Palladium-Catalyzed α-Arylation of Esters: Synthesis of the Tetrahydroisoquinoline Ring
Abstract
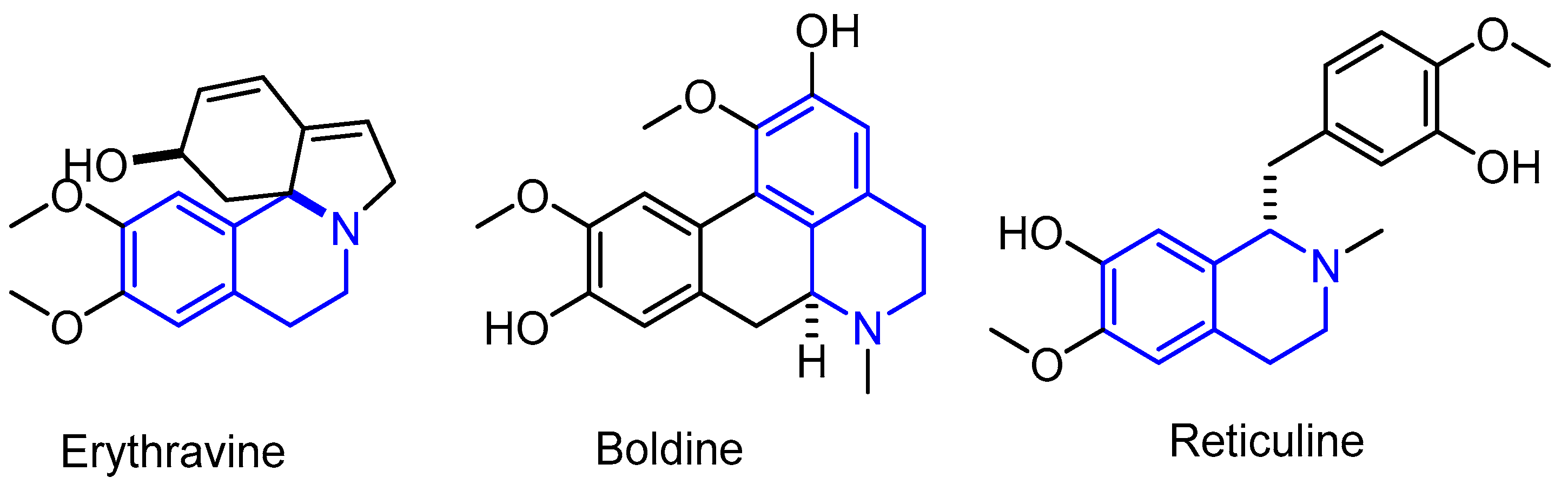
1. Introduction
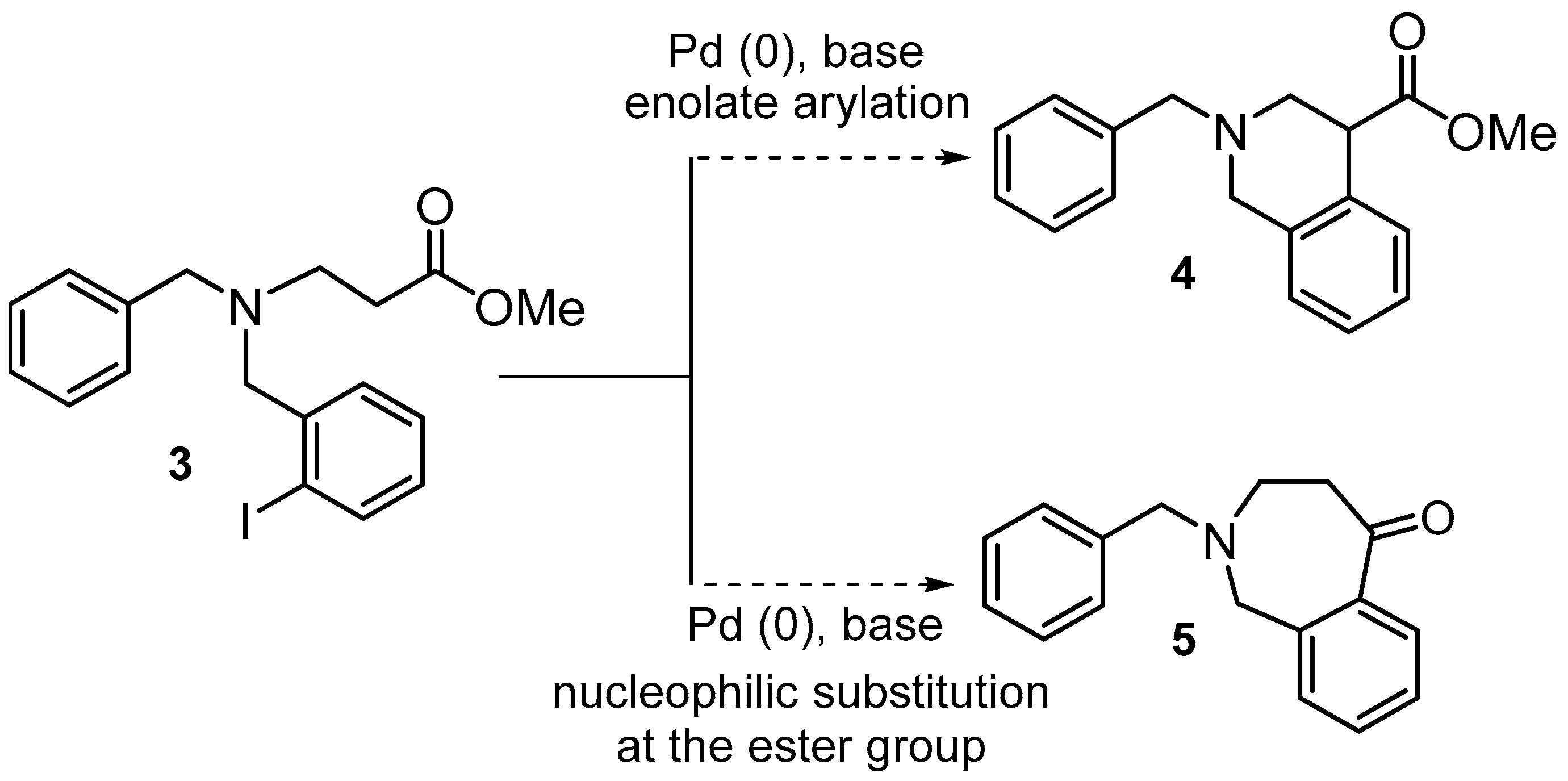
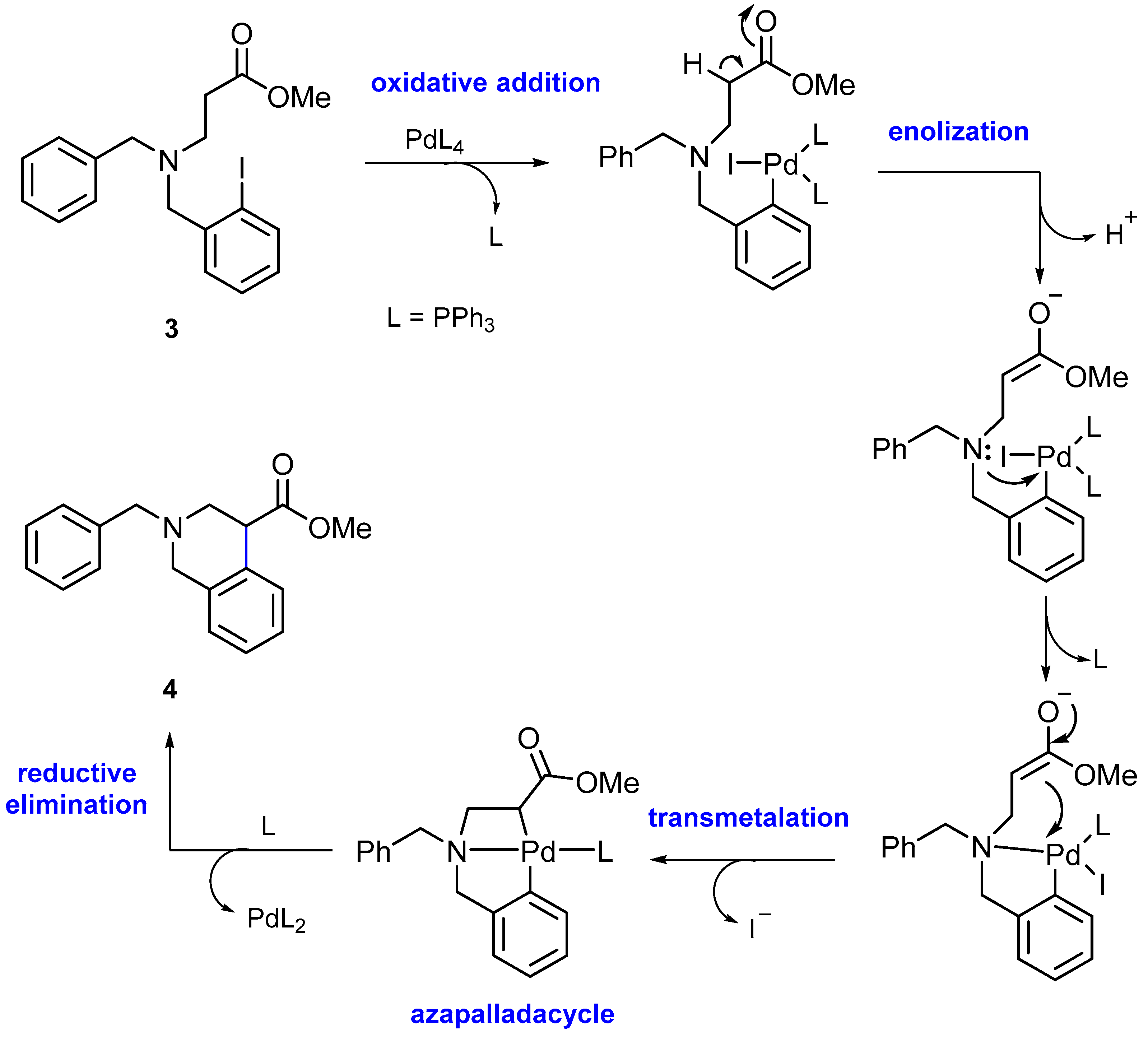
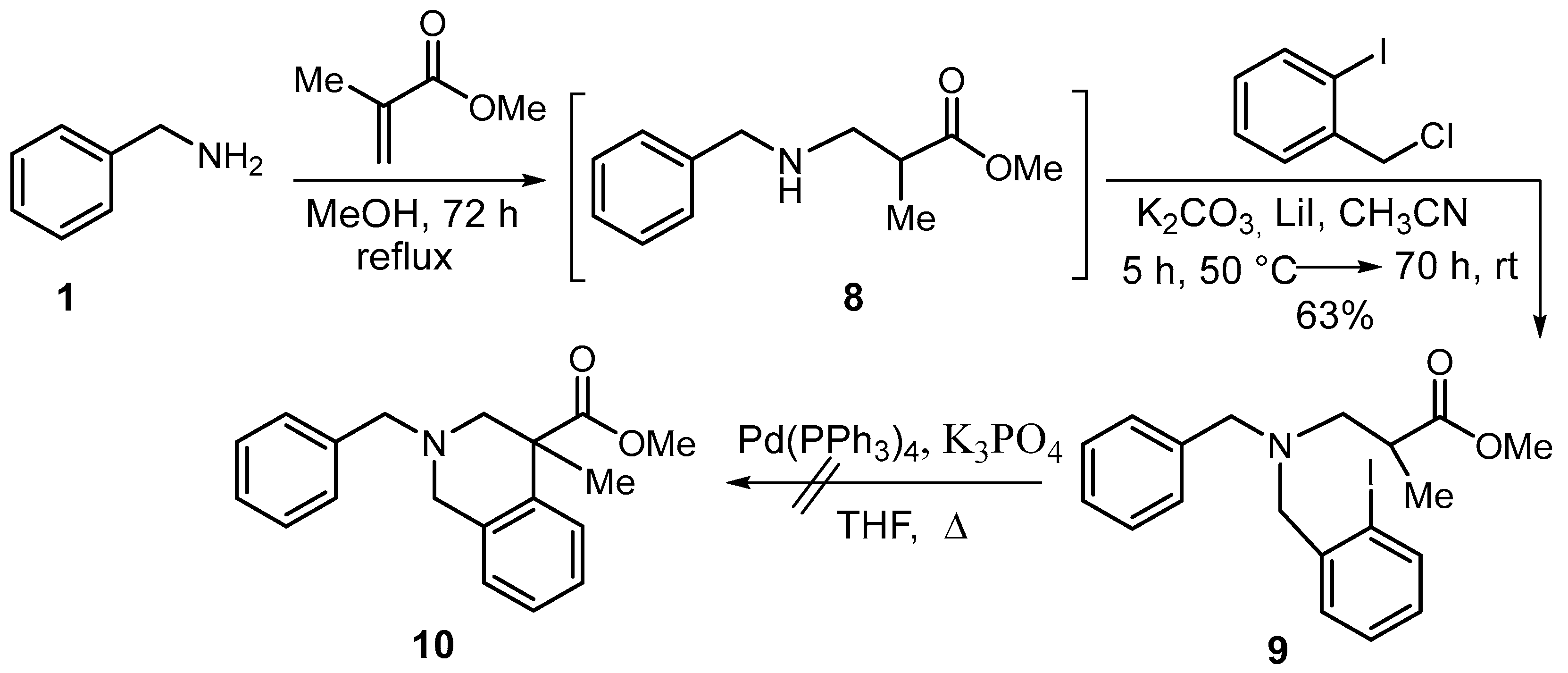
2. Results and Discussion
3. Conclusions
4. Experimental Section
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Amin, A.; Qadir, T.; Sharma, P.K.; Jeelani, I.; Abe, H. A review on the medicinal and industrial applications of N-containing heterocycles. Open Med. Chem. J. 2022, 16, e187410452209010. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Singab, A.N. Alkaloids of genus Erythrina: An updated review. Nat. Prod. Res. 2020, 34, 1891–1912. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Zhu, N.; Zhou, F.; Lin, D.X. Natural aporphine alkaloids with potential to impact metabolic syndrome. Molecules 2021, 26, 6117. [Google Scholar] [CrossRef] [PubMed]
- Unti, E.; Ceravolo, R.; Bonucelli, U. Apomorphine hydrochloride for the treatment of Parkinson’s disease. Expert Rev. Neurother. 2015, 15, 723–732. [Google Scholar] [CrossRef]
- Dargan, P.I.; Button, J.; Hawkins, L.; Archer, J.R.H.; Ovaska, H.; Lidder, S.; Ramsey, J.; Holt, D.W.; Wood, D.M. Detection of the pharmaceutical agent ‘Glaucine’ as a recreational drug. Eur. J. Clin. Pharmacol. 2008, 64, 553–554. [Google Scholar] [CrossRef]
- Wang, J.B.; Mantsch, J.R. L-Tetrahydropalamatine: A potential new medication for the treatment of cocaine addiction. Future Med. Chem. 2012, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.G.; Cassels, B.K. Alkaloids from the genus Duguetia. Alkaloids Chem. Biol. 2010, 68, 83–156. [Google Scholar] [CrossRef]
- Zang, Q.; Javed, S.; Porubsky, P.; Ullah, F.; Neuenswander, B.; Lushington, G.H.; Basha, F.Z.; Organ, M.G.; Hanson, P.R. Synthesis of a unique isoindoline/tetrahydroisoquinoline-based tricyclic sultam library utilizing a Heck-aza-Michael strategy. ACS Comb. Sci. 2012, 14, 211–217. [Google Scholar] [CrossRef]
- Katz, Y.; Weizman, A.; Pick, C.G.; Pasternak, G.W.; Liu, L.; Fonia, O.; Gavish, M. Interactions between laudanosine, GABA, and opioid subtype receptors: Implication for laudanosine seizure activity. Brain Res. 1994, 646, 235–241. [Google Scholar] [CrossRef]
- Exley, R.; Iturriaga-Vasquez, P.; Lukas, R.J.; Sher, E.; Cassels, B.K.; Bermudez, I. Evaluation of benzyltetrahydroisoquinolines as ligands for neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2005, 146, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mahiou, V.; Roblot, F.; Fournet, A.; Hocquemiller, R. Bisbenzylisoquinoline alkaloids from Guatteria boliviana (Annonaceae). Phytochemistry 2000, 54, 709–716. [Google Scholar] [CrossRef]
- Kim, A.N.; Ngamnithiporn, A.; Du, E.; Stoltz, B.M. Recent advances in the total synthesis of the tetrahydroisoquinoline alkaloids (2002−2020). Chem. Rev. 2023, 123, 9447–9496. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, W.; Song, J.; Ding, W.; Zhao, Q.F.; Peng, C.; Song, W.W.; Tang, G.L.; Liu, W. Characterization of the saframycin A gene cluster from Streptomyces lavendulae NRRL 11002 revealing a nonribosomal peptide synthetase system for assembling the unusual tetrapeptidyl skeleton in an iterative manner. J. Bacteriol. 2008, 190, 251–263. [Google Scholar] [CrossRef]
- Cuevas, C.; Perez, M.; Martin, M.J.; Chicharro, J.L.; Fernandez-Rivas, C.; Flores, M.; Francesch, A.; Gallego, P.; Zarzuelo, M.; de La Calle, F.; et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org. Lett. 2000, 2, 2545–2548. [Google Scholar] [CrossRef]
- Scott, J.D.; Williams, R.M. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 2002, 102, 1669–1730. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Metobo, S.; Williams, R.M. Synthetic studies on ecteinascidin-743: Constructing a versatile pentacyclic intermediate for the synthesis of ecteinascidins and saframycins. Org. Lett. 2003, 5, 2095–2098. [Google Scholar] [CrossRef]
- Le, V.H.; Inai, M.; Williams, R.M.; Kan, T. Ecteinascidins. A review of the chemistry, biology and clinical utility of potent tetrahydroisoquinoline antitumor antibiotics. Nat. Prod. Rep. 2015, 32, 328–347. [Google Scholar] [CrossRef]
- Leyva-Peralta, M.A.; Robles-Zepeda, R.E.; Garibay-Escobar, A.; Ruiz-Bustos, E.; Alvarez-Berber, L.P.; Galvez-Ruiz, J.C. In vitro anti-proliferative activity of Argemone gracilenta and identification of some active components. BMC Complement. Altern. Med. 2015, 15, 13. [Google Scholar] [CrossRef]
- Chang, G.J.; Su, M.J.; Hung, L.M.; Lee, S.S. Cardiac electrophysiologic and antiarrhythmic actions of a pavine alkaloid derivative, O-methyl-neocaryachine, in rat heart. Br. J. Pharmacol. 2002, 136, 459–471. [Google Scholar] [CrossRef]
- Rebets, Y.; Nadmid, S.; Paulus, C.; Dahlem, C.; Herrmann, J.; Hubner, H.; Ruckert, C.; Kiemer, A.K.; Gmeiner, P.; Kalinowski, J.; et al. Perquinolines A-C: Unprecedented bacterial tetrahydroisoquinolines involving an intriguing biosynthesis. Angew. Chem. Int. Ed. 2019, 58, 12930–12934. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, A.; Mengozzi, L.; Manoni, E.; Cozzi, P.G. Stereoselective organocatalytic addition of nucleophiles to isoquinolinium and 3,4-dihydroisoquinolinium ions: A simple approach for the synthesis of isoquinoline alkaloids. Catal. Lett. 2015, 145, 398–419. [Google Scholar] [CrossRef]
- Joshi, H.C.; Salil, A.; Bughani, U.; Naik, P.K. Noscapinoids: A new class of anticancer drugs demand biotechnological intervention. In Medicinal Plant Biotechnology; Arora, R., Ed.; CABI Digital Library: Wallingford, UK, 2010; pp. 303–320. [Google Scholar]
- Zhou, Y.D.; Kim, Y.P.; Mohammed, K.A.; Jones, D.K.; Muhammad, I.; Dunbar, D.C.; Nagle, D.G. Terpenoid tetrahydroisoquinoline alkaloids emetine, klugine, and isocephaeline inhibit the activation of hypoxia-inducible factor-1 in breast tumor cells. J. Nat. Prod. 2005, 68, 947–950. [Google Scholar] [CrossRef]
- Boss, C.; Brisbare-Roch, C.; Jenck, F. Biomedical application of orexin/hypocretin receptor ligands in neuroscience. J. Med. Chem. 2009, 52, 891–903. [Google Scholar] [CrossRef]
- Charifson, P.S.; Wyrick, S.D.; Hoffman, A.J.; Simmons, R.M.; Bowen, J.P.; McDougald, D.L.; Mailman, R.B. Synthesis and pharmacological characterization of 1-phenyl-, 4-phenyl-, and 1-benzyl-1,2,3,4-tetrahydroisoquinolines as dopamine receptor ligands. J. Med. Chem. 1988, 31, 1941–1946. [Google Scholar] [CrossRef]
- El Aouad, N.; Berenguer, I.; Romero, V.; Marın, P.; Serrano, A.; Andujar, S.; Suvire, F.; Bermejo, A.; Ivorra, M.D.; Enriz, R.D.; et al. Structure-activity relationship of dopaminergic halogenated 1-benzyl-tetrahydroisoquinoline derivatives. Eur. J. Med. Chem. 2009, 44, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.; Polak, D.; Romanska, I.; Michaluk, J.; Antkiewicz-Michaluk, L. The impact of 1MeTIQ on the dopaminergic system function in the 6-OHDA model of Parkinson’s disease. Pharmacol. Rep. 2016, 68, 1205–1213. [Google Scholar] [CrossRef]
- Mozdzen, E.; Babinska, I.; Wojcikowski, J.; Antkiewicz-Michaluk, L. 1-Methyl-1,2,3,4-tetrahydroisoquinoline—The toxicological research on an exo/endogenous amine with antidepressant-like activity—In vivo, in vitro and in silico studies. Pharmacol. Rep. 2019, 71, 1140–1146. [Google Scholar] [CrossRef]
- Jovanovic, D.; Filipovic, A.; Janjic, G.; Lazarevic-Pasti, T.; Dzambaski, Z.; Bondzic, B.P.; Bondzic, A.M. Targeting Alzheimer’s disease: Evaluating the efficacy of C-1 functionalized N-aryl tetrahydroisoquinolines as cholinergic enzyme inhibitors and promising therapeutic candidates. Int. J. Mol. Sci. 2024, 25, 1033. [Google Scholar] [CrossRef]
- Abo-Elmagd, M.I.; Hassan, R.M.; Aboutabl, M.E.; Amin, K.M.; El-Azzouny, A.A.; Aboul-Enein, M.N. Design, synthesis and anti-inflammatory assessment of certain substituted 1,2,4-triazoles bearing tetrahydroisoquinoline scaffold as COX 1/2-inhibitors. Bioorganic Chem. 2024, 150, 107577. [Google Scholar] [CrossRef]
- Guo, J. Recent advances in the synthesis and activity of analogues of bistetrahydroisoquinoline alkaloids as antitumor agents. Eur. J. Med. Chem. 2023, 262, 115917. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Andersson, K.E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007, 100, 987–1006. [Google Scholar] [CrossRef]
- Faheem, M.; Kumar, B.K.; Chandra Sekhar, K.V.G.; Chander, S.; Kunjiappan, S.; Murugesan, S. Medicinal chemistry perspectives of 1,2,3,4 tetrahydroisoquinoline analogs—Biological activities and SAR studies. RSC Adv. 2021, 11, 12254–12287. [Google Scholar] [CrossRef] [PubMed]
- Murugavel, S.; Manikandan, N.; Lakshmanan, D.; Naveen, K.; Perumal, P.T. Synthesis, crystal structure, DFT and antibacterial activity studies of (E)-2-benzyl-3-(furan-3-yl)-6,7-dimethoxy-4-(2-phenyl-1H-inden-1-ylidene)-1,2,3,4-tetrahydroisoquinoline. J. Chil. Chem. Soc. 2015, 60, 3015–3020. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Al-Majed, A.R.A.; Aljohar, H.; El-Emam, A.; Pathak, S.K.; Sachan, A.K.; Prasad, O.; Leena, S. First Principle study of a potential bioactive molecule with tetrahydroisoquinoline, carbothiomide and adamantane scaffolds. J. Mol. Struct. 2017, 1143, 204–216. [Google Scholar] [CrossRef]
- Heravi, M.; Zadsirjan, V.; Malmir, M. Application of the asymmetric Pictet–Spengler reaction in the total synthesis of natural products and relevant biologically active compounds Majid. Molecules 2018, 23, 943. [Google Scholar] [CrossRef]
- Ruff, B.M.; Brase, S.; O’Connor, S.E. Biocatalytic production of tetrahydroisoquinolines. Tetrahedron Lett. 2012, 53, 1071–1074. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Multicomponent synthesis of tetrahydroisoquinolines. Org. Lett. 2018, 20, 3460–3464. [Google Scholar] [CrossRef]
- Priebbenow, D.L.; Pfeffer, F.M.; Stewart, S.G. A one-pot, three-component approach to functionalised tetrahydroisoquinolines using domino Heck–aza-Michael reactions. Eur. J. Org. Chem. 2011, 9, 1632–1635. [Google Scholar] [CrossRef]
- Sole, D.; Perez-Janer, F.; Mancuso, R. Pd(0)-catalyzed intramolecular α-arylation of sulfones: Domino reactions in the synthesis of functionalized tetrahydroisoquinolines. Chem. Eur. J. 2015, 21, 4580–4584. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Jeyachandran, V.; Perumal, S.; Menendez, J.C. A three-component domino protocol for the facile synthesis of highly functionalized tetrahydroisoquinolines by creation of their benzene ring. Tetrahedron 2011, 67, 1432–1437. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Wang, Z.; Zhu, G.; Sun, J. Complex bioactive alkaloid-type polycycles through efficient catalytic asymmetric multicomponent Aza-Diels-Alder reaction of indoles with oxetane as directing group. Angew. Chem. Int. Ed. 2013, 52, 2027–2031. [Google Scholar] [CrossRef]
- Shinde, A.H.; Archith, N.; Malipatel, S.; Sharada, D.S. A facile one-pot protocol for the synthesis of tetrazolyl-tetrahydroisoquinolines via novel domino intramolecular cyclization/Ugi-azide sequence. Tetrahedron Lett. 2014, 55, 6821–6826. [Google Scholar] [CrossRef]
- Serban, G. 5-Arylamino-1,3,4-thiadiazol-2-yl acetic acid esters as intermediates for the synthesis of new bisheterocyclic compounds. Farmacia 2015, 63, 146–149. [Google Scholar]
- Serban, G.; Cuc, S.; Egri, E.; Salvan, A. Synthesis of some 2-R-5-formyl-1,3,4-thiadiazole derivatives by Sommelet reaction. Farmacia 2010, 58, 818–824. [Google Scholar]
- Serban, G.; Abe, H.; Takeuchi, Y.; Harayama, T. A new approach to the benzopyridoxepine core by metal mediated intramolecular biaryl ether formation. Heterocycles 2008, 75, 2949–2958. [Google Scholar] [CrossRef]
- Serban, G. 2-Amino-1,3,4-thiadiazoles as prospective agents in trypanosomiasis and other parasitoses. Acta Pharm. 2020, 70, 259–290. [Google Scholar] [CrossRef]
- Serban, G.; Shigeta, Y.; Nishioka, H.; Abe, H.; Takeuchi, Y.; Harayama, T. Studies toward the synthesis of toddaquinoline by intramolecular cyclization. Heterocycles 2007, 71, 1623–1630. [Google Scholar] [CrossRef]
- Gaertzen, O.; Buchwald, S.L. Palladium-catalyzed intramolecular alpha-arylation of alpha-amino acid esters. J. Org. Chem. 2002, 67, 465–475. [Google Scholar] [CrossRef]
- Krishna Reddy, A.G.; Krishna, J.; Satyanarayana, G. Palladium-mediated intramolecular Buchwald–Hartwig α-arylation of β-amino esters: Synthesis of functionalized tetrahydroisoquinolines. Synlett. 2011, 12, 1756–1760. [Google Scholar] [CrossRef]
- Krishna Reddy, A.G.; Satyanarayana, G. A simple efficient sequential one-pot intermolecular aza-Michael addition and intramolecular Buchwald-Hartwig α-arylation of amines: Synthesis of functionalized tetrahydroisoquinolines. Tetrahedron 2012, 68, 8003–8010. [Google Scholar] [CrossRef]
- Nara, S.; Toshima, H.; Ichihara, A. Asymmetric total syntheses of (+)-coronafacic acid and (+)-coronatine, phytotoxins isolated from Pseudomonas syringae pathovars. Tetrahedron 1997, 53, 9509–9524. [Google Scholar] [CrossRef]
- Sole, D.; Serrano, O. Intramolecular Pd(0)-catalyzed reactions of beta-(2-iodoanilino) carboxamides: Enolate arylation and nucleophilic substitution at the carboxamide group. J. Org. Chem. 2008, 73, 9372–9378. [Google Scholar] [CrossRef]
- Sole, D.; Serrano, O. Synthesis of indole-3-carboxylic acid derivatives by Pd(0)-catalyzed intramolecular α-arylation of β-(2-iodoanilino) esters. J. Org. Chem. 2008, 73, 2476–2479. [Google Scholar] [CrossRef]
- Sole, D.; Serrano, O. Palladium-catalyzed intramolecular nucleophilic substitution at the alkoxycarbonyl group. Angew. Chem. Int. Ed. 2007, 46, 7270–7272. [Google Scholar] [CrossRef]


 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst (equiv) | Additive (equiv) | Base (equiv) | Temp (°C) | Time (h) | Yield (%) | ||
| 3 | 4 | 6 | ||||||
| 1 | Pd(PPh3)4 (0.05) | PhOH (3) | t-BuOK (2.5) | reflux | 48 | 67 | - | 31 |
| 2 | Pd(PPh3)4 (0.2) | PhOH (3) | t-BuOK (2.5) | reflux | 48 | 31 | - | - |
| 3 | Pd(PPh3)4 (0.2) | - | K3PO4 (3) | 110 | 72 | - | 84 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, G.; Diaba, F. Palladium-Catalyzed α-Arylation of Esters: Synthesis of the Tetrahydroisoquinoline Ring. Reactions 2025, 6, 17. https://doi.org/10.3390/reactions6010017
Serban G, Diaba F. Palladium-Catalyzed α-Arylation of Esters: Synthesis of the Tetrahydroisoquinoline Ring. Reactions. 2025; 6(1):17. https://doi.org/10.3390/reactions6010017
Chicago/Turabian StyleSerban, Georgeta, and Faïza Diaba. 2025. "Palladium-Catalyzed α-Arylation of Esters: Synthesis of the Tetrahydroisoquinoline Ring" Reactions 6, no. 1: 17. https://doi.org/10.3390/reactions6010017
APA StyleSerban, G., & Diaba, F. (2025). Palladium-Catalyzed α-Arylation of Esters: Synthesis of the Tetrahydroisoquinoline Ring. Reactions, 6(1), 17. https://doi.org/10.3390/reactions6010017






