Dose Limits and Countermeasures for Mitigating Radiation Risk in Moon and Mars Exploration
Abstract
:1. Introduction
2. Dose Limits
2.1. NASA
2.2. RSA
2.3. ESA
2.4. JAXA
2.5. CSA
3. ICRP
4. Countermeasures
- -
- nuclear propulsion,
- -
- plasma,
- -
- ionic thrusters.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chancellor, J.; Scott, G.; Sutton, J. Space Radiation: The number one risk to astronaut health beyond low Earth orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Tessa, C.; Sivertz, M.; Chiang, I.H.; Lowenstein, D.; Rusek, A. Overview of the NASA space radiation laboratory. Life Sci. Space Res. 2016, 11, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraudo, M.; Schuy, C.; Weber, U.; Rovituso, M.; Santin, G.; Norbury, J.W.; Tracino, E.; Menicucci, A.; Bocchini, L.; Lobascio, C.; et al. Accelerator-based tests of shielding effectiveness of different materials and multilayers using high-energy light and heavy ions. Radiat. Res. 2018, 190, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Schneider, U.; Fogtman, A.; Kausch, C.; McKenna-Lawlor, S.; Narici, L.; Ngo-Anh, J.; Reitz, G.; Sabatier, L.; Santin, G.; et al. Research plans in Europe for radiation health hazard assessment in exploratory space missions. Life Sci. Space Res. 2019, 21, 73–82. [Google Scholar] [CrossRef]
- Mullenders, L.; Atkinson, M.; Paretzke, H.; Sabatier, L.; Bouffler, S. Assessing cancer risks of low-dose radiation. Nat. Rev. Cancer 2009, 9, 596–604. [Google Scholar] [CrossRef] [PubMed]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann. ICRP 2007, 37, 1–332. Available online: https://www.sciencedirect.com/journal/annals-of-the-icrp/vol/37/issue/2 (accessed on 25 January 2022).
- Preston, D.L.; Shimizu, Y.; Pierce, D.A.; Mabuchi, K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat. Res. 2003, 407, 381–407. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Physical basis of radiation protection in space travel. Rev. Mod. Phys. 2011, 83, 1245–1281. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef]
- Straube, U.; Berger, T.; Reitz, G.; Facius, R.; Fuglesang, C.; Reiter, T.; Damann, V.; Tognini, M. Operational radiation protection for astronauts and cosmonauts and correlated activities of ESA medical operations. Acta Astronaut. 2010, 66, 963–973. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [Green Version]
- Rühm, W.; Woloschak, G.E.; Shore, R.E.; Azizova, T.V.; Grosche, B.; Niwa, O.; Akiba, S.; Ono, T.; Suzuki, K.; Iwasaki, T.; et al. Dose and dose-rate effects of ionizing radiation: A discussion in the light of radiological protection. Radiat. Environ. Biophys. 2015, 54, 379–401. [Google Scholar] [CrossRef]
- Durante, M. Space radiation protection: Destination Mars. Life Sci. Space Res. 2014, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Little, M.P.; Day, S.; Charvat, J.; Blattnig, S.; Huff, J.; Patel, Z.S. Cancer incidence and mortality in the USA Astronaut Corps, 1959–2017. Occup. Environ. Med. 2021, 78, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Kronenberg, A. Ground-based research with heavy ions for space radiation protection. Adv. Space Res. 2005, 35, 180–184. [Google Scholar] [CrossRef]
- Ozasa, K.; Shimizu, Y.; Suyama, A.; Kasagi, F.; Soda, M.; Grant, E.J. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat. Res. 2012, 243, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Grant, E.J.; Brenner, A.; Sugiyama, H.; Sakata, R.; Sadakane, A.; Utada, M.; Cahoon, E.K.; Milder, C.M.; Soda, M.; Cullings, H.M.; et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958–2009. Radiat. Res. 2017, 187, 513–537. [Google Scholar] [CrossRef] [Green Version]
- Shuchman, M. Striving for Mars: What are acceptable risks? CMAJ 2014, 186, E7–E8. [Google Scholar] [CrossRef] [Green Version]
- Kahn, J.; Liverman, C.T.; Mccoy, M.A. Health Standards for Long Duration and Exploration Spaceflight; National Academies Press: Washington, DC, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222144/ (accessed on 25 January 2022).
- Luoni, F.; Horst, F.; Reidel, C.A.; Quarz, A.; Bagnale, L.; Sihver, L.; Weber, U.; Norman, R.B.; de Wet, W.; Giraudo, M.; et al. Total nuclear reaction cross-section database for radiation protection in space and heavy-ion therapy applications. New J. Phys. 2021, 23, 101201. [Google Scholar] [CrossRef]
- Tinganelli, W.; Luoni, F.; Durante, M. What can space radiation protection learn from radiation oncology? Life Sci. Space Res. 2021, 30, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Kim, M.H.Y.; Chappell, L.J. Space Radiation Cancer Risk Projections and Uncertainties—2012. Report NASA/TP-2013-217375; The NASA STI Program Office: Hanover, MD, USA, 2013. Available online: https://three.jsc.nasa.gov/articles/TP_2013_CancerRisk.pdf (accessed on 25 January 2022).
- NCRP. Radiation Protection Guidance for Activities in Low-Earth Orbit (2000); NCRP Report No. 132; NCRP: Bethesda, MD, USA, 2000; Available online: https://ncrponline.org/shop/reports/report-no-132 (accessed on 25 January 2022).
- NCRP. Information Needed to Make Radiation Protection Recommendations for Space Missions Beyond Low-Earth Orbit (2006); NCRP Report No. 153; NCRP: Bethesda, MD, USA, 2006; Available online: https://ncrponline.org/shop/reports/report-no-153 (accessed on 25 January 2022).
- McKenna-Lawlor, S.; Bhardwaj, A.; Ferrari, F.; Kuznetsov, N.; Lal, A.K.; Li, Y.; Nagamatsu, A.; Nymmik, R.; Panasyuk, M.; Petrov, V.; et al. Feasibility study of astronaut standardized career dose limits in LEO and the outlook for BLEO. Acta Astronaut. 2014, 104, 565–573. [Google Scholar] [CrossRef]
- National Academy of Sciences. Space Radiation and Astronaut Health; The National Academies Press: Washington, DC, USA, 2021. [CrossRef]
- Cucinotta, F.A.; Schimmerling, W.; Blakely, E.A.; Hei, T.K. A proposed change to astronaut exposures limits is a giant leap backwards for radiation protection. Life Sci. Space Res. 2021, 31, 59–70. [Google Scholar] [CrossRef]
- Petrov, V.M.; Kovalev, E.E.; Sakovich, V.A. Radiation: Risk and protection in manned space flight. Acta Astronaut. 1981, 8, 1091–1097. [Google Scholar] [CrossRef]
- Shafirkin, A.V.; Petrov, V.M.; Kolomensky, A.V.; Shurshakov, V.A. Lifetime total radiation risk of cosmonauts for orbotal and interplanetary flights. Adv. Space Res. 2002, 30, 999–1003. [Google Scholar] [CrossRef]
- Petrov, V.M. Radiation risk during long-term spaceflight. Adv. Space Res. 2002, 30, 989–994. [Google Scholar] [CrossRef]
- Menzel, H.-G.; Harrison, J. Effective dose: A radiation protection quantity. Ann. ICRP 2012, 41, 117–123. [Google Scholar] [CrossRef]
- ROSCOSMOS. Limitation of Cosmonaut Exposure during Near-Earth Space Flights; ROSCOSMOS: Moscow, Russia, 2021. [Google Scholar]
- ICRP. Recommendations of the International Commission on Radiological Protection. Publication 60. Ann. ICRP 1991, 21, 1–201. Available online: https://www.sciencedirect.com/journal/annals-of-the-icrp/vol/21/issue/1 (accessed on 25 January 2022).
- Dietze, G.; Bartlett, D.T.; Cool, D.A.; Cucinotta, F.A.; Jia, X.; McAulay, I.R.; Pelliccioni, M.; Petrov, V.; Reitz, G.; Sato, T. ICRP Publication 123: Assessment of radiation exposure of astronauts in space. Ann. ICRP 2013, 42, 1–339. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Krämer, M.; Durante, M. Ion beam transport calculations and treatment plans in particle therapy. Eur. Phys. J. D 2010, 60, 195–202. [Google Scholar] [CrossRef]
- Friedrich, T.; Scholz, U.; Elsässer, T.; Durante, M.; Scholz, M. Calculation of the biological effects of ion beams based on the microscopic spatial damage distribution pattern. Int. J. Radiat. Biol. 2012, 88, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chauvie, S.; Francis, Z.; Guatelli, S.; Incerti, S.; Mascialino, B.; Moretto, P.; Nieminen, P.; Pia, M.G. Geant4 physics processes for microdosimetry simulation: Design foundation and implementation of the first set of models. IEEE Trans. Nucl. Sci. 2007, 54, 2619–2628. [Google Scholar] [CrossRef] [Green Version]
- Schneider, U.; Walsh, L. Risk of secondary cancers: Bridging epidemiology and modeling. Phys. Med. 2017, 42, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Hafner, L.; Straube, U.; Ulanowski, A.; Fogtman, A.; Durante, M.; Weerts, G.; Schneider, U. A bespoke health risk assessment methodology for the radiation protection of astronauts. Radiat. Environ. Biophys. 2021, 60, 213–231. [Google Scholar] [CrossRef]
- Ulanowski, A.; Kaiser, J.C.; Schneider, U.; Walsh, L. Lifetime radiation risk of stochastic effects—Prospective evaluation for space flight or medicine. Ann. ICRP 2020, 49, 200–212. [Google Scholar] [CrossRef]
- Shkolnikov, V.; Barbieri, M.; Wilmoth, J. Human Mortality Databse. Available online: https://www.mortality.org (accessed on 25 January 2022).
- Komiyama, T. Practicalities of dose management for Japanese astronauts staying at the International Space Station. Ann. ICRP 2020, 49, 194–199. [Google Scholar] [CrossRef]
- Devleesschauwer, B.; Havelaar, A.H.; Maertens de Noordhout, C.; Haagsma, J.A.; Praet, N.; Dorny, P.; Duchateau, L.; Torgerson, P.R.; Van Oyen, H.; Speybroeck, N. Calculating disability-adjusted life years to quantify burden of disease. Int. J. Public Health 2014, 59, 565–569. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Lopez, A.D. Measuring the Global burden of disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Kai, M. Calculating disability-adjusted life years (DALY) as a measure of excess cancer risk following radiation exposure. J. Radiol. Prot. 2015, 35, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breckow, J. Do we really need the “detriment” for radiation protection? Radiat. Environ. Biophys. 2020, 59, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A. Space radiation risks for astronauts on multiple international space station missions. PLoS ONE 2014, 9, e96099. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Cosmic Rays: Hurdles on the Road to Mars. Nucl. Phys. News 2014, 24, 32–34. [Google Scholar] [CrossRef]
- ISECG. The Global Exploration Roadmap. January 2018; NASA: Washington, DC, USA, 2018. Available online: https://www.nasa.gov/sites/default/files/atoms/files/ger_2018_small_mobile.pdf (accessed on 25 January 2022).
- Little, M.P.; Azizova, T.V.; Hamada, N. Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: A review of the epidemiology. Int. J. Radiat. Biol. 2021, 97, 782–803. [Google Scholar] [CrossRef]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2017, 15, 167–180. [Google Scholar] [CrossRef]
- NCRP. Potential for Central Nervous System Effects from Radiation Exposure During Space Activities Phase I: Overview (2016); NCRP Commentary No. 25; NCRP: Bethesda, MD, USA, 2016; Available online: https://ncrponline.org/shop/commentaries/potential-for-central-nervous-system-effects-from-radiation-exposure-during-space-activities-phase-i-overview-2016/ (accessed on 25 January).
- NCRP. Potential Impact of Individual Genetic Susceptibility and Previous Radiation Exposure on Radiation Risk for Astronauts (2010); NCRP Report No. 167; NCRP: Bethesda, MD, USA, 2010; Available online: https://ncrponline.org/shop/reports/report-no-167 (accessed on 25 January).
- Kennedy, A.R. Biological effects of space radiation and development of effective countermeasures. Life Sci. Space Res. 2014, 1, 10–43. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, P.; Casolino, M.; Durante, M.; Mueller-Mellin, R.; Reitz, G.; Rossi, L.; Shurshakov, V.; Sorbi, M. Shielding from cosmic radiation for interplanetary missions: Active and passive methods. Radiat. Meas. 2007, 42, 14–23. [Google Scholar] [CrossRef]
- Wilson, J.W.; Cucinotta, F.; Shinn, J.; Simonsen, L.; Dubey, R.; Jordan, W.; Jones, T.; Chang, C.; Kim, M. Shielding from solar particle event exposures in deep space. Radiat. Meas. 1999, 30, 361–382. [Google Scholar] [CrossRef]
- Dobynde, M.I.; Shprits, Y.Y.; Drozdov, A.Y.; Hoffman, J.; Li, J. Beating 1 Sievert: Optimal radiation shielding of astronauts on a mission to Mars. Space Weather. 2021, 19, e2021SW002749. [Google Scholar] [CrossRef]
- Miller, J.; Zeitlin, C.; Cucinotta, F.A.; Heilbronn, L.; Stephens, D.; Wilson, J.W. Benchmark studies of the effectiveness of structural and internal materials as radiation shielding for the international space station. Radiat. Res. 2003, 159, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Schuy, C.; La Tessa, C.; Horst, F.; Rovituso, M.; Durante, M.; Giraudo, M.; Bocchini, L.; Baricco, M.; Castellero, A.; Fioreh, G.; et al. Experimental assessment of Lithium hydride’s space radiation shielding performance and Monte Carlo benchmarking. Radiat. Res. 2018, 191, 154. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Guetersloh, S.; Heilbronn, L.; Miller, J.; Elkhayari, N.; Empl, A.; LeBourgeois, M.; Mayes, B.W.; Pinsky, L.; Christl, M.; et al. Shielding experiments with high-energy heavy ions for spaceflight applications. New J. Phys. 2008, 10, 075007. [Google Scholar] [CrossRef]
- Viúdez-Moreiras, D. The ultraviolet radiation environment and shielding in pit craters and cave skylights on Mars. Icarus 2021, 370, 114658. [Google Scholar] [CrossRef]
- Czysz, P.A.; Bruno, C.; Chudoba, B. Future Spacecraft Propulsion Systems and Integration; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- National Academy of Sciences. Space Nuclear Propulsion for Human Mars Exploration; National Academies Press: Washington, DC, USA, 2021. [CrossRef]
- Durante, M.; Bruno, C. Impact of rocket propulsion technology on the radiation risk in missions to Mars. Eur. Phys. J. D 2010, 60, 215–218. [Google Scholar] [CrossRef]
- Ebrahimi, F. An Alfvenic reconnecting plasmoid thruster. J. Plasma Phys. 2020, 86, 905860614. [Google Scholar] [CrossRef]
- Mazouffre, S. Electric propulsion for satellites and spacecraft: Established technologies and novel approaches. Plasma Sources Sci. Technol. 2016, 25, 033002. [Google Scholar] [CrossRef]
- Rafalskyi, D.; Martínez, J.M.; Habl, L.; Zorzoli Rossi, E.; Proynov, P.; Boré, A.; Baret, T.; Poyet, A.; Lafleur, T.; Dudin, S.; et al. In-orbit demonstration of an iodine electric propulsion system. Nature 2021, 599, 411–415. [Google Scholar] [CrossRef] [PubMed]

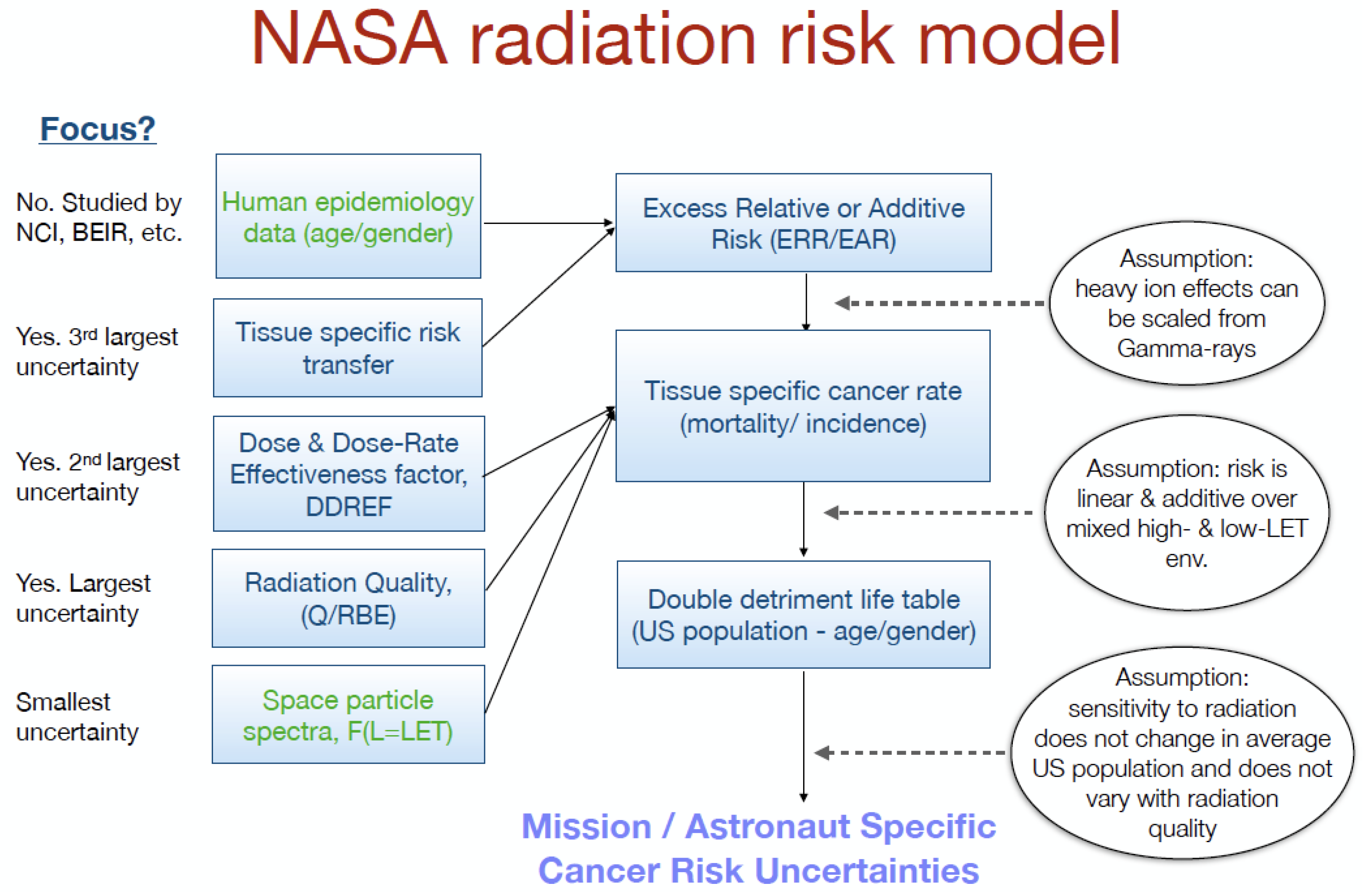

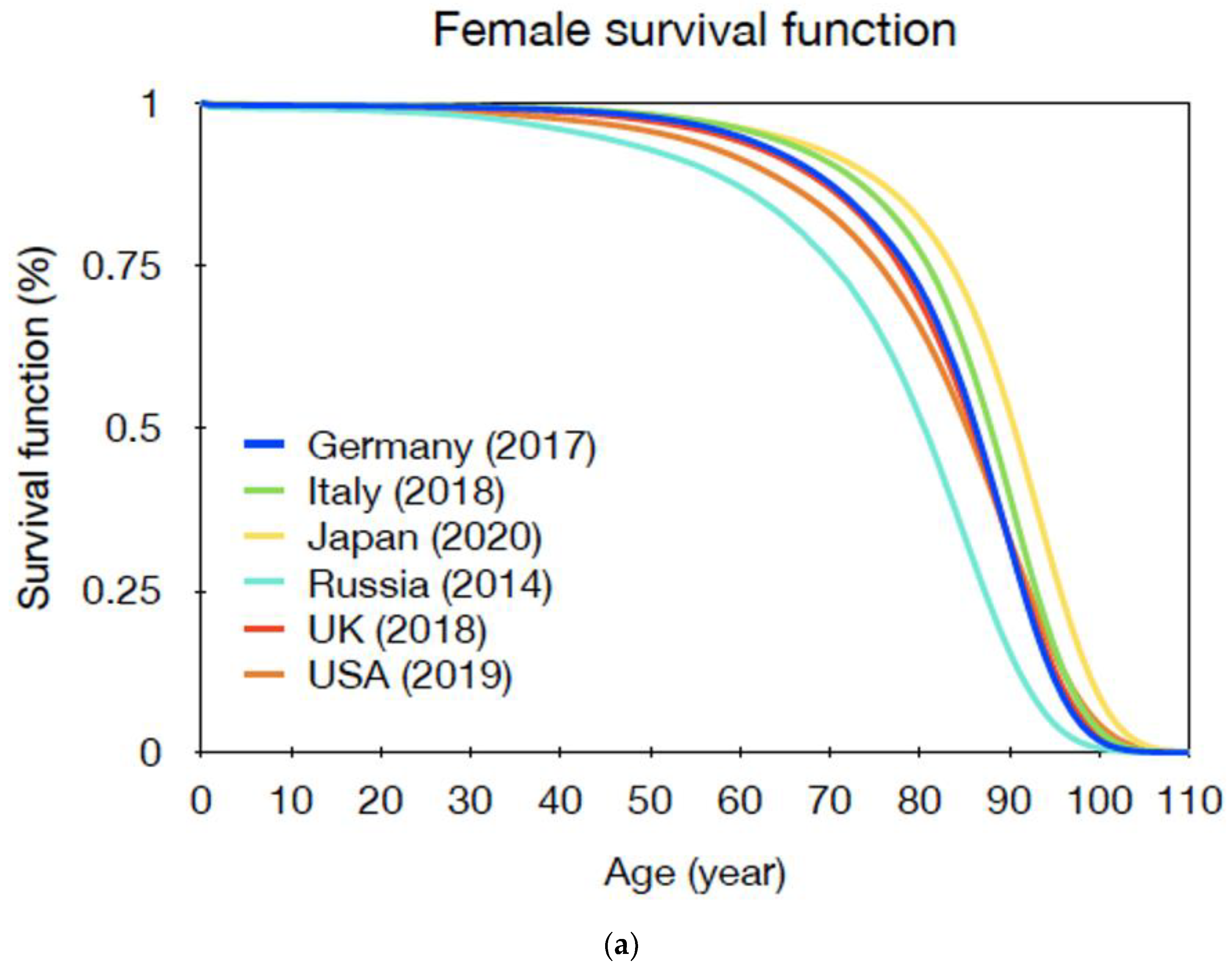
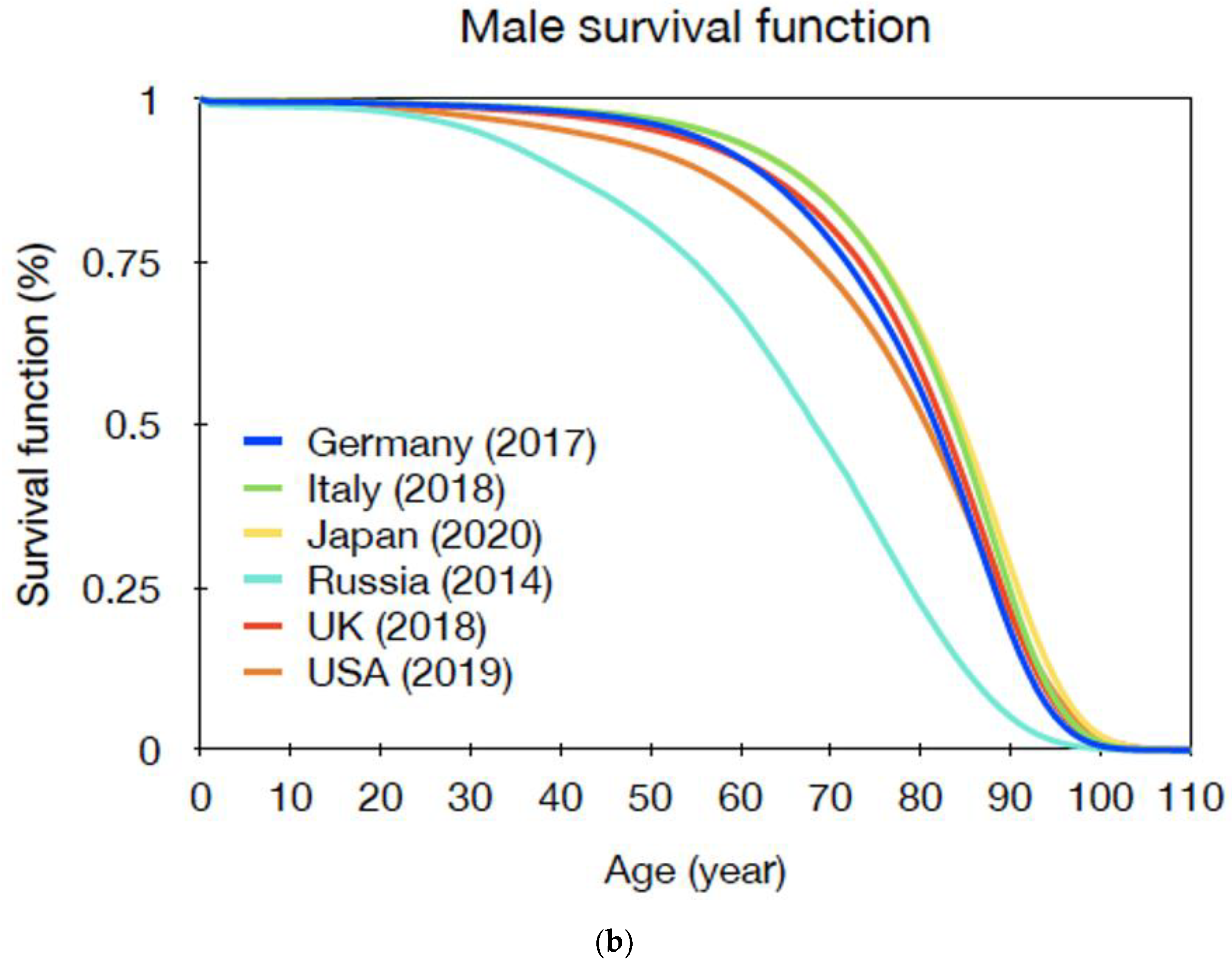
| Gender | Age at Exposure | |||
|---|---|---|---|---|
| 30 | 40 | 50 | 60 | |
| Female | 0.60 | 0.70 | 0.82 | 0.98 |
| Male | 0.78 | 0.88 | 1.00 | 1.17 |
| Calculated Values | Age [y] | Mean Tissue Equivalent Dose [cSv] | ||
|---|---|---|---|---|
| 100 | 125 | 150 | ||
| Generalized dose [cSv] | - | 53.8 | 65.6 | 100 |
| Total radiation risk [%] | - | 7.00 | 8.53 | 12.0 |
| Radiation risk of cancer [%] | 30 | 3.60 | 4.50 | 5.40 |
| 40 | 2.36 | 2.95 | 3.54 | |
| 50 | 1.83 | 2.29 | 2.74 | |
| Mean lifetime reduction [y] | 30 | 2.42 | 2.95 | 3.49 |
| 40 | 2.16 | 2.63 | 3.10 | |
| 50 | 1.89 | 2.30 | 2.71 | |
| Age at the First Space Flight | Female | Male |
|---|---|---|
| 27–30 | 0.5 | 0.6 |
| 31–35 | 0.6 | 0.7 |
| 36–40 | 0.65 | 0.8 |
| 41–45 | 0.75 | 0.95 |
| >45 | 0.8 | 1.0 |
| DALY(c,s,a,t) = TLD(c,s,a,t) + YLL(c,s,a,t) |
| YLL(c,s,a,t) = N(c,s,a,t) × LE(s,a) |
| YLD(c,s,a,t) = I(c,s,a,t) × DW(c,s,a) × LD(c,s,a,t) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscolo, D.; Durante, M. Dose Limits and Countermeasures for Mitigating Radiation Risk in Moon and Mars Exploration. Physics 2022, 4, 172-184. https://doi.org/10.3390/physics4010013
Boscolo D, Durante M. Dose Limits and Countermeasures for Mitigating Radiation Risk in Moon and Mars Exploration. Physics. 2022; 4(1):172-184. https://doi.org/10.3390/physics4010013
Chicago/Turabian StyleBoscolo, Daria, and Marco Durante. 2022. "Dose Limits and Countermeasures for Mitigating Radiation Risk in Moon and Mars Exploration" Physics 4, no. 1: 172-184. https://doi.org/10.3390/physics4010013
APA StyleBoscolo, D., & Durante, M. (2022). Dose Limits and Countermeasures for Mitigating Radiation Risk in Moon and Mars Exploration. Physics, 4(1), 172-184. https://doi.org/10.3390/physics4010013






