Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic
Abstract
:1. Introduction
2. Principle of Cancer Theranostics
3. Application of Cancer Theranostics
3.1. Quantum Dots
3.2. Liposomes
3.3. Extracellular Vesicles and Exosomes
3.4. Polymeric Nanoparticles
3.5. Radioisotopes
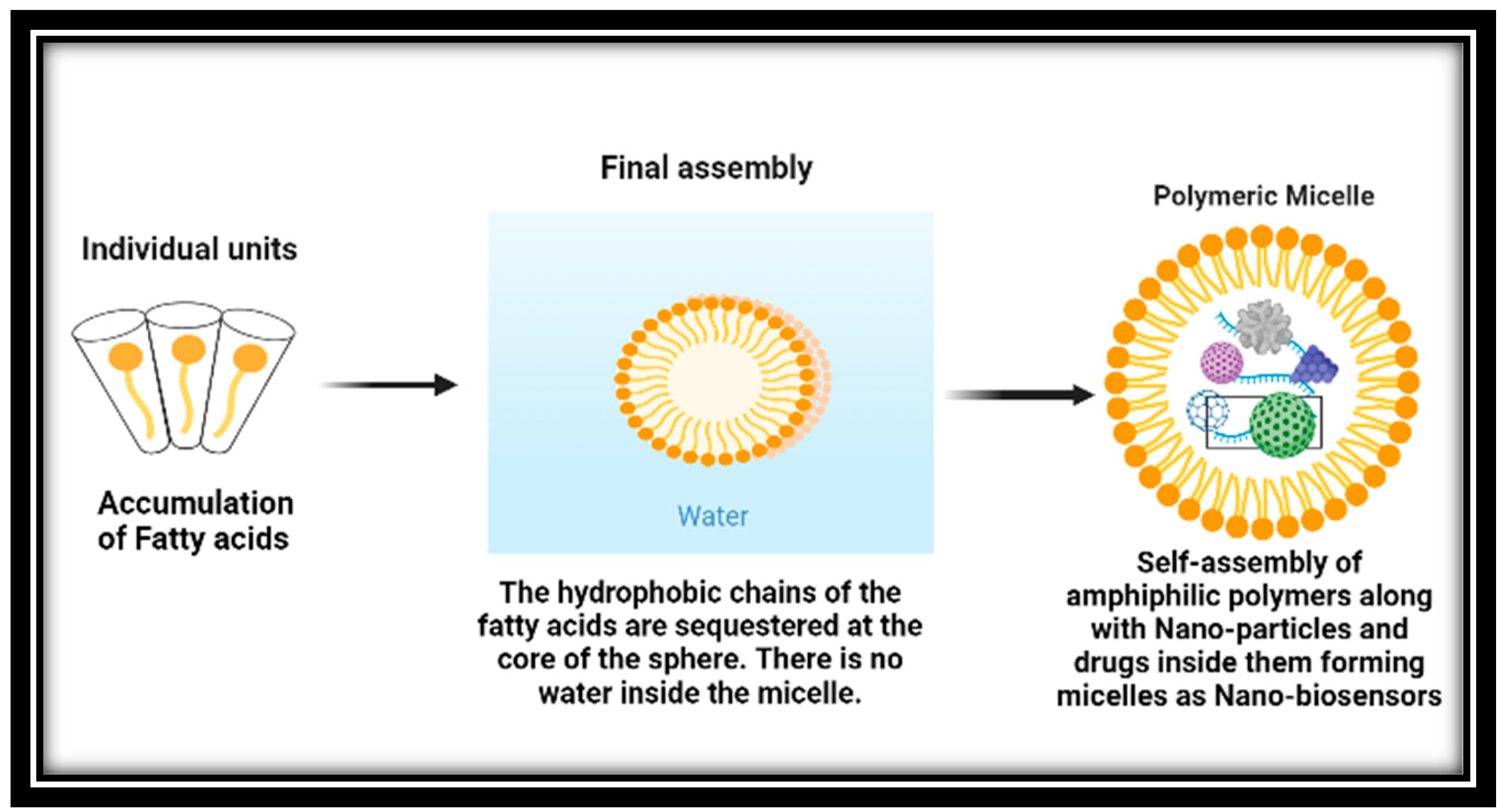
3.6. Micelles
3.7. Nanobubbles
3.8. Diagnosis Using Cancer Theranostics
4. Clinical Trials of Cancer Theranostics
| Project | Consortium | Aim |
|---|---|---|
| Vibrant | 10 groups | Contrast agent for pancreatic beta-cell imaging in diabetes Mellitus type I |
| Magnifyco | 11 groups | Magnetic nanoparticles have the potential to be used theranostically to treat ovarian cancer. |
| SaveMe | 19 groups | Nano core platforms to advance cancer treatment and diagnosis |
| Nicotinamide Adenine Dinucleotide (NAD) | 19 groups | Alzheimers’ treatment |
| Namdiatream | 22 groups | Molecular biomarker and detection |
| Multifun | 15 groups | Breast and pancreatic cancer early detection using iron oxide nanoparticles with cancer stem cells |
| Nanomagdye | 8 groups | Iron oxide nanoparticles as a new contrast agent in cancer patients’ lymph node imaging |
5. Limitations and Challenges
6. Discussion
7. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Singh, N.; Kim, J.; Kim, J.; Lee, K.; Zunbul, Z.; Lee, I.; Kim, E.; Chi, S.G.; Kim, J.S. Covalent organic framework nanomedicines: Biocompatibility for advanced nanocarriers and cancer theranostics applications. Bioact. Mater. 2023, 21, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Li, M.; Ma, K.; Wang, D.; Su, L.; Zhang, X.; Tang, B.Z. Nanolab in a Cell: Crystallization-Induced In Situ Self-Assembly for Cancer Theranostic Amplification. J. Am. Chem. Soc. 2022, 144, 14388–14395. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zhao, L.; Wang, Z.G.; Liu, S.L.; Pang, D.W. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small 2022, 18, 2104567. [Google Scholar] [CrossRef] [PubMed]
- Barati, F.; Avatefi, M.; Moghadam, N.B.; Asghari, S.; Ekrami, E.; Mahmoudifard, M. A review of graphene quantum dots and their potential biomedical applications. J. Biomater. Appl. 2023, 37, 1137–1158. [Google Scholar] [CrossRef]
- Campora, S.; Ghersi, G. Recent developments and applications of smart nanoparticles in biomedicine. Nanotechnol. Rev. 2022, 11, 2595–2631. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-loaded liposomes for anticancer therapy: An updated review. Nanomedicine 2022, 17, 547–568. [Google Scholar] [CrossRef]
- Karges, J. Clinical development of metal complexes as photosensitizers for photodynamic therapy of cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef]
- Mou, Y.; Zhang, P.; Lai, W.F.; Zhang, D. Design and applications of liposome-in-gel as carriers for cancer therapy. Drug Deliv. 2022, 29, 3245–3255. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Tian, W.; Jiang, S.; Zhao, J.; Zhou, J.; He, Q.; Tang, Z.; Shen, W.; Wang, J. A dual drug delivery platform based on thermo-responsive polymeric micelle capped mesoporous silica nanoparticles for cancer therapy. Microporous Mesoporous Mater. 2022, 338, 111943. [Google Scholar] [CrossRef]
- Gautam, P.; Choudhary, S. Physicochemical insights into the micelle-based drug-delivery of bioactive compounds to the carrier protein. New J. Chem. 2022, 46, 19124–19135. [Google Scholar]
- Mishra, S.; Streeter, P.R. Micelle-Based Nanocarriers for Targeted Delivery of Cargo to Pancreas. In Type-1 Diabetes: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 175–184. [Google Scholar]
- Siddique, S.; Chow, J.C. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. February. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 69, pp. 166–177. [Google Scholar]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Ying, N.; Lin, X.; Xie, M.; Zeng, D. Effect of surface ligand modification on the properties of anti-tumor nanocarrier. Colloids Surf. B Biointerfaces 2022, 220, 112944. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.E.; Oh, S.W.; Park, W. Hybrid nanovesicle of chimeric antigen receptor (CAR)-engineered cell-derived vesicle and drug-encapsulated liposome for effective cancer treatment. J. Ind. Eng. Chem. 2023, 122, 127–137. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2022, 221, 112999. [Google Scholar] [CrossRef]
- Soman, S.; Kulkarni, S.; Pandey, A.; Dhas, N.; Subramanian, S.; Mukherjee, A.; Mutalik, S. 2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements. Biosensors 2022, 12, 1009. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef] [Green Version]
- Nasirmoghadas, P.; Mousakhani, A.; Behzad, F.; Beheshtkhoo, N.; Hassanzadeh, A.; Nikoo, M.; Mehrabi, M.; Kouhbanani, M.A.J. Nanoparticles in cancer immunotherapies: An innovative strategy. Biotechnol. Prog. 2021, 37, e3070. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, M.S. Cytology of a spontaneous triploid Coffea canephora Pierre ex Froehner. Caryologia 1981, 34, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Jortner, J.; Ratner, M.A. (Eds.) Molecular Electronics; Blackwell Science: Oxford, UK, 1997; p. 255. [Google Scholar]
- Kastner, M.A. Mesoscopic physics and artificial atoms. AIP Conf. Proc. 1993, 275, 573–586. [Google Scholar]
- Collier, C.P.; Vossmeyer, T.; Heath, J.R. Nanoparticles Superlattices. Anu. Rev. Phys. Phys. Chem. 1998, 49, 371. [Google Scholar] [CrossRef]
- Tiwari, K.P.; Sahu, M.; Kumar, G.; Ashourian, M. Pivotal Role of Quantum Dots in the Advancement of Healthcare Research. Comput. Intell. Neurosci. 2021, 2021, 2096208. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Sundarraj, S.; Ganeshbabu, M.; Kumar, P.; Selvan, R.K. Green synthesis of multifunctional carbon quantum dots: An approach in cancer theranostics. Biomater. Adv. 2022, 136, 212756. [Google Scholar] [CrossRef]
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Soman, S.; Mutalik, S.; Barnwal, R.P.; Singh, G.; et al. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Liu, H.R.; Lou, Q.; Wang, F.; Liu, K.K.; Dong, L.; Shan, C.X. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics 2022, 12, 2860. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Kaushik, D.; Verma, R.; Bhatt, S.; Sahoo, B.M.; Bhattacharya, T.; Alshehri, S.; et al. Quantum dots: An emerging approach for cancer therapy. Front. Mater. 2022, 8, 585. [Google Scholar] [CrossRef]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef]
- Gao, A.; Hu, X.L.; Saeed, M.; Chen, B.F.; Li, Y.P.; Yu, H.J. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019, 40, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Lim, H.J.; Masin, D.; Madden, T.D.; Bally, M.B. Influence of drug release characteristics on the therapeutic activity of liposomal mitoxantrone. J. Pharmacol. Exp. Ther. 1997, 281, 566–573. [Google Scholar]
- Forssen, E.A.; Male-Brune, R.; Adler-Moore, J.P.; Lee, M.J.A.; Schmidt, P.G.; Krasieva, T.B.; Shimizu, S.; Tromberg, B.J. Fluorescence imaging studies for the disposition of daunorubicin liposomes (DaunoXome) within tumor tissue. Cancer Res. 1996, 56, 2066–2075. [Google Scholar]
- Ali, A.; Ahmad, Z.; Ahmad, U.; Khan, M.M.; Haider, M.F.; Akhtar, J. Integrating Nanotherapeutic Platforms to Image Guided Approaches for Management of Cancer. In Molecular Pharmacology; Catala, A., Ahmad, U., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Krishna, R.; Webb, M.S.; Onge, G.S.; Mayer, L.D. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 2001, 298, 1206–1212. [Google Scholar] [PubMed]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Ozols, R.F.; Myers, C.E. The anthracycline antineoplastic drugs. N. Engl. J. Med. 1981, 305, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Deamer, D.W. Catecholamine uptake and concentration by liposomes maintaining pH gradients. Biochim. Biophys. Acta BBA Biomembr. 1976, 455, 269–271. [Google Scholar] [CrossRef]
- Mayer, L.D.; Bally, M.B.; Hope, M.J.; Cullis, P.R. Uptake of antineoplastic agents into large unilamellar vesicles in response to a membrane potential. Biochim. Biophys. Acta BBA Biomembr. 1985, 816, 294–302. [Google Scholar] [CrossRef]
- Madden, T.D.; Harrigan, P.R.; Tai, L.C.; Bally, M.B.; Mayer, L.D.; Redelmeier, T.E.; Loughrey, H.C.; Tilcock, C.P.; Reinish, L.W.; Cullis, P.R. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: A survey. Chem. Phys. Lipids 1990, 53, 37–46. [Google Scholar] [CrossRef]
- Lasic, D.D.; Frederik, P.M.; Stuart, M.C.A.; Barenholz, Y.; McIntosh, T.J. Gelation of liposome interior A novel method for drug encapsulation. FEBS Lett. 1992, 312, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.A. A few notions involved in the clinical use of radioisotopes. Ann. Intern. Med. 1957, 47, 922–938. [Google Scholar]
- Otuka, N.; Takács, S. Definitions of radioisotope thick target yields. Radiochim. Acta 2015, 103, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pinnaduwage, D.S.; Srivastava, S.P.; Yan, X.; Jani, S.; Brachman, D.G.; Sorensen, S.P. Dosimetric impacts of source migration, radioisotope type, and decay with permanent implantable collagen tile brachytherapy for brain tumors. Technol. Cancer Res. Treat. 2022, 21, 15330338221106852. [Google Scholar] [CrossRef]
- Niculae, D.; Dusman, R.; Leonte, R.A.; Chilug, L.E.; Dragoi, C.M.; Nicolae, A.; Serban, R.M.; Niculae, D.A.; Dumitrescu, I.B.; Draganescu, D. Biological pathways as substantiation of the use of copper radioisotopes in cancer theranostics. Front. Phys. 2021, 8, 568296. [Google Scholar] [CrossRef]
- Vretenar, M.; Mamaras, A.; Bisoffi, G.; Foka, P. Production of radioisotopes for cancer imaging and treatment with compact linear accelerators. J. Phys. Conf. Ser. 2023, 2420, 012104. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Multifunctionality of lipid-core micelles for drug delivery and tumour targeting. Mol. Membr. Biol. 2010, 27, 232–246. [Google Scholar] [CrossRef]
- Keskin, D.; Tezcaner, A. Micelles as delivery system for cancer treatment. Curr. Pharm. Des. 2017, 23, 5230–5241. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, R.; Moharil, P.; Liu, Y.; Chen, J.; Sun, R.; Song, X.; Ao, Q. Polymeric micelles in cancer immunotherapy. Molecules 2021, 26, 1220. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A.Z. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1456. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D. Plasmonic nanobubbles as tunable cellular probes for cancer theranostics. Cancers 2011, 3, 802–840. [Google Scholar] [CrossRef] [Green Version]
- Jose, A.D.; Wu, Z.; Thakur, S.S. A comprehensive update of micro-and nanobubbles as theranostics in oncology. Eur. J. Pharm. Biopharm. 2022, 172, 123–133. [Google Scholar] [CrossRef]
- Yan, W.C.; Chua, Q.W.; Ong, X.J.; Sharma, V.K.; Tong, Y.W.; Wang, C.H. Fabrication of ultrasound-responsive microbubbles via coaxial electrohydrodynamic atomization for triggered release of tPA. J. Colloid Interface Sci. 2017, 501, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Wande, D.P.; Trevaskis, N.; Farooq, M.A.; Jabeen, A.; Nayak, A.K. Theranostic nanostructures as nanomedicines: Benefits, costs, and future challenges. Des. Appl. Theranostic Nanomed. 2023, 1, 3–24. [Google Scholar]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for antimetastatic treatment. Trends Cancer 2020, 6, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—Application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660. [Google Scholar] [CrossRef] [Green Version]
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.U. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J. Nanomater. 2019, 2019, 1526186. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials 2019, 194, 105–116. [Google Scholar] [CrossRef]
- Panigrahi, B.K.; Nayak, A.K. Carbon nanotubes: An emerging drug delivery carrier in cancer therapeutics. Curr. Drug Deliv. 2020, 17, 558–576. [Google Scholar] [CrossRef]
- Costa, P.M.; Wang, J.T.W.; Morfin, J.F.; Khanum, T.; To, W.; Sosabowski, J.; Tóth, E.; Al-Jamal, K.T. Functionalised carbon nanotubes enhance brain delivery of amyloid-targeting Pittsburgh compound B (PiB)-derived ligands. Nanotheranostics 2018, 2, 168. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Hodgins, N.O.; Maher, J.; Sosabowski, J.K.; Al-Jamal, K.T. Organ biodistribution of radiolabelled γδ T cells following liposomal alendronate administration in different mouse tumour models. Nanotheranostics 2020, 4, 71. [Google Scholar] [CrossRef]
- Medalsy, I.; Dgany, O.; Sowwan, M.; Cohen, H.; Yukashevska, A.; Wolf, S.G.; Wolf, A.; Koster, A.; Almog, O.; Marton, I.; et al. SP1 protein-based nanostructures and arrays. Nano Lett. 2008, 8, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wu, Y.; Zhu, J.; Wen, S.; Shen, M.; Shi, X. Multifunctional lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: Investigating the role played by PEG spacer. ACS Appl. Mater. Interfaces 2014, 6, 16416–16425. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Meza, L.R.; Greer, F.; Greer, J.R. Fabrication and deformation of three-dimensional hollow ceramic nanostructures. Nat. Mater. 2013, 12, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardhian, D.F.; Vrynas, A.; Storm, G.; Bansal, R.; Prakash, J. FGF2 engineered SPIONs attenuate tumor stroma and potentiate the effect of chemotherapy in 3D heterospheroidal model of pancreatic tumor. Nanotheranostics 2020, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Deng, J.; Guo, D.; Zhang, J.; Yang, L.; Wu, F. A folate-conjugated platinum porphyrin complex as a new cancer-targeting photosensitizer for photodynamic therapy. Org. Biomol. Chem. 2019, 17, 5367–5374. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Yong, T.; Wang, D.; Li, X.; Yan, Y.; Hu, J.; Gan, L.; Yang, X. Extracellular vesicles for tumor targeting delivery based on five features principle. J. Control. Release 2020, 322, 555–565. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv. Mater. 2019, 31, 1802896. [Google Scholar] [CrossRef]
- Tran, P.H.; Xiang, D.; Tran, T.T.; Yin, W.; Zhang, Y.; Kong, L.; Chen, K.; Sun, M.; Li, Y.; Hou, Y.; et al. Exosomes and nanoengineering: A match made for precision therapeutics. Adv. Mater. 2020, 32, 1904040. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kalluri, R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Cho, J.Y. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019, 17, 5. [Google Scholar] [CrossRef] [Green Version]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, F.; Zhong, J.Y.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Yuan, L.Q. Exosomes as mediators of cell-to-cell communication in thyroid disease. Int. J. Endocrinol. 2020, 2020, 4378345. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Peng, Y.; Yu, S.; Liu, J.; Wu, L.; Zhang, L.; Wu, Q.; Chang, X.; Yu, X.; et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget 2018, 9, 2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Tian, T.; Zhu, Y.; Ali, D.J.; Hu, F.; Qi, Y.; Sun, B.; Xiao, Z. Exosomes transfer among different species cells and mediating miRNAs delivery. J. Cell. Biochem. 2017, 118, 4267–4274. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Drug Deliv. 2010, 55–86. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, L.; Liu, T.; Zhang, L.; Yao, Y.; Yu, D.; Wang, L.; Zhang, N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231–3242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, M.; Yuan, Z. Immunoscore guided cold tumors to acquire “temperature” through integrating physicochemical and biological methods. BIO Integr. 2020, 1, 6–14. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: Ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef] [Green Version]
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853. [Google Scholar]
- Li, X.; He, G.; Jin, H.; Tao, J.; Li, X.; Zhai, C.; Luo, Y.; Liu, X. Dual-therapeutics-loaded mesoporous silica nanoparticles applied for breast tumor therapy. ACS Appl. Mater. Interfaces 2019, 11, 46497–46503. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, Q.; Osada, K.; Liu, X.; Tockary, T.A.; Uchida, S.; Dirisala, A.; Ishii, T.; Nomoto, T.; Toh, K.; et al. Targeted gene delivery by polyplex micelles with crowded PEG palisade and cRGD moiety for systemic treatment of pancreatic tumors. Biomaterials 2014, 35, 3416–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitanis, A.; Staal, S. Liposomal doxorubicin and nab-paclitaxel: Nanoparticle cancer chemotherapy in current clinical use. Cancer Nanotechnol. Methods Protoc. 2010, 624, 385–392. [Google Scholar]
- Ambasta, R.K.; Sharma, A.; Kumar, P. Nanoparticle mediated targeting of VEGFR and cancer stem cells for cancer therapy. Vasc. Cell 2011, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-based immunotherapy for cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Purushottamachar, P.; Godbole, A.M.; Gediya, L.K.; Martin, M.S.; Vasaitis, T.S.; Kwegyir-Afful, A.K.; Ramalingam, S.; Ates-Alagoz, Z.; Njar, V.C. Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer. J. Med. Chem. 2013, 56, 4880–4898. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.Y.; Su, Y.L.; Cheng, W.; Hu, P.F.; Chiang, C.S.; Chen, W.T.; Hu, S.H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2018, 19, 69–81. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite nanoparticles for improved cancer theranostics. J. Funct. Biomater. 2022, 13, 100. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-black-phosphorus-reinforced 3D-printed scaffolds: A stepwise countermeasure for osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Tang, W.; Hu, L.; Chen, X.; Su, Y.; Zou, C.; Wang, J.; Lu, W.W.; Zhen, W.; et al. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small 2019, 15, 1901560. [Google Scholar] [CrossRef]
- Geng, S.; Pan, T.; Zhou, W.; Cui, H.; Wu, L.; Li, Z.; Chu, P.K.; Yu, X.F. Bioactive phospho-therapy with black phosphorus for in vivo tumor suppression. Theranostics 2020, 10, 4720. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Xiao, X.; Liang, D.; Liang, X.; Mi, L.; Wang, J.; Liu, J. Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors. Acta Biomater. 2020, 107, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-functionalized black phosphorus quantum dots for cancer theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Gaikwad, G.; Rohra, N.; Kumar, C.; Jadhav, S.; Sarma, H.D.; Borade, L.; Chakraborty, S.; Bhagwat, S.; Dandekar, P.; Jain, R.; et al. A facile strategy for synthesis of a broad palette of intrinsically radiolabeled chitosan nanoparticles for potential use in cancer theranostics. J. Drug Deliv. Sci. Technol. 2021, 63, 102485. [Google Scholar] [CrossRef]
- Li, Y.; Feng, P.; Wang, C.; Miao, W.; Huang, H. Black phosphorus nanophototherapeutics with enhanced stability and safety for breast cancer treatment. Chem. Eng. J. 2020, 400, 125851. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef]
- Stern, S.T.; Hall, J.B.; Lee, L.Y.; Wood, L.J.; Paciotti, G.F.; Tamarkin, L.; Long, S.E.; McNeil, S.E. Translational considerations for cancer nanomedicine. J. Control. Release 2010, 146, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Jain, S.; Hirst, D.G.; O’Sullivan, J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Staves, B. Pilot Study of AurolaseTM Therapy in Refractory and/or Recurrent Tumors of the Head and Neck,. 2010. ClinicalTrials.gov, Identifier: NCT00848042. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00848042 (accessed on 17 May 2023).
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr. Oncol. Rep. 2010, 12, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, D.J.; Jung, S.E.; Cho, S.H.; Hahn, S.T.; Lee, J.M. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma <5 cm: Comparison with transarterial chemoembolisation monotherapy—Preliminary observations. Br. J. Radiol. 2012, 85, e940–e946. [Google Scholar] [PubMed] [Green Version]
- Mokhosi, S.R.; Mdlalose, W.; Nhlapo, A.; Singh, M. Advances in the synthesis and application of magnetic ferrite nanoparticles for cancer therapy. Pharmaceutics 2022, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 602–623. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent progress in lipid nanoparticles for cancer theranostics: Opportunity and challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N. Superparamagnetic nanoparticles for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Cambridge, UK, 2018; pp. 843–859. [Google Scholar]
- Nayak, V.; Singh, K.R.; Verma, R.; Pandey, M.D.; Singh, J.; Singh, R.P. Recent advancements of biogenic iron nanoparticles in cancer theranostics. Mater. Lett. 2022, 313, 131769. [Google Scholar] [CrossRef]
- Cheng, H.W.; Tsao, H.Y.; Chiang, C.S.; Chen, S.Y. Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 2021, 10, 2001451. [Google Scholar] [CrossRef]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, A.; Canete, M.; Roca, A.G.; Calero, M.; Veintemillas-Verdaguer, S.; Serna, C.J.; del Puerto Morales, M.; Miranda, R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 2009, 20, 115103. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and future perspectives of multifunctional magnetic nanoparticles based controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2022, 19, 321–335. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y. Gene and oligonucleotide delivery via micro-and nanobubbles by ultrasound exposure. Drug Metab. Pharmacokinet. 2022, 44, 100445. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, M.; Katz, S.; Mano, T.; Aronovich, R.; Hershkovitz, D.; Exner, A.A.; Ilovitsh, T. Low frequency nanobubble-enhanced ultrasound mechanotherapy for noninvasive cancer surgery. Nanoscale 2022, 14, 13614–13627. [Google Scholar] [CrossRef] [PubMed]
- Ghafary, S.M.; Rahimjazi, E.; Hamzehil, H.; Mousavi, S.M.M.; Nikkhah, M.; Hosseinkhani, S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of quaorubicin/curcumin and graphene quantum dots. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102544. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Webster, T.J.; Li, L. Theranostic safe quantum dots for anticancer and bioimaging applications. Micro Nano Bio Asp. 2022, 1, 1–11. [Google Scholar]
- Song, H.; Wang, J.; Xiong, B.; Hu, J.; Zeng, P.; Liu, X.; Liang, H. Biologically Safe, Versatile, and Smart Bismuthene Functionalized with a Drug Delivery System Based on Red Phosphorus Quantum Dots for Cancer Theranostics. Angew. Chem. 2022, 134, e202117679. [Google Scholar] [CrossRef]
- Salkho, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Photo-induced drug release from polymeric micelles and liposomes: Phototriggering mechanisms in drug delivery systems. Polymers 2022, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. Micro-and nanoformulations of paclitaxel based on micelles, liposomes, cubosomes, and lipid nanoparticles: Recent advances and challenges. Drug Discov. Today 2022, 27, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [PubMed]
- Yang, L.; Zhang, Y.; Zhang, Y.; Xu, Y.; Li, Y.; Xie, Z.; Wang, H.; Lin, Y.; Lin, Q.; Gong, T.; et al. Live macrophage-delivered doxorubicin-loaded liposomes effectively treat triple-negative breast cancer. ACS Nano 2022, 16, 9799–9809. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Su, H.; Zhu, X.Y.; Li, X.Y.; Li, J.; Chen, X.; Wang, Q.; Hao, R.Y.; Yan, X.Y. Long-circulating doxorubicin and schizandrin A liposome with drug-resistant liver cancer activity: Preparation, characterization, and pharmacokinetic. J. Liposome Res. 2022, 32, 107–118. [Google Scholar] [CrossRef]
- De Oliveira Silva, J.; Fernandes, R.S.; Oda, C.M.R.; Ferreira, T.H.; Botelho, A.F.M.; Melo, M.M.; de Miranda, M.C.; Gomes, D.A.; Cassali, G.D.; Townsend, D.M.; et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef]
- Nandi, U.; Onyesom, I.; Douroumis, D. Anti-cancer activity of sirolimus loaded liposomes in prostate cancer cell lines. J. Drug Deliv. Sci. Technol. 2021, 61, 102200. [Google Scholar] [CrossRef]
- Hu, Y.J.; Ju, R.J.; Zeng, F.; Qi, X.R.; Lu, W.L. Liposomes in drug delivery: Status and advances. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–24. [Google Scholar]
- Terrisse, S.; Karamouza, E.; Parker, C.C.; Sartor, A.O.; James, N.D.; Pirrie, S.; Collette, L.; Tombal, B.F.; Chahoud, J.; Smeland, S.; et al. Overall survival in men with bone metastases from castration-resistant prostate cancer treated with bone-targeting radioisotopes: A meta-analysis of individual patient data from randomized clinical trials. JAMA Oncol. 2020, 6, 206–216. [Google Scholar] [CrossRef]
- Pei, P.; Liu, T.; Shen, W.; Liu, Z.; Yang, K. Biomaterial-mediated internal radioisotope therapy. Mater. Horiz. 2021, 8, 1348–1366. [Google Scholar] [CrossRef]
- Liang, C.; Chao, Y.; Yi, X.; Xu, J.; Feng, L.; Zhao, Q.; Yang, K.; Liu, Z. Nanoparticle-mediated internal radioisotope therapy to locally increase the tumor vasculature permeability for synergistically improved cancer therapies. Biomaterials 2019, 197, 368–379. [Google Scholar] [CrossRef]
- Xia, L.; Meng, X.; Wen, L.; Zhou, N.; Liu, T.; Xu, X.; Wang, F.; Cheng, Z.; Yang, Z.; Zhu, H. A Highly Specific Multiple Enhancement Theranostic Nanoprobe for PET/MRI/PAI Image-Guided Radioisotope Combined Photothermal Therapy in Prostate Cancer. Small 2021, 17, 2100378. [Google Scholar] [CrossRef] [PubMed]
- Choiński, J.; Łyczko, M. Prospects for the production of radioisotopes and radiobioconjugates for theranostics. Bio-Algorithms Med-Syst. 2021, 17, 241–257. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Naskar, N.; Lahiri, S. Theranostic terbium radioisotopes: Challenges in production for clinical application. Front. Med. 2021, 8, 675014. [Google Scholar] [CrossRef]
- Karadag, S.N.; Ustun, O.; Yilmaz, A.; Yilmaz, M. The fabrication of excitation-dependent fluorescence boron/nitrogen co-doped carbon quantum dots and their employment in bioimaging. Chem. Phys. 2022, 562, 111678. [Google Scholar] [CrossRef]
- Yeroslavsky, G.; Umezawa, M.; Okubo, K.; Nigoghossian, K.; Dung, D.T.K.; Miyata, K.; Kamimura, M.; Soga, K. Stabilization of indocyanine green dye in polymeric micelles for NIR-II fluorescence imaging and cancer treatment. Biomater. Sci. 2020, 8, 2245–2254. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Picchio, M.L.; Gugliotta, A.; Bürgi, M.; Ronco, L.I.; Calderón, M.; Etcheverrigaray, M.; Igarzabal, C.I.A.; Minari, R.J.; Gugliotta, L.M. Crosslinked casein micelles bound paclitaxel as enzyme activated intracellular drug delivery systems for cancer therapy. Eur. Polym. J. 2021, 145, 110237. [Google Scholar] [CrossRef]
- Cavalcante, C.H.; Fernandes, R.S.; de Oliveira Silva, J.; Oda, C.M.R.; Leite, E.A.; Cassali, G.D.; Charlie-Silva, I.; Fernandes, B.H.V.; Ferreira, L.A.M.; de Barros, A.L.B. Doxorubicin-loaded pH-sensitive micelles: A promising alternative to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2021, 134, 111076. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Pouyafar, A.; Salehi, R.; Rahbarghazi, R. Chrysin and docetaxel loaded biodegradable micelle for combination chemotherapy of cancer stem cell. Pharm. Res. 2019, 36, 165. [Google Scholar] [CrossRef]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ning, Q.; Mo, Z.; Tang, S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1476–1487. [Google Scholar] [CrossRef]
- Gao, M.; Deng, J.; Liu, F.; Fan, A.; Wang, Y.; Wu, H.; Ding, D.; Kong, D.; Wang, Z.; Peer, D.; et al. Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials 2019, 223, 119486. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Kolokithas-Ntoukas, A.; Fytas, C.; Avgoustakis, K. Folic acid-functionalized, condensed magnetic nanoparticles for targeted delivery of doxorubicin to tumor cancer cells overexpressing the folate receptor. ACS Omega 2019, 4, 22214–22227. [Google Scholar] [CrossRef] [Green Version]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, X.; Si, J.; Mou, X.; Dong, X. All-in-One Nanomedicine: Multifunctional Single-Component Nanoparticles for Cancer Theranostics. Small 2021, 17, 2103072. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, J.; Miao, Q.; Zhu, R.; Wen, L.; Zeng, J.; Gao, M. Biodegradable inorganic nanoparticles for cancer theranostics: Insights into the degradation behavior. Bioconjugate Chem. 2019, 31, 315–331. [Google Scholar] [CrossRef]
- Zavaleta, C.; Ho, D.; Chung, E.J. Theranostic nanoparticles for tracking and monitoring disease state. SLAS Technol. 2018, 23, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic nanoparticles for RNA-based cancer treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef]
- Boehnke, N.; Correa, S.; Hao, L.; Wang, W.; Straehla, J.P.; Bhatia, S.N.; Hammond, P.T. Theranostic Layer-by-Layer Nanoparticles for Simultaneous Tumor Detection and Gene Silencing. Angew. Chem. 2020, 132, 2798–2805. [Google Scholar] [CrossRef]
- Bukhari, S.Z.; Zeth, K.; Iftikhar, M.; Rehman, M.; Munir, M.U.; Khan, W.S.; Ihsan, A. Supramolecular lipid nanoparticles as delivery carriers for non-invasive cancer theranostics. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.; Belmonte-Reche, E.; Gallo, J.; Baltazar, F.; Bañobre-López, M. Magnetic solid nanoparticles and their counterparts: Recent advances towards cancer theranostics. Pharmaceutics 2022, 14, 506. [Google Scholar] [CrossRef] [PubMed]
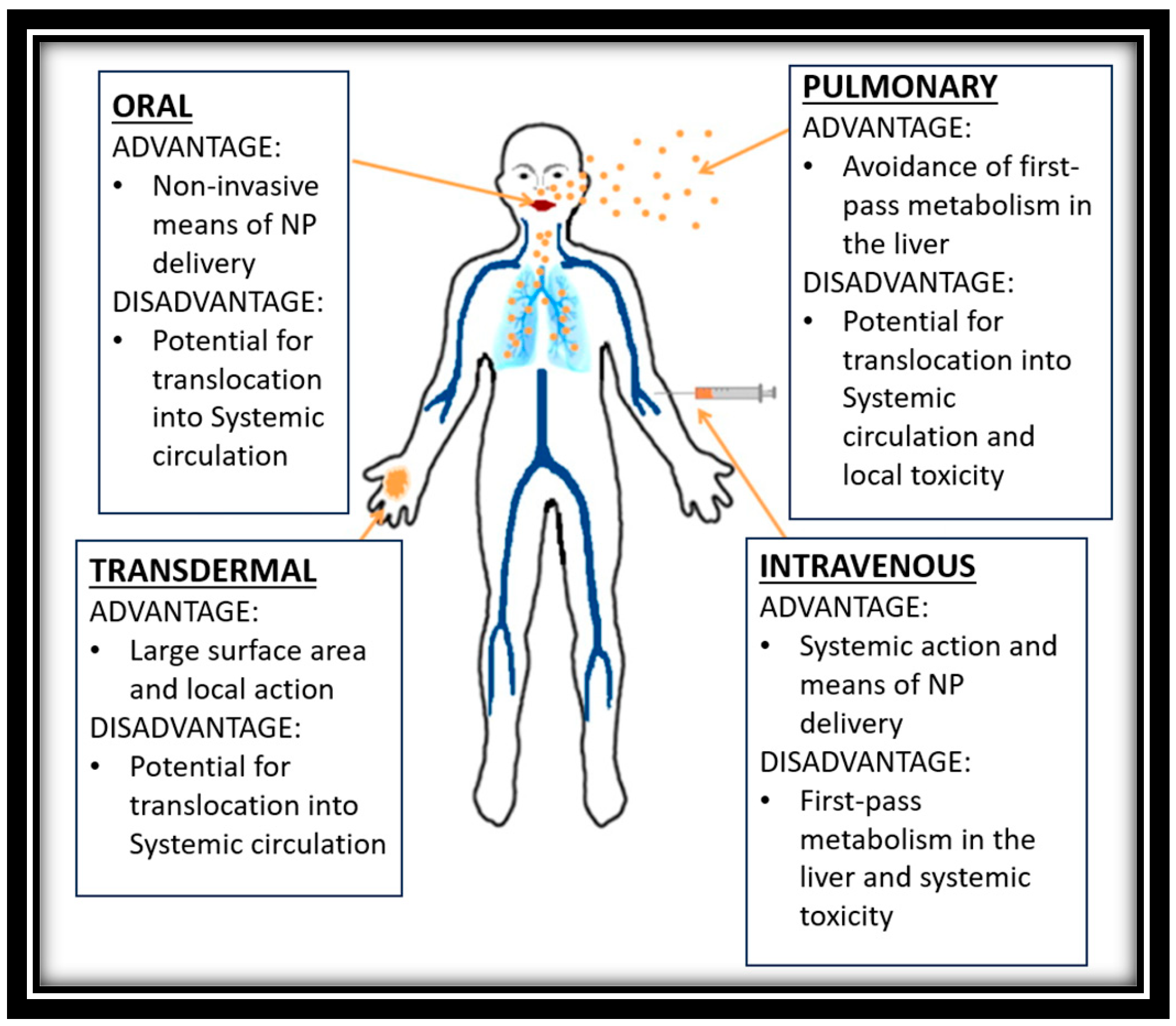



| Contrast Agent | Drug Used | Applications |
|---|---|---|
| Gold | Doxorubicin (DOX) | Diagnosis, tumor targeting, and PTT |
| Silica | Pyro pheophorbide (HPPH), DOX | Drug carrier, X-ray/CT imaging, and photodynamic therapy |
| Manganese oxide | siRNA | MRI plus RNA delivery |
| CNTs | DNA plasmid, DOX, PTX | Diagnosis, DNA, and drug delivery |
| QDs | DOX, MTX | Imaging, therapy, and sensing |
| Iron oxide | siRNA, DOX, docetaxel | Targeting, MRI, and therapy |
| Responses | TACE Group (n = 32) | TACE + HIFU Group (n = 25) |
|---|---|---|
| SD | 4 (13%) | 5 (20%) |
| CR | 9 (28%) | 5 (20%) |
| PD | 17 (53%) | 13 (52%) |
| PR | 2 (6%) | 2 (8%) |
| Disease control rate | 15/32 (47%) | 12/25 (48%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, D.; Hajra, K.; Maity, D. Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic. J. Nanotheranostics 2023, 4, 299-322. https://doi.org/10.3390/jnt4030014
Tripathi D, Hajra K, Maity D. Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic. Journal of Nanotheranostics. 2023; 4(3):299-322. https://doi.org/10.3390/jnt4030014
Chicago/Turabian StyleTripathi, Divya, Kasturee Hajra, and Dipak Maity. 2023. "Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic" Journal of Nanotheranostics 4, no. 3: 299-322. https://doi.org/10.3390/jnt4030014
APA StyleTripathi, D., Hajra, K., & Maity, D. (2023). Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic. Journal of Nanotheranostics, 4(3), 299-322. https://doi.org/10.3390/jnt4030014








