Low-Dimensional Nanostructures for Electrochemical Energy Applications
Abstract
:1. Introduction
2. Zero-Dimensional Graphene Material for the Counter Electrode of Dye-Sensitized Solar Cells
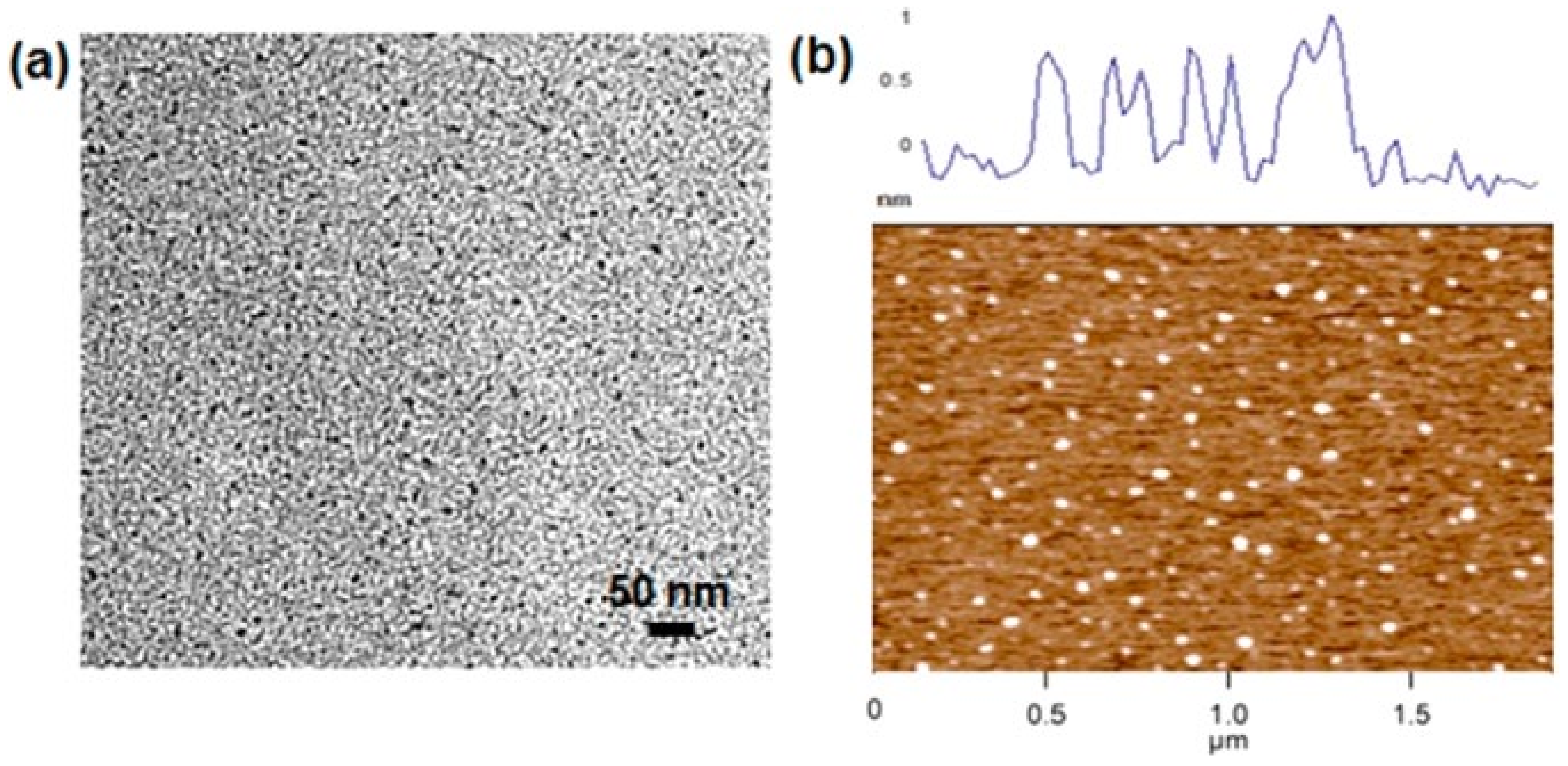
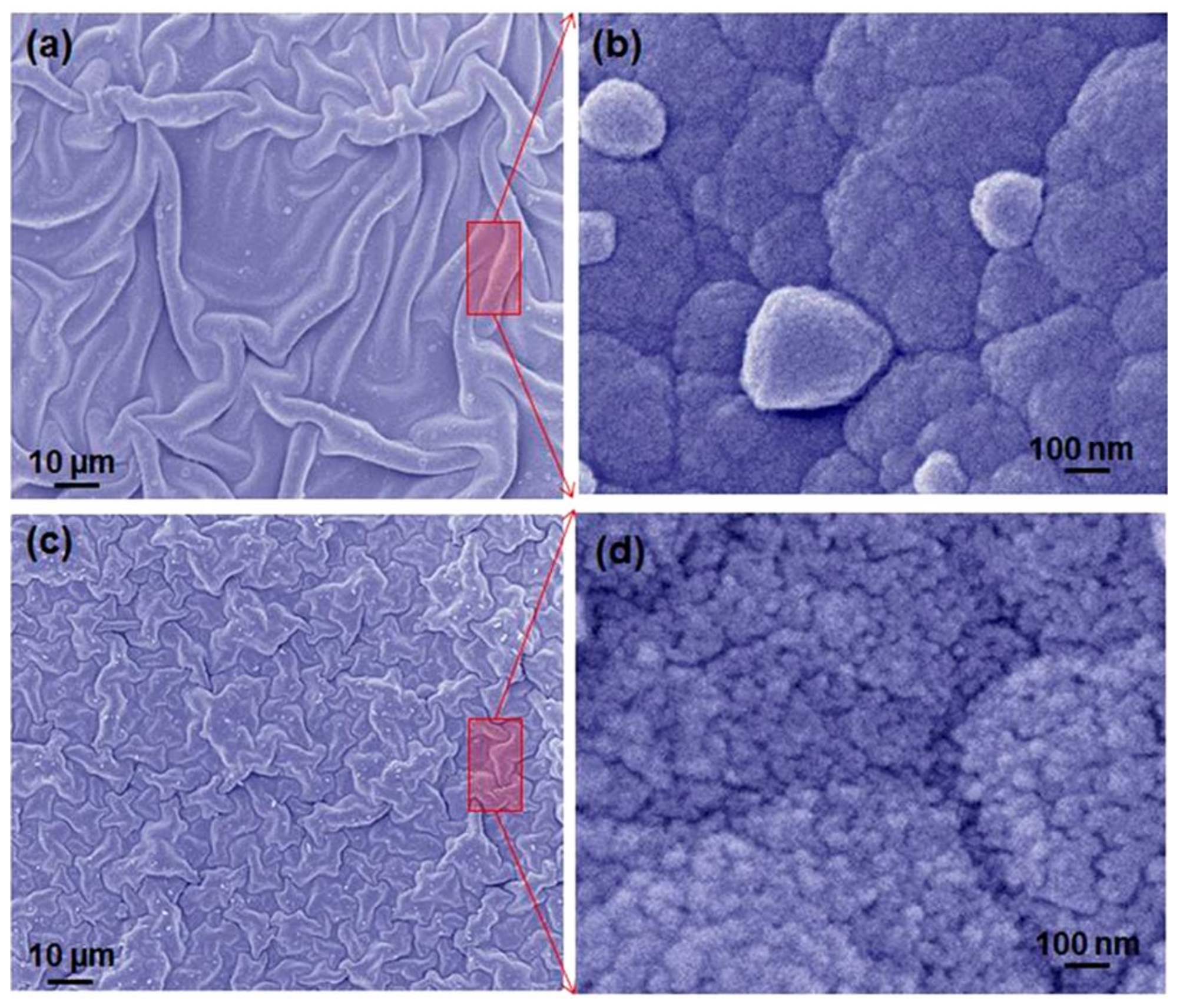
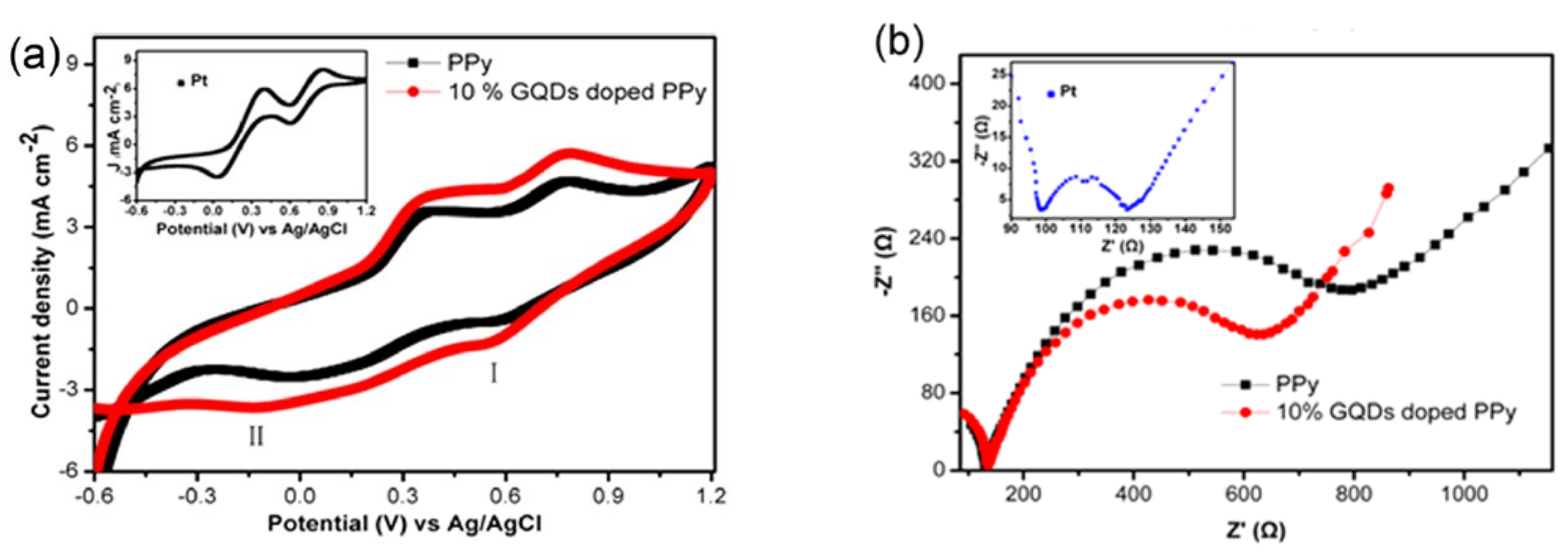
2.1. Graphene Quantum Dot-Doped Polypyrrole
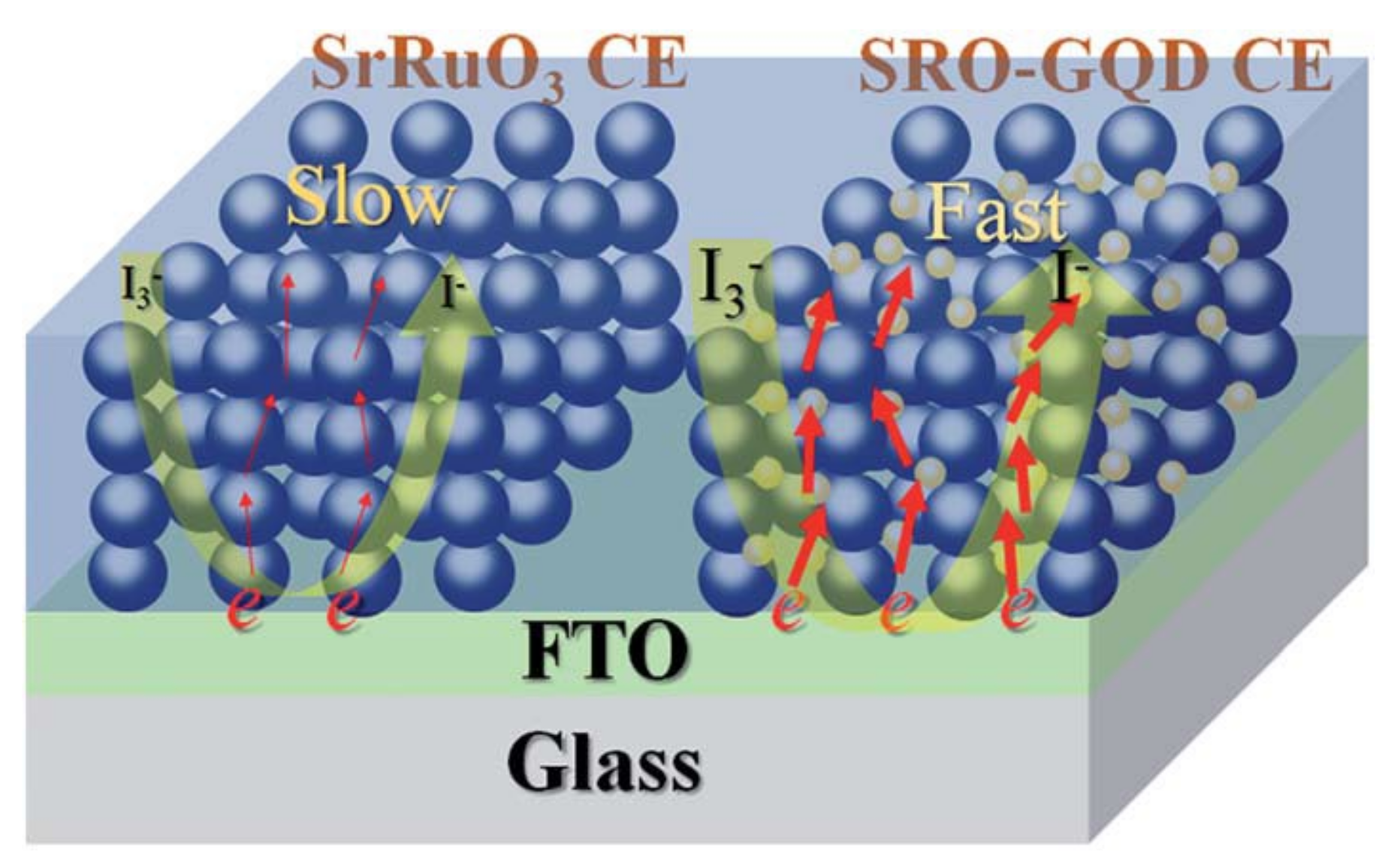
2.2. Graphene Quantum Dot-Decorated Strontium Ruthenate (SrRuO3) Mesoporous Film
2.3. Graphene Dots (GDs)/poly(3,4-Ethylene Dioxythiophene):poly(4-Styrene Sulfonate) (PEDOT:PSS) Composite Film
3. One-Dimensional Materials for Hydrogen Evolution Reaction (HER)
3.1. One-Dimensional Nanowire
3.2. One-Dimensional Nanotube
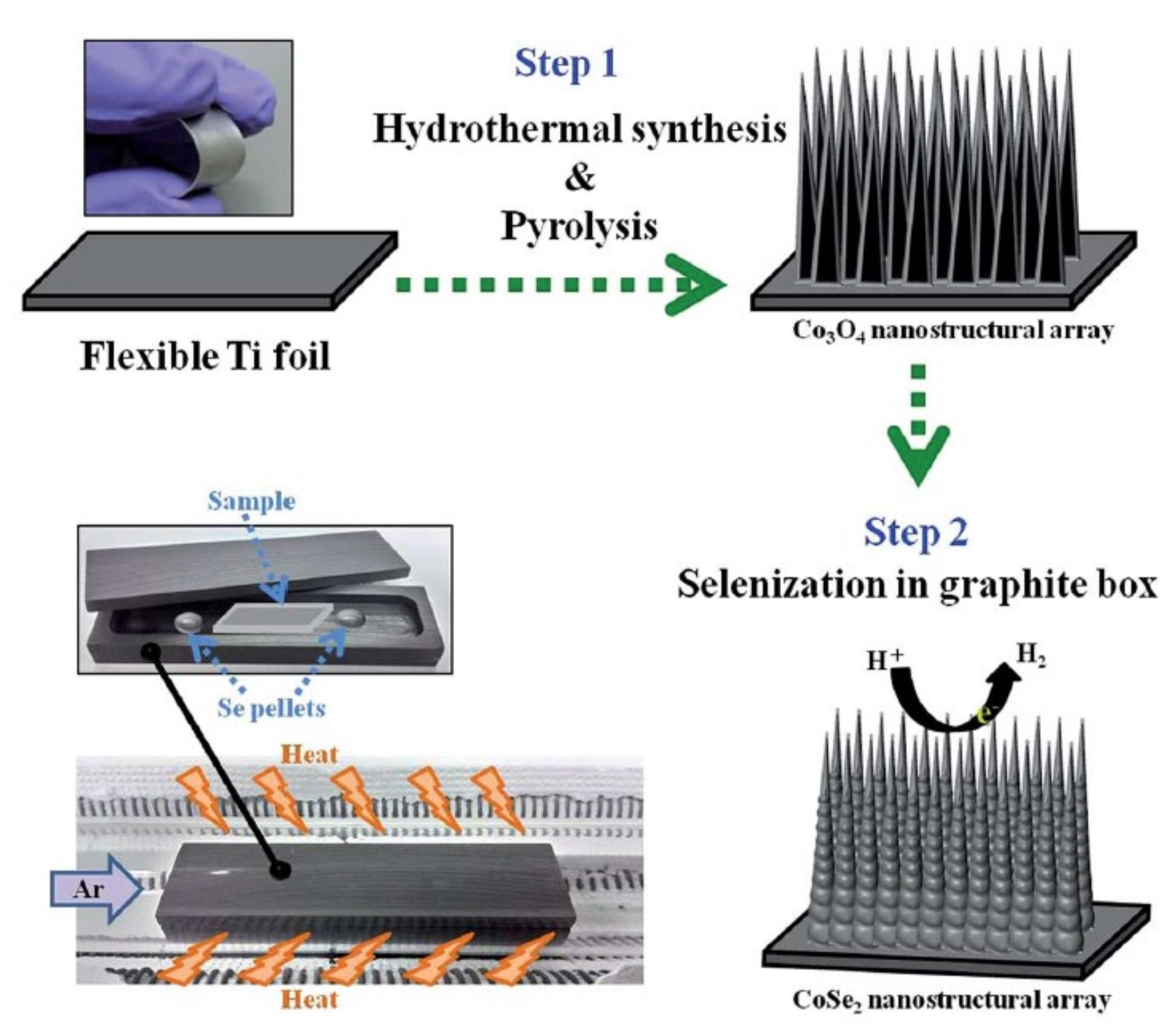
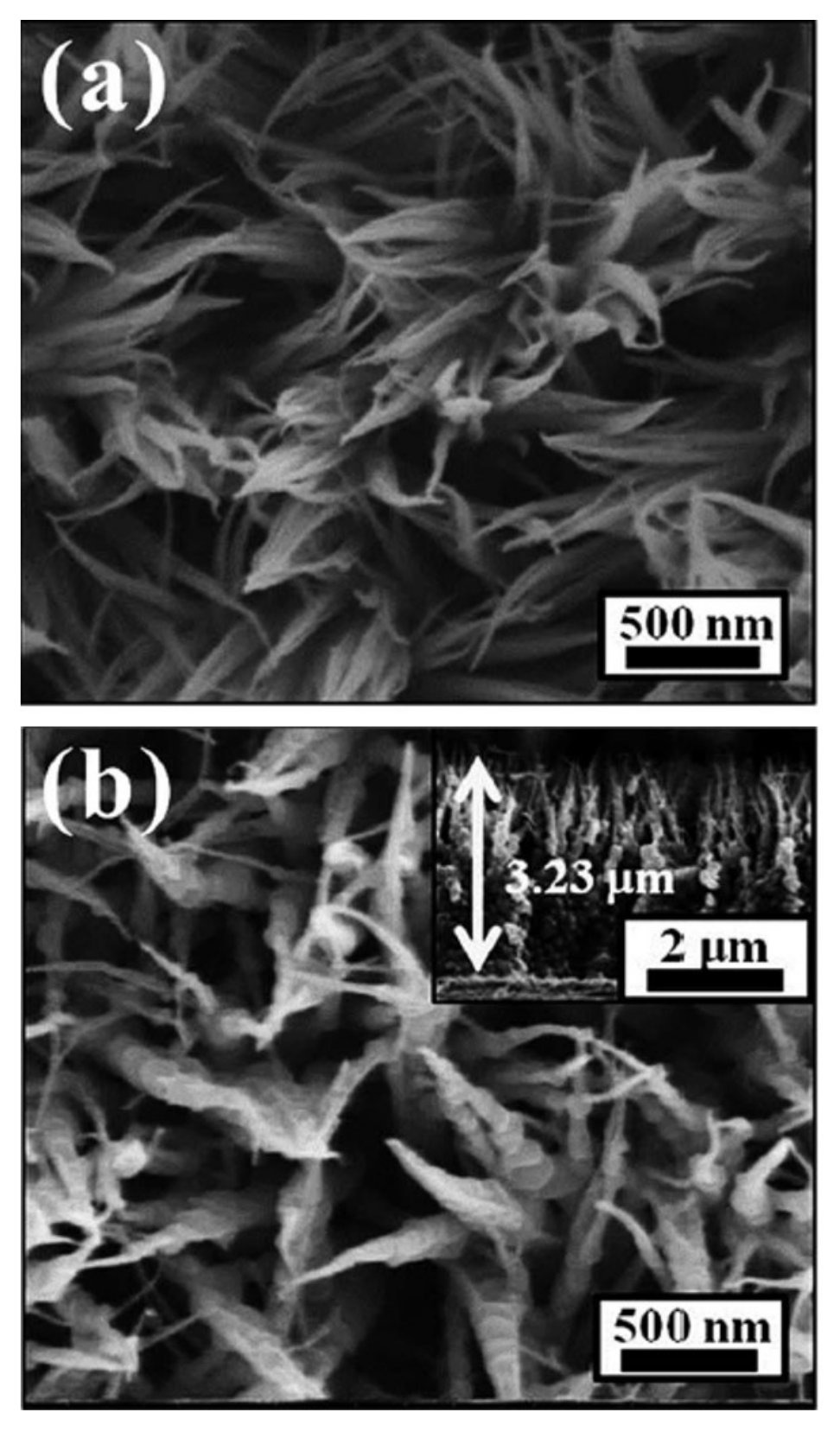
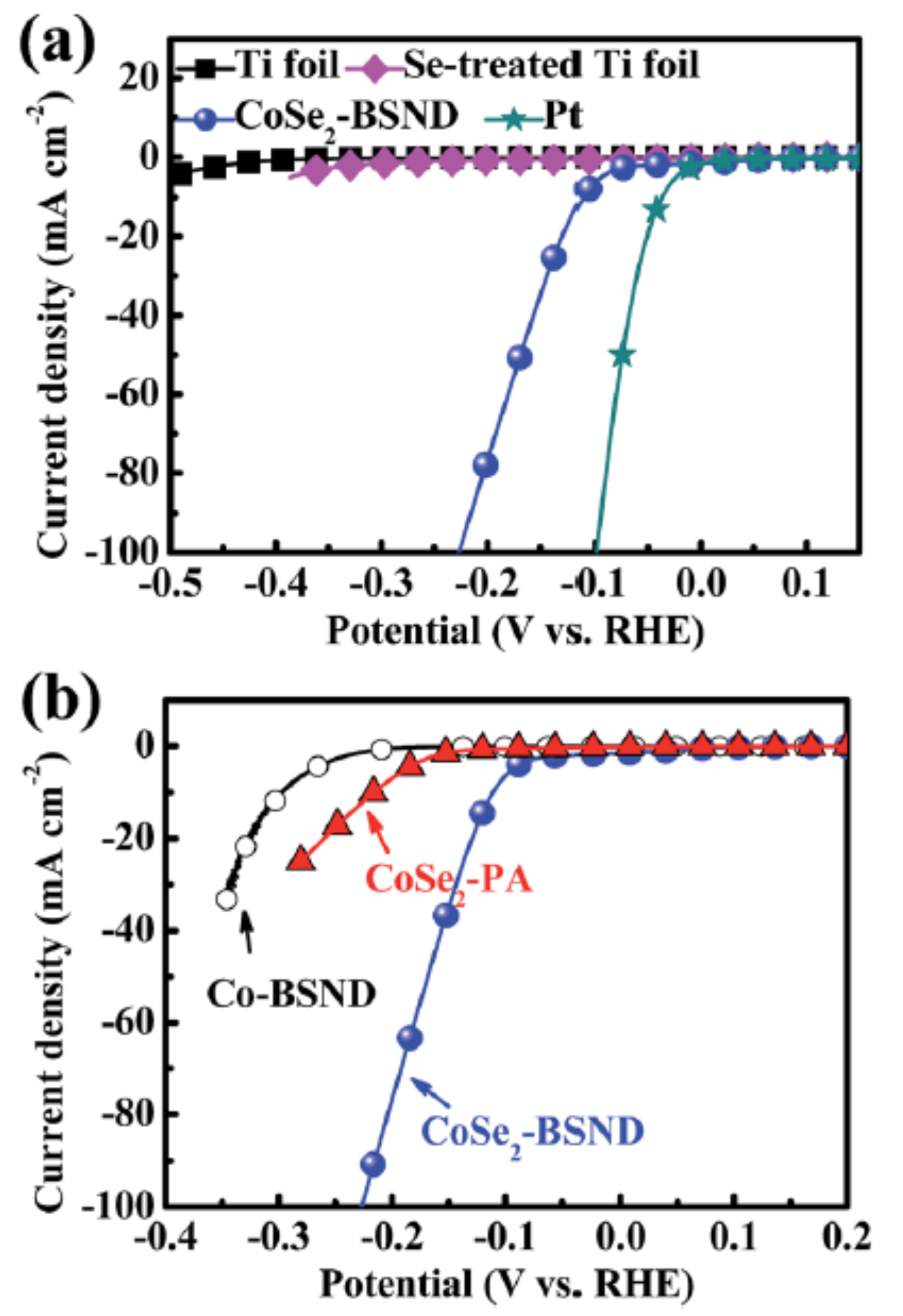
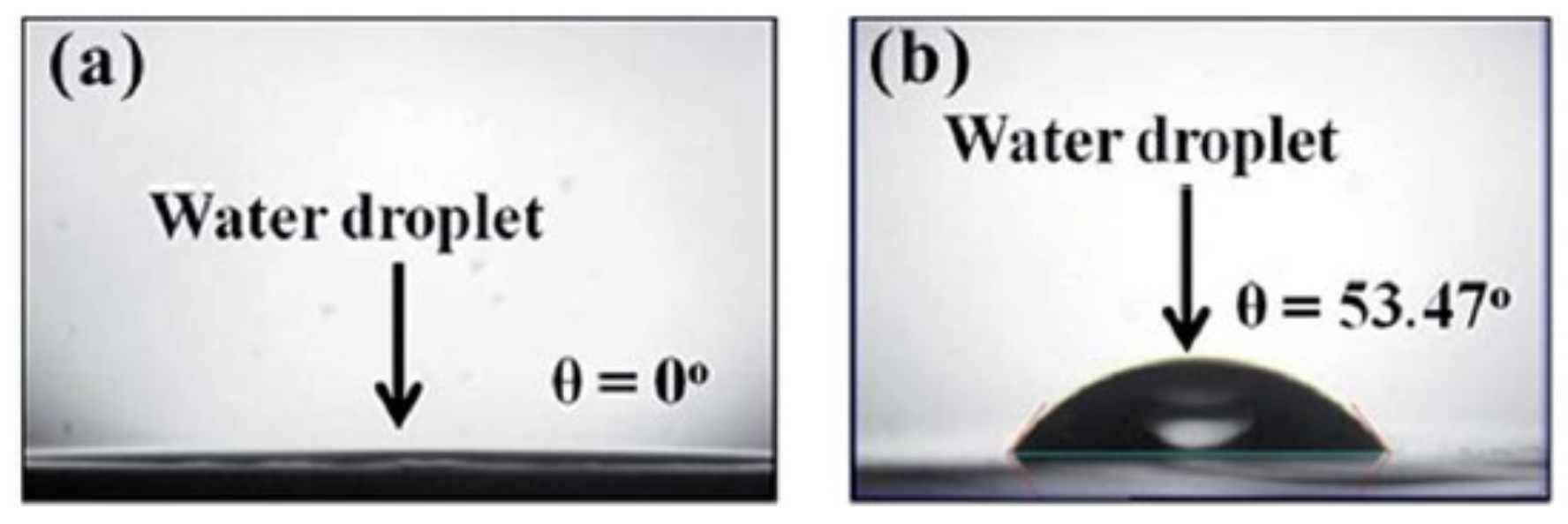
3.3. One-Dimensional Nanoneedle
4. Two-/Three-Dimensional Materials for the Counter Electrode of Dye-Sensitized Solar Cells
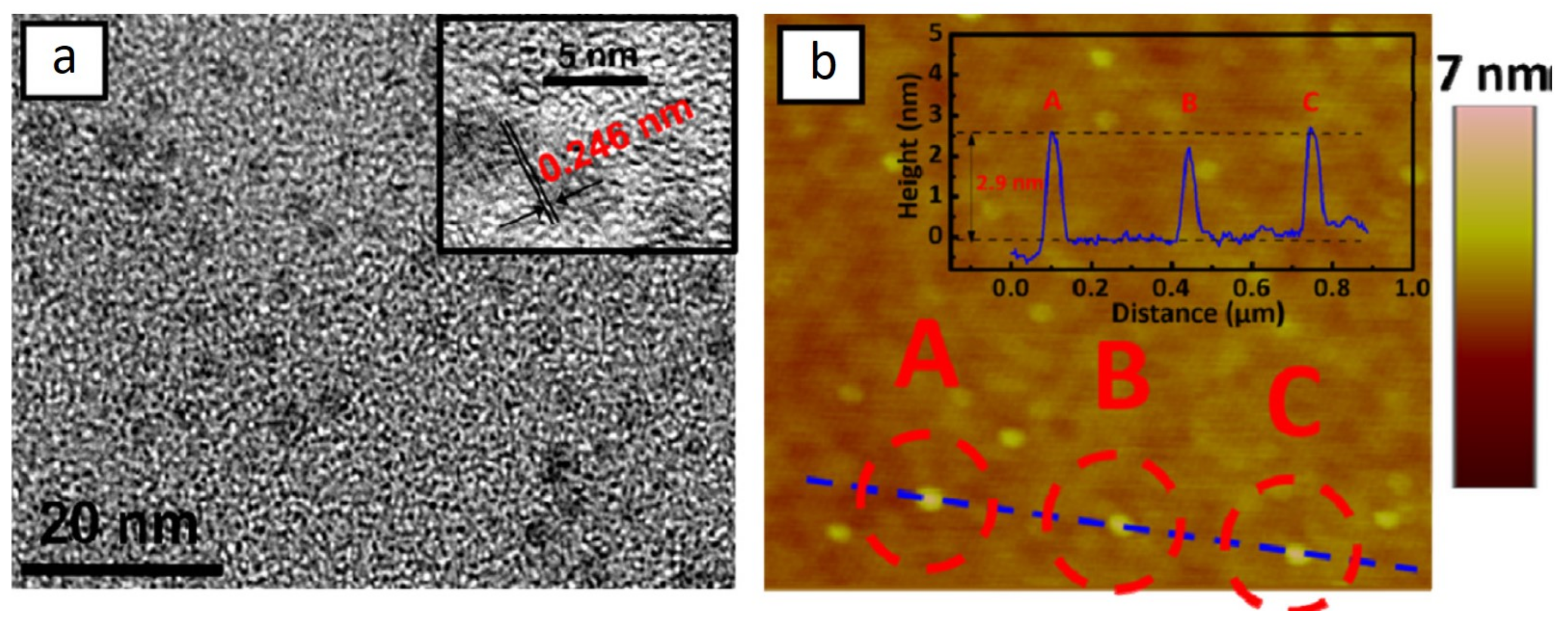
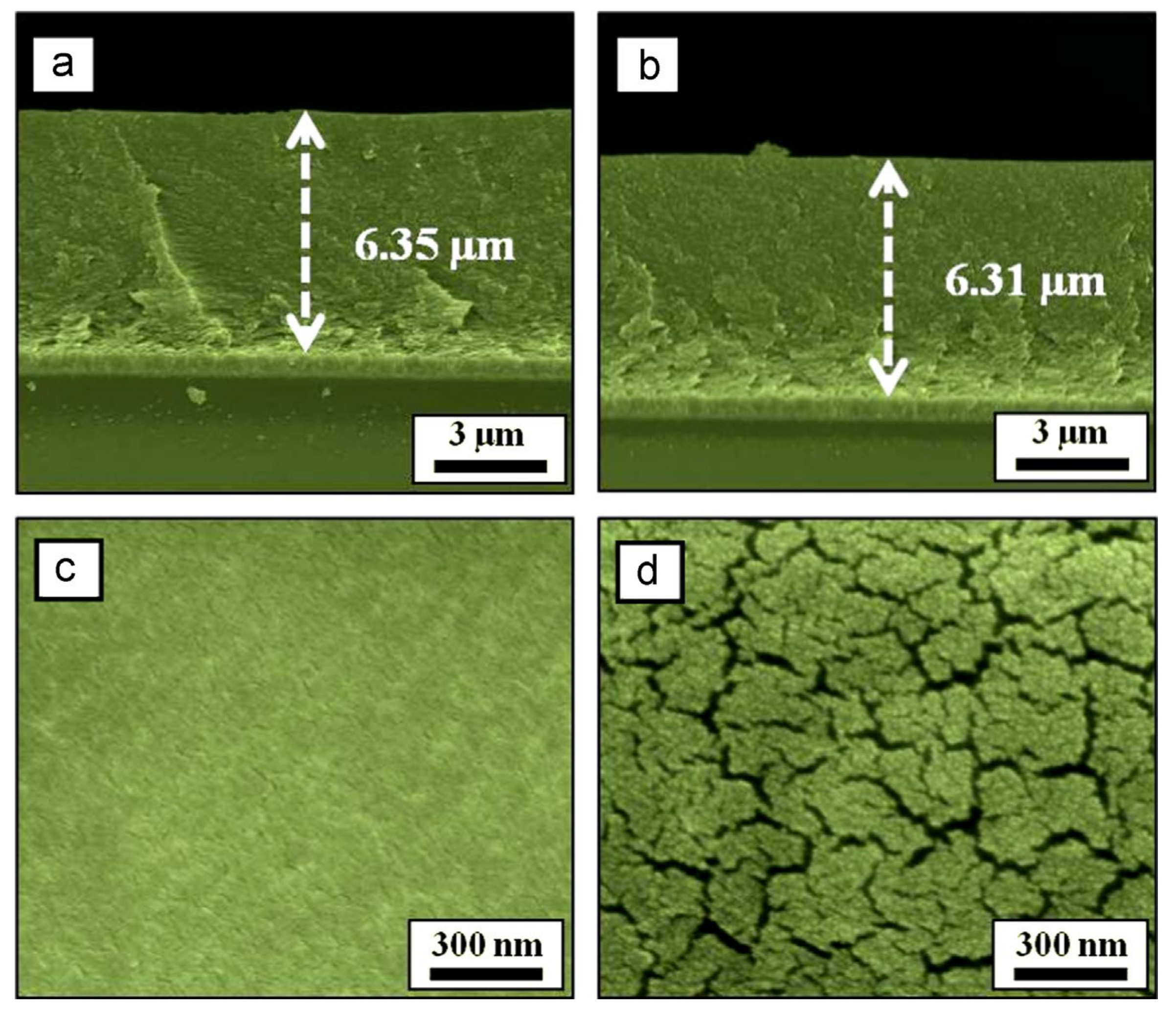
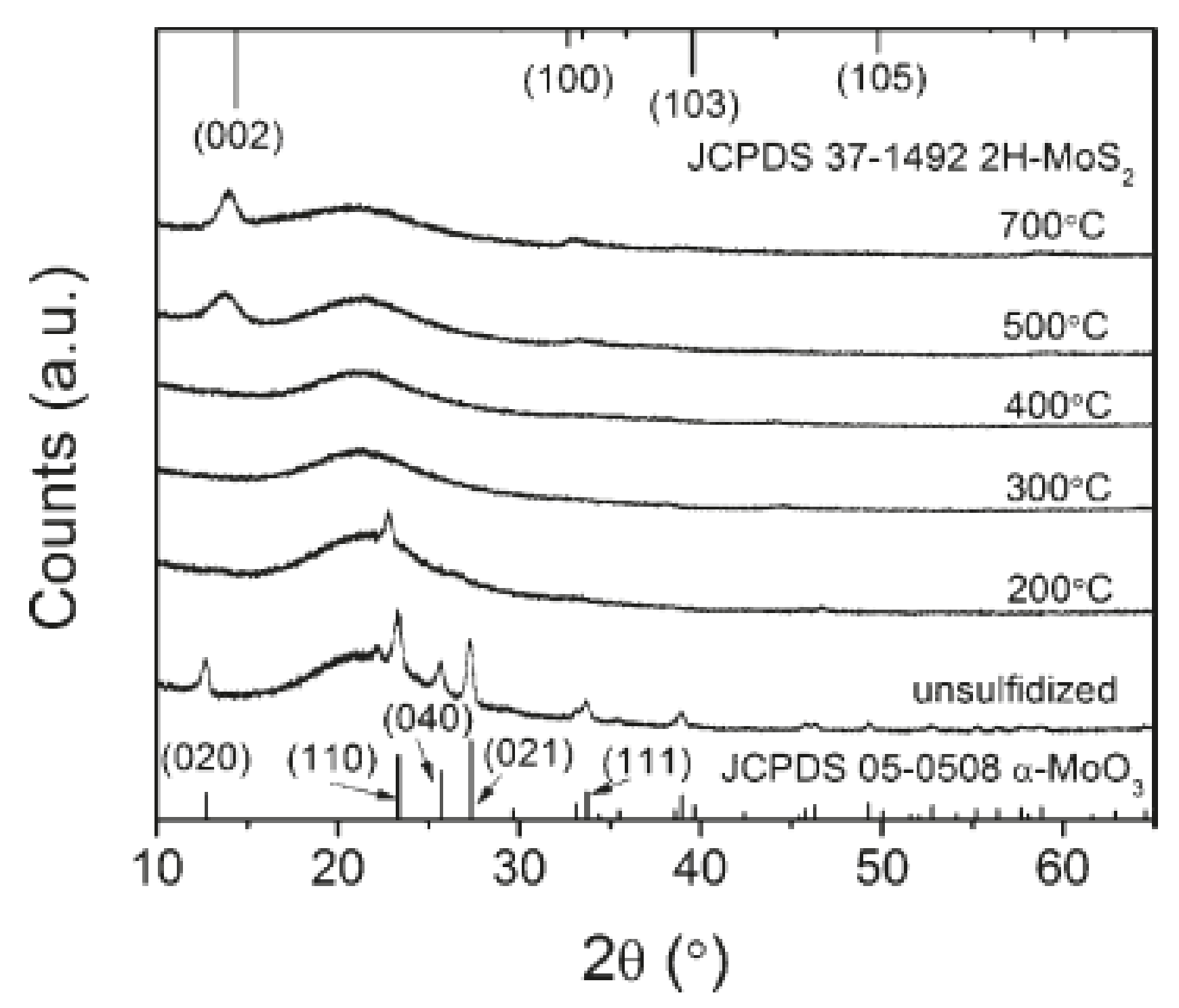
4.1. Molybdenum Disulfide (MoS2)
4.2. MoS2/Graphene Composite
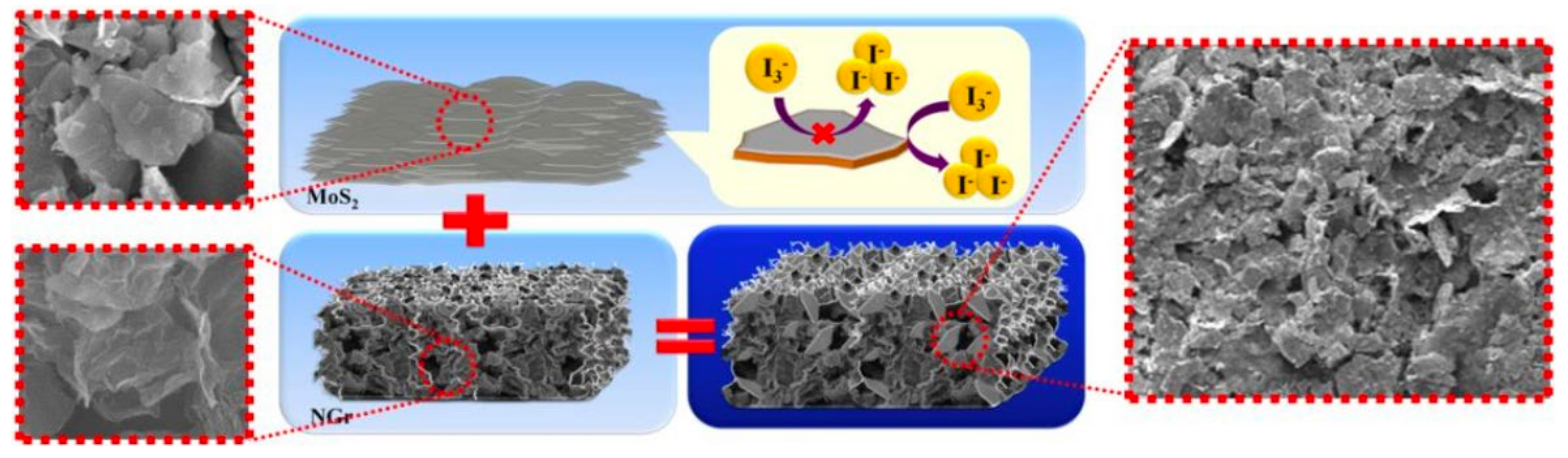
4.3. MoS2/N-Doped Graphene Composite
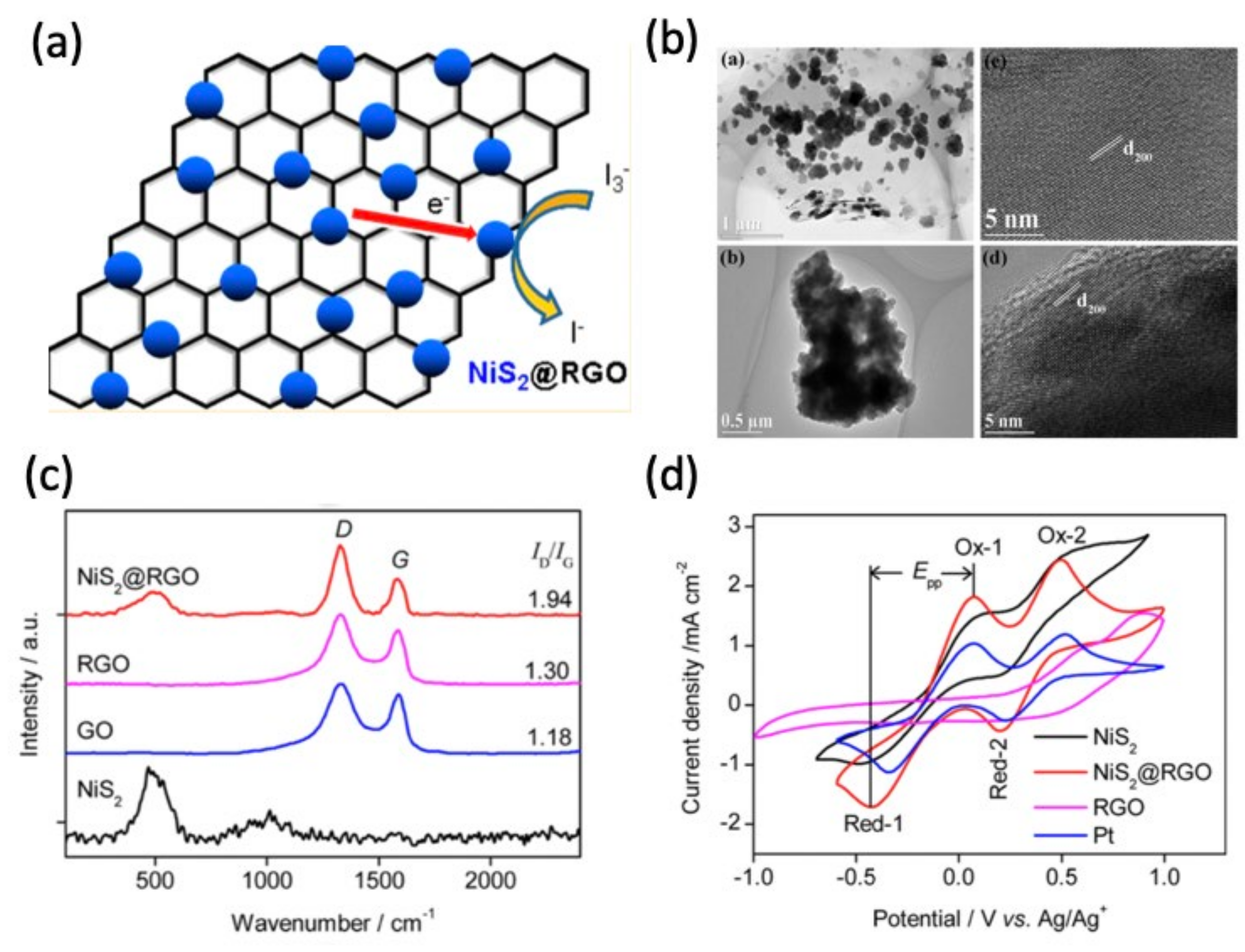
4.4. Nickel Disulfide (NiS2)
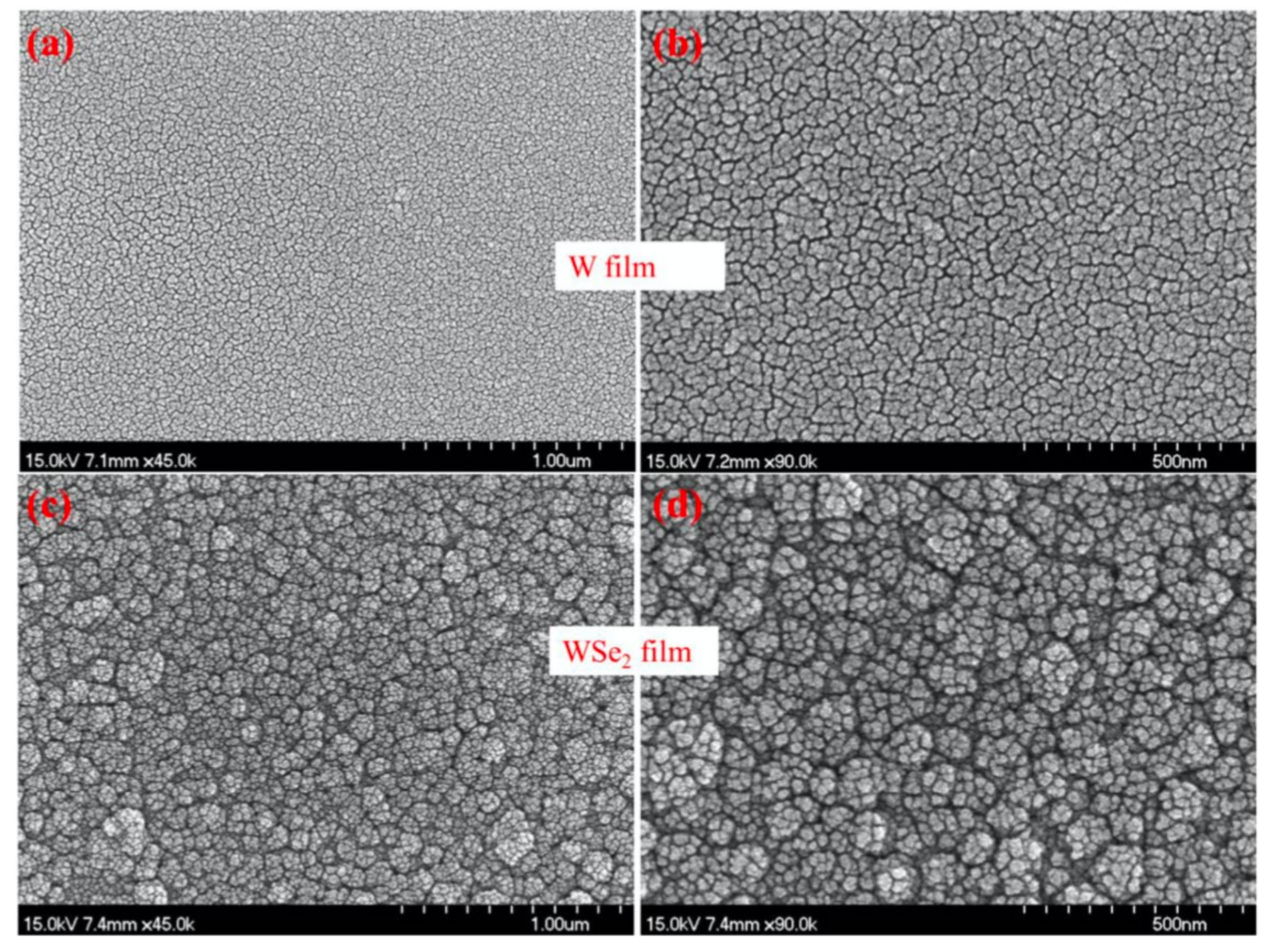
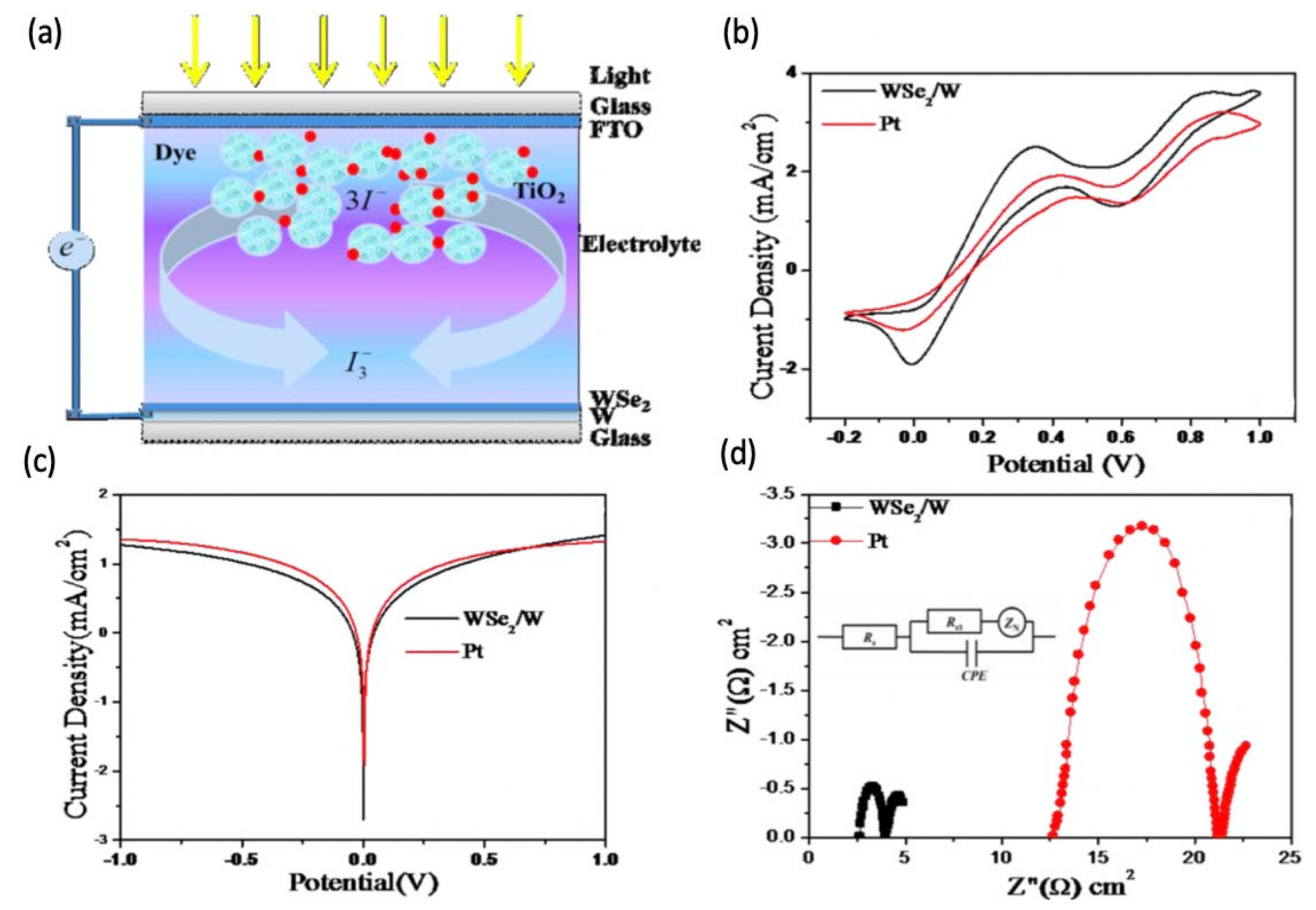
4.5. Tungsten Diselenide (WSe2)
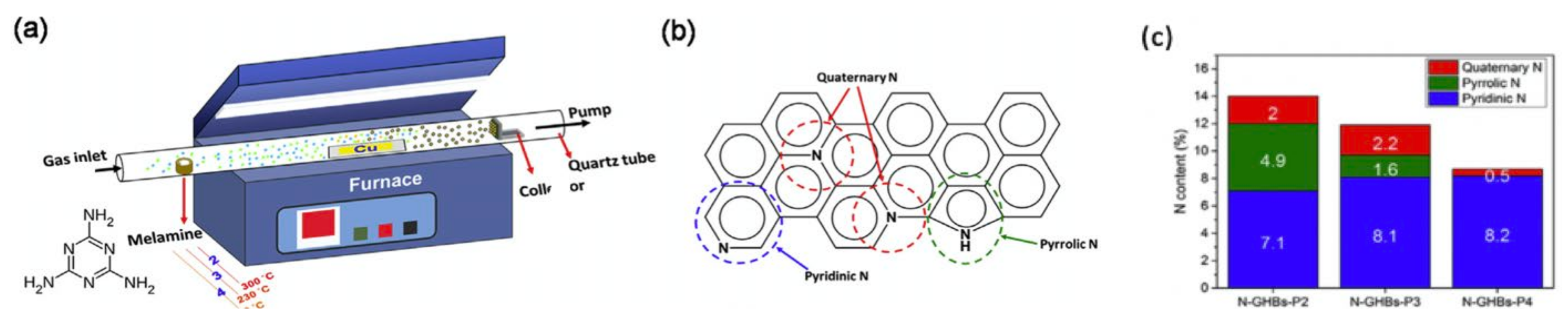
4.6. Nitrogen-Doped Graphene Hollow Nanoballs (N-GHBs)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gomez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Comprehensive Application of Graphene: Emphasis on Biomedical Concerns; Springer: Berlin/Heidelberg, Germany, 2019; Volume 11. [Google Scholar]
- Wang, C.C.; Lu, S.Y. Carbon black-derived graphene quantum dots composited with carbon aerogel as a highly efficient and stable reduction catalyst for the iodide/tri-iodide couple. Nanoscale 2015, 7, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Maity, N.; Kuila, A.; Das, S.; Mandal, D.; Shit, A.; Nandi, A.K. Optoelectronic and photovoltaic properties of graphene quantum dot–polyaniline nanostructures. J. Mater. Chem. A 2015, 3, 20736–20748. [Google Scholar] [CrossRef]
- Tseng, C.A.; Lee, C.P.; Huang, Y.J.; Pang, H.W.; Ho, K.C.; Chen, Y.T. One-step synthesis of graphene hollow nanoballs with various nitrogen-doped states for electrocatalysis in dye-sensitized solar cells. Mater. Today Energy 2018, 8, 15–21. [Google Scholar] [CrossRef]
- Nethravathi, C.; Jeffery, A.A.; Rajamathi, M.; Kawamoto, N.; Tenne, R.; Golberg, D.; Bando, Y. Chemical Unzipping of WS2 Nanotubes. ACS Nano 2013, 7, 7311–7317. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Zibouche, N.; Wahiduzzaman, M.; Oliveira, A.F.; Kuc, A.; Heine, T. Electromechanics in MoS2 and WS2: Nanotubes vs. monolayers. Sci. Rep. 2013, 3, 2961. [Google Scholar] [CrossRef] [Green Version]
- Levi, R.; Bitton, O.; Leitus, G.; Tenne, R.; Joselevich, E. Field-Effect Transistors Based on WS2 Nanotubes with High Current-Carrying Capacity. Nano Lett. 2013, 13, 3736–3741. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.J. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 14971–15005. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, D.; Shuai, Z. Indirect-to-Direct Band Gap Crossover in Few-Layer Transition Metal Dichalcogenides: A Theoretical Prediction. J. Phys. Chem. C 2016, 120, 21866–21870. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.; Zhang, Q.; Lei, Y.; Xie, J.; Ee, S.; Guai, G.; Song, Q.; Li, C.M. Graphene Quantum-Dot-Doped Polypyrrole Counter Electrode for High-Performance Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 2047–2052. [Google Scholar] [CrossRef]
- Saranya, K.; Sivasankar, N.; Subramania, A. Microwave-assisted exfoliation method to develop platinum-decorated graphene nanosheets as a low cost counter electrode for dye-sensitized solar cells. RSC Adv. 2014, 4, 36226–36233. [Google Scholar] [CrossRef]
- Liu, T.; Yu, K.; Gao, L.; Chen, H.; Wang, N.; Hao, L.; Li, T.; He, H.; Guo, Z. A graphene quantum dot decorated SrRuO3mesoporous film as an efficient counter electrode for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 17848–17855. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef]
- Yue, G.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M. Low cost poly(3,4-ethylenedioxythiophene): Polystyrenesulfonate/carbon black counter electrode for dye-sensitized solar cells. Electrochim. Acta 2012, 67, 113–118. [Google Scholar] [CrossRef]
- Lee, C.P.; Lin, C.A.; Wei, T.C.; Tsai, M.L.; Meng, Y.; Li, C.T.; Ho, K.C.; Wu, C.I.; Lau, S.P.; He, J.H. Economical low-light photovoltaics by using the Pt-free dye-sensitized solar cell with graphene dot/PEDOT: PSS counter electrodes. Nano Energy 2015, 18, 109–117. [Google Scholar] [CrossRef]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum Dots as Efficient Catalyst Materials for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; Sun, Y.; Chen, X.; Zhong, W.; Du, Y. Monolayer MoS2 quantum dots as catalysts for efficient hydrogen evolution. RSC Adv. 2015, 5, 97696–97701. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.L.; Sunkara, M.K.; Jaramillo, T.F. Core–shell MoO3–MoS2 Nanowires for Hydrogen Evolution: A Functional Design for Electrocatalytic Materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
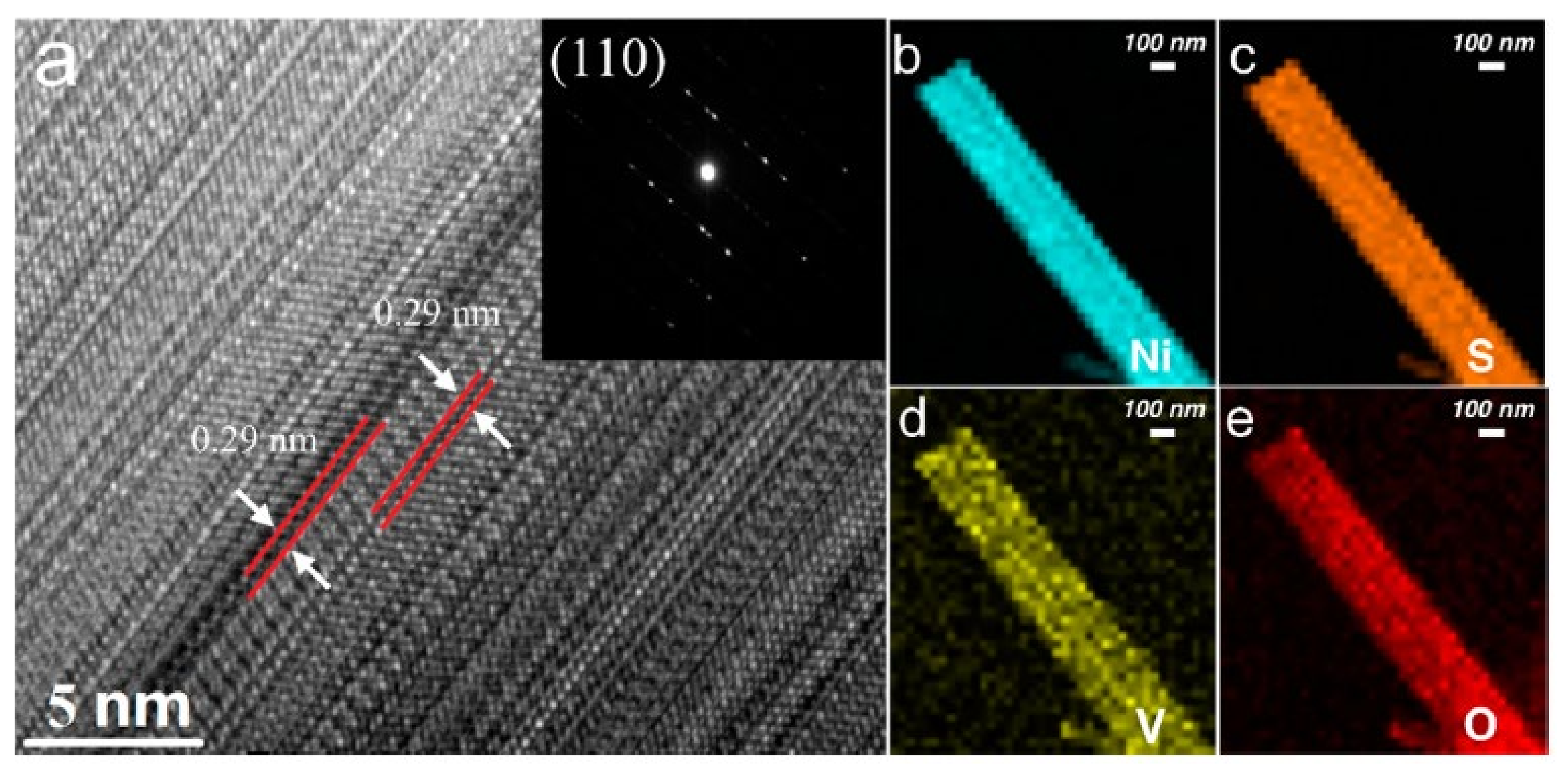
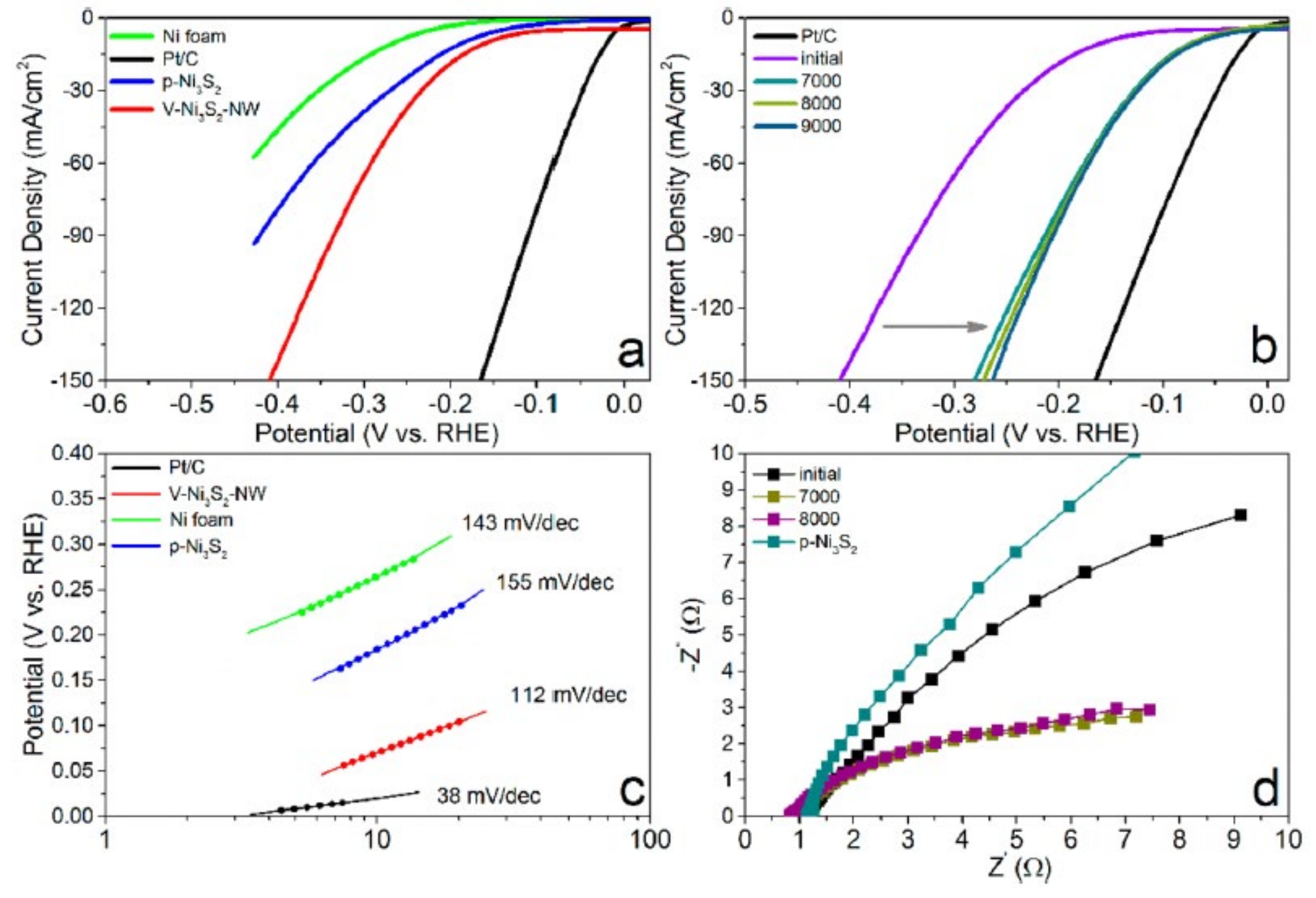
- Qu, Y.; Yang, M.; Chai, J.; Tang, Z.; Shao, M.; Kwok, C.T.; Yang, M.; Wang, Z.; Chua, D.; Wang, S.; et al. Facile Synthesis of Vanadium-Doped Ni3S2 Nanowire Arrays as Active Electrocatalyst for Hydrogen. ACS Appl. Mater. Interfaces 2017, 9, 5959–5967. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.D.; Feng, X. Interface Engineering of MoS2/Ni3S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Angew. Chem. Int. Ed. 2016, 55, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, G.D.; Liu, Y.; Yang, L.; Lian, X.; Asefac, T.; Zou, X. Overall Water Splitting Catalyzed Efficiently by an Ultrathin Nanosheet-Built, Hollow Ni3S2-Based Electrocatalyst. Adv. Funct. Mater. 2016, 26, 4839–4847. [Google Scholar] [CrossRef]
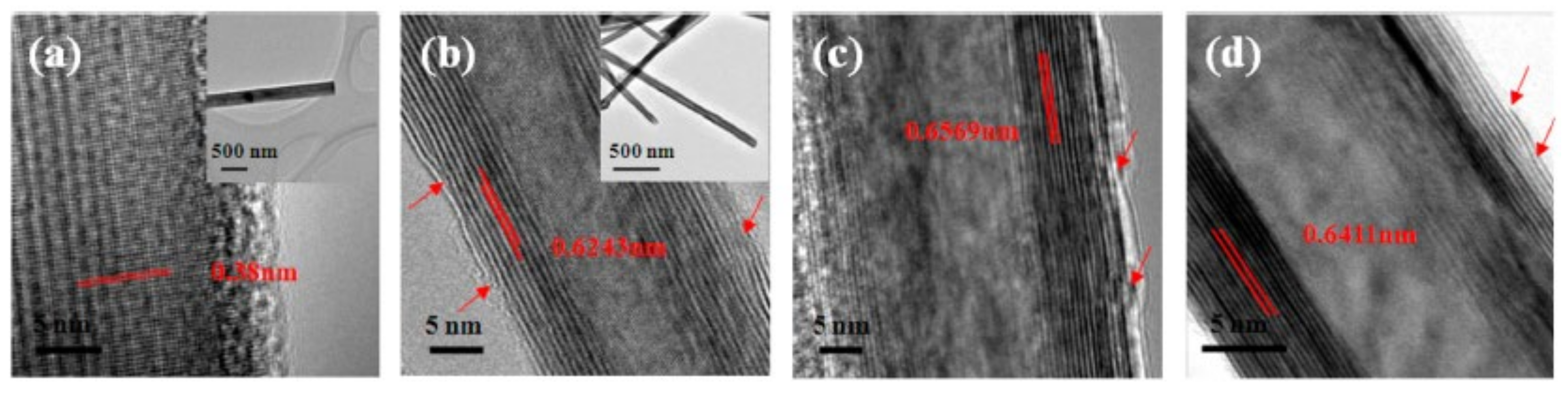
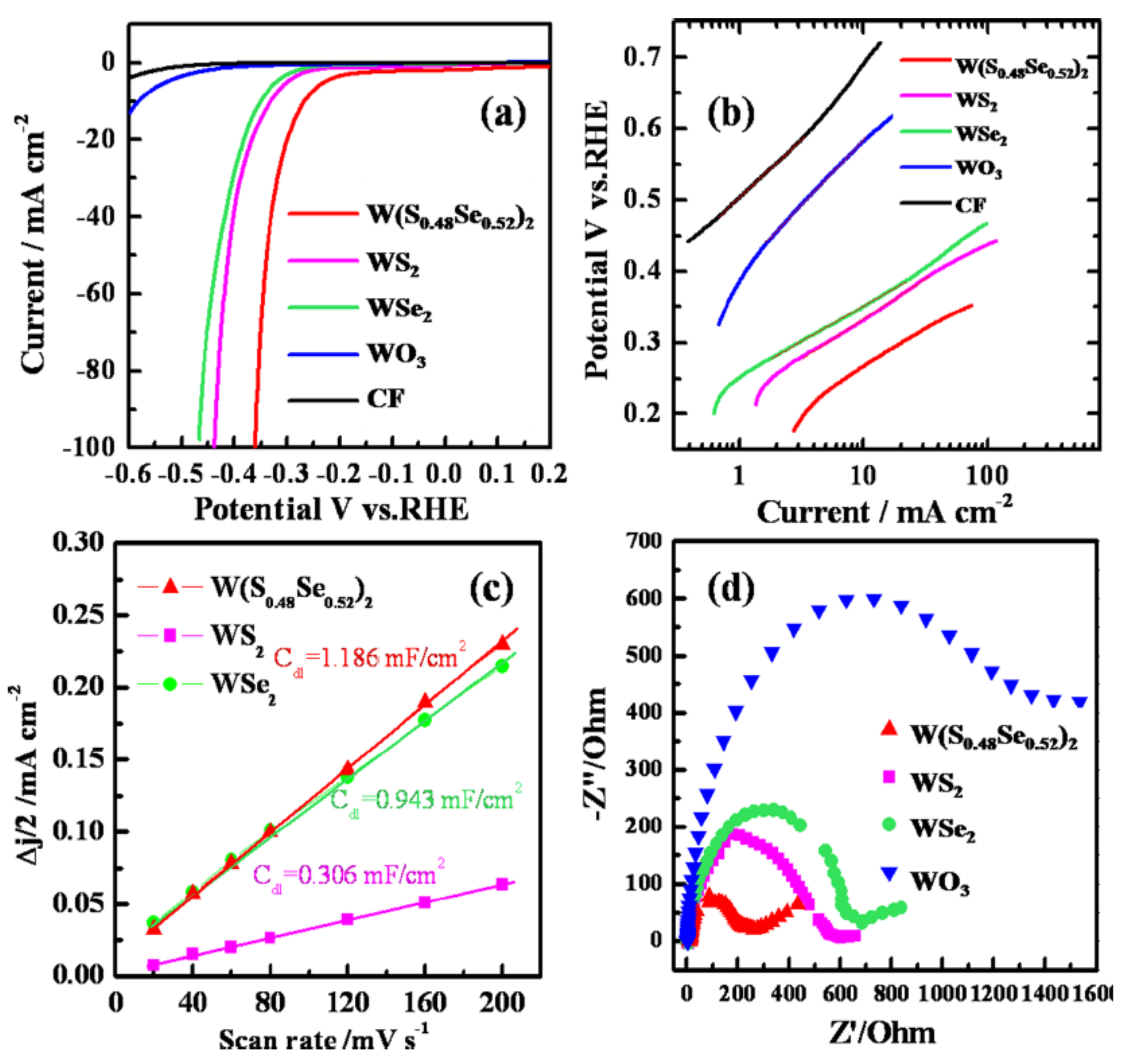
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1–x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Z.; Lupini, A.R.; Shi, G.; Lin, J.; Najmaei, S.; Lin, Z.; Elías, A.L.; Berkdemir, A.; You, G.; et al. Band Gap Engineering and Layer-by-Layer Mapping of Selenium-Doped Molybdenum Disulfide. Nano Lett. 2013, 14, 442–449. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Ham, D.J.; Hong, S.J.; Thongtem, S.; Lee, J.S. Synthesis of hexagonal WO3 nanowires by microwave-assisted hydrothermal method and their electrocatalytic activities for hydrogen evolution reaction. J. Mater. Chem. 2010, 20, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- Zibouche, N.; Ghorbani-Asl, M.; Heine, T.; Kuc, A. Electromechanical Properties of Small Transition-Metal Dichalcogenide Nanotubes. Inorganics 2014, 2, 155–167. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.C.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Chen, W.F.; Billo, T.; Lin, Y.G.; Fu, F.Y.; Samireddi, S.; Lee, C.H.; Hwang, J.S.; Chen, L.C.; Chen, L.-C. Beaded stream-like CoSe2 nanoneedle array for efficient hydrogen evolution electrocatalysis. J. Mater. Chem. A 2016, 4, 4553–4561. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.; Lin, J.Y.; Tai, S.Y.; Xiao, Y.; Lan, Z. A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim. Acta 2012, 85, 162–168. [Google Scholar] [CrossRef]
- Fan, M.S.; Lee, C.P.; Li, C.T.; Huang, Y.J.; Vittal, R.; Ho, K.C. Nitrogen-doped graphene/molybdenum disulfide composite as the electrocatalytic film for dye-sensitized solar cells. Electrochim. Acta 2016, 211, 164–172. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tseng, C.A.; Lee, C.P.; Ann, S.B.; Huang, Y.J.; Ho, K.C.; Chen, Y.T. N- and S-codoped graphene hollow nanoballs as an efficient Pt-free electrocatalyst for dye-sensitized solar cells. J. Power Sources 2020, 449, 227470. [Google Scholar] [CrossRef]
- Jamil, A.; Batool, S.S.; Sher, F.; Rafiq, M.A. Determination of density of states, conduction mechanisms and dielectric properties of nickel disulfide nanoparticles. AIP Adv. 2016, 6, 55120. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yao, H.B.; Gao, M.R.; Yu, S.H. Monodisperse cubic pyrite NiS2 dodecahedrons and microspheres synthesized by a solvothermal process in a mixed solvent: Thermal stability and magnetic properties. CrystEngComm 2009, 11, 1383–1390. [Google Scholar] [CrossRef]
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Two-dimensional transition metal dichalcogenide-based counter electrodes for dye-sensitized solar cells. RSC Adv. 2017, 7, 28234–28290. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gong, F.; Zhou, G.; Wang, Z.S. NiS2/Reduced Graphene Oxide Nanocomposites for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 6561–6566. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, Y.; Tian, T.; Hu, X.; Zeng, K.; Zhang, Y.W.; Zhang, Y.W. Structure, Stability, and Kinetics of Vacancy Defects in Monolayer PtSe2: A First-Principles Study. ACS Omega 2017, 2, 8640–8648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miro, P.; Heine, T.; Ghorbani-Asl, M. Two Dimensional Materials beyond MoS2: Noble-Transition-Metal Dichalcogenides. Angew. Chem. Int. Ed. 2014, 53, 3015–3018. [Google Scholar] [CrossRef]
- Hussain, S.; Patil, S.A.; Vikraman, D.; Arbab, A.A.; Jeong, S.H.; Kim, H.S.; Choi, H. Growth of a WSe2/W counter electrode by sputtering and selenization annealing for high-efficiency dye-sensitized solar cells. Appl. Surf. Sci. 2017, 406, 84–90. [Google Scholar] [CrossRef]










| GQD Ratio (V/V) (%) | PCE (%) | Voc (V) | Isc (mA cm−2) | FF (%) |
|---|---|---|---|---|
| 0 | 4.46 | 0.72 | 12.83 | 48.60 |
| 3 | 4.89 | 0.74 | 13.81 | 47.90 |
| 10 | 5.27 | 0.72 | 14.36 | 50.80 |
| 15 | 5.00 | 0.73 | 13.80 | 49.80 |
| 20 | 4.94 | 0.73 | 12.23 | 55.60 |
| 25 | 4.67 | 0.70 | 12.07 | 55.60 |
| 30 | 4.62 | 0.71 | 11.41 | 57.30 |
| Pt | 6.02 | 0.70 | 12.81 | 67.50 |
| CE with | Cathodic Current Density a (mA cm−2) | ΔEp a (mV) | Rctb (ohm) |
|---|---|---|---|
| PEDOT:PSS | −0.39 | 436 | 10.38 |
| GD-PEDOT:PSS | −1.58 | 347 | 7.92 |
| DSSC with | η (%) | Voc (mV) | Jsc (mA cm−2) | FF |
|---|---|---|---|---|
| PEDOT:PSS CE | 5.14 | 668 | 12.82 | 0.60 |
| GD-PEDOT:PSS CE | 7.36 | 718 | 14.70 | 0.70 |
| Catalyst | Morphology | Overpotential (mV) | Corresponding Tafel Slope (mV dec−1) | Current Density (mA cm−2) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|
| Core-shell MoO3-MoS2 | nanowire | 150–200 | 50–60 | N/A | 0.5 M H2SO4 | [23] |
| V-doped Ni3S2 | nanowire | 157 | 112 | N/A | 1 M KOH | [24] |
| WS2(1-x)Se2x /CFs | nanotube | 150–200 | 105 | 0.029 | 1 M H2SO4 | [27] |
| WSe2/CFs | nanotube | 250–300 | 99 | 0.003 | 1 M H2SO4 | [27] |
| WS2/CFs | nanotube | 250–300 | 113 | 0.0012 | 1 M H2SO4 | [27] |
| CoSe2-BSND | nanoneedle | 125 | 48 | 0.043 | 0.5 M H2SO4 | [33] |
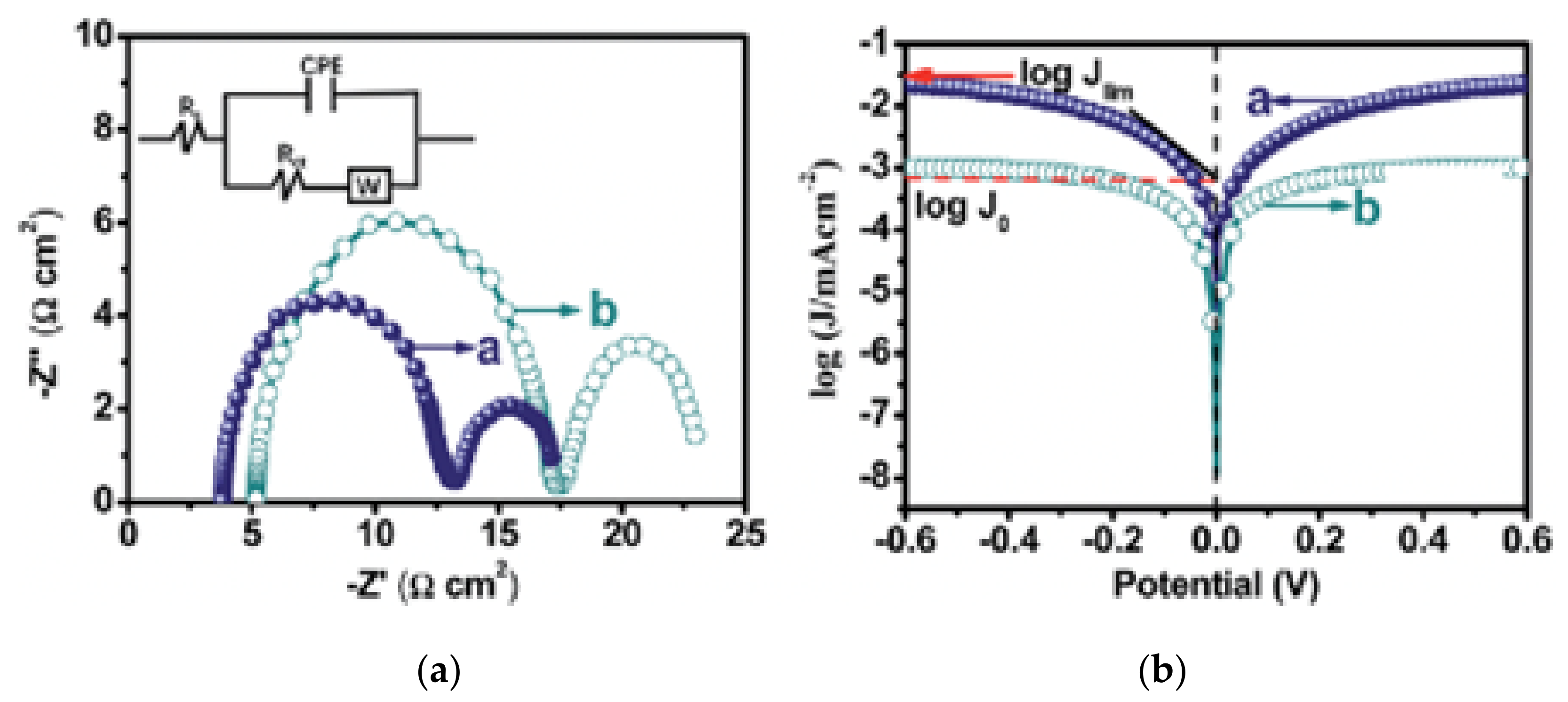
| CE | Jpc (mA cm−2) | ΔEp (V) | Jsc-IPCE (mA cm−2) | k0 (cm s−1) | Ae (cm2) | Rs (Ω cm2) | Rct (Ω cm2) | ZW (Ω cm2) | Rct-Tafel (Ω cm2) | J0 (mA cm−2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | 2.55 | 0.38 | 12.82 | 3.75 × 10–3 | 0.196 | 15.23 | 10.15 | 8.49 | 9.88 | 1.30 |
| NGr | 0.49 | 0.72 | 10.16 | 1.34 × 10–3 | 0.390 | 16.31 | 30.17 | 35.46 | 33.93 | 0.38 |
| MoS2 | 0.52 | 0.91 | 10.89 | 7.04 × 10–4 | 0.136 | 21.14 | 24.93 | 27.20 | 24.61 | 0.52 |
| NM-8 | 2.06 | 0.58 | 12.03 | 2.40 × 10–3 | 0.730 | 15.60 | 16.73 | 18.12 | 16.15 | 0.80 |
| CEs | Voc (mV) | Jsc (mA cm−2) | FF | PCE (%) | Rs (Ω cm2) | Rct (Ω cm2) | Epp (V) |
|---|---|---|---|---|---|---|---|
| NiS2 | 738 | 14.42 | 0.66 | 7.02 | 5.10 | 8.80 | 0.65 |
| NiS2@RGO | 749 | 16.55 | 0.69 | 8.55 | 6.40 | 2.90 | 0.50 |
| RGO | 716 | 10.98 | 0.40 | 3.14 | 14.20 | 100.20 | N/A |
| Pt | 739 | 15.75 | 0.70 | 8.15 | 2.20 | 0.50 | 0.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Xiao, Y.-H.; Chen, L.-J.; Tseng, C.-A.; Lee, C.-P. Low-Dimensional Nanostructures for Electrochemical Energy Applications. Physics 2020, 2, 481-502. https://doi.org/10.3390/physics2030027
Chen H-Y, Xiao Y-H, Chen L-J, Tseng C-A, Lee C-P. Low-Dimensional Nanostructures for Electrochemical Energy Applications. Physics. 2020; 2(3):481-502. https://doi.org/10.3390/physics2030027
Chicago/Turabian StyleChen, Hsin-Yu, Yi-Hong Xiao, Lin-Jiun Chen, Chi-Ang Tseng, and Chuan-Pei Lee. 2020. "Low-Dimensional Nanostructures for Electrochemical Energy Applications" Physics 2, no. 3: 481-502. https://doi.org/10.3390/physics2030027
APA StyleChen, H.-Y., Xiao, Y.-H., Chen, L.-J., Tseng, C.-A., & Lee, C.-P. (2020). Low-Dimensional Nanostructures for Electrochemical Energy Applications. Physics, 2(3), 481-502. https://doi.org/10.3390/physics2030027






