Simultaneous Thermal and Spectroscopic Screening of Morphologically Complex Theranostic Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gold Nanostars’ Synthesis
2.2.2. Gold Nanoplates’ Synthesis
2.2.3. Surface Modification of NPs for SERS Study
2.2.4. Characterization of AuNSs and AuNPLs
2.2.5. Setup for Simultaneous Photothermal Conversion and SERS Study
2.2.6. Simultaneous Measurement of Heat Output and SERS Spectra
2.2.7. Time-Dependent Stability of the NPs
2.2.8. Effect of Heating on NP Morphology
2.2.9. Effect of Sample Volume on the Heat Output
2.2.10. NP Exposure to Multiple Heat/Cool Cycles
3. Results
3.1. Characterization of Gold Nanostars and Nanoplates
3.2. Effect of Volume on Heat Output
3.3. Stability—UV–vis Analysis
3.4. Change in Morphology after Heating
3.5. Exposure to Multiple Heating and Cooling Cycles
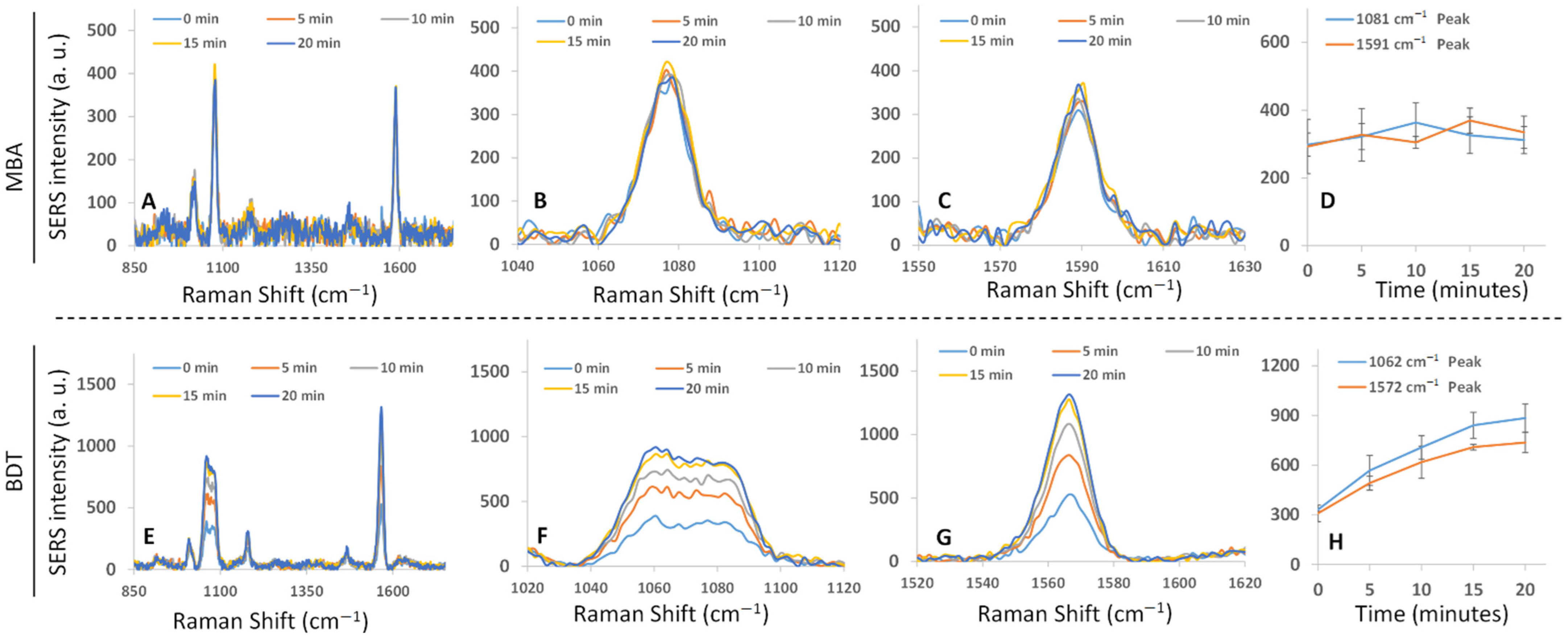
3.6. SERS Enhancement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Govorov, A.O.; Richardson, H.H. Generating Heat with Metal Nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Rashidi-Huyeh, M.; Palpant, B. Thermal Response of Nanocomposite Materials under Pulsed Laser Excitation. J. Appl. Phys. 2004, 96, 4475–4482. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 9, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Plech, A.; Kotaidis, V.; Grésillon, S.; Dahmen, C.; Von Plessen, G. Laser-Induced Heating and Melting of Gold Nanoparticles Studied by Time-Resolved x-Ray Scattering. Phys. Rev. B—Condens. Matter Mater. Phys. 2004, 70, 195423. [Google Scholar] [CrossRef]
- Wilson, O.M.; Hu, X.; Cahill, D.G.; Braun, P.V. Colloidal Metal Particles as Probes of Nanoscale Thermal Transport in Fluids. Phys. Rev. B—Condens. Matter Mater. Phys. 2002, 66, 224301. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Hartland, G.V. Heat Dissipation for Au Particles in Aqueous Solution: Relaxation Time versus Size. J. Phys. Chem. B 2002, 106, 7029–7033. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (Epr) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In Vivo Particle Tracking and Photothermal Ablation Using Plasmon-Resonant Gold Nanostars. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A Plasmonic Gold Nanostar Theranostic Probe for in Vivo Tumor Imaging and Photothermal Therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Nam, S.H.; Lim, D.K.; Suh, Y.D. SERS-Based Particle Tracking and Molecular Imaging in Live Cells: Toward the Monitoring of Intracellular Dynamics. Nanoscale 2019, 11, 21724–21727. [Google Scholar] [CrossRef]
- Guo, H.; He, L.; Xing, B. Applications of Surface-Enhanced Raman Spectroscopy in the Analysis of Nanoparticles in the Environment. Environ. Sci. Nano 2017, 4, 2093–2107. [Google Scholar] [CrossRef]
- Bodelón, G.; Pastoriza-Santos, I. Recent Progress in Surface-Enhanced Raman Scattering for the Detection of Chemical Contaminants in Water. Front. Chem. 2020, 8, 478. [Google Scholar] [CrossRef]
- Chavva, S.R.; Pramanik, A.; Nellore, B.P.V.; Sinha, S.S.; Yust, B.; Kanchanapally, R.; Fan, Z.; Crouch, R.A.; Singh, A.K.; Neyland, B.; et al. Theranostic Graphene Oxide for Prostate Cancer Detection and Treatment. Part. Part. Syst. Charact. 2014, 31, 1252–1259. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- West, C.L.; Doughty, A.C.V.; Liu, K.; Chen, W.R. Monitoring Tissue Temperature during Photothermal Therapy for Cancer. J. Bio-X Res. 2019, 2, 159–168. [Google Scholar] [CrossRef]
- Nicolson, F.; Jamieson, L.E.; Mabbott, S.; Plakas, K.; Shand, N.C.; Detty, M.R.; Graham, D.; Faulds, K. Multiplex Imaging of Live Breast Cancer Tumour Models through Tissue Using Handheld Surface Enhanced Spatially Offset Resonance Raman Spectroscopy (SESORRS). Chem. Commun. 2018, 54, 8530–8533. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, F.; Jamieson, L.E.; Mabbott, S.; Plakas, K.; Shand, N.C.; Detty, M.R.; Graham, D.; Faulds, K. Through Tissue Imaging of a Live Breast Cancer Tumour Model Using Handheld Surface Enhanced Spatially Offset Resonance Raman Spectroscopy (SESORRS). Chem. Sci. 2018, 9, 3788–3792. [Google Scholar] [CrossRef] [Green Version]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal Nanoparticle Solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Richardson, H.H.; Hickman, Z.N.; Govorov, A.O.; Thomas, A.C.; Zhang, W.; Kordesch, M.E. Thermooptical Properties of Gold Nanoparticles Embedded in Ice: Characterization of Heat Generation and Melting. Nano Lett. 2006, 6, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, I.L.; Akchurin, G.G.; Khlebtsov, B.N.; Terentyuk, G.S.; Akchurin, G.G.; Ermolaev, I.A.; Skaptsov, A.A.; Soboleva, E.P.; Khlebtsov, N.G.; Tuchin, V.V. Near-Infrared Laser Photothermal Therapy of Cancer by Using Gold Nanoparticles: Computer Simulations and Experiment. Med. Laser Appl. 2007, 22, 199–206. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper Selenide Nanocrystals for Photothermal Therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef] [Green Version]
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple Experimental Procedures to Distinguish Photothermal from Hot-Carrier Processes in Plasmonics. Light Sci. Appl. 2020, 9, 108. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 75102. [Google Scholar] [CrossRef] [Green Version]
- Alfranca, G.; Artiga, Á.; Stepien, G.; Moros, M.; Mitchell, S.G.; De La Fuente, J.M. Gold Nanoprism-Nanorod Face off: Comparing the Heating Efficiency, Cellular Internalization and Thermoablation Capacity. Nanomedicine 2016, 11, 2903–2916. [Google Scholar] [CrossRef] [Green Version]
- Vega, M.M.; Bonifacio, A.; Lughi, V.; Marsi, S.; Carrato, S.; Sergo, V. Long-Term Stability of Surfactant-Free Gold Nanostars. J. Nanoparticle Res. 2014, 16, 2729. [Google Scholar] [CrossRef]
- Matsuo, N.; Muto, H.; Miyajima, K.; Mafuné, F. Single Laser Pulse Induced Aggregation of Gold Nanoparticles. Phys. Chem. Chem. Phys. 2007, 9, 6027–6031. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Dissociation and Aggregation of Gold Nanoparticles under Laser Irradiation. J. Phys. Chem. B 2001, 105, 9050–9056. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying Thiol-Gold Interactions towards the Efficient Strength Control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Duan, N.; Wu, S.; Zou, Y.; Wang, Z. Polydimethylsiloxane Gold Nanoparticle Composite Film as Structure for Aptamer-Based Detection of Vibrio Parahaemolyticus by Surface-Enhanced Raman Spectroscopy. Food Anal. Methods 2019, 12, 595–603. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Shen, Y. Hydrothermal Synthesis of Gold Nanoplates and Their Structure-Dependent LSPR Properties. J. Mater. Res. 2018, 33, 2671–2679. [Google Scholar] [CrossRef]
- Rycenga, M.; Kim, M.H.; Camargo, P.H.C.; Cobley, C.; Li, Z.Y.; Xia, Y. Surface-Enhanced Raman Scattering: Comparison of Three Different Molecules on Single-Crystal Nanocubes and Nanospheres of Silver. J. Phys. Chem. A 2009, 113, 3932–3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho De La Rosa, A.; Becerril, D.; Gómez-Farfán, G.; Esquivel-Sirvent, R. Time-Harmonic Photothermal Heating by Nanoparticles in a Non-Fourier Medium. J. Phys. Chem. C 2021, 125, 22856–22862. [Google Scholar] [CrossRef]
- Breitenborn, H.; Dong, J.; Piccoli, R.; Bruhacs, A.; Besteiro, L.V.; Skripka, A.; Wang, Z.M.; Govorov, A.O.; Razzari, L.; Vetrone, F.; et al. Quantifying the Photothermal Conversion Efficiency of Plasmonic Nanoparticles by Means of Terahertz Radiation. APL Photonics 2019, 4, 126106. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, M.; Huang, M.H. Direct Synthesis of Branched Gold Nanocrystals and Their Transformation into Spherical Nanoparticles. J. Phys. Chem. B 2006, 110, 19291–19294. [Google Scholar] [CrossRef]
- Blazhynska, M.M.; Kyrychenko, A.; Kalugin, O.N. Molecular Dynamics Simulation of the Size-Dependent Morphological Stability of Cubic Shape Silver Nanoparticles. Mol. Simul. 2018, 44, 981–991. [Google Scholar] [CrossRef]
- Van De Broek, B.; Frederix, F.; Bonroy, K.; Jans, H.; Jans, K.; Borghs, G.; Maes, G. Shape-Controlled Synthesis of NIR Absorbing Branched Gold Nanoparticles and Morphology Stabilization with Alkanethiols. Nanotechnology 2011, 22, 15601. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Zhu, D.; Zheng, Z.; Luo, G.; Suo, M.; Li, X.; Duo, Y.; Tang, B.Z. Single Injection and Multiple Treatments: An Injectable Nanozyme Hydrogel as AIEgen Reservoir and Release Controller for Efficient Tumor Therapy. Nano Today 2021, 37, 101091. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef] [PubMed]
- Higbee-Dempsey, E.; Amirshaghaghi, A.; Case, M.J.; Miller, J.; Busch, T.M.; Tsourkas, A. Indocyanine Green–Coated Gold Nanoclusters for Photoacoustic Imaging and Photothermal Therapy. Adv. Ther. 2019, 2, 1900088. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhou, J.; Song, Z.; Mei, H.; Zhou, M.; Fu, Z.F.; Han, H.; Zhao, L. Aptamer and RVG Functionalized Gold Nanorods for Targeted Photothermal Therapy of Neurotropic Virus Infection in the Mouse Brain. Chem. Eng. J. 2021, 411, 128557. [Google Scholar] [CrossRef]
- Ding, S.; Ma, L.; Feng, J.; Chen, Y.; Yang, D.; Wang, Q. Surface-Roughness-Adjustable Au Nanorods with Strong Plasmon Absorption and Abundant Hotspots for Improved SERS and Photothermal Performances. Nano Res. 2022, 15, 2715–2721. [Google Scholar] [CrossRef]
- Puente, C.; Sánchez-Domínguez, M.; Brosseau, C.L.; López, I. Silver-Chitosan and Gold-Chitosan Substrates for Surface-Enhanced Raman Spectroscopy (SERS): Effect of Nanoparticle Morphology on SERS Performance. Mater. Chem. Phys. 2021, 260, 124107. [Google Scholar] [CrossRef]
- Sun, C.; Gao, M.; Zhang, X. Surface-Enhanced Raman Scattering (SERS) Imaging-Guided Real-Time Photothermal Ablation of Target Cancer Cells Using Polydopamine-Encapsulated Gold Nanorods as Multifunctional Agents. Anal. Bioanal. Chem. 2017, 409, 4915–4926. [Google Scholar] [CrossRef]
- Guarise, C.; Pasquato, L.; Scrimin, P. Reversible Aggregation/Deaggregation of Gold Nanoparticles Induced by a Cleavable Dithiol Linker. Langmuir 2005, 21, 5537–5541. [Google Scholar] [CrossRef]
- Ikbal, M.; Balogh, D.; Mervinetsky, E.; Sfez, R.; Yitzchaik, S. Light-Induced Aggregation of Gold Nanoparticles and Photoswitching of Silicon Surface Potential. J. Phys. Chem. C 2017, 121, 27176–27181. [Google Scholar] [CrossRef]
- Jung, S.; Nam, J.; Hwang, S.; Park, J.; Hur, J.; Im, K.; Park, N.; Kim, S. Theragnostic PH-Sensitive Gold Nanoparticles for the Selective Surface Enhanced Raman Scattering and Photothermal Cancer Therapy. Anal. Chem. 2013, 85, 7674–7681. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, Z.; Zhang, D.; Xiao, W.; Qing, M.; Liu, X. Research on the Temperature Effect Characteristics of SERS Enhancement Factor. Optik 2016, 127, 9926–9931. [Google Scholar] [CrossRef]
- Rycenga, M.; Wang, Z.; Gordon, E.; Cobley, C.M.; Schwartz, A.G.; Lo, C.S.; Xia, Y. Probing the Photothermal Effect of Gold-Based Nanocages with Surface-Enhanced Raman Scattering (SERS). Angew. Chem.—Int. Ed. 2009, 48, 9924–9927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, M.D.; Khadka, S.; Craig, G.A.; Mason, M.D. Effect of Local Heating on the SERS Efficiency of Optically Trapped Prismatic Nanoparticles. J. Phys. Chem. C 2008, 112, 11751–11757. [Google Scholar] [CrossRef]
- Canpean, V.; Astilean, S. Temperature Effect on the SERS Signature of P-Aminothiophenol: A New Evidence for the Production of p,p’-Dimercaptoazobenzene on Metallic Nanostructures. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2012, 96, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Hidehiro, K.; Motoyuki, I. Surface Modification and Characterization for Dispersion Stability of Inorganic Nanometer-Scaled Particles in Liquid Media. Sci. Technol. Adv. Mater. 2010, 11, 44304. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavva, S.R.; Bhat, N.; San Juan, A.M.T.; Jaitpal, S.; Mabbott, S. Simultaneous Thermal and Spectroscopic Screening of Morphologically Complex Theranostic Gold Nanoparticles. J. Nanotheranostics 2022, 3, 102-116. https://doi.org/10.3390/jnt3020007
Chavva SR, Bhat N, San Juan AMT, Jaitpal S, Mabbott S. Simultaneous Thermal and Spectroscopic Screening of Morphologically Complex Theranostic Gold Nanoparticles. Journal of Nanotheranostics. 2022; 3(2):102-116. https://doi.org/10.3390/jnt3020007
Chicago/Turabian StyleChavva, Suhash Reddy, Namratha Bhat, Angela Michelle T. San Juan, Siddhant Jaitpal, and Samuel Mabbott. 2022. "Simultaneous Thermal and Spectroscopic Screening of Morphologically Complex Theranostic Gold Nanoparticles" Journal of Nanotheranostics 3, no. 2: 102-116. https://doi.org/10.3390/jnt3020007
APA StyleChavva, S. R., Bhat, N., San Juan, A. M. T., Jaitpal, S., & Mabbott, S. (2022). Simultaneous Thermal and Spectroscopic Screening of Morphologically Complex Theranostic Gold Nanoparticles. Journal of Nanotheranostics, 3(2), 102-116. https://doi.org/10.3390/jnt3020007





