Novel Biophotonic Techniques for Phototherapy Enhancement: Cerenkov Radiation as a Bridge between Ionizing and Non-Ionizing Radiation Treatment
Abstract
:1. Introductory Remarks
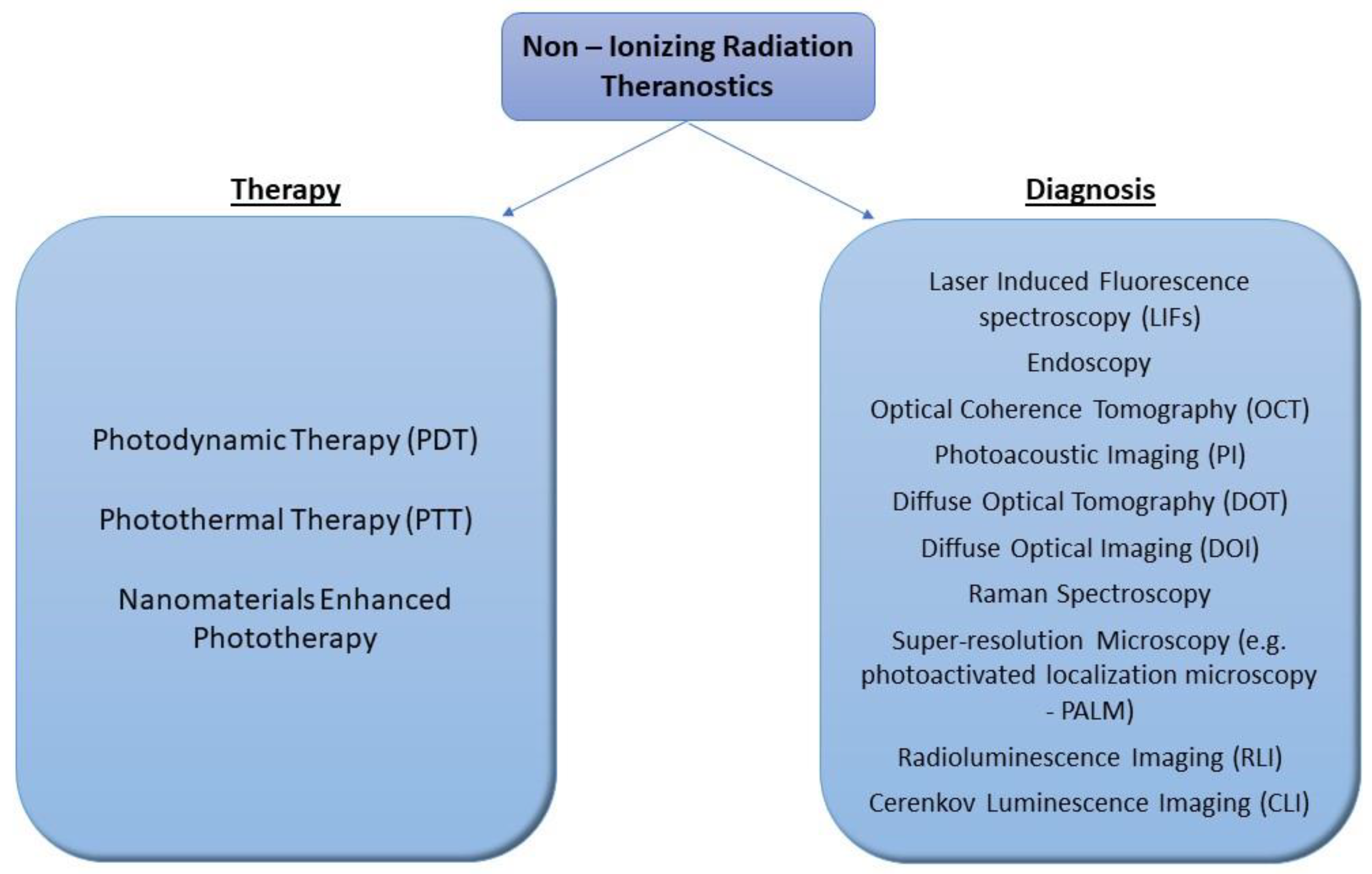
2. Novelties in Cancer Diagnosis
3. New Trends in Cancer Therapy: A SHIFT to Non-Ionizing Radiation
3.1. Photodynamic Therapy (PDT)
3.2. Photothermal Therapy (PTT)
4. Radioluminescence Combined with Nanoparticles in Imaging and Phototherapy
Cerenkov Radiation Coupled with Nanoparticles Mediates Imaging and Phototherapy Enhancement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/330745 (accessed on 1 December 2022).
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef] [PubMed]
- Personalized Medicine. Available online: https://www.genome.gov/genetics-glossary/Personalized-Medicine (accessed on 30 November 2022).
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.; Kamel-Reid, S.; Batist, G.; Chia, S.; Blanke, C.; Moore, M.; Sawyer, M.B.; Desjardins, C.; Dubois, A.; Pun, J.; et al. Benefits, issues, and recommendations for personalized medicine in oncology in Canada. Curr. Oncol. 2013, 20, e475–e483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kareliotis, G.; Tremi, I.; Kaitatzi, M.; Drakaki, E.; Serafetinides, A.A.; Makropoulou, M.; Georgakilas, A.G. Combined radiation strategies for novel and enhanced cancer treatment. Int. J. Radiat. Biol. 2020, 96, 1087–1103. [Google Scholar] [CrossRef]
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in biomedicine: Physics, applications, and models. Phys. Med. Biol. 2019, 64, 04tr01. [Google Scholar] [CrossRef]
- Shaffer, T.M.; Pratt, E.C.; Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 2017, 12, 106–117. [Google Scholar] [CrossRef]
- Amirrashedi, M.; Zaidi, H.; Ay, M.R. Towards quantitative small-animal imaging on hybrid PET/CT and PET/MRI systems. Clin. Transl. Imaging 2020, 8, 243–263. [Google Scholar] [CrossRef]
- van Zandwijk, J.K.; Simonis, F.F.J.; Heslinga, F.G.; Hofmeijer, E.I.S.; Geelkerken, R.H.; Ten Haken, B. Comparing the signal enhancement of a gadolinium based and an iron-oxide based contrast agent in low-field MRI. PLoS ONE 2021, 16, e0256252. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [Green Version]
- Optical Imaging Fact Sheet; National Institute of Health: Bethesda, MA, USA, 2019. Available online: www.nibib.nih.gov (accessed on 2 December 2020).
- Shu, X.; Beckmann, L.; Zhang, H. Visible-Light optical coherence tomography: A review. J. Biomed. Opt. 2017, 22, 121707. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Akbari Sari, A.; Mobinizadeh, M.; Azadbakht, M. A systematic review of the effects of diffuse optical imaging in breast diseases. Iran. J. Cancer Prev. 2013, 6, 44–51. [Google Scholar] [PubMed]
- Kouri, M.A.; Spyratou, E.; Karnachoriti, M.; Kalatzis, D.; Danias, N.; Arkadopoulos, N.; Seimenis, I.; Raptis, Y.S.; Kontos, A.G.; Efstathopoulos, E.P. Raman Spectroscopy: A Personalized Decision-Making Tool on Clinicians & rsquo; Hands for In Situ Cancer Diagnosis and Surgery Guidance. Cancers 2022, 14, 1144. [Google Scholar] [PubMed]
- Zhong, H. Photoactivated localization microscopy (PALM): An optical technique for achieving ~10-nm resolution. Cold Spring Harb. Protoc. 2010, 2010, pdb.top91. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yuan, S.; Wierwille, J.; Naphas, R.; Li, Q.; Blackwell, T.R.; Winnard, P.T.; Raman, V.; Glunde, K. Integrated Optical Coherence Tomography (OCT) and Fluorescence Laminar Optical Tomography (FLOT). IEEE J. Sel. Top. Quantum Electron. 2010, 16, 755–766. [Google Scholar] [CrossRef]
- ICNIRP Statement on Diagnostic Devices Using Non-ionizing Radiation: Existing Regulations and Potential Health Risks. Health Phys. 2017, 112, 305–321. [CrossRef]
- Pogue, B.W.; Wilson, B.C. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 121610. [Google Scholar] [CrossRef]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Noltes, M.E.; van Dam, G.M.; Nagengast, W.B.; van der Zaag, P.J.; Slart, R.; Szymanski, W.; Kruijff, S.; Dierckx, R. Let’s embrace optical imaging: A growing branch on the clinical molecular imaging tree. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4120–4128. [Google Scholar] [CrossRef]
- Youn, H.; Hong, K.-J. In Vivo Noninvasive Small Animal Molecular Imaging. Osong Public Health Res. Perspect. 2012, 3, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laura, M.; Stephen, A.B.; Mark, R.H.; Jürgen, P.; Brian, C.W. Biophotonics: The big picture. J. Biomed. Opt. 2017, 23, 021103. [Google Scholar] [CrossRef] [Green Version]
- Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles. Cancers 2017, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremi, I.; Spyratou, E.; Souli, M.; Efstathopoulos, E.P.; Makropoulou, M.; Georgakilas, A.G.; Sihver, L. Requirements for Designing an Effective Metallic Nanoparticle (NP)-Boosted Radiation Therapy (RT). Cancers 2021, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Radiotherapy Overview. Available online: https://www.nhs.uk/conditions/radiotherapy/ (accessed on 1 December 2022).
- Makropoulou, M. Cancer and electromagnetic radiation therapy: Quo Vadis? arXiv 2016. [Google Scholar] [CrossRef]
- Kareliotis, G.; Chronopoulou, E.; Makropoulou, M. In Silico, Combined Plasmonic Photothermal and Photodynamic Therapy in Mice. J. Nanotheranostics 2022, 3, 4. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Makropoulou, M.; Kareliotis, G.; Spyratou, E.; Drakaki, E.; Serafetinides, A.A.; Efstathopoulos, E. Non-ionizing, laser radiation in Theranostics: The need for dosimetry and the role of Medical Physics. Phys. Med. 2019, 63, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Kareliotis, G.; Xanthopoulos, S.; Drakaki, E.; Papachristou, M.; Datseris, I.; Bouziotis, P.; Makropoulou, M. Photodynamic Therapy of 4T1 tumors in NOD-SCID mice. arXiv 2020. [Google Scholar] [CrossRef]
- Kareliotis, G.; Liossi, S.; Makropoulou, M. Assessment of singlet oxygen dosimetry concepts in photodynamic therapy through computational modeling. Photodiagnosis Photodyn. Ther. 2018, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Baochang, L.; Thomas, J.F.; Michael, S.P. Comparison of photodynamic therapy with different excitation wavelengths using a dynamic model of aminolevulinic acid-photodynamic therapy of human skin. J. Biomed. Opt. 2012, 17, 088001. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.K.-H.; Finlay, J.C.; Busch, T.M.; Hahn, S.M.; Zhu, T.C. Explicit dosimetry for photodynamic therapy: Macroscopic singlet oxygen modeling. J. Biophotonics 2010, 3, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.C.; Kim, M.M.; Liang, X.; Finlay, J.C.; Busch, T.M. In-Vivo singlet oxygen threshold doses for PDT. Photonics Lasers Med. 2015, 4, 59–71. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; Brooks, S.; van der Ploeg-van den Heuvel, A.; ten Hagen, T.L.M.; de Haas, E.R.M.; Robinson, D.J. Light Fractionation Significantly Increases the Efficacy of Photodynamic Therapy Using BF-200 ALA in Normal Mouse Skin. PLoS ONE 2016, 11, e0148850. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Mpourazanis, G.; Konschake, W.; Vogiatzis, R.; Papalexis, P.; Georgakopoulou, V.E.; Ntritsos, G.; Sklapani, P.; Trakas, N. The Role and Effectiveness of Photodynamic Therapy on Patients With Actinic Keratosis: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e26390. [Google Scholar] [CrossRef]
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006, 24, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinten, M. Optical Properties of Nanoparticle Systems: Mie and Beyond; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Wrigglesworth, E.; Johnston, J. Mie theory and the dichroic effect for spherical gold nanoparticles: An experimental approach. Nanoscale Adv. 2021, 3, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Hergert, W.; Wriedt, T. The Mie Theory; Springer: Berlin/Heidelberg, Germany, 2012; Volume 169. [Google Scholar]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Zhang, L.; Sun, T.; Zhang, Y.; Liu, Y.; Gong, M.; Xu, Z.; Du, M.; Liu, Y.; Liu, G.; et al. Activatable NIR-II Plasmonic Nanotheranostics for Efficient Photoacoustic Imaging and Photothermal Cancer Therapy. Adv. Mater. 2021, 33, e2006532. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Ether, D.S.; Pires, L.B.; Umrath, S.; Martinez, D.; Ayala, Y.; Pontes, B.; Araujo, G.D.S.; Frases, S.; Ingold, G.-L.; Rosa, F.S.S.; et al. Probing the Casimir force with optical tweezers. Europhys. Lett. 2015, 112, 44001. [Google Scholar] [CrossRef] [Green Version]
- Machta, B.B.; Veatch, S.L.; Sethna, J.P. Critical Casimir Forces in Cellular Membranes. Phys. Rev. Lett. 2012, 109, 138101. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Zvyagin, A.V.; Roy, I. Theranostic Applications of Nanoparticle-Mediated Photoactivated Therapies. J. Nanotheranostics 2021, 2, 9. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, R.; Soenen, S.; Braeckmans, K.; Skirtach, A. Towards Theranostic Multicompartment Microcapsules: In-Situ Diagnostics and Laser-induced Treatment. Theranostics 2013, 3, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lee, H.-L.; Chiou, J.-F.; Lo, L.-W. Recent Advances in Gold Nanomaterials for Photothermal Therapy. J. Nanotheranostics 2022, 3, 8. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Zhang, J.S.; Yang, F.; Zhou, Q.L.; Liao, Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed. Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef] [Green Version]
- Pizzuti, V.J.; Viswanath, D.; Torregrosa-Allen, S.E.; Currie, M.P.; Elzey, B.D.; Won, Y.Y. Bilirubin-Coated Radioluminescent Particles for Radiation-Induced Photodynamic Therapy. ACS Appl. Bio. Mater. 2020, 3, 4858–4872. [Google Scholar] [CrossRef]
- Ciarrocchi, E.; Belcari, N. Cerenkov luminescence imaging: Physics principles and potential applications in biomedical sciences. EJNMMI Phys. 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, A.E.; Boschi, F. Photodynamic Therapy Using Cerenkov and Radioluminescence Light. Front. Phys. 2021, 9, 637120. [Google Scholar] [CrossRef]
- Jelley, J.V. SPECIAL ARTICLE: Cerenkov radiation and its applications. Br. J. Appl. Phys. 1955, 6, 227–232. [Google Scholar] [CrossRef]
- Fazio, G.G.; Jelley, J.V.; Charman, W.N. Generation of Cherenkov Light Flashes by Cosmic Radiation within the Eyes of the Apollo Astronauts. Nature 1970, 228, 260–264. [Google Scholar] [CrossRef]
- Tendler, I.I.; Hartford, A.; Jermyn, M.; LaRochelle, E.; Cao, X.; Borza, V.; Alexander, D.; Bruza, P.; Hoopes, J.; Moodie, K.; et al. Experimentally Observed Cherenkov Light Generation in the Eye During Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Hachadorian, R.L.; Jermyn, M.; Bruza, P.; Alexander, D.A.; Tendler, I.I.; Williams, B.B.; Gladstone, D.J.; Schaner, P.E.; Zaki, B.I.; et al. Initial Clinical Experience of Cherenkov Imaging in External Beam Radiation Therapy Identifies Opportunities to Improve Treatment Delivery. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Holt, R.W.; Zhang, R.; Esipova, T.V.; Vinogradov, S.A.; Glaser, A.K.; Gladstone, D.J.; Pogue, B.W. Cherenkov excited phosphorescence-based pO2 estimation during multi-beam radiation therapy: Phantom and simulation studies. Phys. Med. Biol. 2014, 59, 5317–5328. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Rao Allu, S.; Jiang, S.; Jia, M.; Gunn, J.R.; Yao, C.; LaRochelle, E.P.; Shell, J.R.; Bruza, P.; Gladstone, D.J.; et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat. Commun. 2020, 11, 573. [Google Scholar] [CrossRef] [Green Version]
- Pogue, B.W.; Feng, J.; LaRochelle, E.P.; Bruža, P.; Lin, H.; Zhang, R.; Shell, J.R.; Dehghani, H.; Davis, S.C.; Vinogradov, S.A.; et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nat. Biomed. Eng. 2018, 2, 254–264. [Google Scholar] [CrossRef]
- Zhang, R.; Glaser, A.; Esipova, T.V.; Kanick, S.C.; Davis, S.C.; Vinogradov, S.; Gladstone, D.; Pogue, B.W. Čerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies. Biomed. Opt. Express 2012, 3, 2381–2394. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Gunn, J.R.; Allu, S.R.; Bruza, P.; Jiang, S.; Vinogradov, S.A.; Pogue, B.W. Implantable sensor for local Cherenkov-excited luminescence imaging of tumor pO2 during radiotherapy. J. Biomed. Opt. 2020, 25, 112704. [Google Scholar] [CrossRef]
- Keen, C.E. Visualizing the Treatment Beam Improves Radiation Therapy Delivery; Physics World: Bristol, UK, 2021. [Google Scholar]
- Cherenkov Emission Imaging and Spectroscopy Using a Pulsed Linear Accelerator and the Subsequent Deep-Tissue Imaging of Molecular Oxygen Sensors in a Human Body Phantom; © 2018 Princeton Instruments, Inc. Available online: https://www.princetoninstruments.com/wp-content/uploads/2020/04/AppNote_CherenkovDeepTissueMolecularOxygenHumanPhantom.pdf (accessed on 1 December 2022).
- Glaser, A.K.; Zhang, R.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms. Opt. Lett. 2012, 37, 1193–1195. [Google Scholar] [CrossRef]
- Pratt, E.C. Cerenkov Imaging. Available online: https://www.mskcc.org/research/ski/labs/jan-grimm/cerenkov-imaging (accessed on 11 November 2022).
- Robertson, R.; Germanos, M.S.; Li, C.; Mitchell, G.S.; Cherry, S.R.; Silva, M.D. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 2009, 54, N355–N365. [Google Scholar] [CrossRef] [PubMed]
- Boschi, F.; Spinelli, A. Cerenkov Luminescence Imaging at a Glance. Curr. Mol. Imaging 2014, 3, 106–117. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xing, B.; Han, P.; Gambhir, S.S.; Cheng, Z. Radiation-Luminescence-Excited Quantum Dots for in vivo Multiplexed Optical Imaging. Small 2010, 6, 1087–1091. [Google Scholar] [CrossRef]
- Zhang, A.-W.; Guo, W.-H.; Qi, Y.-F.; Wang, J.-Z.; Ma, X.-X.; Yu, D.-X. Synergistic Effects of Gold Nanocages in Hyperthermia and Radiotherapy Treatment. Nanoscale Res. Lett. 2016, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Ni, K.; Veroneau, S.S.; Luo, T.; You, E.; Lin, W. Nanoscale Metal-Organic Framework Hierarchically Combines High-Z Components for Multifarious Radio-Enhancement. J. Am. Chem. Soc. 2019, 141, 6859–6863. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [Green Version]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Yang, W.; He, Z.; Jia, H.; Wang, H.; Zhao, W.; Gao, L.; Zhang, Z.; Gao, F.; Gao, X. A chlorin-lipid nanovesicle nucleus drug for amplified therapeutic effects of lung cancer by internal radiotherapy combined with the Cerenkov radiation-induced photodynamic therapy. Biomater. Sci. 2020, 8, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ni, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Wei, H.; Ferreira, C.A.; Lan, X.; Engle, J.W.; He, Q.; Yu, F.; et al. A “Missile-Detonation” Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv. Mater. 2019, 31, e1904894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Li, J.; Wang, P.; Lang, J.; Yang, Y. Cherenkov Luminescence in Tumor Diagnosis and Treatment: A Review. Photonics 2022, 9, 390. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO(2) Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef]
- Qian, R.; Wang, K.; Guo, Y.; Li, H.; Zhu, Z.; Huang, X.; Gong, C.; Gao, Y.; Guo, R.; Yang, B.; et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J. Nanobiotechnol. 2022, 20, 203. [Google Scholar] [CrossRef]
- Goel, S.; Ferreira, C.A.; Chen, F.; Ellison, P.A.; Siamof, C.M.; Barnhart, T.E.; Cai, W. Activatable Hybrid Nanotheranostics for Tetramodal Imaging and Synergistic Photothermal/Photodynamic Therapy. Adv. Mater. 2018, 30, 1704367. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef] [Green Version]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.A.; Raliya, R.; Tang, R.; Xu, B.; Mixdorf, M.; Achilefu, S.; Biswas, P. Electrospray Functionalization of Titanium Dioxide Nanoparticles with Transferrin for Cerenkov Radiation Induced Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zheleznyak, A.; Mixdorf, M.; Ghai, A.; Prior, J.; Black, K.C.L.; Shokeen, M.; Reed, N.; Biswas, P.; Achilefu, S. Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma. ACS Nano 2020, 14, 4255–4264. [Google Scholar] [CrossRef]
- Ouyang, Z.; Liu, B.; Yasmin-Karim, S.; Sajo, E.; Ngwa, W. Nanoparticle-aided external beam radiotherapy leveraging the Cerenkov effect. Phys. Med. 2016, 32, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Shen, C.; Li, H.; Goldys, E.M.; Deng, W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J. Nanobiotechnol. 2020, 18, 87. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Zhou, W.; Qu, G.; Wang, J.; Yang, N.; Gao, J.; Chen, T.; Chu, P.K.; Yu, X.F. Stable black phosphorus/Bi2O3 heterostructures for synergistic cancer radiotherapy. Biomaterials 2018, 171, 12–22. [Google Scholar] [CrossRef]
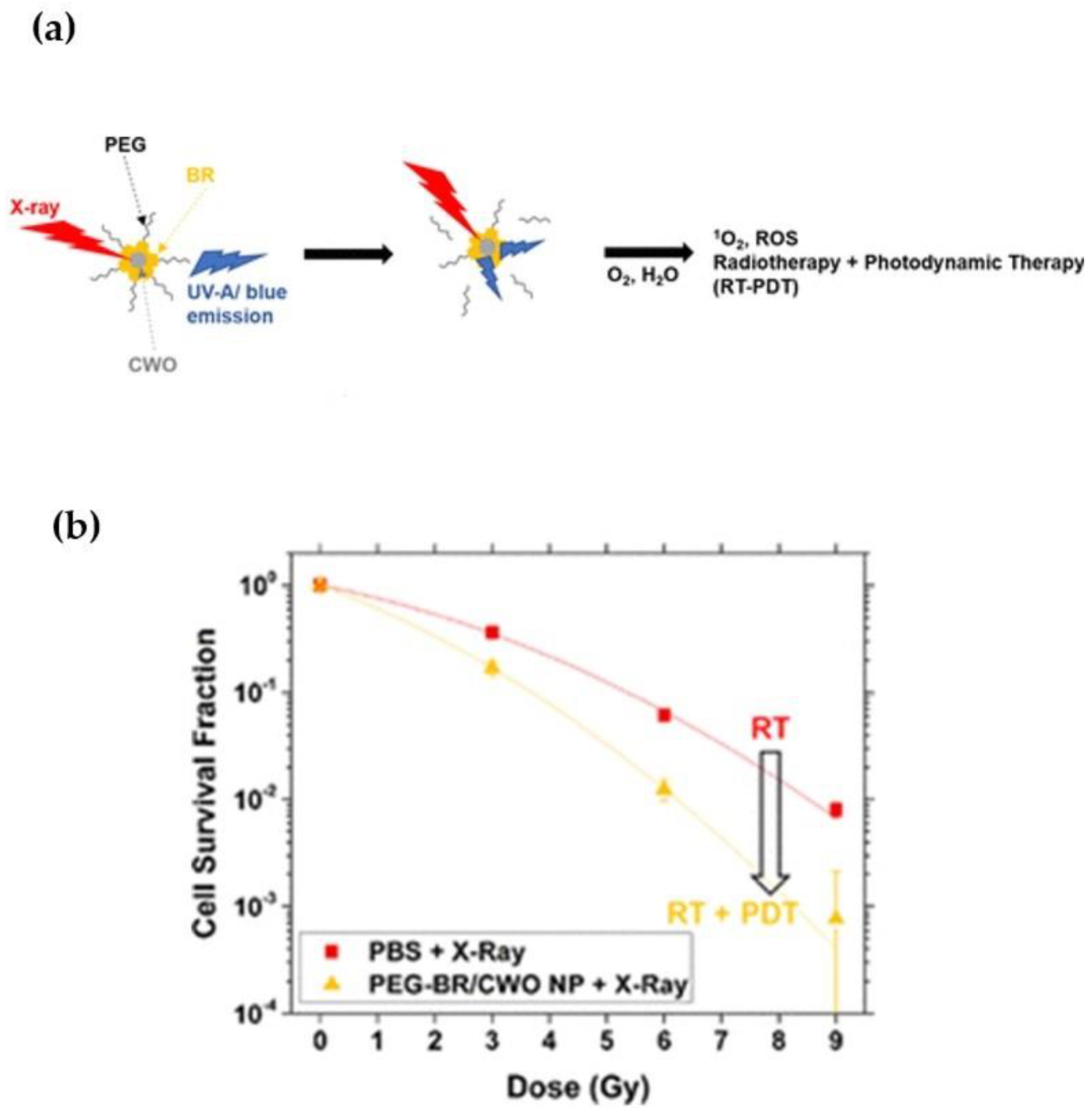





| Cell line/Application | NPs | Source | Effects | Ref. |
|---|---|---|---|---|
| Human fibrosarcoma cell tumors (HT1080) in mice/in vivo | Polyethyleneglycol-coated TiO2 NPs (TiO2–PEG NPs) (type: small nanoclusters) | 64Cu | Remarkable tumor volume shrinkage in 3 days and complete tumor regression by 30 days | [95] |
| Human fibrosarcoma cell tumors (HT1080) in mice/in vivo and in vitro | Apo-transferrin (devoid of iron and abbreviated as Tf)-coated TiO2 NPs (TiO2-Tf) | 18 FDG | Effects related to necrosis were observed in vitro, with significant suppression of tumors in vivo. | [95] |
| Breast cancer—4T1 cells in mice/in vivo | Dextran-modified TiO2 nanoparticles (D-TiO2 NPs) | 68Ga | Strong DNA damage to tumor cells | [96] |
| Breast cancer cells—4T1/in vitro and 4T1 tumors in Balb/c mice/in vivo | Chlorin e6 induced hollow mesoporous silica NPs (HMSN-Ce6) radiolabeled with 89Zr | 89Zr (bound to the NPs) | Both in vivo and in vitro results confirmed the PDT effects on cells. | [100] |
| Breast cancer cells—4T1/in vitro | Magnetic nanoparticles (MNPs) with porphyrin molecule (TCPP) surface modification for magnetic tumor targeting and MNP-PEG (average diameter ~20nm). | 89Zr (bound to the NPs) | Large amount of ROS generation in cells treated with 89Zr-MNP/TCPP was observed. | [101] |
| 4T1 murine breast tumor-bearing mice/in vivo | CuS NPs on the surface of [89Zr]-labeled hollow mesoporous silica nanoshells filled with porphyrin molecules. | 89Zr (bound to the NPs) | Hyperthermia and photodynamic therapy result in the complete elimination of tumors with no side effects | [98] |
| Human multiple myeloma—MM1.S cancer cell line and HT1080 cells/in vitro | Titanium dioxide (TiO2) nanoparticles coated with protein transferrin (Tf) [Tf/TiO2] and average diameter of 25 nm (±3.2 nm). | 18FDG | Electrospray-fabricated NPs improved cell killing from 23% to 57% compared to NPs produced with other methods. | [102] |
| Multiple Myeloma MM1.S cells/in vivo, in vitro, ex vivo | Transferin-coated titanium dioxide NPs (Tf/TiO2) with average diameter of 122 nm ± 16 nm | 89Zr (bound to the NPs) | Higher levels of ROS at all time points were generated compared to either 89Zr alone or TiO2-Tf NPs. Overall, 89Zr-TiO2-Tf is capable of generating sufficient ROS to cause MM cell death. | [103] |
| Human lung A549 cancer cells/in vitro | Titania NPs with average size of 2 nm. | 6MV radiation | There were 20% more cancer cells killed when radiation and NPs were combined compared to radiation alone. | [104] |
| Human colorectal cancer cells (HCT116)/in vitro | Liposome nanocarriers containing gold NPs with diameter of 10 nm and 5 nm and verteporfin and conjugated with TPP to target cells’ mitochondria. | 4 Gy of X-ray radiation. | Liposomes with 10 nm NPs produced the highest amount of ROS. Gold NPs were able to amplify the radiation doses in tumor tissue. Μitochondrial damage was induced and activated the mechanisms for cancer cell death. | [105] |
| Melanoma A375 cells and cardiomyocyte H9C2 cells/in vitro and in vivo | Bi2O3 nanoparticles with a size of 5 ± 3 nm turned onto Black Phosphorus (BP) nanosheets | 4 Gy of X-ray radiation. | Efficient production of X-ray-PDT effect to induce cell apoptosis and cell cycle arrest | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyratou, E.; Kokkinogoulis, K.; Tsigaridas, G.; Kareliotis, G.; Platoni, K.; Makropoulou, M.; Efstathopoulos, E.P. Novel Biophotonic Techniques for Phototherapy Enhancement: Cerenkov Radiation as a Bridge between Ionizing and Non-Ionizing Radiation Treatment. J. Nanotheranostics 2023, 4, 86-105. https://doi.org/10.3390/jnt4010005
Spyratou E, Kokkinogoulis K, Tsigaridas G, Kareliotis G, Platoni K, Makropoulou M, Efstathopoulos EP. Novel Biophotonic Techniques for Phototherapy Enhancement: Cerenkov Radiation as a Bridge between Ionizing and Non-Ionizing Radiation Treatment. Journal of Nanotheranostics. 2023; 4(1):86-105. https://doi.org/10.3390/jnt4010005
Chicago/Turabian StyleSpyratou, Ellas, Kyriakos Kokkinogoulis, Georgios Tsigaridas, Georgios Kareliotis, Kalliopi Platoni, Mersini Makropoulou, and Efstathios P. Efstathopoulos. 2023. "Novel Biophotonic Techniques for Phototherapy Enhancement: Cerenkov Radiation as a Bridge between Ionizing and Non-Ionizing Radiation Treatment" Journal of Nanotheranostics 4, no. 1: 86-105. https://doi.org/10.3390/jnt4010005








