Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts
Abstract
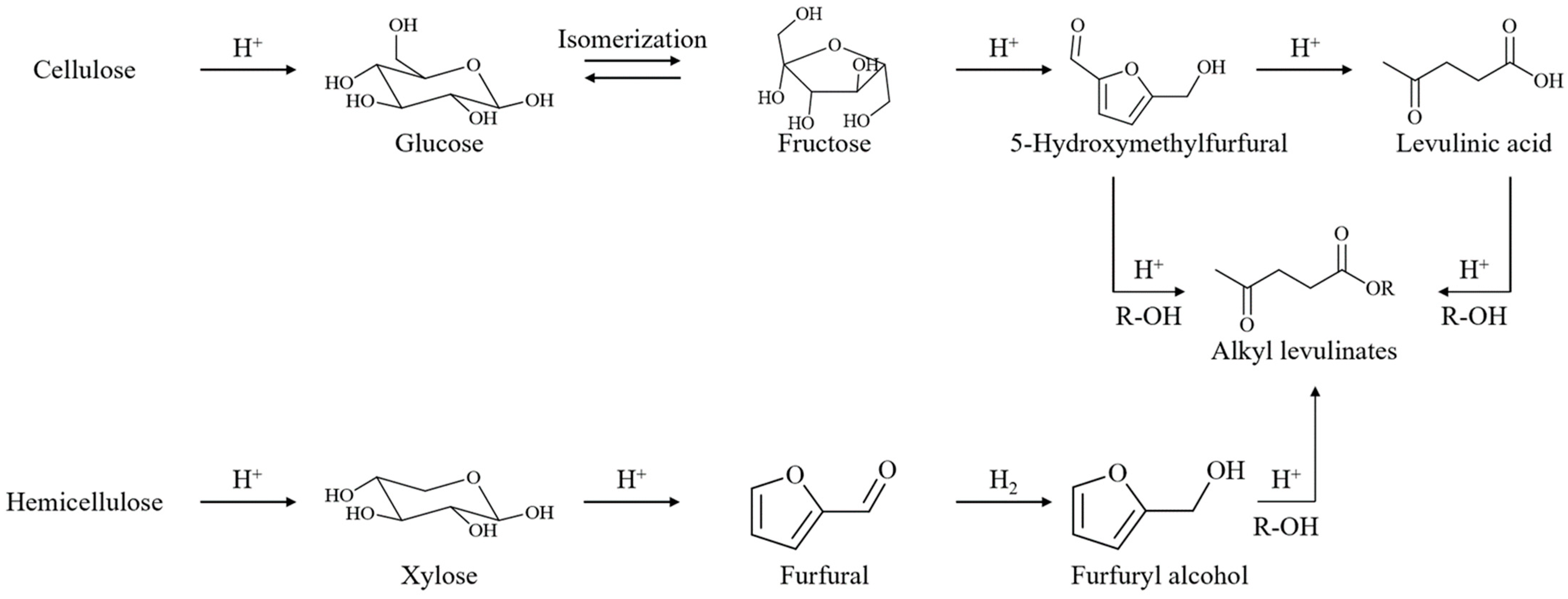
:1. Introduction
2. Heterogeneous Catalyst-Driven Conversion of Biomass-Derived Molecules into Alkyl Levulinates
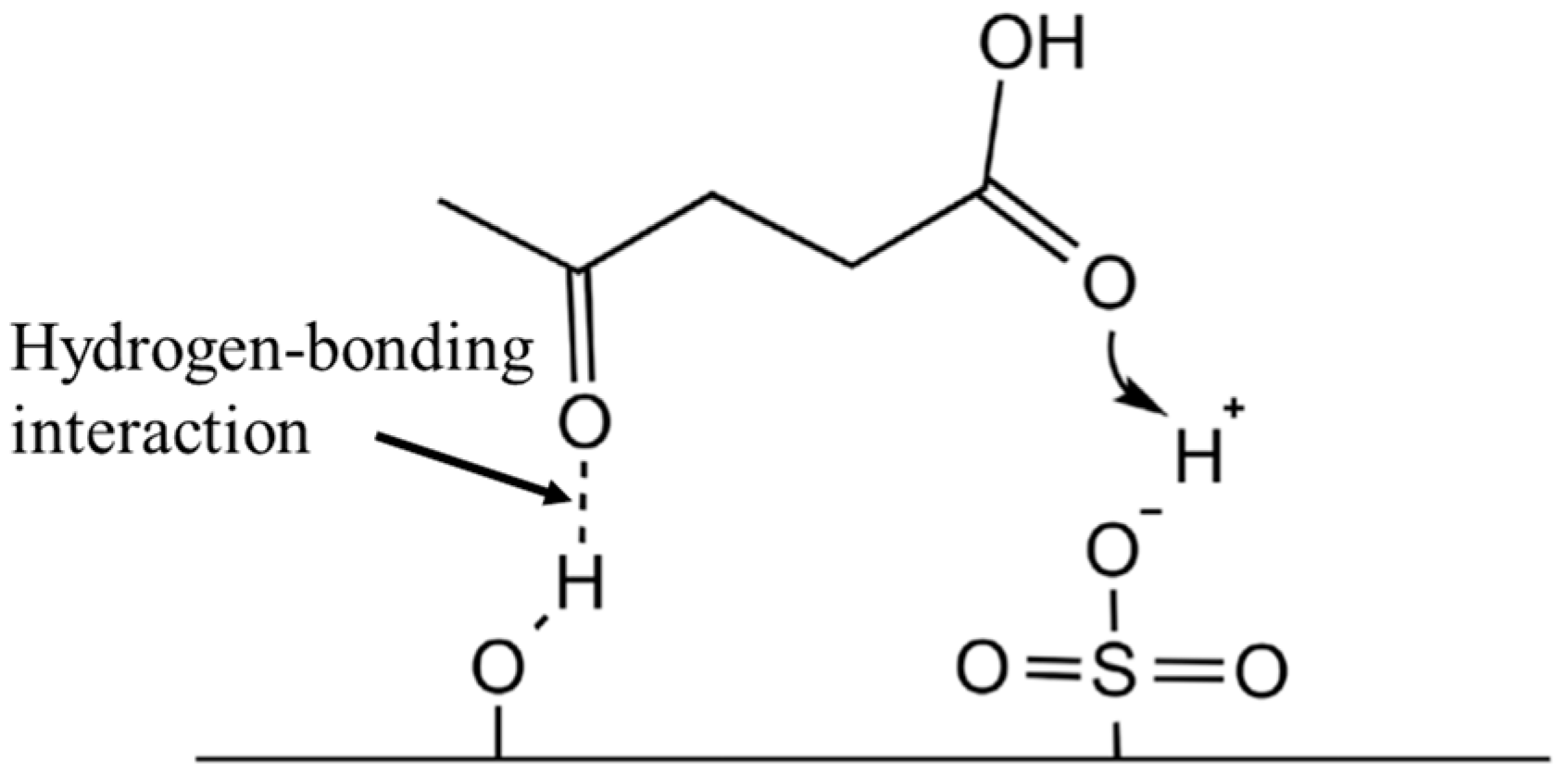
2.1. Levulinic Acid
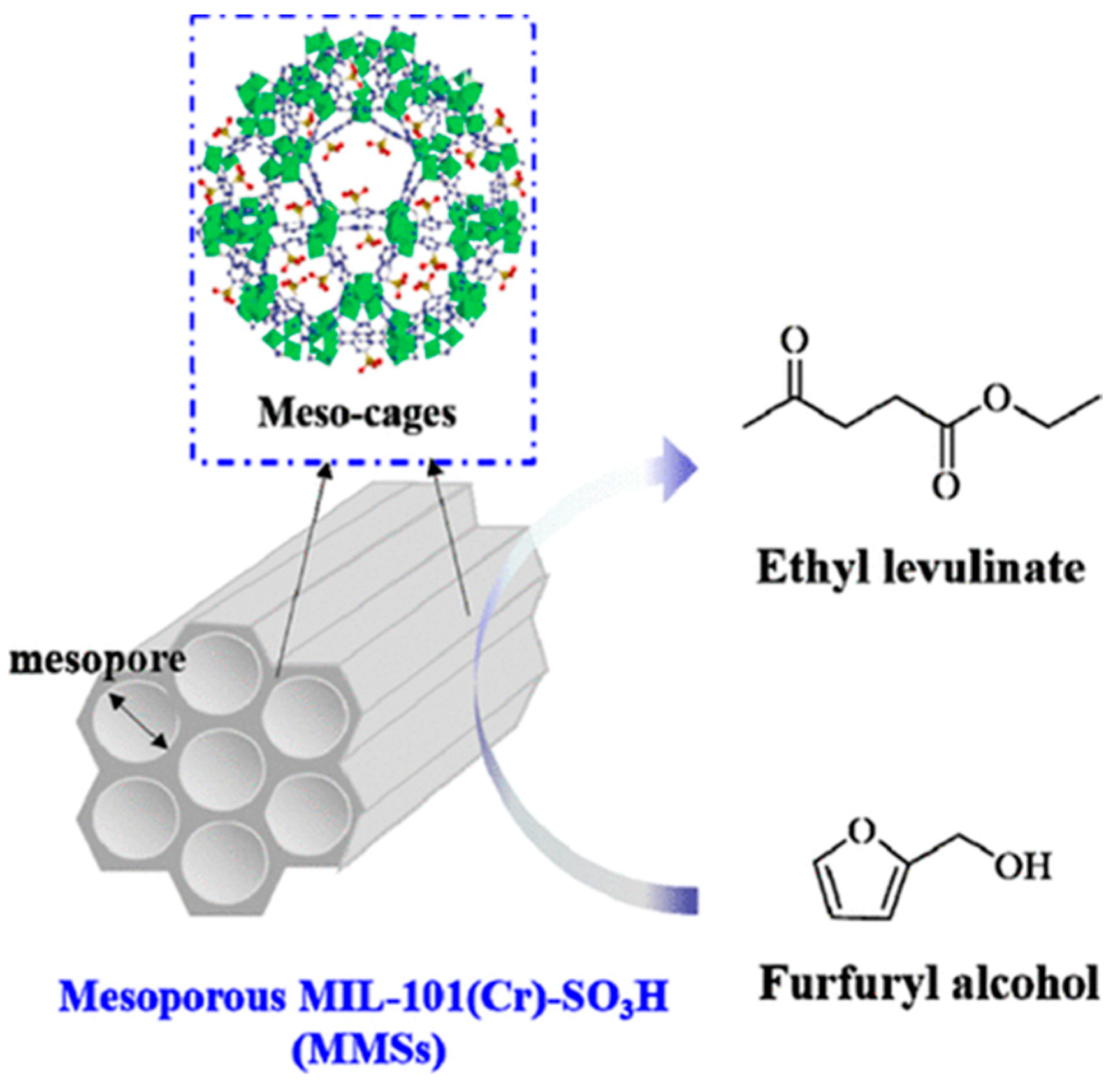
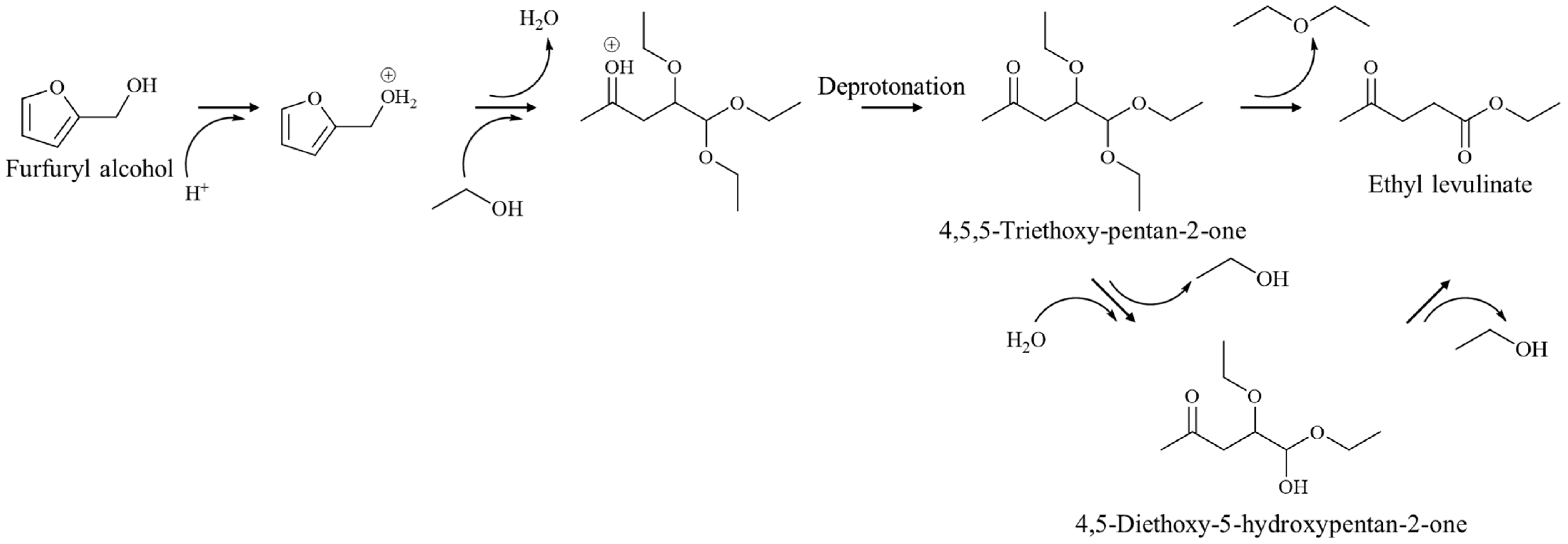
2.2. Furfuryl Alcohol
| Entry | Catalyst | Temp. (°C) | Time (h) | Alkyl Group | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | H-ZSM-5-50 | 150 | 5 min | Me | 60 | [31] |
| 2 | Purolite CT151 | 80–120 | 5 | Me to sec-Bu | 30–71 | [8] |
| 3 | α-Fe2O3 | 250 | 40 min–80 min | Me to n-Bu | 73–86 | [32] |
| 4 | GCC | 150 | 1 | Et | 67.1 | [33] |
| 5 | TPA/SBA-16 | 110 | 3 | Me to n-Bu | 8–97 | [20] |
| 6 | [(HSO3-p)2im][HSO4] | 110 | 2 | Me to n-Pe | 80–95 | [35] |
| 7 | [BMIm-SH][HSO4] | 130 | 2 | Me to n-Bu | 68–99 | [36] |
| 8 | Al-TUD-1 | 140 | 24 | Et | 80 | [37] |
| 9 | SBA-15-SO3H | 110 | 4 | n-Bu | 96 | [38] |
| 10 | MMS(0.3)-0.15 | 160 | 2 | Et | 83.8 | [39] |
| 11 | Graphene oxide | 120 | 6 | Me to n-Hex | 78.2–95.5 | [40] |
| 12 | γ-Fe3O4/H-ZSM5 | 130 | 8 | sec-Bu | Not available | [41] |
2.3. Furfural
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Ding, H.; Zhu, J.; Liu, X.; Xu, Q.; Yin, D. Esterification of levulinic acid into n-butyl levulinate catalyzed by sulfonic acid-functionalized lignin-montmorillonite complex. J. Bioresour. Bioprod. 2020, 5, 291–299. [Google Scholar] [CrossRef]
- Yu, F.; Zhong, R.; Chong, H.; Smet, M.; Dehaen, W.; Sels, B.F. Fast catalytic conversion of recalcitrant cellulose into alkyl levulinates and levulinic acid in the presence of soluble and recoverable sulfonated hyperbranched poly(arylene oxindole)s. Green. Chem. 2017, 19, 153–163. [Google Scholar] [CrossRef]
- Yamanaka, N.; Abe, D.; Miwaka-Saiga, M.; Yasunaga, K.; Yamada, H.; Shimazu, S. One-pot two-step synthesis of alkyl levulinates directly from furfural by combining Ni3Sn2 alloy nanoparticles and montmorillonite K10. Sustain. Energy Fuels. 2022, 6, 5153–5159. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis of applications of alkyl levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, A.; Liu, F.; Zhang, J.; Xia, T.; Zeng, X.; Fan, W.; Zhang, Y. Catalytic conversion network for lignocellulosic biomass valorization: A panoramic view. Ind. Chem. Mater. 2023, 1, 188–206. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.-M. Levulinic acid biorefineries: New challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Song, Y.; Wu, L.; Gholizadeh, M.; Li, C.-Z. One-pot synthesis of levulinic acid/esters from C5 carbohydrates in a methanol medium. ACS Sustain. Chem. Eng. 2013, 1, 1593–1599. [Google Scholar] [CrossRef]
- Annatelli, M.; Trapasso, G.; Lena, L.; Aricò, F. Alkyl levulinates from furfuryl alcohol using CT151 Purolite as heterogeneous catalysts: Optimization, purification, and recycling. Sustain. Chem. 2021, 2, 493–505. [Google Scholar] [CrossRef]
- Belguendouz, M.N.E.H.; Gancedo, J.; Rapado, P.; Ursueguía, D.; Patiño, Y.; Faba, L.; Bahmani, A.; Díaz, E.; Ordóñez, S. Selective synthesis of g-valerolactone from levulinic and formic acid over ZnAl mixed oxide. Chem. Eng. J. 2021, 414, 128902. [Google Scholar] [CrossRef]
- Pasquale, G.; Vázquez, P.; Romanelli, G.; Baronetti, G. Catalytic upgrading of levulinic acid to ethyl levulinate using reusable silica-included Wells-Dawson heteropolyacid as catalyst. Catal. Commun. 2012, 18, 115–120. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, L.; Bai, J.; Lei, M.; Long, M.; Huang, K. Catalytic esterification of levulinic acid into the biofuel n-butyl levulinate over nanosized TiO2 particles. Nanomaterials 2022, 12, 3870. [Google Scholar] [CrossRef]
- Dharne, S.; Bokade, V.V. Esterification of levulinic acid to n-butyl levulinate over heteropolyacid supported on acid-treated clay. J. Nat. Gas Chem. 2011, 20, 18–24. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Li, B.; Zhang, H. Advances in the catalytic production of valuable levulinic acid derivatives. ChemCatChem 2012, 4, 1230–1237. [Google Scholar] [CrossRef]
- Rodiansono, R.; Astuti, M.D.; Hara, T.; Ichikuni, N.; Shimazu, S. Efficient hydrogenation of levulinic acid in water using a supported Ni-Sn alloy on aluminium hydroxide catalysts. Catal. Sci. Technol. 2016, 6, 2955–2961. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Zhang, L.; Zhang, S. Efficient production of n-butyl levulinate fuel additive from levulinic acid using amorphous carbon enriched with oxygenated groups. Catalysts 2018, 8, 14. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Fulignati, S.; Licursi, D. Direct alcoholysis of carbohydrate precursors and real cellulosic biomasses to alkyl levulinates: A critical review. Catalysts 2020, 10, 1221. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, Z.; Chen, X.; Lyu, Q.; Mei, J.; Han, L.; Liu, D.; Xiao, W. Understanding the mechanism of enhanced alcoholysis of biomass carbohydrates to alkyl levulinates over bifunctional catalysts: Does it resemble that in water? Green Chem. 2023, 25, 5222–5232. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. A review on catalytic synthesis of energy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process. Technol. 2020, 197, 106213. [Google Scholar] [CrossRef]
- Bernal, H.G.; Benito, P.; Rodríguez-Castellón, E.; Galletti, A.M.R.; Funaioli, T. Synthesis of isopropyl levulinate from furfural: Insights on a cascade production perspective. Appl. Catal. A 2019, 575, 111–119. [Google Scholar] [CrossRef]
- Sankar, E.S.; Reddy, K.S.; Jyothi, Y.; Raju, B.D.; Rao, K.S.R. Alcoholysis of furfuryl alcohol into n-butyl levulinate over SBA-16 supported heteropoly acid catalyst. Catal. Lett. 2017, 147, 2807–2816. [Google Scholar] [CrossRef]
- Zhu, S.; Cen, Y.; Guo, J.; Chai, J.; Wang, J.; Fan, W. One-pot conversion of furfural to alkyl levulinates over bifunctional Au-H4SiW12O40/ZrO2 without external H2. Green. Chem. 2016, 18, 5667–5675. [Google Scholar] [CrossRef]
- Ehrenthal, I.; Montgomery, R.; Smith, F. The carbohydrates of Gramineae. II. The constitution of the hemicelluloses of wheat straw and corn cobs. J. Am. Chem. Soc. 1954, 76, 5509–5514. [Google Scholar] [CrossRef]
- Soleimani, M.; Tabil, L.G.; Panigrahi, S. A kinetic study of xylose recovery from a hemicellulose-rich biomass for xylitol fermentative production. Chem. Eng. Commun. 2018, 206, 193–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, S.; Chen, J.-M.; Hammer, S.K.; Luttinger, J.; Yang, L.; Jin, Y.-S.; Avalos, J.L. Xylose utilization stimulates mitochondrial production of isobutanol and 2-methyl-1-butanol in Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Garedew, M.; Lin, F.; Song, B.; DeWinter, T.M.; Jackson, J.E.; Saffron, C.M.; Lam, C.H.; Anastas, P.T. Greener routes to biomass waste valorization: Lignin transformation through electrocatalysis for renewable chemicals and fuels production. ChemSusChem 2020, 13, 4214–4237. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, J.; Diehl, B.N.; Kilgore, K.; Lomenzo, S.A.; Trudell, M.L. Halloysite-catalyzed esterification of bio-mass derived acids. ACS Omega 2019, 4, 19437–19441. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Ma, L.; Song, D.; Zhang, X.; Guo, Y. Design of a highly ordered mesoporous H3PW12O40/ZrO2-Si(Ph)Si hybrid catalyst for methyl levulinate synthesis. Green. Chem. 2013, 15, 885–890. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Sonar, S.K.; Niphadkar, P.S.; Joshi, P.N.; Deshpande, S.S.; Patil, V.S.; Bokade, V.V. Catalytic upgrading of renewable levulinic acid to ethyl levulinate biodiesel dodecatungstophosphoric acid supported on desilicated H-ZSM-5 as catalyst. Appl. Catal. A 2013, 460–461, 90–98. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Zhao, D.; Prinsen, P.; Wang, Y.; Ouyang, W.; Delbecq, F.; Len, C.; Luque, R. Continuous flow alcoholysis of furfuryl alcohol to alkyl levulinates using zeolites. ACS Sustain. Chem. Eng. 2018, 6, 6901–6909. [Google Scholar] [CrossRef]
- Ren, D.; Fu, J.; Li, L.; Liu, Y.; Jin, F.; Huo, Z. Efficient conversion of biomass-derived furfuryl alcohol to levulinate esters over commercial a-Fe2O3. RSC Adv. 2016, 6, 22174–22178. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, M.; Xia, X.; Li, L.; Xu, B. Conversion of furfuryl alcohol into ethyl levulinate over glucose-derived carbon-based solid acid in ethanol. Molecules 2019, 24, 1881. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Peng, L.; Li, H.; Chen, K. Formation of humin and alkyl levulinate in the acid-catalyzed conversion of biomass-derived furfuryl alcohol. BioResources 2015, 10, 6548–6564. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Song, L. Efficient and selective alcoholysis of furfuryl alcohol to alkyl levulinates catalyzed by double SO3H-functionalized ionic liquids. Green. Chem. 2014, 16, 1436–1443. [Google Scholar] [CrossRef]
- Hengne, A.M.; Kamble, S.B.; Rode, C.V. Single one-pot conversion of furfuryl alcohol to levulinic esters and g-valerolactone in the presence of sulfonic acid functionalized ILs and metal catalysts. Green. Chem. 2013, 15, 2540–2547. [Google Scholar] [CrossRef]
- Neves, P.; Lima, S.; Pillinger, M.; Rocha, S.M.; Rocha, J.; Valente, A.A. Conversion of furfuryl alcohol to ethyl levulinate using porous aluminosilicate acid catalysts. Catal. Today 2013, 218–219, 76–84. [Google Scholar] [CrossRef]
- Carà, P.D.; Ciriminna, R.; Shiju, N.R.; Rothenberg, G.; Pagliaro, M. Enhanced heterogeneous catalytic conversion of furfuryl alcohol into butyl levulinate. ChemSusChem 2014, 7, 835–840. [Google Scholar] [CrossRef]
- Liu, X.; Pan, H.; Zhang, H.; Li, H.; Yang, S. Efficient catalytic upgradation of bio-based furfuryl alcohol to ethyl levulinate using mesoporous acidic MIL-101(Cr). ACS Omega 2019, 4, 8390–8399. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, C.; Xue, Y.; Wu, J.; Wang, J.; Fan, W. Graphene oxide: An efficient catalyst for alcoholysis and esterification reactions. ChemCatChem 2014, 6, 3080–3083. [Google Scholar] [CrossRef]
- Lima, T.M.; Lima, C.G.S.; Rathi, A.K.; Gawande, M.B.; Tucek, J.; Urquieta-González, E.A.; Zbořil, R.; Paixão, M.W.; Varma, R.S. Magnetic ZSM-5 zeolite: A selective catalyst for the valorization of furfuryl alcohol to g-valerolactone, alkyl levulinates or levulinic acid. Green. Chem. 2016, 18, 5586–5593. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Yu, X.; Li, H.; Zhang, J.; He, L. Facile and high-yield synthesis of alkyl levulinate directly from furfural by combining Zr-MCM-41 and Amberlyst-15 without external H2. Energy Fuels 2019, 33, 330–339. [Google Scholar] [CrossRef]
- Tang, K.; Xie, S.; Cofield, G.R.; Yang, X.; Tian, E.; Lin, H. Catalytic transfer hydrogenation of furfural for the production of ethyl levulinate: Interplay of Lewis and Brønsted acidities. Energy Technol. 2018, 6, 1826–1831. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Lu, T.; Yuan, Y.; Yuan, G. Integrated catalytic process to directly convert furfural to levulinate ester with high selectivity. ChemSusChem 2014, 7, 202–209. [Google Scholar] [CrossRef] [PubMed]


| Entry | Catalyst | Temp. (°C) | Time (h) | Alkyl Group | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Halloysite | 170 | 24 | Me to n-Bu | 97–99 | [27] |
| 2 | LMT-SO3H | 120 | 4 | n-Pr to n-Hep | 9.5–99.3 | [1] |
| 3 | DTPA/K10 | 120 | 4 | n-Bu | 97 | [12] |
| 4 | 40WD-S | 78 | 10 | Et | 76 | [10] |
| 5 | H3PW12O40/ZrO2-Si(Ph)Si | 65 | 3 | Me | 99.9 | [28] |
| 6 | TiO2 | 120 | 8 | n-Bu | 87.5 | [11] |
| 7 | DTPA/DH-ZSM-5 | 78 | 4 | Et | 94 | [29] |
| 8 | GC400 | 100 | 4 | n-Bu | 90.5 | [15] |
| Entry | Catalyst | Temp. (°C) | Time (h) | Alkyl Group | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cu-Fe3O4 | 185 | 4 | iso-Pr | 84 | [19] |
| Amberlyst-70 | 120 | |||||
| 2 | Ni3Sn2 | 180 | 16–42 | Me to n-Bu | 37–71 | [3] |
| Montmorillonite K10 | 120 | |||||
| 3 | Zr-MCM-41 + Amberlyst-15 | 130 | 24 | Me to iso-Bu | 85.3 (iso-Pr) | [42] |
| 4 | Zr-SBA-15 + ZSM-5 | 180 | 18 | Et | 55 | [43] |
| 5 | Pt/ZrNbO4 | 130 | 6 | Et to tert-Bu | 75.67 (Et) | [44] |
| 6 | Au-H4SiW12O40/ZrO2 | 120 | 24 | Et to sec-Bu | 50.2–80.2 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, N.; Shimazu, S. Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts. Reactions 2023, 4, 667-678. https://doi.org/10.3390/reactions4040038
Yamanaka N, Shimazu S. Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts. Reactions. 2023; 4(4):667-678. https://doi.org/10.3390/reactions4040038
Chicago/Turabian StyleYamanaka, Nobutaka, and Shogo Shimazu. 2023. "Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts" Reactions 4, no. 4: 667-678. https://doi.org/10.3390/reactions4040038
APA StyleYamanaka, N., & Shimazu, S. (2023). Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts. Reactions, 4(4), 667-678. https://doi.org/10.3390/reactions4040038






