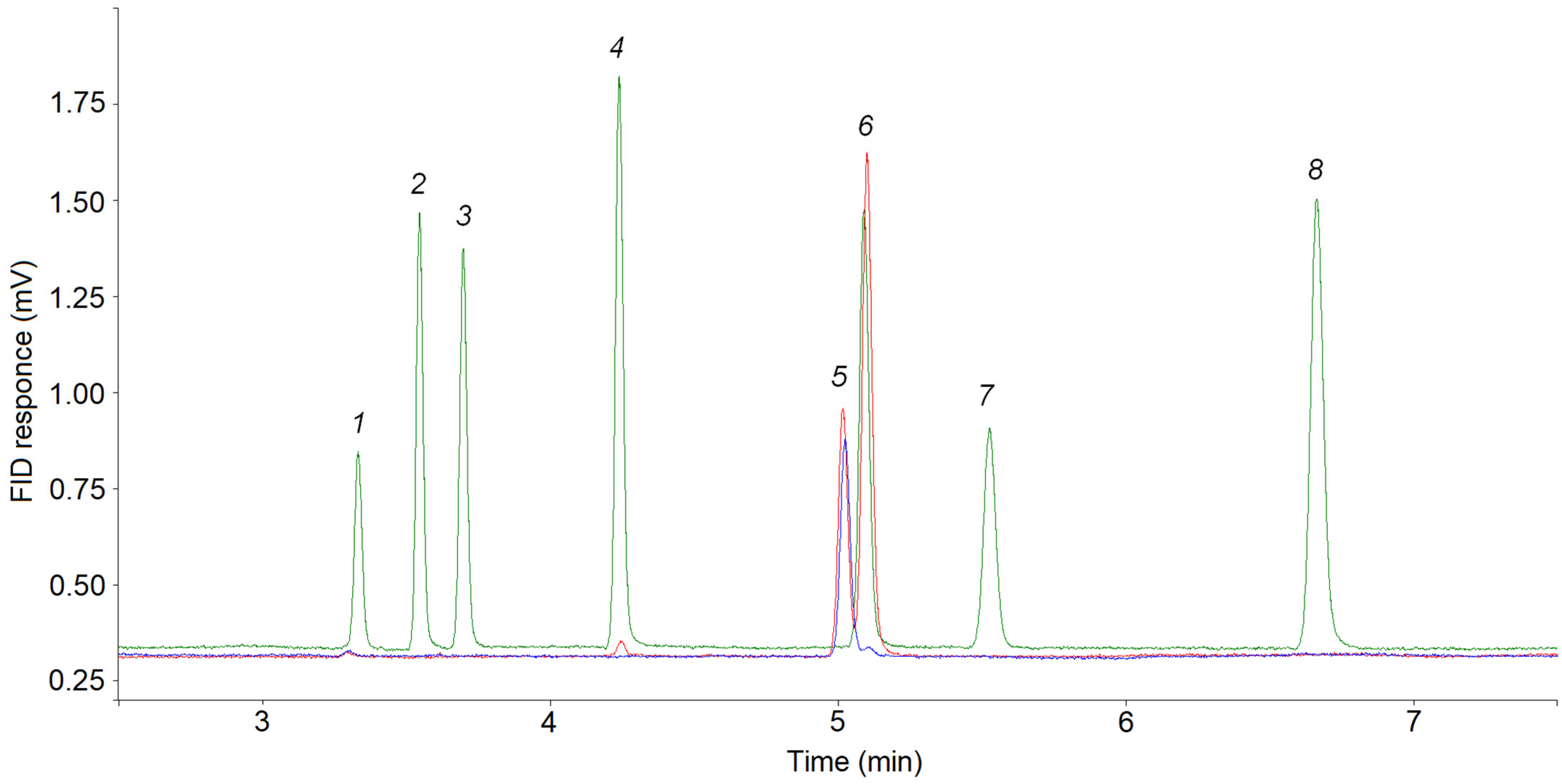
2.4. GC Analysis
3. Results and Discussion
3.1. Pressure–Time History and Calculation of Tmax
3.2. Choosing of Temperature Programmes for GC Analysis
3.3. Cyclopropane Content in a Mixture of Pyrolysis Products of C4–C5 Alkanes
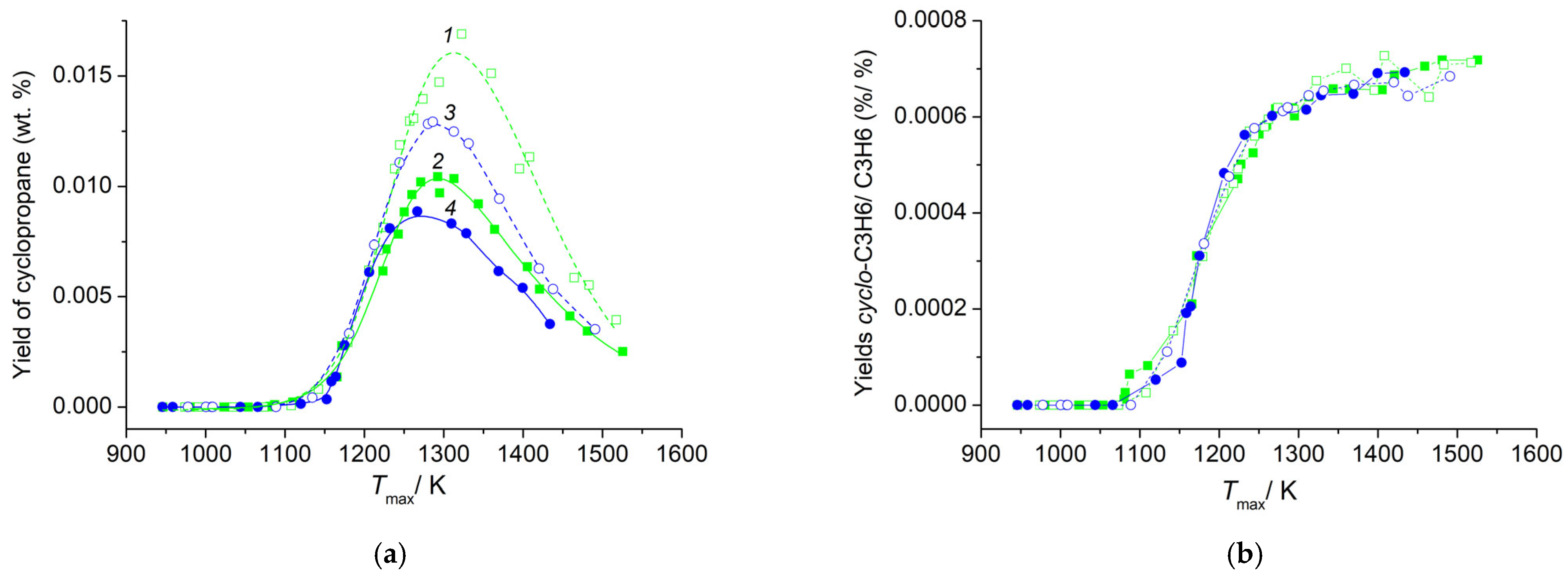

4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Longwell, P.A.; Reamer, H.H.; Wilburn, N.P.; Sage, B.H. Ballistic piston for investigating gas phase reactions. Ind. Eng. Chem. 1958, 50, 603–610. [Google Scholar] [CrossRef]
- Markevich, A.M.; Azatyan, V.V.; Sokolova, N.A. Adiabatic compression as a method for studying chemical processes under nonstationary conditions. Kinet. Catal. 1962, 3, 431–438. [Google Scholar]
- Volokhonovich, I.E.; Markevich, A.M.; Masterovoy, I.F.; Azatyan, V.V. Non-isothermal processes. Thermal cracking of methane. Dokl. USSR 1962, 146, 387–390. [Google Scholar]
- Kondratiev, V.N. Determination of the rate constant for thermal cracking of methane by means of adiabatic compression and expansion. Symp. Int. Combust. 1965, 10, 319–322. [Google Scholar] [CrossRef]
- Buravtsev, N.N.; Grigor’ev, A.S.; Kolbanovskii, Y.A. Kinetics of the cyclodimerization of tetrafluoroethylene and the thermal decomposition of octafluorocyclobutane. Kinet. Catal. 1985, 26, 1–7. [Google Scholar]
- Buravtsev, N.N.; Grigor’ev, A.S.; Kolbanovskii, Y.A. Kinetics and mechanism of pyrolysis of hexafluoropropylene. Kinet. Catal. 1989, 30, 13–22. [Google Scholar]
- Kuptsova, T.S.; Buravtsev, N.N.; Chernyak, N.Y.; Shapiro, E.A.; Kolbanovskii, Y.u.A.; Nefedov, O.M. Thermal isomerization of 3,3-dimethylcyclopropene under adiabatic-compression conditions. Russ. Chem. Bull. 1989, 38, 1845–1847. [Google Scholar] [CrossRef]
- Kolbanovskiy, Y.A. Adiabatic compression in studies on the kinetics and mechanism of reactions involving fluorine-containing carbenes. Russ. Chem. Rev. 1989, 58, 1024–1032. [Google Scholar] [CrossRef]
- Ryabinin, Y.N. Gases at High Densities and Temperatures; Pergamon Press, Inc.: New York, NY, USA, 1961; 52p. [Google Scholar]
- Lalos, G.T.; Hammond, G.L. The Ballistic Compression and High Temperature Properties of Dense Gases. In Experimental Thermodynamics, Volume II. Experimental Thermodynamics of Non-Reacting Fluids; Le Neindre, B., Vodar, B., Eds.; Springer Science + Business Media: New York, NY, USA, 1975; pp. 1193–1218. [Google Scholar]
- Kolbanovskiy, Y.A.; Shchipachev, V.S.; Chernyak, N.Y.; Chernyshova, A.S.; Grigor’ev, A.S. Impulsive Compression of Gases in Chemistry and Technology; Science: Moscow, Russia, 1982; 240p. (In Russian) [Google Scholar]
- Buravtsev, N.N.; German, L.S.; Grigor’ev, A.S.; Kolbanovskii, Y.A.; Ovsyannikov, A.A.; Volkonskii, A.Y. Trifluoromethylfluorocarbene Formation and Reactions under C2F5SiF3 Pulsed Adiabatic Compression Pyrolysis. Mendeleev Commun. 1993, 3, 133–134. [Google Scholar] [CrossRef]
- Slotboom, Y.; Roosjen, S.; Kronberg, A.; Glushenkov, M.; Kersten, S.R.A. Methane to ethylene by pulsed compression. Chem. Eng. J. 2021, 414, 128821. [Google Scholar] [CrossRef]
- Drost, S.; Schießl, R.; Maas, U. Multi compression–expansion process for chemical energy conversion: Transformation of methane to unsaturated hydrocarbons and hydrogen. Appl. Energy Combust. Sci. 2023, 14, 100129. [Google Scholar] [CrossRef]
- Porras, S.; Kaczmarek, D.; Herzler, J.; Drost, S.; Werler, M.; Kasper, T.; Fikri, M.; Schießl, R.; Atakan, B.; Schulz, C.; et al. An experimental and modeling study on the reactivity of extremely fuel-rich methane/dimethyl ether mixtures. Combust. Flame 2020, 212, 107–122. [Google Scholar] [CrossRef]
- Ezdin, B.S.; Vasiljev, S.A.; Yatsenko, D.A.; Fedorov, V.E.; Ivanova, M.N.; Kalyada, V.V.; Pakharukov, Y.V.; Shabiev, F.K.; Zarvin, A.E. The Synthesis of Carbon Nanoparticles in a Compression Reactor in the Atmosphere of Buffer Gases. Sib. J. Phys. 2022, 17, 29–46. [Google Scholar] [CrossRef]
- Rudolph, C.; Atakan, B. Pyrolysis of Methane and Ethane in a Compression–Expansion Process as a New Concept for Chemical Energy Storage: A Kinetic and Exergetic Investigation. Energy Technol. 2021, 9, 2000948. [Google Scholar] [CrossRef]
- Ashok, A.; Katebah, M.A.; Linke, P.; Kumar, D.; Arora, D.; Fischer, K.; Jacobs, T.; Al-Rawashdeh, M. Review of piston reactors for the production of chemicals. Rev. Chem. Eng. 2023, 39, 1–30. [Google Scholar] [CrossRef]
- Bilera, I.V.; Buravtsev, N.N. The homogeneous pyrolysis of isobutane under pulsed adiabatic compression. Gorenie Vzryv. Mosk.—Combust. Explos. 2013, 6, 37–40. [Google Scholar]
- Bilera, I.V. The homogeneous pyrolysis of n-butane under pulsed adiabatic compression. Gorenie Vzryv. Mosk.—Combust. Explos. 2014, 7, 35–41. [Google Scholar]
- Bilera, I.V. The homogeneous pyrolysis of n-pentane under pulsed adiabatic compression. Gorenie Vzryv. Mosk.—Combust. Explos. 2015, 8, 97–105. [Google Scholar]
- Bilera, I.V.; Buravtsev, N.N. The homogeneous pyrolysis of isopentane under pulsed adiabatic compression. Gorenie Vzryv. Mosk.—Combust. Explos. 2016, 9, 74–82. [Google Scholar]
- ScanView—Online eLibrary (Agilent Technologies). The Fully Updated 2018 Version Is Available and Is Hosted in Agilent Community. Available online: https://community.agilent.com (accessed on 16 October 2018).
- Allara, D.L.; Edelson, D. A Computational Modeling Study of the Low-Temperature Pyrolysis of n-Alkanes; Mechanisms of Propane, n-Butane, and n-Pentane Pyrolyses. Int. J. Chem. Kinet. 1975, 7, 479–507. [Google Scholar] [CrossRef]
- Safarik, I.; Strausz, O.P. The thermal decomposition of hydrocarbons. Part 1. n-alkanes (C ≥ 5). Res. Chem. Intermed. 1996, 22, 275–314. [Google Scholar] [CrossRef]
- Dente, M.; Bozzano, G.; Faravelli, T.; Marongiu, A.; Pierucci, S.; Ranzi, E. Kinetic Modelling of Pyrolysis Processes in Gas and Condensed Phase. Adv. Chem. Eng. 2007, 32, 51–166. [Google Scholar] [CrossRef]
- Hou, X.; Song, C.; Ma, Z.; Chen, B.; Zhao, L.; Huang, J.; Yuan, E.; Cui, T. Universality analysis of the reaction pathway and product distribution in C5–C10 n-alkanes pyrolysis. J. Anal. Appl. Pyrolysis 2022, 162, 105451. [Google Scholar] [CrossRef]
- Zamostny, P.; Belohlav, Z.; Starkbaumova, L.; Patera, J. Experimental study of hydrocarbon structure effects on the composition of its pyrolysis products. J. Anal. Appl. Pyrolysis 2010, 87, 207–216. [Google Scholar] [CrossRef]
- Yanovskaya, L.A.; Dombrovskii, V.A. Functionally Substituted Cyclopropanes. Russ. Chem. Rev. 1975, 44, 154–169. [Google Scholar] [CrossRef]
- Lebel, H.; Marcoux, J.-F.; Molinaro, C.; Charette, A.B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. [Google Scholar] [CrossRef]
- Vogel, E. Kleine Kohlenstoff-Ringe. Angew. Chem. 1960, 72, 4–26. [Google Scholar] [CrossRef]
- Engel, P.S. Mechanism of the thermal and photochemical decomposition of azoalkanes. Chem. Rev. 1980, 80, 99–150. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeeva, E.O.; Vereshchagin, A.N.; Nikishin, G.I. Electrochemical synthesis of cyclopropanes. Russ. Chem. Rev. 2015, 84, 485–497. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef]
- Lott, W.A.; Christiansen, W.G. The preparation of cyclopropane. J. Am. Pharm. Assoc. 1930, 19, 341–344. [Google Scholar] [CrossRef]
- Setser, D.W.; Rabinovitch, B.S. Singlet methylene from thermal decomposition of diazomethane. Unimolecular reactions of chemically activated cyclopropane and dimethylcyclopropane molecules. Can. J. Chem. 1962, 40, 1425–1451. [Google Scholar] [CrossRef]
- Setser, D.W.; Rabinovitch, B.S.; Simons, J.W. Collisional Transition Probabilities for Deactivation of Highly Vibrationally Excited Cyclopropane and Dimethylcyclopropane. J. Chem. Phys. 1964, 40, 1751–1761. [Google Scholar] [CrossRef]
- Crawford, R.J.; Mishra, A. The Mechanism of the Thermal Decomposition of 1-Pyrazolines and Its Relationship to Cyclopropane Isomerizations. J. Am. Chem. Soc. 1966, 88, 3963–3969. [Google Scholar] [CrossRef]
- Campbell, R.J.; Schlag, E.W.; Ristow, B.W. Mechanistic consequences of curved Stern–Volmer plots. The photolysis of cyclobutanone. J. Am. Chem. Soc. 1967, 89, 5098–5102. [Google Scholar] [CrossRef]
- Blades, A.T. Kinetics sf the thermal decomposition of cyclobutanone. Can. J. Chem. 1969, 47, 615–617. [Google Scholar] [CrossRef]
- Tsang, W. Thermal decomposition of cyclopentane and related compounds. Int. J. Chem. Kinet. 1978, 10, 599–617. [Google Scholar] [CrossRef]
- Gusel’nikov, L.E.; Volkova, V.V.; Ivanov, P.E.; Inyushkin, S.V.; Shevelkova, L.V.; Zimmermann, G.; Ziegler, U.; Ondruschka, B. Direct infrared spectroscopic evidence of allyl radical and definition of the initiation step in thermal decomposition of cycloalkanes. A comparison with very low pressure pyrolysis of alkenes. J. Anal. Appl. Pyrolysis 1991, 21, 79–93. [Google Scholar] [CrossRef]
- Tsang, W. Thermal stability of cyclohexane and 1-hexene. Int. J. Chem. Kinet. 1978, 10, 1119–1138. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Z.; Yuan, W.; Cai, J.; Zhang, L.; Zhang, F.; Qi, F.; Wang, J. An experimental and kinetic modeling study of cyclohexane pyrolysis at low pressure. Combust. Flame 2012, 159, 2243–2253. [Google Scholar] [CrossRef] [Green Version]
- Awan, I.A.; McGivern, W.S.; Tsang, W.; Manion, J.A. Decomposition and isomerization of 5-methylhex-1-yl radical. J. Phys. Chem. A 2010, 114, 7832–7846. [Google Scholar] [CrossRef]
- Awan, I.A.; Burgess, D.R., Jr.; Manion, J.A. Pressure Dependence and Branching Ratios in the Decomposition of 1-Pentyl Radicals: Shock Tube Experiments and Master Equation Modeling. J. Phys. Chem. A 2012, 116, 2895–2910. [Google Scholar] [CrossRef]
- Awan, I.A.; Burgess, D.R., Jr.; Tsang, W.; Manion, J.A. Shock tube study of the decomposition of cyclopentyl radicals. Proc. Combust. Inst. 2011, 33, 341–349. [Google Scholar] [CrossRef]
- Manion, J.A.; Awan, I.A. A Shock tube study of H atom addition to cyclopentene. Int. J. Chem. Kinet. 2018, 50, 225–242. [Google Scholar] [CrossRef]
- Markus, M.W.; Woiki, D.; Roth, P. Two-channel thermal decomposition of CH3. Proc. Combust. Inst. 1992, 24, 581–588. [Google Scholar] [CrossRef]
- Saldana, M.H.; Bogin, G.E., Jr. Investigation of n-pentane pyrolysis at elevated temperatures and pressures in a variable pressure flow reactor. J. Anal. Appl. Pyrolysis 2016, 118, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-J.; Yuan, W.-H.; Cai, J.-H.; Zhang, L.-D.; Qi, F.; Li, Y.-Y. Product identification and mass spectrometric analysis of n-butane and i-butane pyrolysis at low pressure. Chin. J. Chem. Phys. 2013, 26, 151–156. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Li, Y.; Li, T.; Zhang, Y.; Cao, C.; Zou, J.; Law, C.K. Experimental and kinetic modeling investigation on pyrolysis and combustion of n-butane and i-butane at various pressures. Combust. Flame 2018, 191, 126–141. [Google Scholar] [CrossRef]
- Goos, E.; Hippler, H.; Hoyermann, K.; Jürges, B. Laser powered homogeneous pyrolysis of butane initiated by methyl radicals in a quasi-wall-free reactor at 750–1000 K. Phys. Chem. Chem. Phys. 2000, 2, 5127–5132. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hellier, P.; Ladommatos, N.; Almaleki, A. Sampling of Gas-Phase Intermediate Pyrolytic Species at Various Temperatures and Residence Times during Pyrolysis of Methane, Ethane, and Butane in a High-Temperature Flow Reactor. Sustainability 2023, 15, 6183. [Google Scholar] [CrossRef]
- Sirjean, B.; Glaude, P.A.; Ruiz-Lopez, M.F.; Fournet, R. Detailed kinetic study of the ring opening of cycloalkanes by CBS-QB3 calculations. J. Phys. Chem. A 2006, 110, 12693–12704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkidas, A.C.; Plett, E.G.; Summerfieldt, M. Performance study of a ballistic compressor. AIAA J. 1976, 14, 1752–1758. [Google Scholar] [CrossRef]
- Price, D.; Lalos, G.T. Adiabatic compressor for P-V-T measurements on gases to 100,000 pounds per square inch. Ind. Eng. Chem. 1957, 49, 1987–1992. [Google Scholar] [CrossRef]
- Castello, G.; Vezzani, S.; Gardella, L. Influence of temperature on the polarity of porous polymer beads stationary phases for gas chromatography. J. Chromatogr. A 1999, 837, 153–170. [Google Scholar] [CrossRef]
- Ji, Z.; Majors, R.E.; Guthrie, E.J. Porous layer open-tubular capillary columns: Preparations, applications and future directions. J. Chromatogr. A 1999, 842, 115–142. [Google Scholar] [CrossRef]
- Grob, R.L.; Kaiser, M.A. Qualitative and Quantitative Analysis by Gas Chromatography. In Modern Practice of Gas Chromatography, 4th ed.; Grob, R.L., Barry, E.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 403–460. [Google Scholar]
- Do, L.; Raulin, F. Gas chromatography of Titan’s atmosphere. III. Analysis of low-molecular-weight hydrocarbons and nitriles with a CP-Sil5 CB WCOT capillary column. J. Chromatogr. 1992, 591, 297–301. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Reid, G.L.; Luong, J. Gas Separations: A Comparison of GasPro™ and Aluminum Oxide PLOT Columns for the Separation of Highly Volatile Compounds. Curr. Sep. 1996, 15, 5–11. Available online: http://www.currentseparations.com/issues/15-1/cs15-1a.pdf (accessed on 16 March 2023).
- Ji, Z.; Chang, I. A New Look at Light Hydrocarbon Separations on Commercial Alumina PLOT Columns: Column Selectivity and Separation. J. High Resolut. Chromatogr. 1996, 19, 32–36. [Google Scholar] [CrossRef]
- Schneider, W.; Frone, J.; Bruderreck, H. Determination of hydrocarbons in the parts per 109 range using glass capillary columns coated with aluminium oxide. J. Chromatogr. 1978, 155, 311–327. [Google Scholar] [CrossRef]
- De Nijs, R.C.M. Analysis of Light Hydrocarbons C1-C5 with Porous Layer Open Tubular Fused Silica Columns of Aluminum Oxide. J. High Resolut. Chromatogr. 1981, 4, 612–615. [Google Scholar] [CrossRef]
- De Nijs, R.C.M.; De Zeeuw, J. Aluminium oxide-coated fused-silica porous-layer open-tubular column for gas-solid chromatography of C1-C10 hydrocarbons. J. Chromatogr. 1983, 279, 41–48. [Google Scholar] [CrossRef]
- De Zeeuw, J.; De Nijs, R.C.M.; Henrich, L.T. Adsorption Chromatography on PLOT (Porous-Layer Open-Tubular) Columns: A New Look at the Future of Capillary GC. J. Chromatogr. Sci. 1987, 25, 71–83. [Google Scholar] [CrossRef]
- Henrich, L.H. Recent Advances in Adsorption Chromatography for Analysis of Light Hydrocarbons in Petrochemical-Related Materials. J. Chromatogr. Sci. 1988, 26, 198–205. [Google Scholar] [CrossRef]
- Schaeffer, H.-J. Gas Chromatographic Analysis of Traces of Light Hydrocarbons. A Review of Different Systems in Practice. J. High Resolut. Chromatogr. 1989, 12, 69–81. [Google Scholar] [CrossRef]
- De Zeeuw, J.; Luong, J. Developments in stationary phase technology for gas chromatography. Trends Anal. Chem. 2002, 21, 594–607. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Tran, Q.; Fellin, P.; Cheng, W.K.; Drummond, I. Determination of C1–C4 hydrocarbons in air. Anal. Chem. 1993, 65, 1932–1935. [Google Scholar] [CrossRef]
- Do, L.; Raulin, F. Gas chromatography of Titan’s atmosphere. I. Analysis of low-molecular-weight hydrocarbons and nitriles with a PoraPLOT Q porous polymer coated open-tubular capillary column. J. Chromatogr. 1989, 481, 45–54. [Google Scholar] [CrossRef]
- Ji, Z.; Hutt, S. A New Bonded Porous Polymer PLOT U Column with Increased Polarity. J. Chromatogr. Sci. 2000, 38, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Z.; Liu, H.J. Preparation of 4-vinylpyridine and divinylbenzene porous-layer open tubular columns by in situ copolymerization. J. Chromatogr. 1995, 693, 79–88. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, C. Recent update of the various sources originating ghost peaks in gas chromatography: A review. J. Chromatogr. A 2022, 1685, 463625. [Google Scholar] [CrossRef]
- Battin-Leclerc, F. Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates. Progr. Energy Combust. Sci. 2008, 34, 440–498. [Google Scholar] [CrossRef] [Green Version]
- Vlasov, P.A.; Garmash, A.A.; Tereza, A.M. Ignition of Cyclopropane in Shock Waves. Russ. J. Phys. Chem. B 2016, 10, 602–614. [Google Scholar] [CrossRef]
- Wang, R.; Xu, P.; Tang, W.; Ding, T.; Zhang, C.; Li, X. An experimental and kinetic study of the ignition delay characteristics of cyclopropane in air. Fuel 2022, 319, 123784. [Google Scholar] [CrossRef]



| GC Column | Application No. |
|---|---|
| CP-Al2O3/KCl PLOT | A00155, A00677, A01099, A01123, A01124, A01545 |
| CP-PoraBOND Q | A01454 |
| CP-PoraPLOT U | A01456, CA000C1128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilera, I.V. The Formation of Small Amounts of Cyclopropane during Pulsed Pyrolysis of C4–C5 Acyclic Alkanes in the Adiabatic Compression Reactor. Reactions 2023, 4, 381-397. https://doi.org/10.3390/reactions4030023
Bilera IV. The Formation of Small Amounts of Cyclopropane during Pulsed Pyrolysis of C4–C5 Acyclic Alkanes in the Adiabatic Compression Reactor. Reactions. 2023; 4(3):381-397. https://doi.org/10.3390/reactions4030023
Chicago/Turabian StyleBilera, Igor V. 2023. "The Formation of Small Amounts of Cyclopropane during Pulsed Pyrolysis of C4–C5 Acyclic Alkanes in the Adiabatic Compression Reactor" Reactions 4, no. 3: 381-397. https://doi.org/10.3390/reactions4030023





