Nanocomposite-Supported Polymeric Composites Prepared with Different Deposition Bases: Characterization and Application in X-ray Shielding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetite NPs
2.2. Fabrication of Nanocomposites
2.3. Characterization Techniques
3. Results and Discussion
3.1. Effect of Sedimentation Rules on Structure Description
3.2. Effect of Different Sedimentation Rules on Surface Area
3.3. Effect of Sedimentation Rules on Powder Morphology
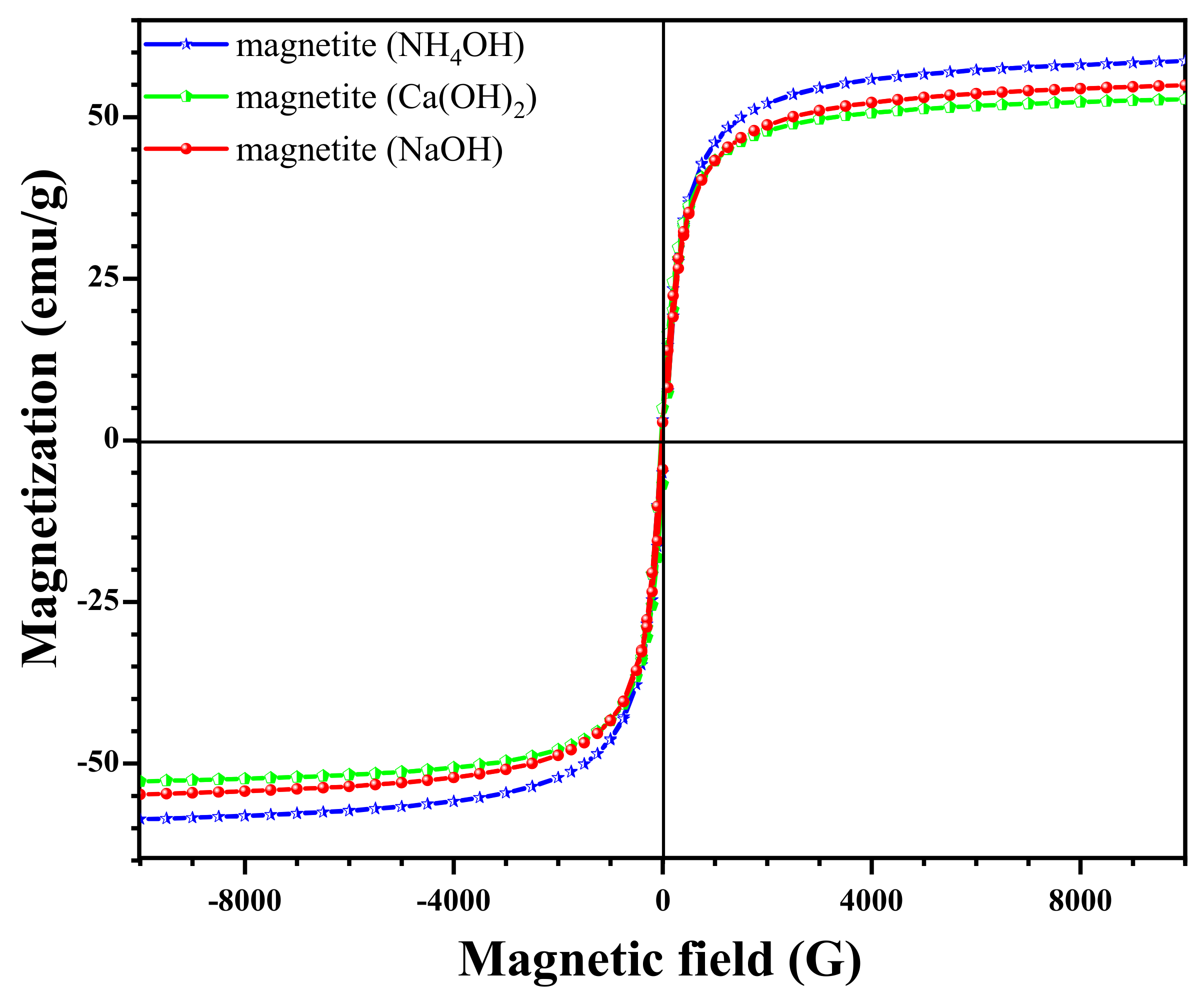
3.4. Effect of Different Sedimentation Rules on Magnetic Properties
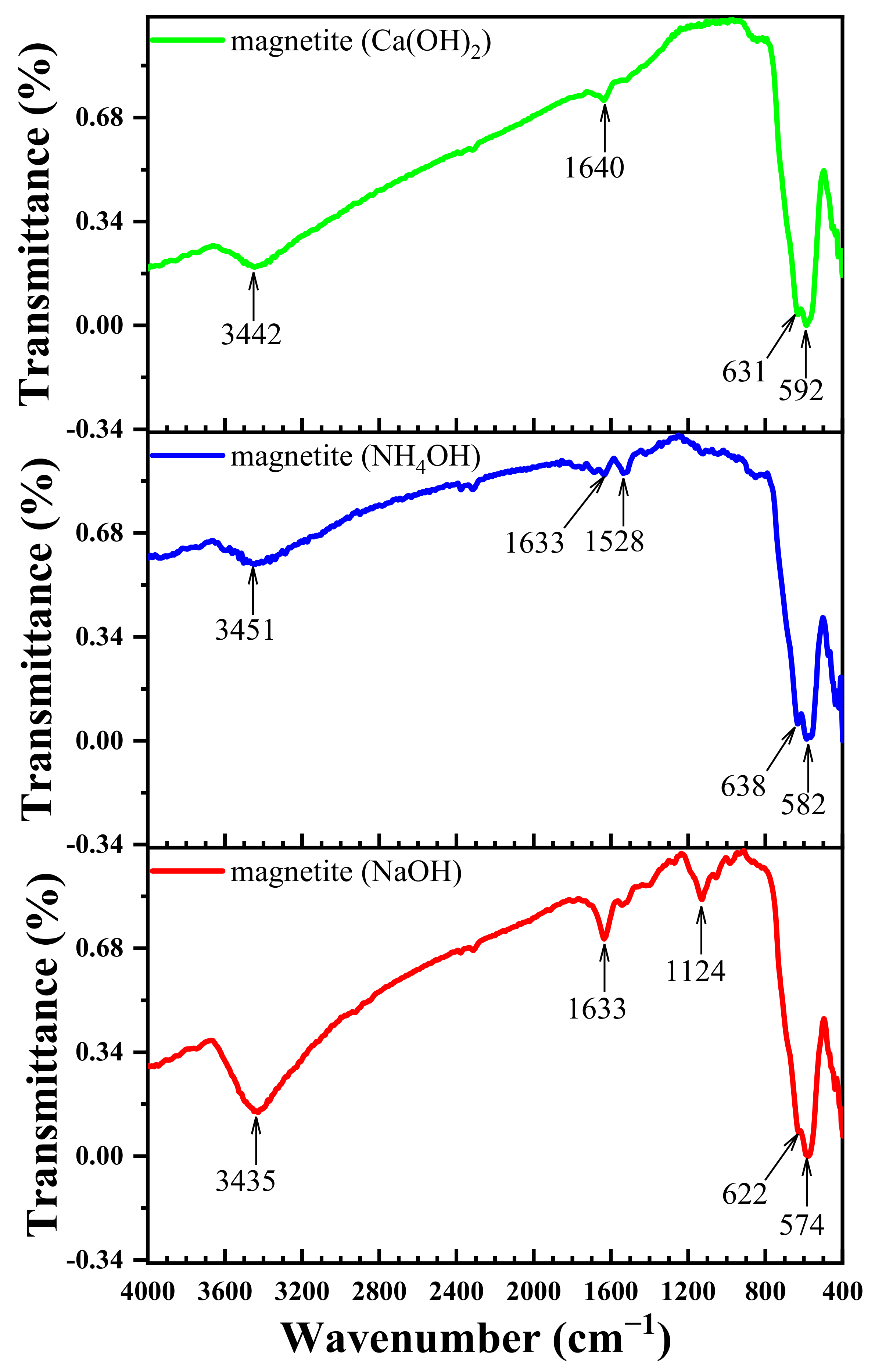
3.5. Effect of Different Deposition Rules on the FT-IR Spectra
3.6. The Effect of the Size of Magnetite NPs on Optical Properties
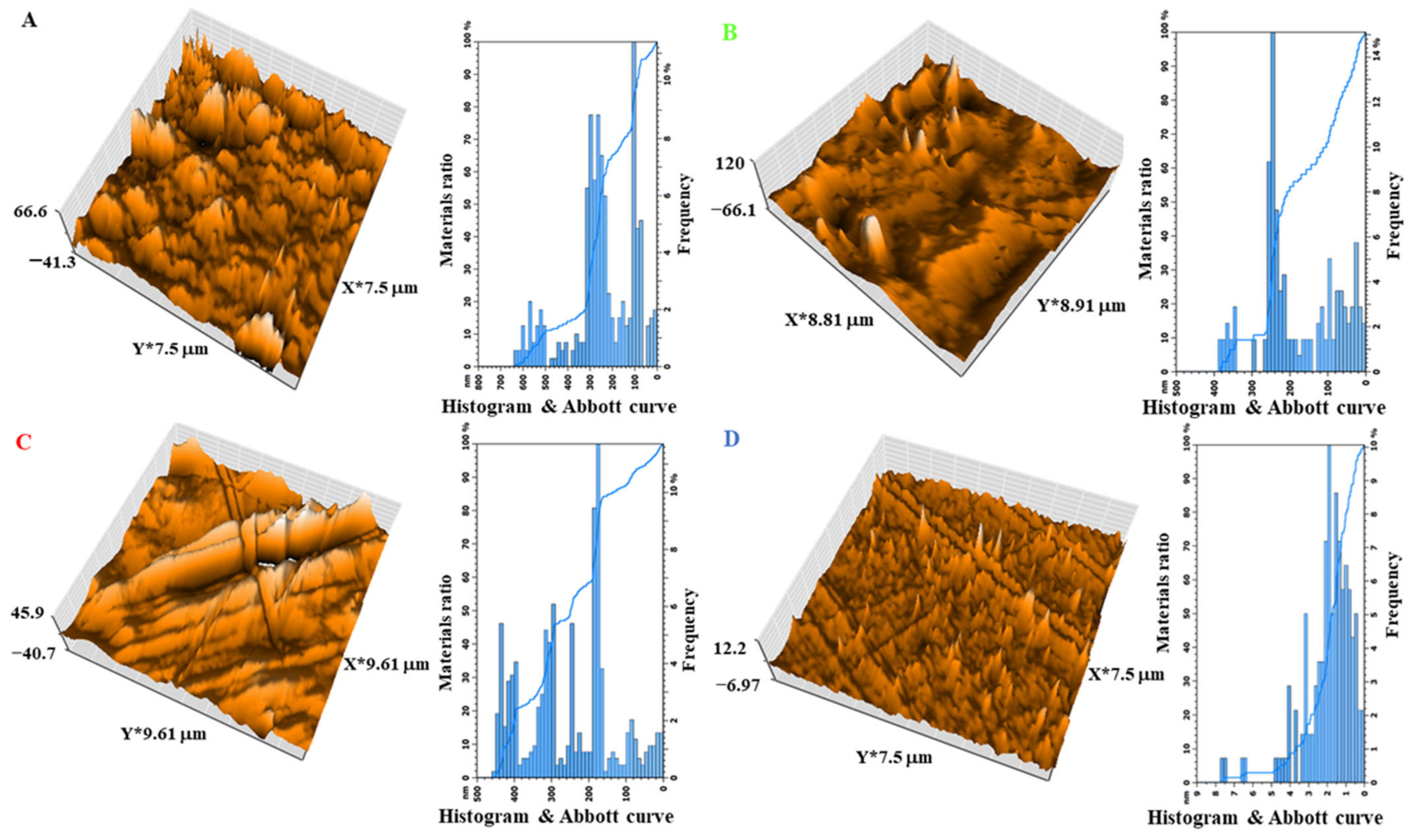
3.7. The Effect of the Size of Magnetite NPs on the XRD and AFM of the Shields
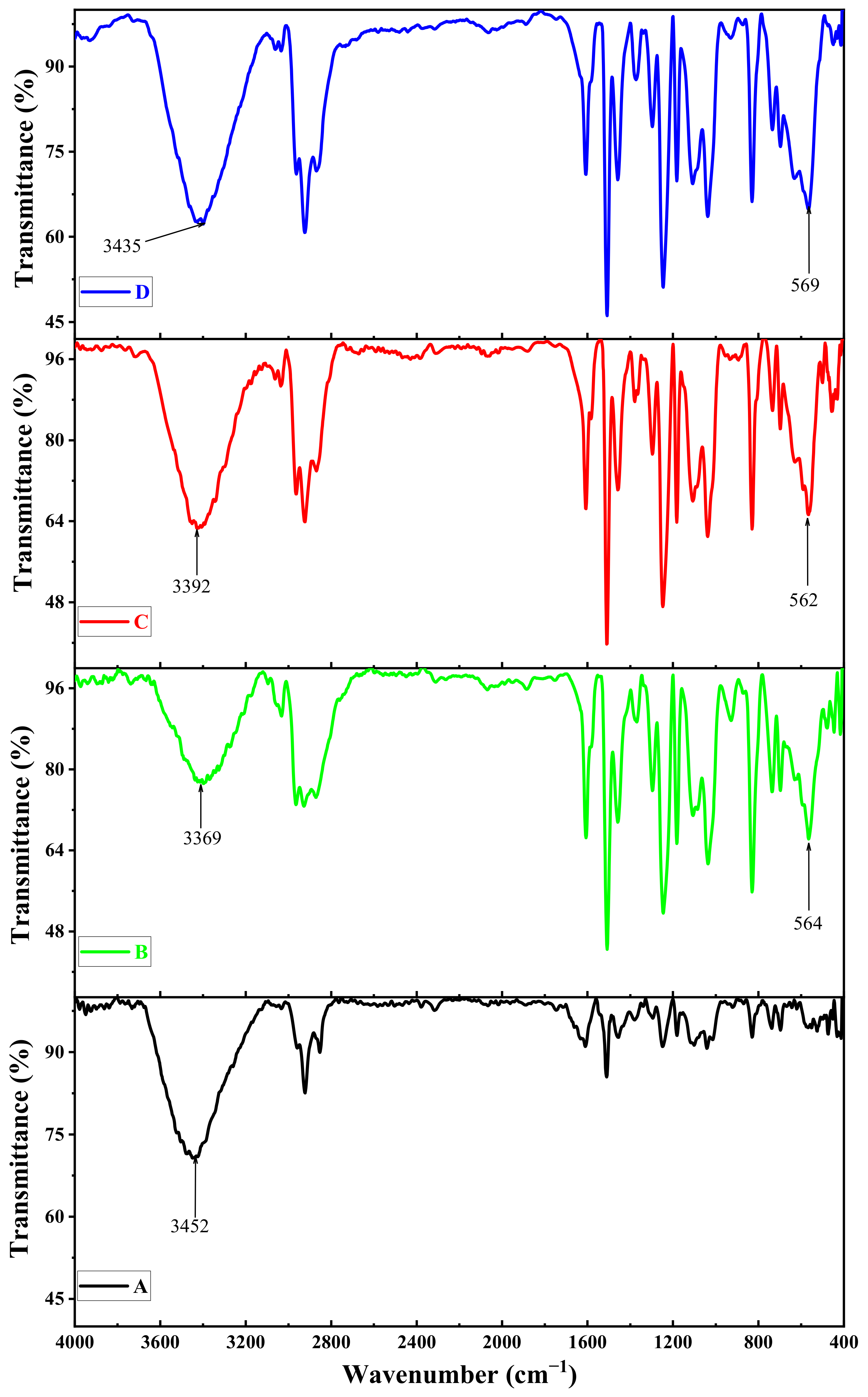
3.8. The Effect of the Size of Magnetite NPs on the FT-IR Spectra of the Shields
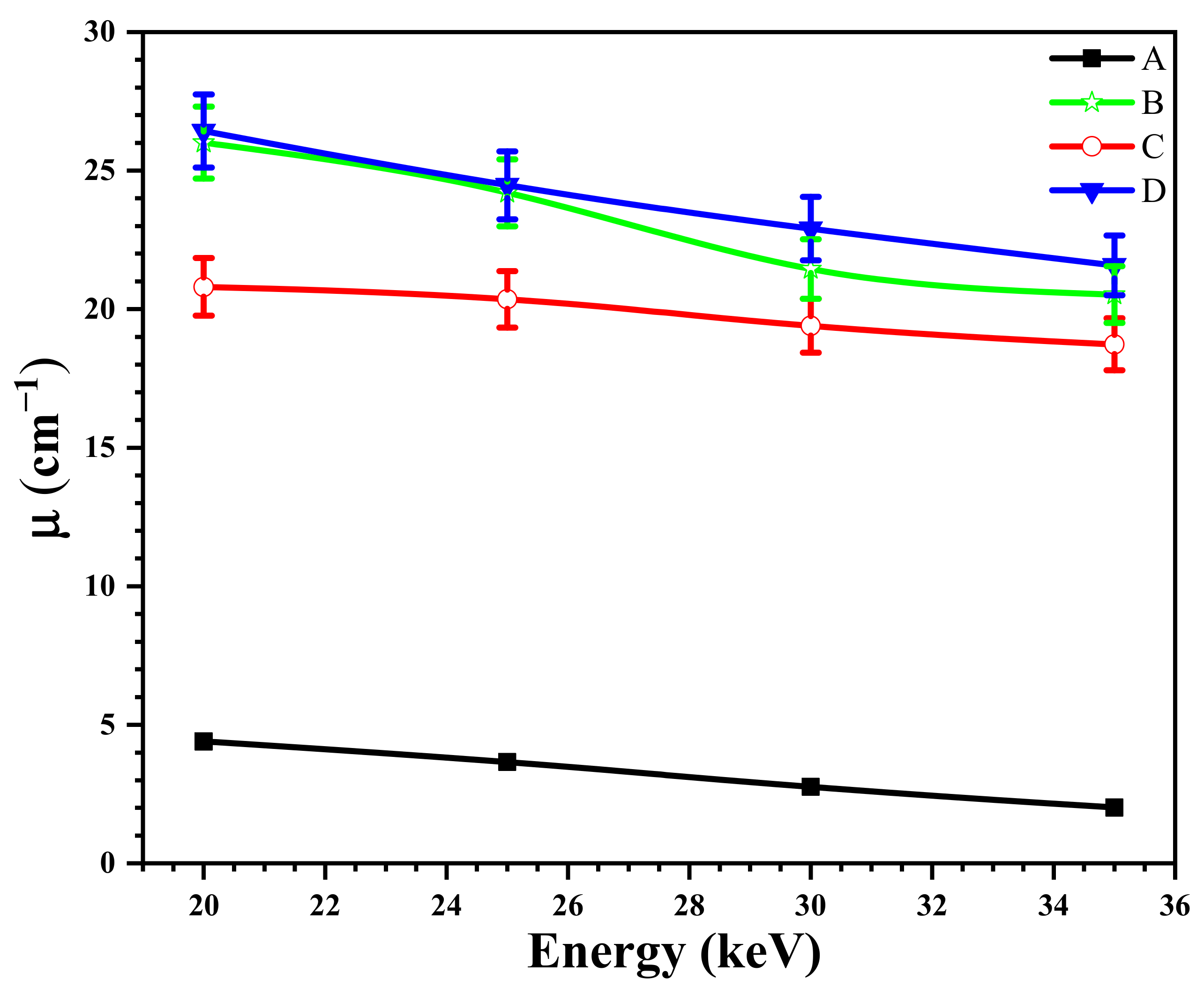
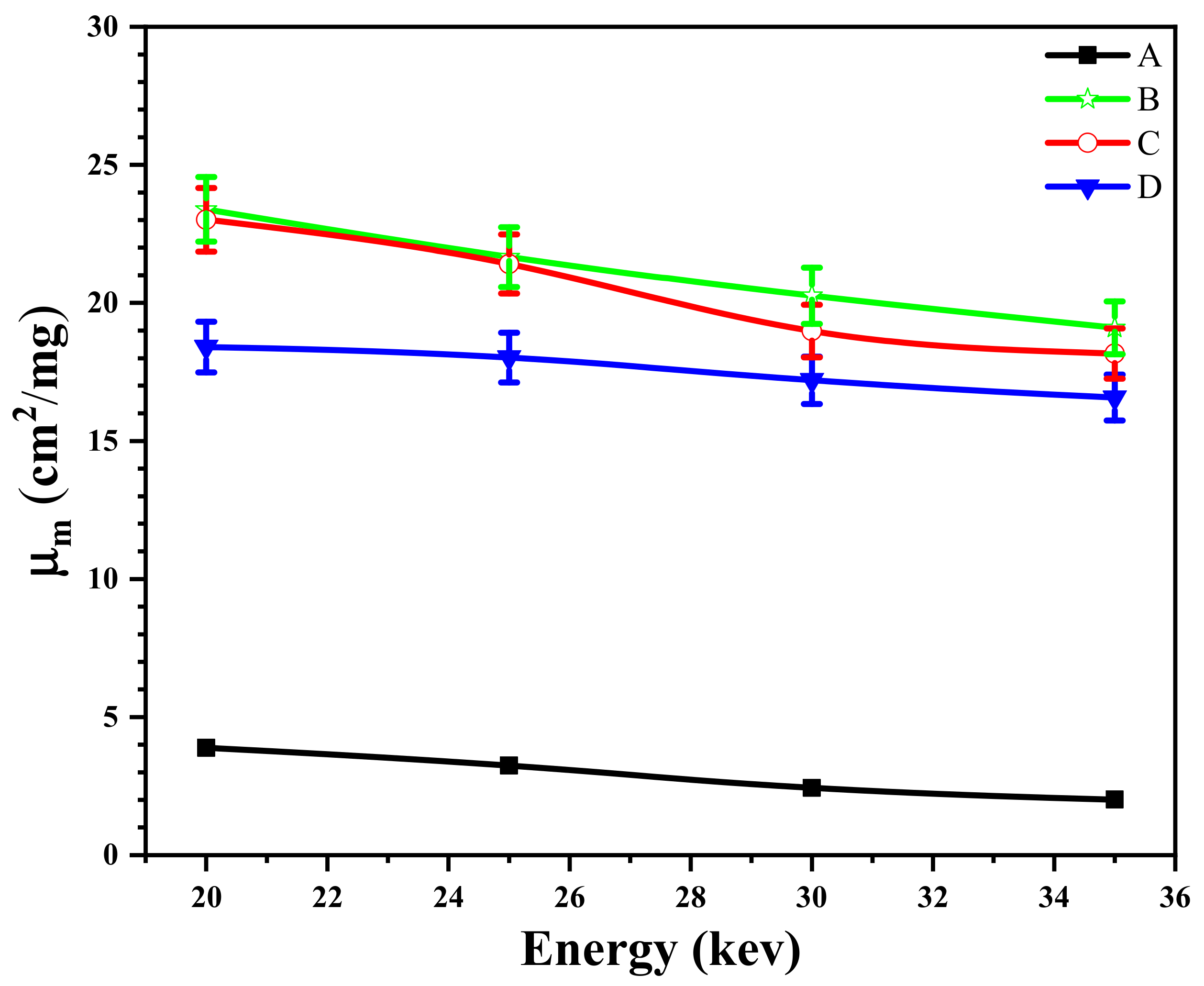
3.9. The Effect of the Size of Magnetite NPs on the X-ray Attenuation Properties of Shields
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pavlenko, V.I.; Cherkashina, N.I.; Yastrebinsky, R.N. Synthesis and radiation shielding properties of polyimide/Bi2O3 composites. Heliyon 2019, 5, e01703. [Google Scholar] [CrossRef]
- Aghaz, A.; Faghihi, R.; Mortazavi, S.M.J.; Haghparast, A.; Mehdizadeh, S.; Sina, S. Radiation attenuation properties of shields containing micro and Nano WO3 in diagnostic X-ray energy range. Int. J. Radiat. Res. 2016, 14, 127–131. [Google Scholar] [CrossRef]
- Afkham, Y.; Mesbahi, A.; Alemi, A.; Zolfagharpour, F.; Jabbari, N. Design and fabrication of a nano-based neutron shield for fast neutrons from medical linear accelerators in radiation therapy. Radiat. Oncol. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Saravanan, T.; Philip, J. Preparation, characterization and X-ray attenuation property of Gd2O3-based nanocomposites. Appl. Nanosci. 2017, 7, 919–931. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Ge, M.; Wu, S.; Guan, J.; Tang, J.; Wu, X.; Ritchie, R.O. Facile self-assembly synthesis of γ-Fe2O3/graphene oxide for enhanced photo-Fenton reaction. Eviron. Pollut. 2019, 248, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, X.; Gong, G.; Zhu, F.; Wang, J.; Qi, Y.; Huang, K.; Wu, J.; Chen, R.; Liu, Y. Recent progress in γ-Fe2O3-based catalysts: An overview of the synthesis and applications in environmental remediation. Chem. Eng. J. 2023, 475, 146198. [Google Scholar] [CrossRef]
- Anik, M.I.; Khalid Hossain, M.; Hossain, I.; Mahfuz, A.M.U.B.; Tayebur Rahman, M.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Kadhim, R.M.; Al-Abodi, E.E.; Al-Alawy, A.F. Citrate-coated magnetite nanoparticles as osmotic agent in a forward osmosis process. Desalin. Water Treat. 2018, 115, 45–52. [Google Scholar] [CrossRef]
- Noor Azman, N.Z.; Siddiqui, S.A.; Low, I.M. Characterisation of micro-sized and nano-sized tungsten oxide-epoxy composites for radiation shielding of diagnostic X-rays. Mater. Sci. Eng. C 2013, 33, 4952–4957. [Google Scholar] [CrossRef]
- Al-Saadi, S.N.; Al-Hussainy, R.M.; Al-Zubaydi, J.H.A. Rock Slope Stability Assessment for Rock Cliffs at Tar Al-Sayyed Area (Karbala Governorate Middle of Iraq). J. Kerbala Univ. 2014, 12, 35–52. Available online: https://www.iasj.net/iasj/article/87711 (accessed on 27 December 2023).
- Castellanos-Rubio, I.; Arriortua, O.; Iglesias-Rojas, D.; Barón, A.; Rodrigo, I.; Marcano, L.; Garitaonandia, J.S.; Orue, I.; Fdez-Gubieda, M.L.; Insausti, M. A Milestone in the chemical synthesis of Fe3O4 nanoparticles: Unreported bulklike properties lead to a remarkable magnetic hyperthermia. Chem. Mater. 2021, 33, 8693–8704. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef]
- Daoush, W.M. Co-precipitation and magnetic properties of magnetite nanoparticles for potential biomedical applications. J. Nanomed. Res. 2017, 5, 00018. [Google Scholar] [CrossRef]
- Badawy, S.M.; Abd El-Latif, A.A. Synthesis and characterizations of magnetite nanocomposite films for radiation shielding. Polym. Compos. 2017, 38, 974–980. [Google Scholar] [CrossRef]
- Rashid, H.; Mansoor, M.A.; Haider, B.; Nasir, R.; Abd Hamid, S.B.; Abdulrahman, A. Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method. Sep. Sci. Technol. 2020, 55, 1207–1215. [Google Scholar] [CrossRef]
- Jannah, N.R.; Onggo, D. Synthesis of Fe3O4 nanoparticles for colour removal of printing ink solution. J. Phys. Conf. Ser. 2019, 124, 012040. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haque, M.E.; Akter, M.; Ferdous, H.A. Synthesis of bismuth ferrite nanoparticles by modified Pechini sol-gel method. Int. J. Sci. Eng. Investig. 2020, 9(101), 35–39. Available online: https://www.ijsei.com/archive-910120.htm (accessed on 27 December 2023).
- Sallam, O.I.; Madbouly, A.M.; Ezz-Eldin, F.M. Impact of Nd3+ additive on the radiation shielding competence of borosilicate glasses fabricated from agro-waste materials. J. Non-Cryst. Solids 2022, 590, 121691. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, Z.; Badawi, M.S.; Awad, R.; Thabet, A.A.; El-Khatib, A.M. Study of some γ-ray attenuation parameters for new shielding materials composed of nano ZnO blended with high density polyethylene. Nucl. Technol. Radiat. Prot. 2019, 34, 342–352. [Google Scholar] [CrossRef]
- Alsayed, Z.; Awad, R.; Badawi, M.S. Thermo-mechanical properties of high density polyethylene with zinc oxide as a filler. Iran. Polym. J. 2020, 29, 309–320. [Google Scholar] [CrossRef]
- Alsayed, Z.; Badawi, M.S.; Awad, R. Characterization of zinc ferrite nanoparticles capped with different PVP concentrations. J. Electron. Mater. 2019, 48, 4925–4933. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Doma, A.S.; Badawi, M.S.; Abu-Rayan, A.E.; Aly, N.S.; Alzahrani, J.S.; Abbas, M.I. Conductive natural and waste rubbers composites-loaded with lead powder as environmental flexible gamma radiation shielding material. Mater. Res. Express 2020, 7, 105309. [Google Scholar] [CrossRef]
- More, C.; Pawar, P.P.; Badawi, M.S.; Thabet, A.A. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2020, 35, 138–149. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, A.M.; Yousef, N.S.; Ghatass, Z.F.; Badawi, M.S.; Mohamed, M.M.; Elkhatib, M. Synthesized silver carbon nanotubes and zinc oxide nanoparticles and their ability to remove methylene blue dye. J. Nano Res. 2019, 56, 1–16. [Google Scholar] [CrossRef]
- Osman, A.F.; El Balaa, H.; El Samad, O.; Awad, R.; Badawi, M.S. Assessment of X-ray shielding properties of polystyrene incorporated with different nano-sizes of PbO. Radiat. Environ. Biophys. 2023, 62, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Obeid, A.; El Balaa, H.; El Samad, O.; Awad, R.; Badawi, M.S. Attenuation parameters of HDPE filled with different nano-size and bulk WO3 for X-ray shielding applications. Eur. Phys. J. Plus 2022, 137, 1229. [Google Scholar] [CrossRef]
- Osman, A.F.; El Balaa, H.; El Samad, O.; Alsayed, Z.; Awad, R.; Badawi, M.S. Effect of PbO incorporation with different particle size on X-ray attenuation of polystyrene. Nucl. Technol. Rad. Protect. 2022, 37, 18–30. [Google Scholar] [CrossRef]
- Obeid, A.; El Balaa, H.; El Samad, O.; Alsayed, Z.; Awad, R.; Badawi, M.S. Effects of different nano size and bulk WO3 enriched by HDPE composites on attenuation of the X-ray narrow spectrum. Nucl. Technol. Rad. Protect. 2021, 36, 315–328. Available online: https://www.ntrp.vinca.rs/2021_4/Contents2021_4.html (accessed on 27 December 2023).
- Chang, Q.; Guo, S.; Zhang, X. Sustainable natural biopolymers for biomedical applications. Mater. Des. 2023, 233, 112253. [Google Scholar] [CrossRef]
- National Council on Radiation Protection and Measurements. Structural Shielding Design and Evaluation for Megavoltage X- and Gamma-Ray Radiotherapy Facilities. NCRP Report No. 151; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 2005; Available online: https://www.severin.su/wp-content/uploads/2020/01/ncrp_151_structural_shielding.pdf (accessed on 27 December 2023).
- Stirling, D.A. The Nanotechnology Revolution: A Global Bibliographic Perspective; Jenny Stanford Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Reda, S.M. Gamma ray shielding by a new combination of aluminum, iron, copper and lead using MCNP5. Arab. J. Nucl. Sci. Appl. 2016, 49(4), 211–217. [Google Scholar]
- Al-Alawy, A.F.; Al-Abodi, E.E.; Kadhim, R.M. Synthesis and characterization of magnetic iron oxide nanoparticles by co-precipitation method at different conditions. J. Eng. 2018, 24, 60–72. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Jiang, C.; Ji, V.; Chen, M.; Zhan, K.; Moreira, F. Investigation on microstructure and properties of electrodeposited Ni-Ti-CeO2 composite coating. J. Alloys Compd. 2018, 754, 93–104. [Google Scholar] [CrossRef]
- Ravidhas, C.; Josephine, A.J.; Sudhagar, P.; Devadoss, A.; Terashima, C.; Nakata, K.; Fujishima, A.; Raj, A.M.E.; Sanjeeviraja, C. Facile synthesis of nanostructured monoclinic bismuth vanadate by a co-precipitation method: Structural, optical and photocatalytic properties. Mater. Sci. Semicond. Process. 2015, 30, 343–351. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villarea, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite nanoparticles coated with PEG 3350-Tween 80: In vitro characterization using primary cell cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, T.; Harvey, M. Global marketing networks and the development of trust: A dynamic capabilities perspective. J. Market. Chann. 2004, 11, 21–41. [Google Scholar] [CrossRef]
- Larumbe, S.; Gómez-Polo, C.; Pérez-Landazábal, J.I.; García-Prieto, A.; Alonso, J.; Fdez-Gubieda, M.L.; Cordero, D.; Gómez, J. Ni doped Fe3O4 magnetic nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2652–2660. [Google Scholar] [CrossRef]
- Zukova, A.; Teiserskis, A.; Rohava, Y.; Baranov, A.V.; van Dijken, S.; Gun’ko, Y.K. Deposition of magnetite nanofilms by pulsed injection MOCVD in a magnetic field. Nanomaterials 2018, 8, 1064. [Google Scholar] [CrossRef]
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Effect of co-doping on the optical, magnetic and photocatalytic properties of the Gd modified BiFeO3. J. Mater. Sci. Mater. Electron. 2016, 27, 5699–5706. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M.I.; Waqas, M.; Hussain, M.N.; Abbas, N. Eco-friendly synthesis of magnetite (Fe3O4) nanoparticles with tunable size: Dielectric, magnetic, thermal and optical studies. Mater. Chem. Phys. 2017, 198, 229–235. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Mansouri, E.; Mesbahi, A.; Malekzadeh, R.; Mansouri, A. Shielding characteristics of nanocomposites for protection against X- and gamma rays in medical applications: Effect of particle size, photon energy and nano-particle concentration. Radiat. Environ. Biophys. 2020, 59, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Slimani, S.; Meneghini, C.; Abdolrahimi, M.; Talone, A.; Murillo, J.P.M.; Barucca, G.; Yaacoub, N.; Imperatori, P.; Illés, E.; Smari, M.; et al. Spinel iron oxide by the co-precipitation method: Effect of the reaction atmosphere. Appl. Sci. 2021, 11, 5433. [Google Scholar] [CrossRef]
- Yazid, N.A.; Joon, Y.C. Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater. AIP Conf. Proc. 2019, 2124, 020019. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A. Advanced Inorganic Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 1999; Available online: https://www.scribd.com/document/684184510/Cotton-F-Wilkinson-G-Murillo-C-Bochmann-M-Advanced-Inorganic-Chemistry-John-Wiley-Sons-1999 (accessed on 27 December 2023).
- Peternele, W.S.; Fuentes, V.M.; Fascineli, M.L.; Da Silva, J.R.; Silva, R.C.; Lucci, C.M.; De Azevedo, R.B. Experimental investigation of the coprecipitation method: An approach to obtain magnetite and maghemite nanoparticles with improved properties. J. Nanomater. 2014, 2014, 682985. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Chauhan, P. Influence of annealing temperature on microstructural and magnetic properties of Fe2O3 nanoparticles synthesized via sol-gel method. Inorg. Nano-Met. Chem. 2022, 52, 937–950. [Google Scholar] [CrossRef]
- Namikuchi, E.A.; Gaspar, R.D.L.; Da Silva, D.S.; Raimundo, I.M.; Mazali, I.O. PEG size effect and its interaction with Fe3O4 nanoparticles synthesized by solvothermal method: Morphology and effect of pH on the stability. Nano Express 2021, 2, 020022. [Google Scholar] [CrossRef]
- Abdallah, R.M.; Al-Haddad, R.M.S. Optical and morphology properties of the magnetite (Fe3O4) nanoparticles prepared by Green method. J. Phys. Conf. Ser. 2021, 1829, 012022. [Google Scholar] [CrossRef]
- Botelho, M.Z.; Künzel, R.; Okuno, E.; Levenhagen, R.S.; Basegio, T.; Bergmann, C.P. X-ray transmission through nanostructured and microstructured CuO materials. Appl. Radiat. Isot. 2011, 69, 527–530. [Google Scholar] [CrossRef] [PubMed]

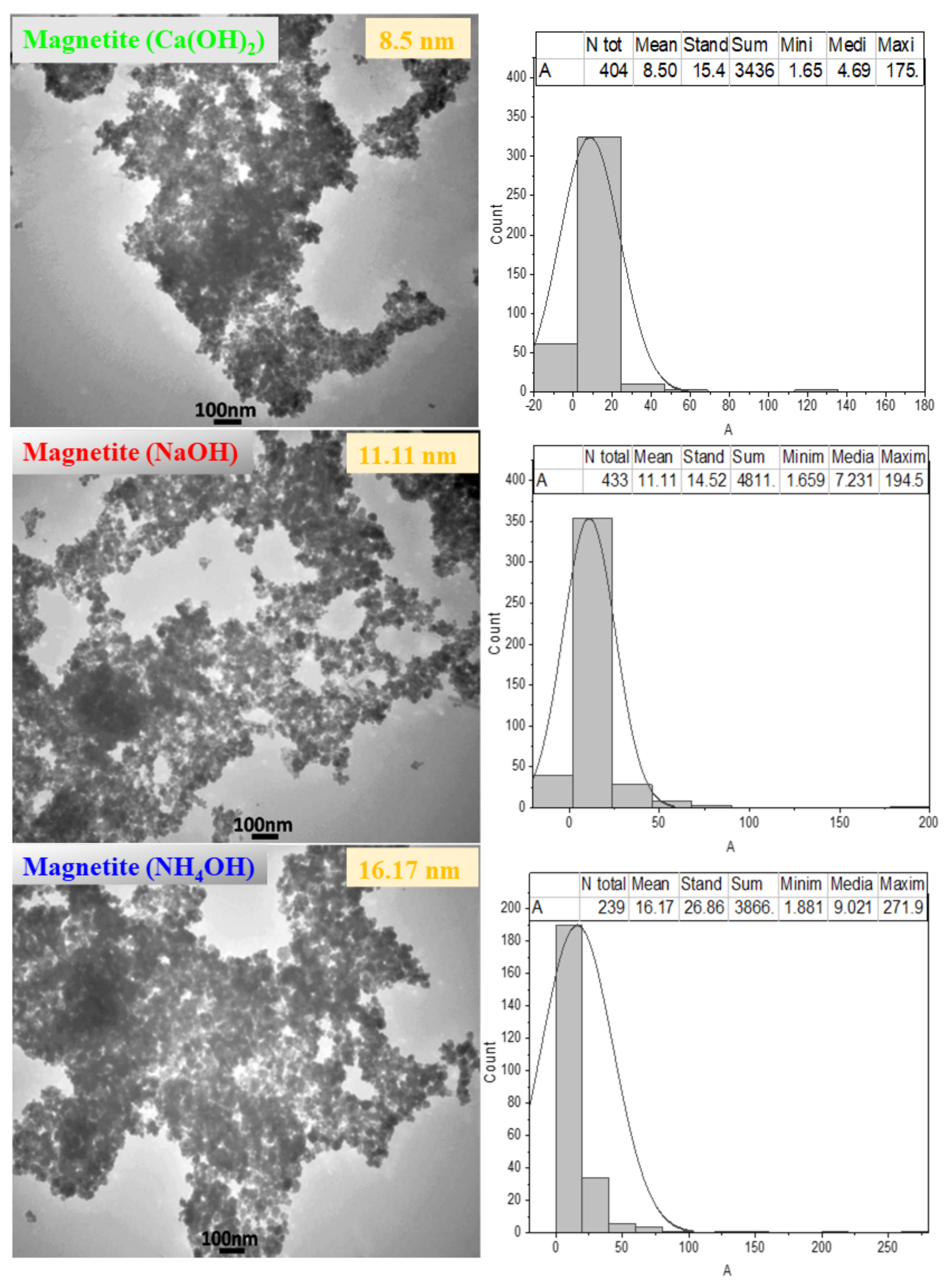

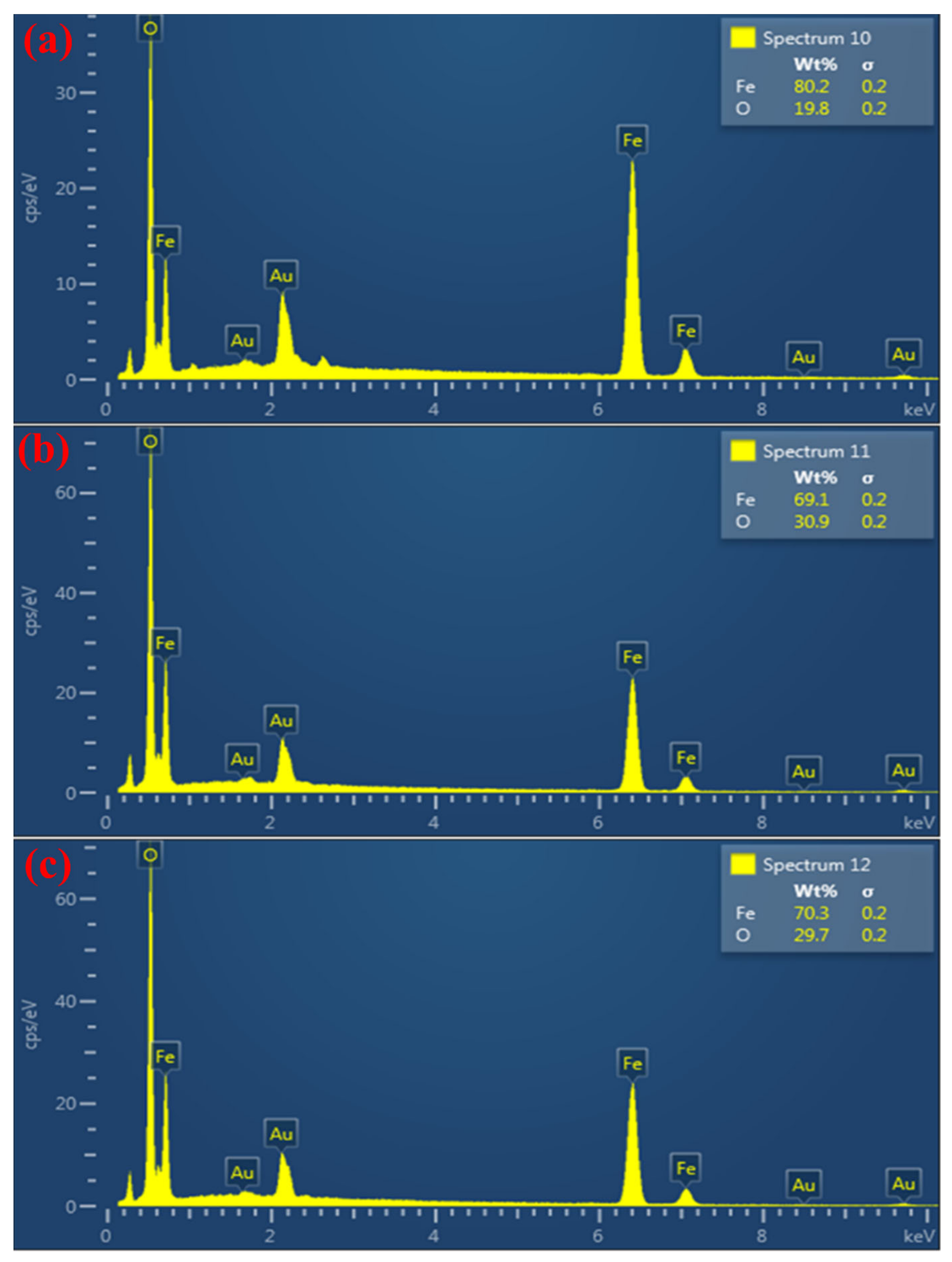





| Material | Chemical Formula | Percentage (%) |
|---|---|---|
| Iron (II) sulfate heptahydrate | FeSO4·7H2O | 98 |
| Ferric chloride | FeCl3 | 97 |
| Hydrochloric acid | HCl | 37 |
| Sodium hydroxide | NaOH | 99 |
| Ammonium hydroxide | NH4OH | 25 |
| Calcium hydroxide | Ca(OH)2 | 99 |
| Nanocomposites | Matrix Material (%) | Reinforcement Materials (%) | ||
|---|---|---|---|---|
| Epoxy | Magnetite (Ca(OH)2) | Magnetite (NaOH) | Magnetite (NH4OH) | |
| A | 100 | 0 | 0 | 0 |
| B | 75 | 25 | 0 | 0 |
| C | 75 | 0 | 25 | 0 |
| D | 75 | 0 | 0 | 25 |
| Sample | S (m2/mg) | DXRD (nm) | DTEM (nm) | DSEM (nm) | Standard Deviation (nm) | a (Å) |
|---|---|---|---|---|---|---|
| Magnetite (Ca(OH)2) | 241 | 4.82 | 8.50 | 22.78 | 8.98 | 8.35 |
| Magnetite (NaOH) | 200 | 5.71 | 11.11 | 28.19 | 11.24 | 8.35 |
| Magnetite (NH4OH) | 130 | 8.2 | 16.17 | 33.51 | 12.50 | 8.35 |
| B | 222 | 5.23 | - | - | - | 8.35 |
| C | 158 | 7.36 | - | - | - | 8.35 |
| D | 105 | 11.03 | - | - | - | 8.35 |
| Fe3O4 Models | S (m2/mg) | V (cm2/mg) | Ms | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T | BJH | Lan | BET | T | BJH | Lan | BET | ||
| Magnetite (Ca(OH)2) | 40.3 | 48.9 | 53.9 | 40.8 | 0 | 0.255 | 12.3 | 9.3 | 52.7 |
| Magnetite (NaOH) | 36.0 | 60.7 | 60.8 | 45.7 | 0 | 0.208 | 13.6 | 10.3 | 54.9 |
| Magnetite (NH4OH) | 55.5 | 65.99 | 75.2 | 57.2 | 0 | 0.283 | 17.2 | 13.1 | 58.6 |
| Nanocomposites | Average Grain Size (nm) | Roughness Average (nm) | Root Mean Square (nm) | Surface Thickness (nm) |
|---|---|---|---|---|
| A | 100 | 672.8 | 93.81 | 66.6 |
| B | 250 | 1093 | 104.2 | 120 |
| C | 180 | 757.3 | 101 | 45.9 |
| D | 2 | 102.4 | 10.24 | 12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayyadh, S.M.; Ben Ahmed, A. Nanocomposite-Supported Polymeric Composites Prepared with Different Deposition Bases: Characterization and Application in X-ray Shielding. Physics 2024, 6, 334-355. https://doi.org/10.3390/physics6010022
Fayyadh SM, Ben Ahmed A. Nanocomposite-Supported Polymeric Composites Prepared with Different Deposition Bases: Characterization and Application in X-ray Shielding. Physics. 2024; 6(1):334-355. https://doi.org/10.3390/physics6010022
Chicago/Turabian StyleFayyadh, Shaymaa Mohammed, and Ali Ben Ahmed. 2024. "Nanocomposite-Supported Polymeric Composites Prepared with Different Deposition Bases: Characterization and Application in X-ray Shielding" Physics 6, no. 1: 334-355. https://doi.org/10.3390/physics6010022





