Paradoxical Roles of Carbon Nanotubes in Cancer Therapy and Carcinogenesis
Abstract
:1. Introduction
2. Applicability of CNTs in Cancer Diagnosis and Treatment
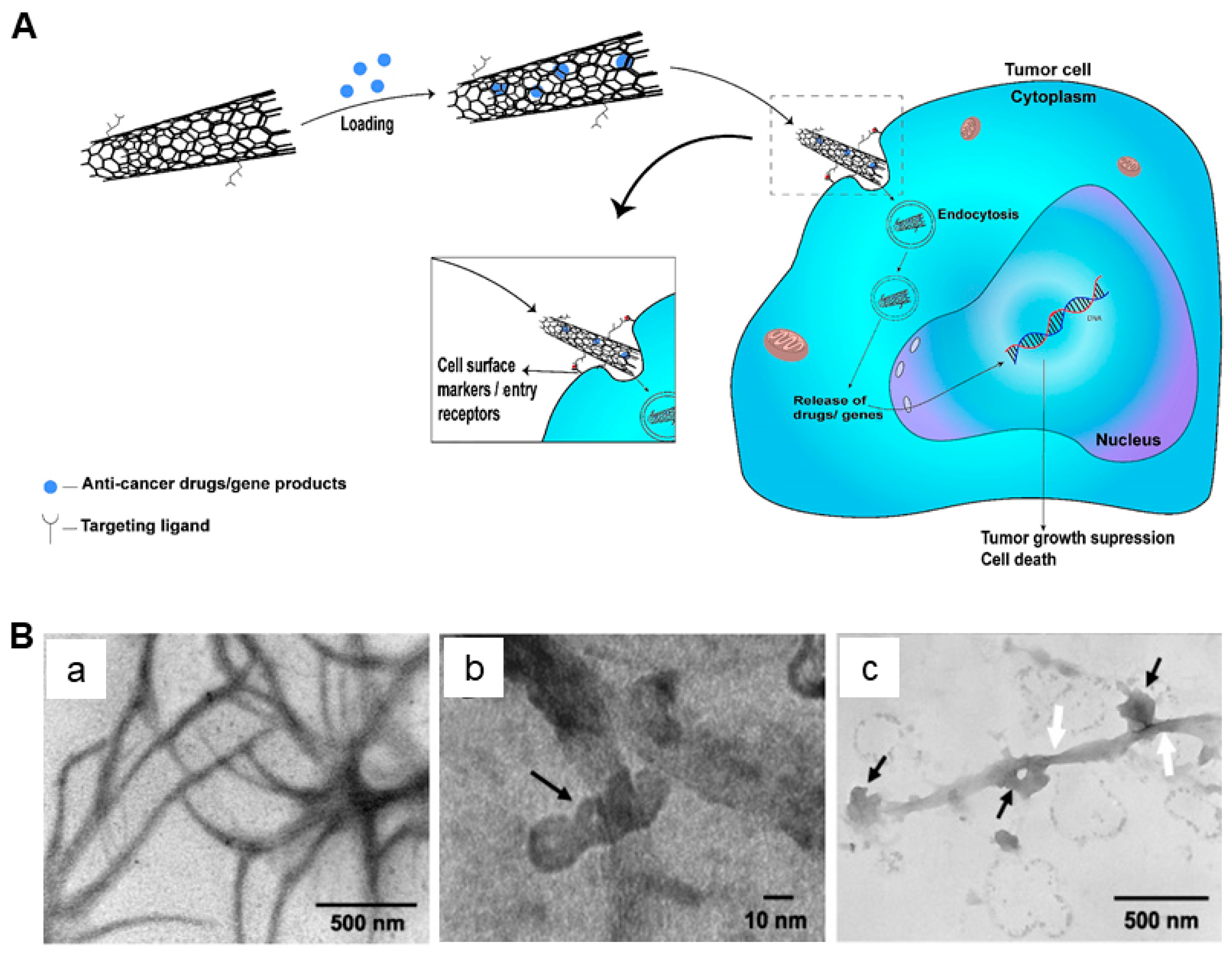
2.1. CNTs as Drug Delivery Vehicles
2.2. CNTs for Cancer Therapy
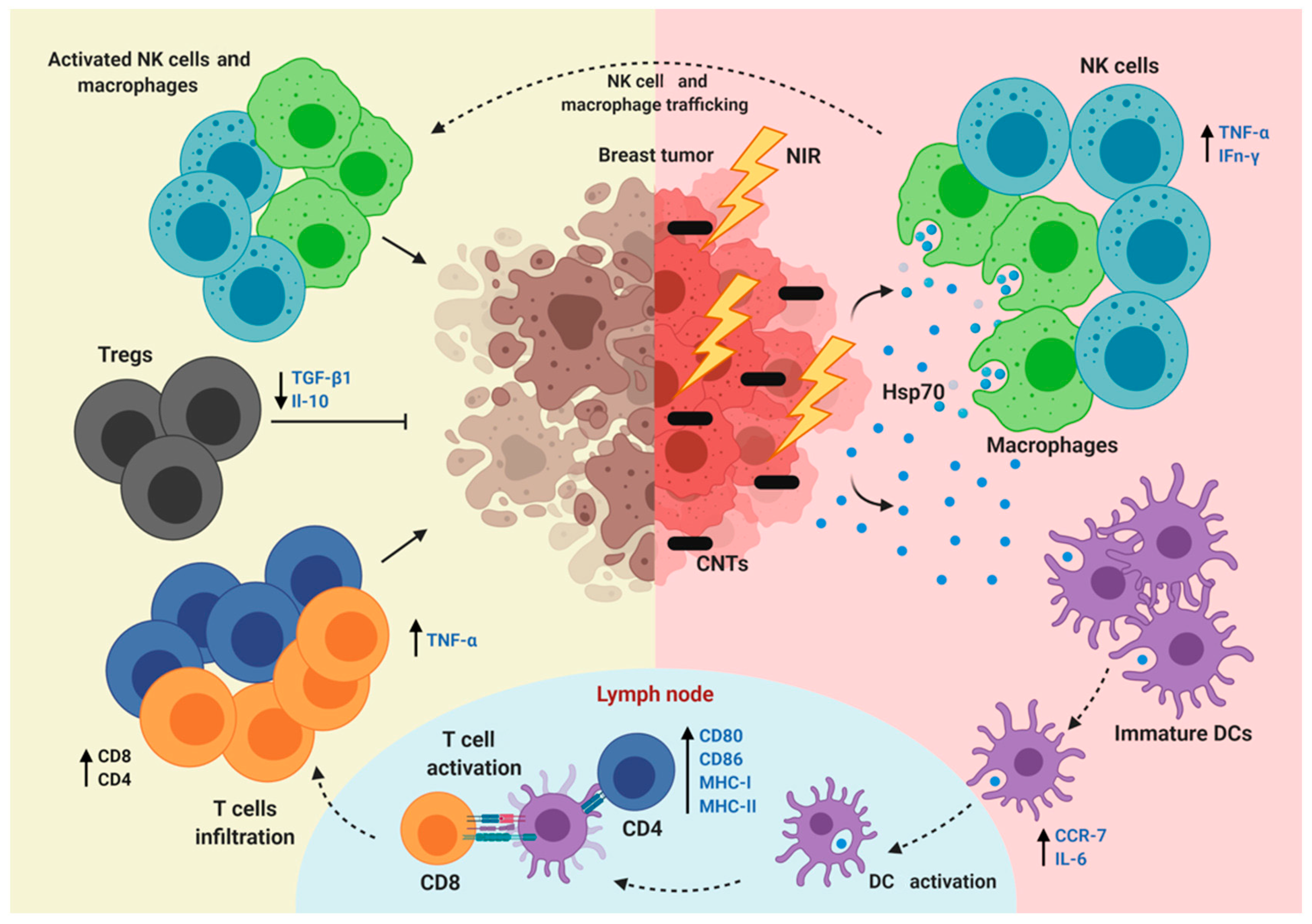
2.3. CNTs for the Regulation of Immunity
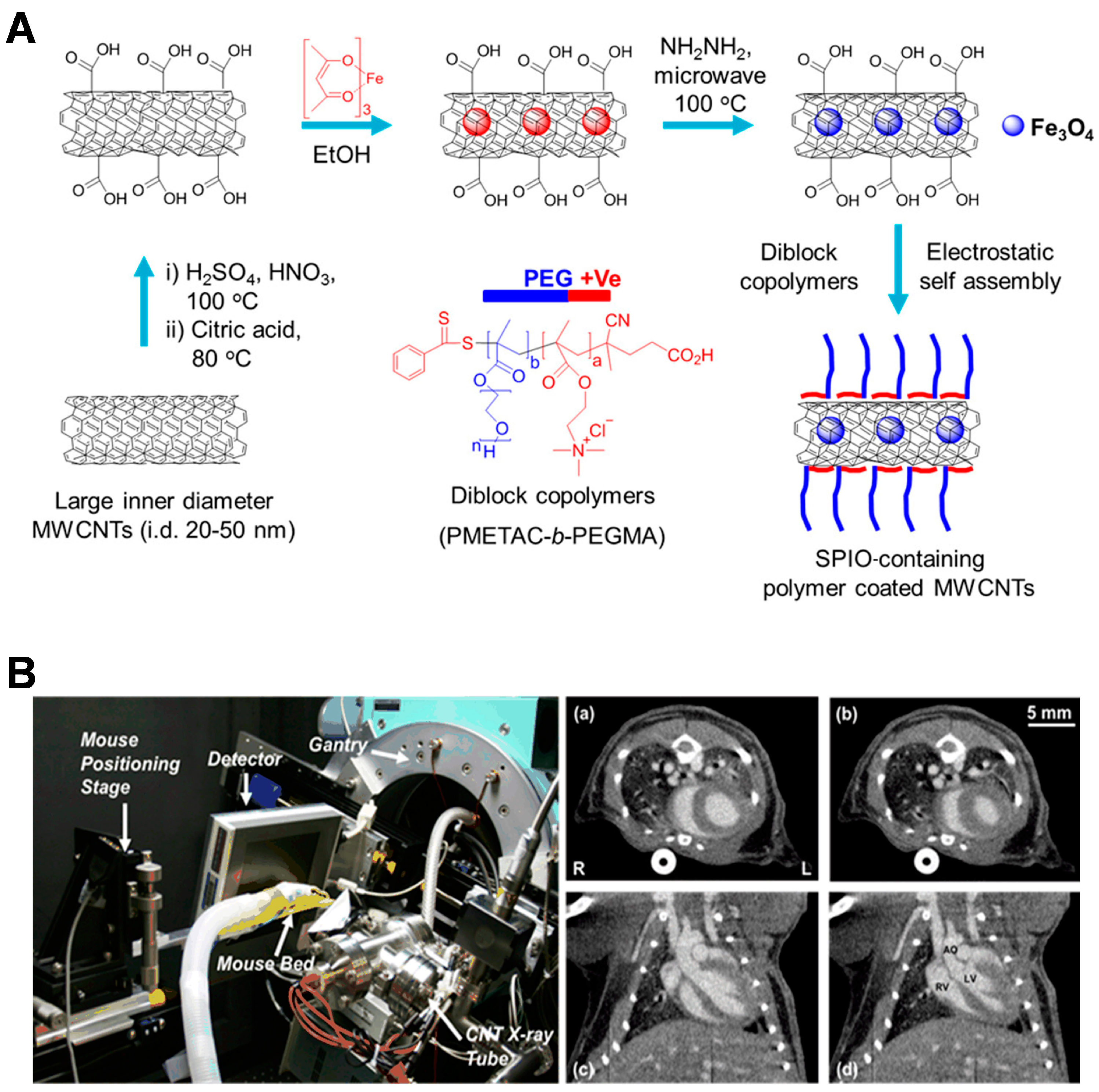
2.4. CNTs for Cancer Diagnosis
3. Carcinogenicity and Potential Mechanisms of CNTs
3.1. ROS
3.2. Inflammation and Fibrosis
3.3. EMT
3.4. Endothelial Leakiness
4. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Aasi, A.; Aasi, E.; Aghaei, S.M.; Panchapakesan, B. CNT biodevices for early liver cancer diagnosis based on biomarkers detection-a promising platform. J. Mol. Graph. Model. 2022, 114, 108208. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A. Anticancer DOX delivery system based on CNTs: Functionalization, targeting and novel technologies. J. Control. Release 2020, 327, 198–224. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Yousefi, F.; Sajadi, S.M.; Munir, M.T.; Abida, O.; Habibzadeh, S.; Mashhadzadeh, A.H.; Rabiee, N.; Mostafaci, E.; Saeb, M.R. Theoretical encapsulation of fluorouracil (5-FU) anti-cancer chemotherapy drug into carbon nanotubes (CNT) and boron nitride nanotubes (BNNT). Molecules 2021, 26, 4920. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials 2020, 10, 264. [Google Scholar] [CrossRef]
- Barbarino, M.; Giordano, A. Assessment of the carcinogenicity of carbon nanotubes in the respiratory system. Cancers 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Wei, Y.; Sun, Y.; Wang, Y.; Zhu, S. Carbon nanotubes as carriers in drug delivery for non-small cell lung cancer, mechanistic analysis of their carcinogenic potential, safety profiling and identification of biomarkers. Int. J. Nanomed. 2022, 17, 6157. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Makoto, O.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M. Biodegradation of carbon nanotubes by macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Dong, J. Signaling pathways implicated in carbon nanotube-induced lung inflammation. Front. Immunol. 2020, 11, 552613. [Google Scholar] [CrossRef]
- Hadrup, N.; Knudsen, K.B.; Carriere, M.; Mayne-L’Hermite, M.; Bobyk, L.; Allard, S.; Miserque, F.; Pibaleau, B.; Pinault, M.; Wallin, H.; et al. Safe-by-design strategies for lowering the genotoxicity and pulmonary inflammation of multiwalled carbon nanotubes: Reduction of length and the introduction of COOH groups. Environ. Toxicol. Pharmacol. 2021, 87, 103702. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, Y.; Bai, R.; Wu, Z.; Qian, W.; Yang, L.; Cai, R.; Yan, H.; Li, T.; Pandey, V.; et al. Long-term pulmonary exposure to multi-walled carbon nanotubes promotes breast cancer metastatic cascades. Nat. Nanotechnol. 2019, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Posypanova, G.A.; Gayduchenko, I.A.; Moskaleva, E.Y.; Fedorov, G.E. Neuronal differentiation of PC12 cells and mouse neural stem cells on carbon nanotube films. Cell Tissue Biol. 2016, 10, 194–201. [Google Scholar] [CrossRef]
- Öner, D.; Moisse, M.; Ghosh, M.; Duca, R.; Poels, K.; Luyts, K.; Putzeys, E.; Cokic, S.; Landuyt, K.L.V.; Lambrechts, D.; et al. Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis 2017, 32, 181–191. [Google Scholar] [CrossRef]
- Panigrahi, B.K.; Nayak, A.K. Carbon nanotubes: An emerging drug delivery carrier in cancer therapeutics. Curr. Drug Deliv. 2020, 17, 558–576. [Google Scholar] [CrossRef]
- Kofoed, A.C.; Khatri, S.; Hansen, J.; Slott, S.; Parvathaneni, R.P.; Mendes, A.C.; Chronakis, L.S.; Hung, S.; Rajasekaran, N.; Ma, Z.; et al. Carbon nanotubes—Potent carriers for targeted drug delivery in rheumatoid arthritis. Pharmaceutics 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, T.; Cao, Y.; Sun, J.; Zhou, Q.; Chen, H.; Guo, S.; Wang, Y.; Zhen, Y.; Liang, X.; et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy. ACS Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef]
- Naief, M.F.; Mohammed, S.N.; Mayouf, H.J.; Mohammed, A.M. A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J. Organomet. Chem. 2023, 999, 122819. [Google Scholar] [CrossRef]
- Ijaz, H.; Mahmood, A.; Abdel-Daim, M.M.; Sarfraz, R.M.; Zaman, M.; Zafar, N.; Alshehery, S.; Salem-Bekhit, M.M.; Ali, M.A.; Eltayeb, L.B.; et al. Review on carbon nanotubes (CNTs) and their chemical and physical characteristics, with particular emphasis on potential applications in biomedicine. Inorg. Chem. Commun. 2023, 155, 111020. [Google Scholar] [CrossRef]
- Behnam, B.; Shier, W.T.; Nia, A.H.; Abnous, K.; Ramezani, M. Non-covalent functionalization of single-walled carbon nanotubes with modified polyethyleneimines for efficient gene delivery. Int. J. Pharm. 2013, 454, 204–215. [Google Scholar] [CrossRef]
- Canal, F.; Vicent, M.J.; Pasut, G.; Schiavon, O. Relevance of folic acid/polymer ratio in targeted PEG–epirubicin conjugates. J. Control. Release 2010, 146, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gao, X.; Taratula, O.; Treado, S.; Urbas, A.; Holbrook, R.D.; E Cavicchi, R.; Avedisian, C.T.; Mitra, S.; Savla, R.; et al. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer 2009, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Lattrich, C.; Juhasz-boess, I.; Ortmann, O.; Treeck, O. Detection of an elevated HER2 expression in MCF-7 breast cancer cells overexpressing estrogen receptor ß1. Oncol. Rep. 2008, 19, 811–817. [Google Scholar] [PubMed]
- Lee, P.C.; Chiou, Y.C.; Wong, J.M.; Peng, C.L.; Shieh, M.J. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials 2013, 34, 8756–8765. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.R.; Kumari, G.K.; Krishnamurthy, P.T. Carbon nanotubes in drug delivery: Focus on anticancer therapies. J. Drug Deliv. Sci. Technol. 2020, 59, 101892. [Google Scholar] [CrossRef]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.P.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew. Chem. Int. Ed. 2004, 43, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Weng, Z.; Wang, C.; Zhu, M.; Lu, Y.; Ding, L.; Wang, Y.; Cheng, X.; Lin, Q.; Wu, K. Increased chemosensitivity and radiosensitivity of human breast cancer cell lines treated with novel functionalized single-walled carbon nanotubes. Oncol. Lett. 2017, 13, 206–214. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Harvey, J.D.; Jena, P.V.; Baker, H.A.; Zerze, G.H.; Williams, R.M.; Galassi, T.V.; Roxbury, D.; Mittal, J.; Heller, D.A. A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 2017, 1, 0041. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Comparetti, E.J.; Pedrosa, V.D.A.; Kaneno, R. Carbon nanotube as a tool for fighting cancer. Bioconjugate Chem. 2017, 29, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, A.; Shaterian, M.; Danafar, H.; Likozar, B.; Šuligoj, A.; Gyergyek, S. The role of single-walled carbon nanotubes functionalized with gold to increase radiosensitivity of cancer cells to X-ray radiation. Appl. Organomet. Chem. 2023, 37, e7265. [Google Scholar] [CrossRef]
- Berber, M.R.; Elkhenany, H.; Hafez, I.H.; El-Badawy, A.; Essawy, M.; El-Badri, N. Efficient tailoring of platinum nanoparticles supported on multiwalled carbon nanotubes for cancer therapy. Nanomedicine 2020, 15, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, H.; Huang, J.; Chen, H.; Wang, J.; Qiu, K.; Zhao, D.; Ji, L.; Chao, H. Noncovalent ruthenium (II) complexes–single-walled carbon nanotube composites for bimodal photothermal and photodynamic therapy with near-infrared irradiation. ACS Appl. Mater. Interfaces 2015, 7, 23278–23290. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.L.; Chen, Y.C.; Hsu, C.Y.; Su, H.L.; Lai, P.S. Carbon nanotube-mediated photothermal disruption of endosomes/lysosomes reverses doxorubicin resistance in MCF-7/ADR cells. J. Biomed. Nanotechnol. 2016, 12, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Radzi, M.R.M.; Johari, N.A.; Zawawi, W.F.A.W.M.; Zawawi, N.A.; Latiff, N.A.; Malek, N.A.N.N.; Wahab, A.A.; Salim, M.I.; Jemon, K. In vivo evaluation of oxidized multiwalled-carbon nanotubes-mediated hyperthermia treatment for breast cancer. Biomater. Adv. 2022, 134, 112586. [Google Scholar] [CrossRef] [PubMed]
- Modo, M.M.; Bulte, J.W. What is molecular and cellular imaging. In Molecular and Cellular MR Imaging; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–9. [Google Scholar]
- Niu, Q.; Lv, W.; Yan, T.; Wang, J.; Yan, B.; Zhou, D. Construction of Durvalumab/carbon nanotube/PEI/aptamer-siRNA chimera for the immunotherapy of hepatocellular carcinoma. Biomed. Mater. 2022, 17, 025015. [Google Scholar]
- Zheng, Y.Y.; Qiu, D.K.; Guo, Z.R.; Gong, Y.M.; Wang, G.X.; Zhu, B. Evaluation of SWCNTs-loaded DNA vaccine encoding predominant antigen epitope VP4-3 against type II GCRV. Aquaculture 2021, 534, 736197. [Google Scholar] [CrossRef]
- Xia, Q.; Gong, C.; Gu, F.; Wang, Z.; Hu, C.; Zhang, L.; Qiang, L.; Ding, X.; Gao, S.; Gao, Y. Functionalized multi-walled carbon nanotubes for targeting delivery of immunostimulatory CpG oligonucleotides against prostate cancer. J. Biomed. Nanotechnol. 2018, 14, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 48, 8154–8162. [Google Scholar] [CrossRef] [PubMed]
- Dizaji, B.F.; Khoshbakht, S.; Farboudi, A.; Azarbaijan, M.H.; Irani, M. Far-reaching advances in the role of carbon nanotubes in cancer therapy. Life Sci. 2020, 257, 118059. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Park, S.H.; Lee, J.W. Applications of functionalized carbon nanotubes for the therapy and diagnosis of cancer. Polymers 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, K.; Lee, S.; Kim, D.H.; Lee, H. Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 23–45. [Google Scholar] [CrossRef]
- Liu, Y.; Muir, B.W.; Waddington, L.J.; Hinton, T.M.; Moffat, B.A.; Hao, X.; Hughes, T.C. Colloidally stabilized magnetic carbon nanotubes providing MRI contrast in mouse liver tumors. Biomacromolecules 2015, 16, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Iancu, C.; Bartos, D.M.; Mahmood, M.W.; Ghosh, A.; Xu, Y.; Dervishi, E.; Collom, S.L.; Khodakovskaya, M.; Mustafa, T.; et al. Raman spectroscopy as a detection and analysis tool for in vitro specific targeting of pancreatic cancer cells by EGF-conjugated, single-walled carbon nanotubes. J. Appl. Toxicol. 2012, 32, 365–375. [Google Scholar] [CrossRef]
- Cao, G. Biomedical X-ray imaging enabled by carbon nanotube X-ray sources. Chin. J. Chem. Phys. 2018, 31, 529–536. [Google Scholar] [CrossRef]
- Dalby, M.J.; Giannaras, D.; Riehle, M.O.; Gadegaard, N.; Affrossman, S.; Curtis, A.S.G. Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography. Biomaterials 2004, 25, 77–83. [Google Scholar] [CrossRef]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Cammisuli, F.; Giordani, S.; Gianoncelli, A.; Rizzardi, C.; Radillo, L.; Zweyer, M.; Da Ros, T.; Salomé, M.; Melato, M.; Pascolo, L. Iron-related toxicity of single-walled carbon nanotubes and crocidolite fibres in human mesothelial cells investigated by Synchrotron XRF microscopy. Sci. Rep. 2018, 8, 706. [Google Scholar] [CrossRef]
- Digifico, E.; Belgiovine, C.; Mantovani, A.; Allavena, P. Microenvironment and immunology of the human pleural malignant mesothelioma. In Mesothelioma: From Research to Clinical Practice; Spring: Berlin/Heidelberg, Germany, 2019; pp. 69–84. [Google Scholar]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The microenvironment of lung cancer and therapeutic implications. Adv. Exp. Med. Biol. 2016, 890, 75–110. [Google Scholar]
- Jiang, T.; Amadei, C.A.; Gou, N.; Lin, Y.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Toxicity of single-walled carbon nanotubes (SWCNTs): Effect of lengths, functional groups and electronic structures revealed by a quantitative toxicogenomics assay. Environ. Sci. Nano 2020, 7, 1348–1364. [Google Scholar] [CrossRef]
- Ghosh, M.; Janssen, L.; Martens, D.S.; Öner, D.; Vlaanderen, J.; Pronk, A.; Kuijpers, E.; Vermeulen, R.; Nawrot, T.S.; Godderis, L.; et al. Increased telomere length and mtDNA copy number induced by multi-walled carbon nanotube exposure in the workplace. J. Hazard. Mater. 2020, 394, 122569. [Google Scholar] [CrossRef]
- Di Cristo, L.; Bianchi, M.G.; Chiu, M.; Taurino, G.; Donato, F.; Garzaro, G.; Bussolati, O.; Bergamaschi, E. Comparative in Vitro Cytotoxicity of Realistic Doses of Benchmark Multi-Walled Carbon Nanotubes towards Macrophages and Airway Epithelial Cells. Nanomaterials 2019, 9, 982. [Google Scholar] [CrossRef]
- Rubio, L.; El Yamani, N.; Kazimirova, A.; Dusinska, M.; Marcos, R. Multi-walled carbon nanotubes (NM401) induce ROS-mediated HPRT mutations in Chinese hamster lung fibroblasts. Environ. Res. 2016, 146, 185–190. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Jaurand, M.C.; Møller, P.; Morimoto, Y.; Kobayashi, N.; Pinkerton, K.E.; Sargent, L.M.; Vermeulen, R.C.; Fubini, B.; Kane, A.B. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit. Rev. Toxicol. 2017, 47, 1–58. [Google Scholar] [CrossRef]
- Kiratipaiboon, C.; Stueckle, T.A.; Ghosh, R.; Rojanasakul, L.W.; Chen, Y.C.; Dinu, C.Z.; Rojanasakul, Y. Acquisition of cancer stem cell-like properties in human small airway epithelial cells after a long-term exposure to carbon nanomaterials. Environ. Sci. Nano 2019, 6, 2152–2170. [Google Scholar] [CrossRef]
- Walker, V.G.; Li, Z.; Hulderman, T.; Schwegler-Berry, D.; Kashon, M.L.; Simeonova, P.P. Potential in vitro effects of carbon nanotubes on human aortic endothelial cells. Toxicol. Appl. Pharmacol. 2009, 236, 319–328. [Google Scholar] [CrossRef]
- Møller, P.; Christophersen, D.V.; Jensen, D.M.; Kermanizadeh, A.; Roursgaard, M.; Jacobsen, N.R.; Hemmingsen, J.G.; Danielsen, P.H.; Cao, Y.; Jantzen, K.; et al. Role of oxidative stress in carbon nanotube-generated health effects. Arch. Toxicol. 2014, 88, 1939–1964. [Google Scholar] [CrossRef]
- Castranova, V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: Role of reactive oxygen/nitrogen species. Free. Radic. Biol. Med. 2004, 37, 916–925. [Google Scholar] [CrossRef]
- He, X.; Young, S.H.; Schwegler-Berry, D.; Chisholm, W.P.; Fernback, J.E.; Ma, Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem. Res. Toxicol. 2011, 24, 2237–2248. [Google Scholar] [CrossRef]
- Ma, D.D.; Yang, W.X. Engineered nanoparticles induce cell apoptosis: Potential for cancer therapy. Oncotarget 2016, 7, 40882. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Soltani, R.; Guo, S.; Bianco, A.; Ménard-Moyon, C. Carbon nanomaterials applied for the treatment of inflammatory diseases: Preclinical evidence. Adv. Ther. 2020, 3, 2000051. [Google Scholar] [CrossRef]
- Lim, C.S.; Porter, D.W.; Orandle, M.S.; Green, B.J.; Barnes, M.A.; Croston, T.L.; Wolfarth, M.G.; Battelli, L.A.; Andrew, M.E.; Beezhold, D.H.; et al. Resolution of pulmonary inflammation induced by carbon nanotubes and fullerenes in mice: Role of macrophage polarization. Front. Immunol. 2020, 11, 1186. [Google Scholar] [CrossRef]
- Huang, X.; Tian, Y.; Shi, W.; Chen, J.; Yan, L.; Ren, L.; Zhang, X.; Zhu, J. Role of inflammation in the malignant transformation of pleural mesothelial cells induced by multi-walled carbon nanotubes. Nanotoxicology 2020, 14, 947–967. [Google Scholar] [CrossRef]
- Wang, P.; Nie, X.; Wang, Y.; Li, Y.; Ge, C.; Zhang, L.; Wang, L.; Bai, R.; Chen, Z.; Zhao, Y.; et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-β/Smad signaling pathway. Small 2013, 9, 3799–3811. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Wang, Y.; Su, L.; Chen, B.; Wu, K.; Xing, Y.; Song, Z.; Dai, R.; Liu, T.; et al. Systematic co-delivery of dual agonists to enhance cancer immunotherapy. Nano Res. 2022, 15, 8326–8335. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reise Sousa, C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018, 172, 1022–1037. [Google Scholar] [CrossRef]
- Long, A.G.; Lundsmith, E.T.; Hamilton, K.E. Inflammation and colorectal cancer. Curr. Color. Cancer Rep. 2017, 13, 341–351. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, D.; Song, H.; Qiu, B.; Tian, D.; Li, Z.; Ji, Y.; Wang, J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e048324. [Google Scholar] [CrossRef]
- He, W.Z.; Jiang, C.; Liu, L.L.; Yin, C.X.; Rong, Y.M.; Hu, W.M.; Yang, L.; Wang, L.; Jin, Y.N.; Lin, X.P.; et al. Association of body composition with survival and inflammatory responses in patients with non-metastatic nasopharyngeal cancer. Oral Oncol. 2020, 108, 104771. [Google Scholar] [CrossRef]
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2015, 13, 27. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Nie, X.; Braïni, C.; Bai, R.; Chen, C. Multiwall carbon nanotubes directly promote fibroblast–myofibroblast and epithelial–mesenchymal transitions through the activation of the TGF-β/Smad signaling pathway. Small 2015, 11, 446–455. [Google Scholar] [CrossRef]
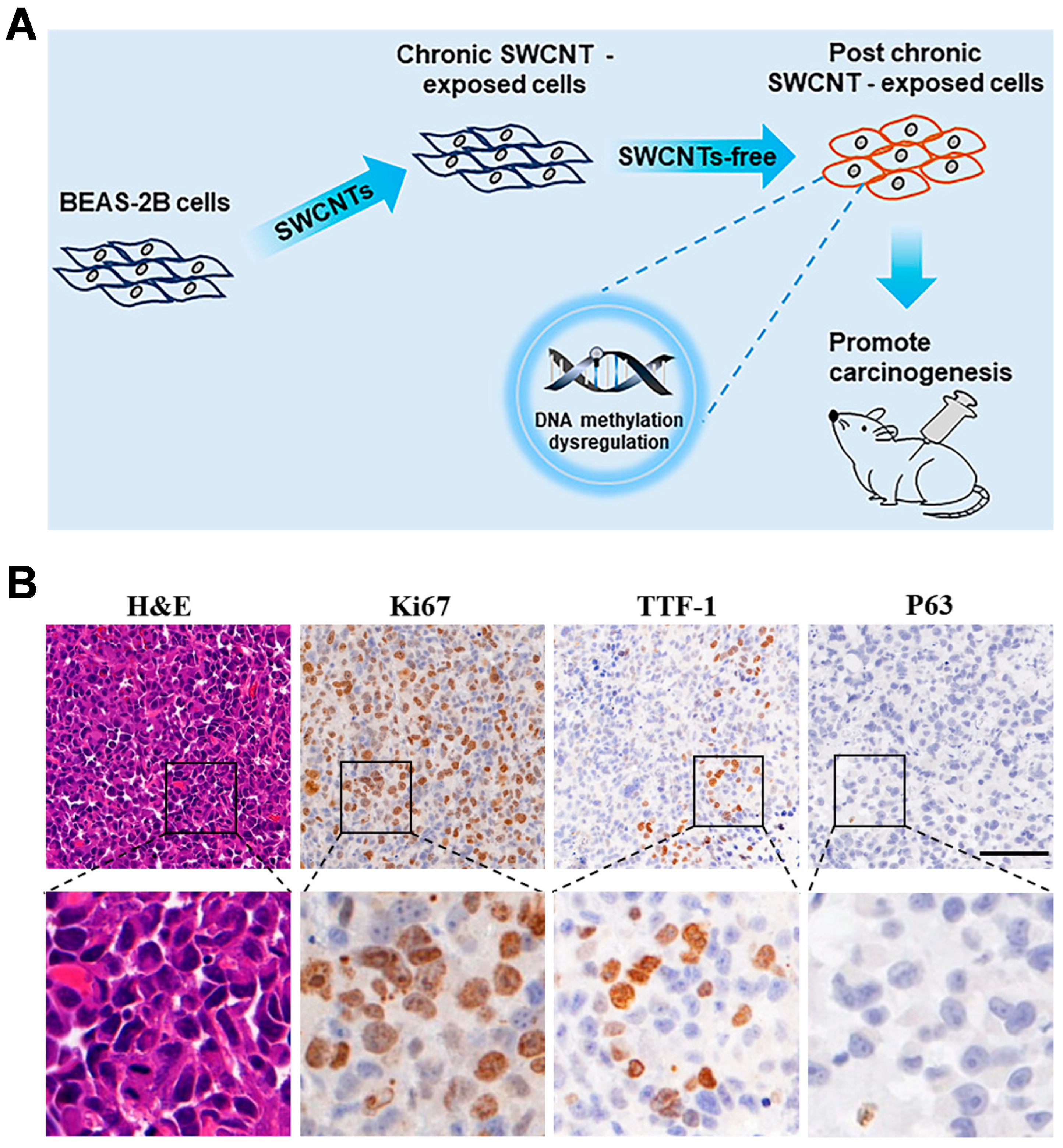
- Wang, J.; Tian, X.; Zhang, J.; Tan, L.; Ouyang, N.; Jia, B.; Chen, C.; Ge, C.; Li, J. Postchronic single-walled carbon nanotube exposure causes irreversible malignant transformation of human bronchial epithelial cells through DNA methylation changes. ACS Nano 2021, 15, 7094–7104. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Castranova, V.; Rojanasakul, Y. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 22. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, M.; Zhang, J.; Yao, Z.; Zhu, J.; Yang, S.; Zhu, Q.; Shen, T. Carbon nanotubes promote alveolar macrophages toward M2 polarization mediated epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Nanotoxicology 2021, 15, 588–604. [Google Scholar] [CrossRef]
- Jin, H.; Gao, S.; Song, D.; Liu, Y.; Chen, X. Intratumorally CpG immunotherapy with carbon nanotubes inhibits local tumor growth and liver metastasis by suppressing the epithelial–mesenchymal transition of colon cancer cells. Anti-Cancer Drugs 2021, 32, 278. [Google Scholar] [CrossRef]
- Yang, D.; Shen, J.; Fan, J.; Chen, Y.; Guo, X. Paracellular permeability changes induced by multi-walled carbon nanotubes in brain endothelial cells and associated roles of hemichannels. Toxicology 2020, 440, 152491. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Gao, G.; Zhao, Q.; Huang, X.; Wang, H.; Liu, B.; Zhi, J. Nanodiamonds inhibit the proliferation and migration of endothelial cells in a tumor/endothelial cells co-culture microfluidic system. Carbon 2024, 218, 118671. [Google Scholar] [CrossRef]
- Lasak, M.; Ciepluch, K. Overview of mechanism and consequences of endothelial leakiness caused by metal and polymeric nanoparticles. Beilstein J. Nanotechnol. 2023, 14, 329–338. [Google Scholar] [CrossRef]
- Wei, W.; Li, Y.; Lee, M.; Andrikopoulos, N.; Lin, S.; Chen, C.; Leong, D.T.; Ding, F.; Song, Y.; Ke, P.C. Anionic nanoplastic exposure induces endothelial leakiness. Nat. Commun. 2022, 13, 4757. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Wang, Q.; Ni, N.; Tee, J.K.; Ariga, K.; Ke, P.C.; Ho, H.K.; Wang, Y.; Leong, D.T. Engineering tumoral vascular leakiness with gold nanoparticles. Nat. Commun. 2023, 14, 4269. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wu, J.; Liu, J.; Kang, Y.; Hu, C.; Feng, X.; Liu, W.; Luo, H.; Chen, A.; et al. Effects of carbon-based nanomaterials on vascular endothelia under physiological and pathological conditions: Interactions, mechanisms and potential therapeutic applications. J. Control. Release 2021, 330, 945–962. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Wu, S.; Wang, Y.; Ji, Y.; Liang, S.; Wang, C.; Tian, X. Paradoxical Roles of Carbon Nanotubes in Cancer Therapy and Carcinogenesis. J. Nanotheranostics 2024, 5, 84-98. https://doi.org/10.3390/jnt5030006
Xu B, Wu S, Wang Y, Ji Y, Liang S, Wang C, Tian X. Paradoxical Roles of Carbon Nanotubes in Cancer Therapy and Carcinogenesis. Journal of Nanotheranostics. 2024; 5(3):84-98. https://doi.org/10.3390/jnt5030006
Chicago/Turabian StyleXu, Bohan, Shunjie Wu, Yiyang Wang, Yuhe Ji, Shufeng Liang, Chunyan Wang, and Xin Tian. 2024. "Paradoxical Roles of Carbon Nanotubes in Cancer Therapy and Carcinogenesis" Journal of Nanotheranostics 5, no. 3: 84-98. https://doi.org/10.3390/jnt5030006
APA StyleXu, B., Wu, S., Wang, Y., Ji, Y., Liang, S., Wang, C., & Tian, X. (2024). Paradoxical Roles of Carbon Nanotubes in Cancer Therapy and Carcinogenesis. Journal of Nanotheranostics, 5(3), 84-98. https://doi.org/10.3390/jnt5030006






