Application of Metal Oxide Nanoparticles in Different Carcinomas
Abstract
:1. Introduction
2. Metal Oxide Nanoparticles for Therapeutic Effect
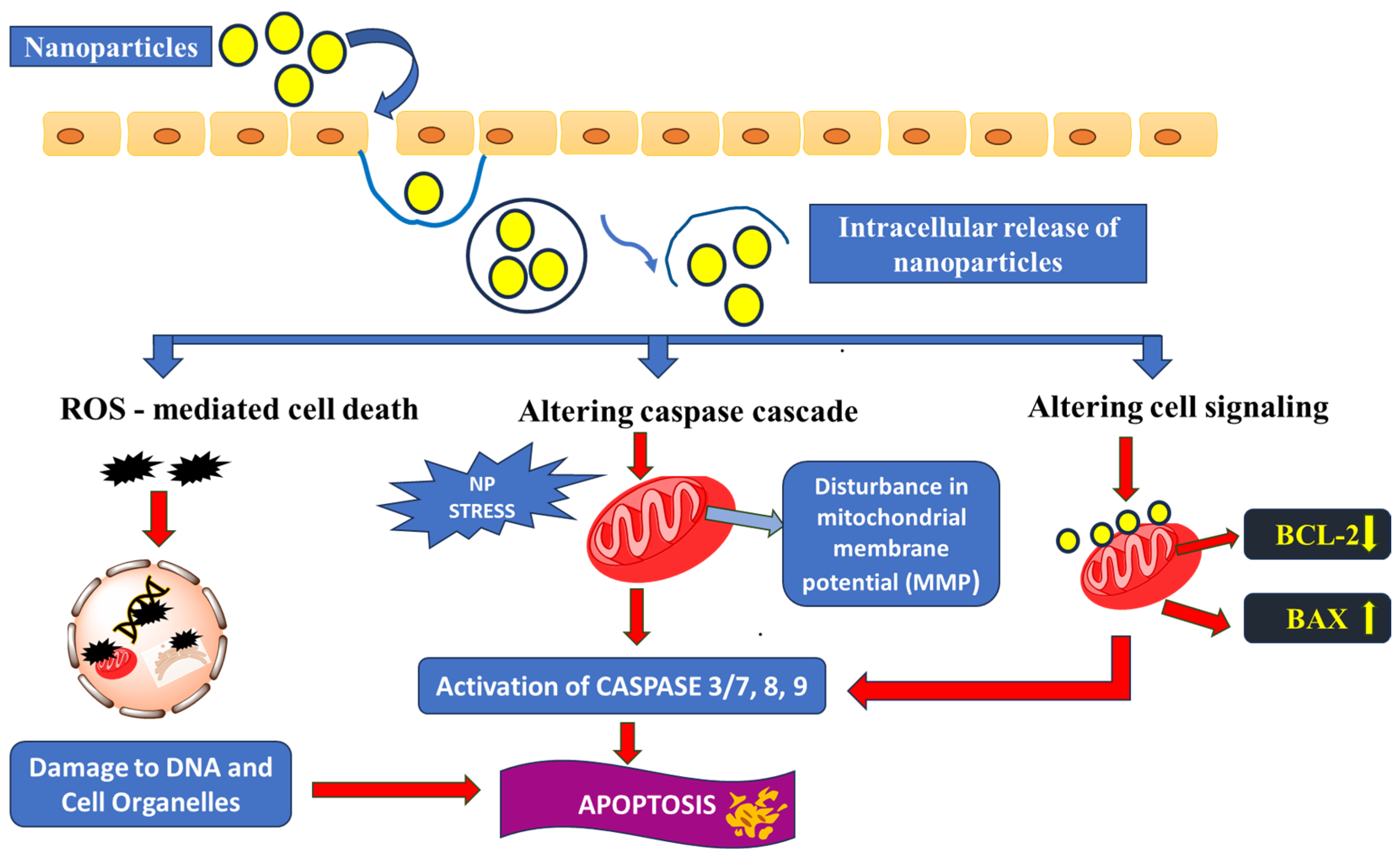
3. Major Apoptosis Mechanisms
3.1. Oxidative Stress Caused by Enhanced ROS Production
3.2. Caspase Cascade
3.3. Disrupting Cell-Signalling Pathways
4. Metal Oxide Nanoparticles for Drug Delivery
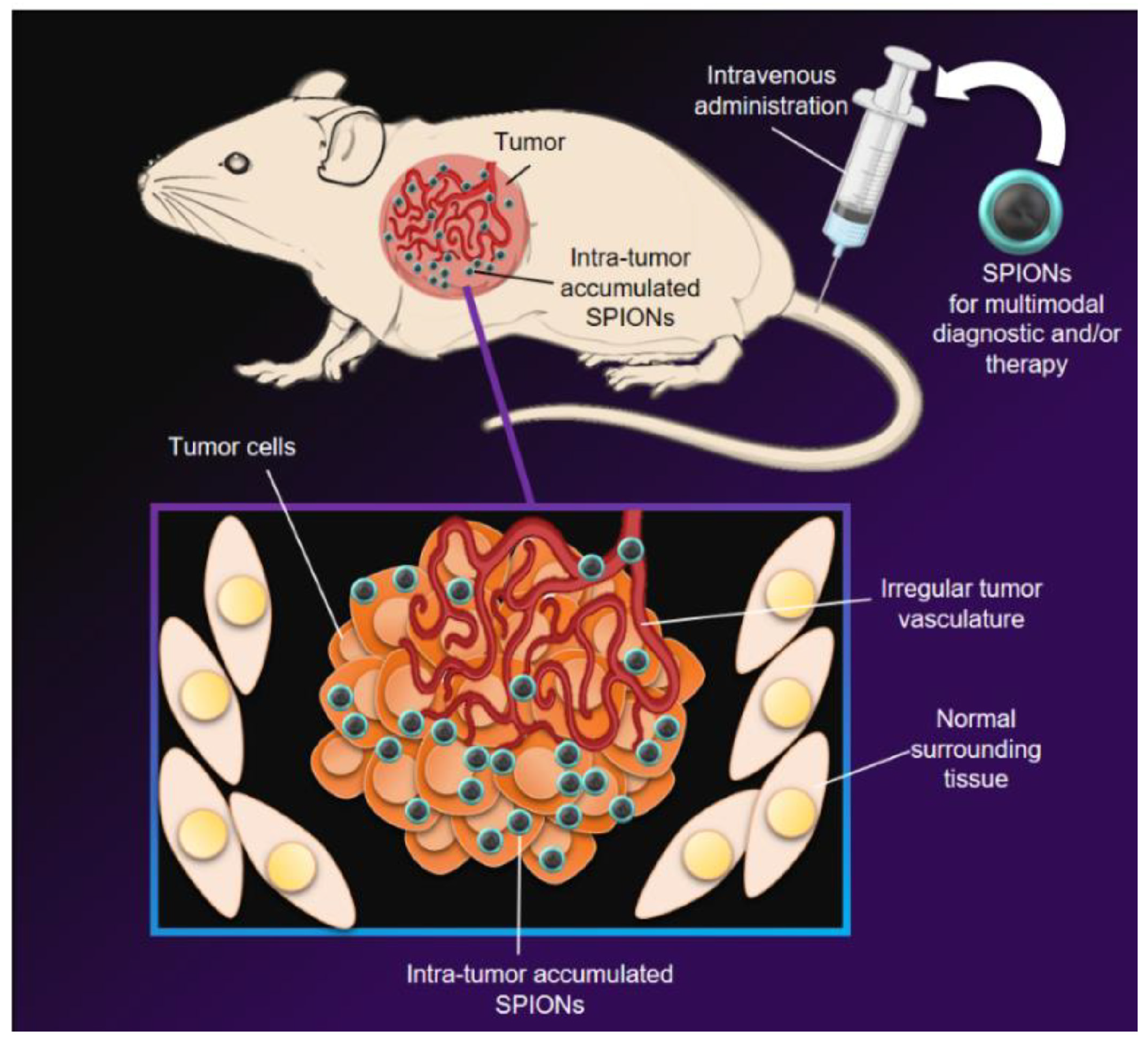
5. Metal Oxide Nanoparticles for Cancer Diagnosis/Imaging
5.1. Magnetic Resonance Imaging (MRI)
5.2. Computed Tomography (CT) Scan Imaging
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer, WHO: Lyon, France, 2020. [Google Scholar]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K.; Ali, R.; Al-Humaid, S.; Alhallaj, A.; Lemine, O.M.; Boudjelal, M.; AlKushi, A. Iron Oxide mesoporous magnetic nanostructures with high surface area for enhanced and selective drug delivery to metastatic cancer cells. Pharmaceutics 2021, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Anzai, Y.; Mclachlan, S.; Morris, M.; Saxton, R.; Lufkin, R.B. Dextran-coated superparamagnetic iron oxide, an MR contrast agent for assessing lymph nodes in the head and neck. Am. J. Neuroradiol. 1994, 15, 87–94. [Google Scholar] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Parashar, S.K.S.; Patro, S.; Suar, M. Mechanistic Insight into Size-Dependent Enhanced Cytotoxicity of Industrial Antibacterial Titanium Oxide Nanoparticles on Colon Cells Because of Reactive Oxygen Species Quenching and Neutral Lipid Alteration. ACS Omega 2018, 3, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, U.R.; Latha, M.S.; Kumar, C.R.; Kiran, M.S.; Onkarappa, H.S.; Betageri, V.S. Potential antidiabetic and anticancer activity of copper oxide nanoparticles synthesised using Areca catechu leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 025008. [Google Scholar] [CrossRef]
- Hesemans, E.; Saffarzadeh, N.; Maksoudian, C.; Izci, M.; Chu, T.; Rios Luci, C.; Wang, Y.; Naatz, H.; Thieme, S.; Richter, C.; et al. Cu-doped TiO2 nanoparticles improve local antitumor immune activation and optimize dendritic cell vaccine strategies. J. Nanobiotechnol. 2023, 21, 87. [Google Scholar] [CrossRef]
- Bai, S.; Yang, N.; Wang, X.; Gong, F.; Dong, Z.; Gong, Y.; Liu, Z.; Cheng, L. Ultrasmall iron-doped titanium oxide nanodots for enhanced sonodynamic and chemodynamic cancer therapy. ACS Nano 2020, 14, 15119–15130. [Google Scholar] [CrossRef]
- Hu, H.; Yu, L.; Ding, Z.; Ding, J.; Hu, Y.; Yin, Z. Chemo-immunotherapy for chemo-resistance and metastasis of triple-negative breast cancer by combination of iron-oxide nanoparticles and dual-targeting doxorubicin liposomes. Chin. Chem. Lett. 2023, 34, 108592. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef]
- Chen, S.; Lv, Y.; Wang, Y.; Kong, D.; Xia, J.; Li, J.; Zhou, Q. Tumor acidic microenvironment-responsive promodulator iron oxide nanoparticles for photothermal-enhanced chemodynamic immunotherapy of cancer. ACS Biomater. Sci. Eng. 2023, 9, 773–783. [Google Scholar] [CrossRef]
- Mahanta, S.; Prathap, S.; Ban, D.K.; Paul, S. Protein functionalization of ZnO nanostructure exhibits selective and enhanced toxicity to breast cancer cells through oxidative stress-based cell death mechanism. J. Photochem. Photobiol. B Biol. 2017, 173, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Amatya, R.; Min, K.A.; Shin, M.C. Liposomal iron oxide nanoparticles loaded with doxorubicin for combined chemo-photothermal cancer therapy. Pharmaceutics 2023, 15, 292. [Google Scholar] [CrossRef] [PubMed]
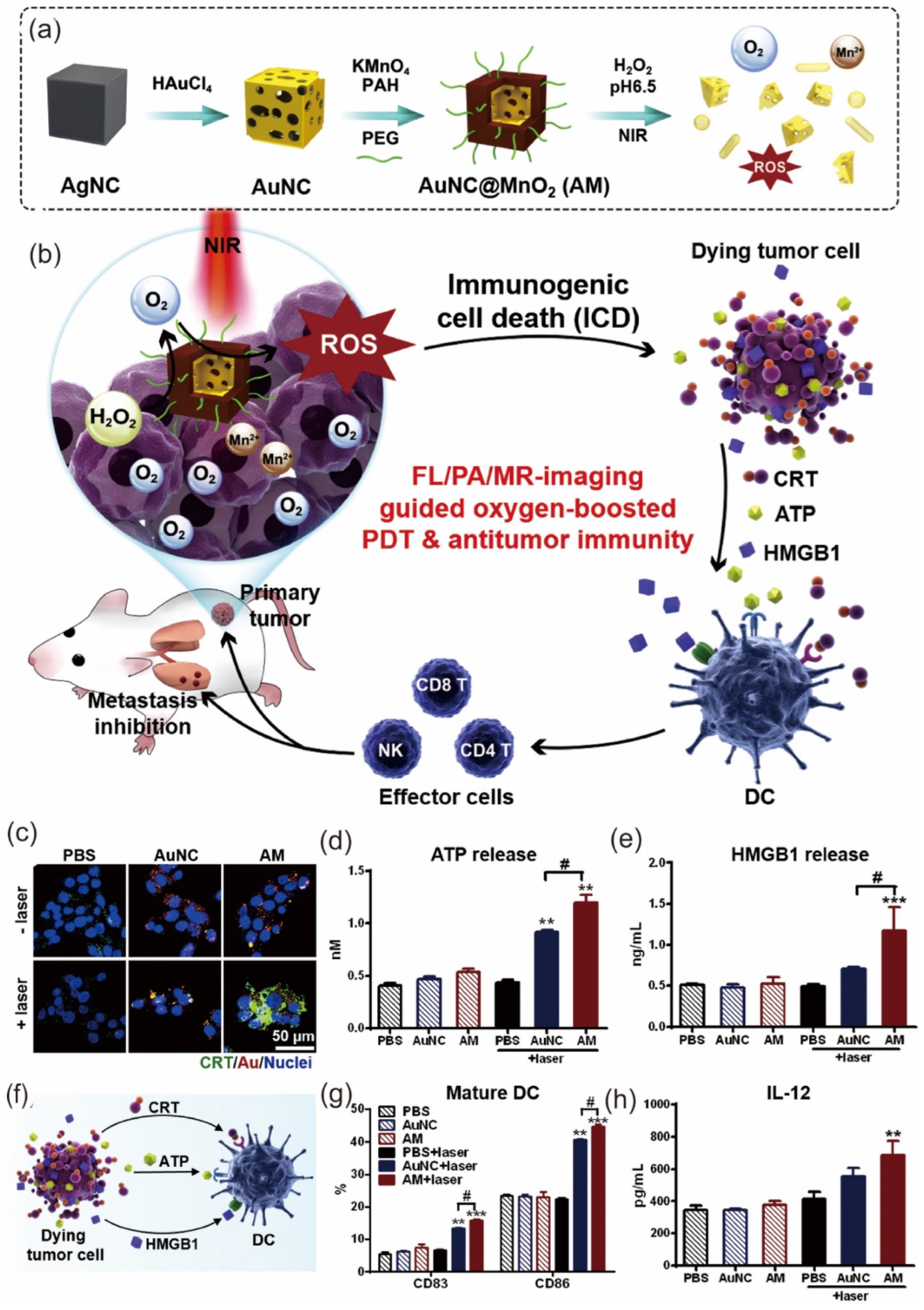
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef]
- Gong, F.; Chen, M.; Yang, N.; Dong, Z.; Tian, L.; Hao, Y.; Zhuo, M.; Liu, Z.; Chen, Q.; Cheng, L. Bimetallic oxide FeWOX nanosheets as multifunctional cascade bioreactors for tumor microenvironment-modulation and enhanced multimodal cancer therapy. Adv. Funct. Mater. 2020, 30, 2002753. [Google Scholar] [CrossRef]
- Koo, S.; Park, O.K.; Kim, J.; Han, S.I.; Yoo, T.Y.; Lee, N.; Kim, Y.G.; Kim, H.; Lim, C.; Bae, J.S.; et al. Enhanced chemodynamic therapy by Cu–Fe peroxide nanoparticles: Tumor microenvironment-mediated synergistic Fenton reaction. ACS Nano 2022, 16, 2535–2545. [Google Scholar] [CrossRef]
- Ma, X.; Lee, C.; Zhang, T.; Cai, J.; Wang, H.; Jiang, F.; Wu, Z.; Xie, J.; Jiang, G.; Li, Z. Image-guided selection of Gd@ C-dots as sensitizers to improve radiotherapy of non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 284. [Google Scholar] [CrossRef]
- Gowdhami, B.; Jaabir, M.; Archunan, G.; Suganthy, N. Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mater. Sci. Eng. C 2019, 103, 109840. [Google Scholar]
- Rahimi Kalateh Shah Mohammad, G.; Seyedi, S.M.R.; Karimi, E.; Homayouni-Tabrizi, M. The cytotoxic properties of zinc oxide nanoparticles on the rat liver and spleen, and its anticancer impacts on human liver cancer cell lines. J. Biochem. Mol. Toxicol. 2019, 33, e22324. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Thamer, N.A.; Barakat, N.T. In Cytotoxic activity of green synthesis copper oxide nanoparticles using cordia myxa L. aqueous extract on some breast cancer cell lines. J. Phys. Conf. Ser. 2019, 1294, 062104. [Google Scholar] [CrossRef]
- Manimaran, K. Synthesis and characterization of Pleurotus citrinopileatus extract–mediated iron oxide (FeO) nanoparticles and its antibacterial and anticancer activity. Biomass Conv. Bioref. 2024, 14, 15011–15020. [Google Scholar] [CrossRef]
- Mohsin, M.H.; Khashan, K.S.; Sulaiman, G.M.; Mohammed, H.A.; Qureshi, K.A.; Aspatwar, A. A novel facile synthesis of metal nitride@metal oxide (BN/Gd2O3) nanocomposite and their antibacterial and anticancer activities. Sci. Rep. 2023, 13, 22749. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.J.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A. Titanium dioxide nanoparticles prepared via laser ablation: Evaluation of their antibacterial and anticancer activity. Surf. Rev. Lett. 2023, 30, 2350066. [Google Scholar] [CrossRef]
- Phalake, S.S.; Somvanshi, S.B.; Tofail, S.A.; Thorat, N.D.; Khot, V.M. Functionalized manganese iron oxide nanoparticles: A dual potential magneto-chemotherapeutic cargo in a 3D breast cancer model. Nanoscale 2023, 15, 15686–15699. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Ghanbarzadeh, S.; Hajiabadi, H.; Mortezazadeh, T.; Yoosefian, M.; Akbari Javar, H. In vitro and in silico characteristics of doxorubicin-loaded four polymeric-based polysaccharides-modified super paramagnetic iron oxide nanoparticles for cancer chemotherapy and magnetic resonance imaging. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 117–130. [Google Scholar] [CrossRef]
- Thevenot, P.; Cho, J.; Wavhal, D.; Timmons, R.B.; Tang, L. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 226–236. [Google Scholar] [CrossRef]
- Lu, S.; Mi, Z.; Liu, P.; Ding, J.; Ma, Y.; Yang, J.; Rong, P.; Zhou, W. Repolarizing neutrophils via MnO2 nanoparticle-activated STING pathway enhances Salmonella-mediated tumor immunotherapy. J. Nanobiotechnol. 2024, 22, 443. [Google Scholar] [CrossRef]
- Du, C.; Zhou, M.; Jia, F.; Ruan, L.; Lu, H.; Zhang, J.; Zhu, B.; Liu, X.; Chen, J.; Chai, Z.; et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials 2021, 269, 120642. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Li, R.; Yang, Y.; Zhang, H.; Tian, B.; Cui, L.; Weng, H.; Wei, F. Anti-CD133 monoclonal antibody conjugated immunomagnetic nanosensor for molecular imaging of targeted cancer stem cells. Sens. Actuators B Chem. 2018, 255, 3447–3457. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Fütterer, J.J.; Takahashi, S. Use of ultrasmall superparamagnetic iron oxide in lymph node MR imaging in prostate cancer patients. Eur. J. Radiol. 2007, 63, 369–372. [Google Scholar] [CrossRef]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Tanino, R.; Amano, Y.; Tong, X.; Sun, R.; Tsubata, Y.; Harada, M.; Fujita, Y.; Isobe, T. Anticancer Activity of ZnO Nanoparticles against Human Small-Cell Lung Cancer in an Orthotopic Mouse Model. Mol. Cancer Ther. 2020, 19, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Qiu, F.; Zhu, F.; Qi, L. Therapeutic Potential of Zinc Oxide-Loaded Syringic Acid Against in vitro and in vivo Model of Lung Cancer. Int. J. Nanomed. 2020, 15, 8249–8260. [Google Scholar] [CrossRef] [PubMed]
- Chabattula, S.C.; Gupta, P.K.; Tripathi, S.K.; Gahtori, R.; Padhi, P.; Mahapatra, S.; Biswal, B.K.; Singh, S.K.; Dua, K.; Ruokolainen, J.; et al. Anticancer therapeutic efficacy of biogenic Am-ZnO nanoparticles on 2D and 3D tumor models. Mater. Today Chem. 2021, 22, 100618. [Google Scholar] [CrossRef]
- Latha, T.S.; Reddy, M.C.; Muthukonda, S.V.; Srikanth, V.V.S.S.; Lomada, D. In vitro and in vivo evaluation of anti-cancer activity: Shape-dependent properties of TiO2 nanostructures. Mater. Sci. Eng. C 2017, 78, 969–977. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, F.; Ma, L.; Yao, H.; Wang, Q.; Wen, L.; Wang, Q.; Gu, N.; Chen, S. Inhibitory effect of magnetic Fe3O4 nanoparticles coloaded with homoharringtonine on human leukemia cells in vivo and in vitro. Int. J. Nanomed. 2016, 11, 4413–4422. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, C.; Yang, Q.; Han, S.; Hu, Q.; Fu, Z. Titanium dioxide nanoparticle stimulating pro-inflammatory responses in vitro and in vivo for inhibited cancer metastasis. Life Sci. 2018, 202, 44–51. [Google Scholar] [CrossRef]
- Ruiz, A.L.; Garcia, C.B.; Gallón, S.N.; Webster, T.J. Novel Silver-Platinum Nanoparticles for Anticancer and Antimicrobial Applications. Int. J. Nanomed. 2020, 15, 169–179. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.A.; Al-Zikri, P.N.H.; Suriyah, W.H.; Soundharrajan, I.; Govindan, N.; Maniam, G.P.; Yusoff, M.M. In Vitro Anticancer Activity of Au, Ag Nanoparticles Synthesized Using Commelina nudiflora L. Aqueous Extract Against HCT-116 Colon Cancer Cells. Biol. Trace Elem. Res. 2016, 173, 297–305. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepic, D.P.; Arsikin, K.M.; Jovanovic, S.P.; Pantovic, A.C.; Dramicanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Shao, W.; Paul, A.; Zhao, B.; Lee, C.; Rodes, L.; Prakash, S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials 2013, 34, 10109–10119. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Evangelou, K.; Falaras, P.; Tsilibary, E.P.C.; Vasileiou, P.V.; Havaki, S.; Angelopoulou, A.; Pavlatou, E.A.; Gorgoulis, V.G. Nanomedicine: Photo-activated nanostructured titanium dioxide, as a promising anticancer agent. Pharmacol. Ther. 2021, 222, 107795. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Lee, C.H.; Lin, M.S.; Chi, C.W.; Chen, Y.J.; Wang, G.S.; Liao, K.W.; Chiu, L.P.; Wu, S.H.; Huang, D.M.; et al. ZnO nanoparticles induced caspase-dependent apoptosis in gingival squamous cell carcinoma through mitochondrial dysfunction and p70S6K signalling pathway. Int. J. Mol. Sci. 2020, 21, 1612. [Google Scholar] [CrossRef]
- Zhang, T.; Du, E.; Liu, Y.; Cheng, J.; Zhang, Z.; Xu, Y.; Qi, S.; Chen, Y. Anticancer effects of zinc oxide nanoparticles through altering the methylation status of histone on bladder cancer cells. Int. J. Nanomed. 2020, 15, 1457–1468. [Google Scholar] [CrossRef]
- Khorsandi, L.; Farasat, M. Zinc oxide nanoparticles enhance expression of maspin in human breast cancer cells. Environ. Sci. Pollut. Res. 2020, 27, 38300–38310. [Google Scholar] [CrossRef]
- Salari, S.; Neamati, A.; Tabrizi, M.H.; Seyedi, S.M.R. Green-synthesized Zinc oxide nanoparticle, an efficient safe anticancer compound for human breast MCF7 cancer cells. Appl. Organomet. Chem. 2020, 34, e5417. [Google Scholar] [CrossRef]
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Deliv. 2022, 19, 199–212. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical applications of iron oxide nanoparticles: Current insights progress and perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Mu, Q.; Yan, B. Nanoparticles in Cancer Therapy-Novel Concepts, Mechanisms, and Applications. Front. Pharmacol. 2019, 9, 1552. [Google Scholar] [CrossRef]
- Karimi Ghezeli, Z.; Hekmati, M.; Veisi, H. Synthesis of Imatinib-loaded chitosan-modified magnetic nanoparticles as an anti-cancer agent for pH responsive targeted drug delivery. Appl. Organomet. Chem. 2019, 33, e4833. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.; Arthanareeswaran, G. Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized zinc oxide nanoparticles for enhanced curcumin delivery and bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Perumalsamy, H.; Castro-Aceituno, V.; Kim, D.; Markus, J.; Lee, S.; Kim, S.; Liu, Y.; Yang, D.C. Photoluminescent and self-assembled hyaluronic acid-zinc oxide-ginsenoside Rh2 nanoparticles and their potential caspase-9 apoptotic mechanism towards cancer cell lines. Int. J. Nanomed. 2019, 14, 8195. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Gai, S.; Yang, P.; Li, C.; Ansari, M.B.; Lin, J. Stimuli responsive drug delivery application of polymer and silica in biomedicine. J. Mater. Chem. B 2015, 3, 8599–8622. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, F.A.; Jasim, S.A.; Atakhanova, N.E.; Majdi, H.S.; Jawad, M.A.; Hasan, M.K.; Borhani, F.; Khatami, M. Drug delivery and anticancer activity of biosynthesised mesoporous Fe2O3 nanoparticles. IET Nanobiotechnol. 2022, 16, 85–91. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Li, K.; Xu, J.; Li, P.; Fan, Y. pH-responsive mesoporous Fe2O3-Au nanomedicine delivery system with magnetic targeting for cancer therapy. Med. Nov. Technol. Devices 2022, 15, 100127. [Google Scholar] [CrossRef]
- Al-Ajmi, M.F.; Hussain, A.; Ahmed, F. Novel synthesis of ZnO nanoparticles and their enhanced anticancer activity: Role of ZnO as a drug carrier. Ceram. Int. 2016, 42, 4462–4469. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Marcu, A.; Pop, S.; Dumitrache, F.; Mocanu, M.; Niculite, C.M.; Gherghiceanu, M.; Lungu, C.P.; Fleaca, C.; Ianchis, R.; Barbut, A.; et al. Magnetic iron oxide nanoparticles as drug delivery system in breast cancer. Appl. Surf. Sci. 2013, 281, 60–65. [Google Scholar] [CrossRef]
- Han, J.; Jang, E.-K.; Ki, M.-R.; Son, R.G.; Kim, S.; Choe, Y.; Pack, S.P.; Chung, S. pH-responsive phototherapeutic poly(acrylic acid)-calcium phosphate passivated TiO2 nanoparticle-based drug delivery system for cancer treatment applications. J. Ind. Eng. Chem. 2022, 112, 258–270. [Google Scholar] [CrossRef]
- Fadeel, D.A.A.; Hanafy, M.S.; Kelany, N.A.; Elywa, M.A. Novel greenly synthesized titanium dioxide nanoparticles compared to liposomes in drug delivery: In vivo investigation on Ehrlich solid tumor model. Heliyon 2021, 7, e07370. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Exploration of polydopamine capped bimetallic oxide (CuO-NiO) nanoparticles inspired by mussels for enhanced and targeted paclitaxel delivery for synergistic breast cancer therapy. Appl. Surf. Sci. 2023, 626, 157266. [Google Scholar] [CrossRef]
- Binu, N.M.; Prema, D.; Prakash, J.; Balagangadharan, K.; Balashanmugam, P.; Selvamurugan, N.; Venkatasubbu, G.D. Folic acid decorated pH sensitive polydopamine coated honeycomb structured nickel oxide nanoparticles for targeted delivery of quercetin to triple negative breast cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127609. [Google Scholar] [CrossRef]
- Sudakaran, S.V.; Venugopal, J.R.; Vijayakumar, G.P.; Abisegapriyan, S.; Grace, A.N.; Ramakrishna, S. Sequel of MgO nanoparticles in PLACL nanofibers for anti-cancer therapy in synergy with curcumin/β-cyclodextrin. Mater. Sci. Eng. C 2017, 71, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Jaisankar, E.; Azarudeen, R.S.; Thirumarimurugan, M. A Study on the Effect of Nanoscale MgO and Hydrogen Bonding in Nanofiber Mats for the Controlled Drug Release along with In Vitro Breast Cancer Cell Line and Antimicrobial Studies. ACS Appl. Bio Mater. 2022, 5, 4327–4341. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Peng, C.; Qiang, S.; Xiong, L.H.; Zhao, Z.; Wang, Z.; Kwok, R.T.; Lam, J.W.; Ma, N.; Tang, B.Z. Less is more: Silver-AIE core@ shell nanoparticles for multimodality cancer imaging and synergistic therapy. Biomaterials 2020, 238, 119834. [Google Scholar] [CrossRef]
- Cui, D.; Lu, X.; Yan, C.; Liu, X.; Hou, M.; Xia, Q.; Xu, Y.; Liu, R. Gastrin-releasing peptide receptor-targeted gadolinium oxide-based multifunctional nanoparticles for dual magnetic resonance/fluorescent molecular imaging of prostate cancer. Int. J. Nanomed. 2017, 12, 6787–6797. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of amino dextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Thangudu, S.; Yu, C.C.; Lee, C.L.; Liao, M.C.; Su, C.H. Magnetic, biocompatible FeCO3 nanoparticles for T2-weighted magnetic resonance imaging of in vivo lung tumors. J. Nanobiotechnol. 2022, 20, 157. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, L.; Lai, S.; Zhang, J.; Wei, J.; Wang, K.; Zhang, W.; Li, C.; Tong, J.; Lei, Z. Green synthesis of glyco-CuInS2 QDs with visible/NIR dual emission for 3D multicellular tumor spheroid and in vivo imaging. J. Nanobiotechnol. 2023, 21, 118. [Google Scholar] [CrossRef]
- Barick, K.C.; Singh, S.; Bahadur, D.; Lawande, M.A.; Patkar, D.P.; Hassan, P.A. Carboxyl decorated Fe3O4 nanoparticles for MRI diagnosis and localized hyperthermia. J. Colloid Interface Sci. 2014, 418, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, H.; Hu, Y.; Bai, L.; Xue, J. MnO nanoparticles with potential application in magnetic resonance imaging and drug delivery for myocardial infarction. Int. J. Nanomed. 2018, 13, 6177–6188. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Zhu, L.; Kurokawa, A.; Takeuchi, H.; Yano, S.; Yanoh, T.; Wada, N.; Taira, S.; Hosokai, Y.; Usui, A.; et al. Synthesis of Gd2O3 nanoparticles for MRI contrast agents. J. Phys. Conf. Ser. 2012, 352, 012008. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, S.; Zhang, Q.; Zhao, B.; Feng, B.; Ji, K.; Yu, L.; Chen, W.; Hou, M.; Xu, Y.; et al. Bicompatible porous Co3O4 nanoplates with intrinsic tumor metastasis inhibition for multimodal imaging and DNA damage–mediated tumor synergetic photothermal/photodynamic therapy. Chem. Eng. J. 2020, 394, 124874. [Google Scholar] [CrossRef]
- Gao, L.; Meng, Y.; Luo, X.; Chen, J.; Wang, X. ZnO Nanoparticles Induced MRI Alterations to the Rat Olfactory Epithelium and Olfactory Bulb after Intranasal Instillation. Toxics 2024, 12, 724. [Google Scholar] [CrossRef]
- Babayevska, N.; Florczak, P.; Wozniak-Budych, M.; Jarek, M.; Nowaczyk, G.; Zalewski, T.; Jurga, S. Functionalized multimodal ZnO@Gd2O3 nanosystems to use as perspective contrast agent for MRI. Appl. Surf. Sci. 2017, 404, 129–137. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Zhang, H.; Xia, Z.; Xie, T.; Li, W.; Zhong, D.; Zhu, H.; Zhou, M. Gram-scale synthesis of highly biocompatible and intravenous injectable hafnium oxide nanocrystal with enhanced radiotherapy efficacy for cancer theranostic. Biomaterials 2020, 226, 119538. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Letelay, L.K.; Gaffar, S.; Wyantuti, S.; Bahti, H.H. Cerium oxide-monoclonal antibody bioconjugate for electrochemical immunosensing of HER2 as a breast cancer biomarker. Sens. Bio-Sens. Res. 2020, 27, 100316. [Google Scholar] [CrossRef]



| Nanoparticles | Cells | Size of Nps | Concentration of Nps | Exposure Duration | Results | Reference |
|---|---|---|---|---|---|---|
| ZnO | N417, H82, H187 Human small-cell Lung cancer | 20 nm | 10 μg/mL | 120 min | Low viability, even in cells orthotopically grafted onto mouse models | [33] |
| ZnO-Loaded Syringic Acid | A549 cells | 120 nm | 12.5 μM | 24 h | The ZnO-Loaded Syringic Acid indued ROS have induced the cell death in A549 cancer cells | [34] |
| Annona muricata-ZnO | A549 and MOLT4 Cells | 80 nm | 0–500 μg/mL | 24 h | Am-ZnO treated cancer cells underwent programmed cell death with depolarization in their MMP. | [35] |
| TiO2 | MDAMB231 cells | 140 nm | 100 μg/mL | 72 h | TiO2 nanostructures inhibited the migration and colony formation of breast cancer MDAMB231 cells. | [36] |
| Fe3O4 nanoparticles coloaded with homoharringtonine | K562, HL-60, SHI-1, and NB4 cells | - | 1.875 μg/mL | 24 h | Fe3O4 nanoparticles coloaded with homoharringtonine had cooperative effect in suppression of tumor cell growth | [37] |
| TiO2 | 4T1 cells (Mice) | 21 nm | 1 μg/mL | 21 days | Peritoneal macrophage exposed to P-25 TiO2 NPs displayed activated M1 macrophage response | [38] |
| AgPt | Detroit 551-CCL-110, #A375 and U 87 cells | ~42 nm | 10–250 μg/mL | 1–3 days | AgPt nanoparticles demonstrated a remarkable and statistically significant ability to reduce the viability of cancer cells | [39] |
| Au | HCT-116 cells | 50 nm | 200 μg/mL | 1 h | target the abnormal growth of HCT-116 colon cancer cells | [40] |
| Ag | HCT-116 cells | 24–80 nm | 100 μg/mL | 1 h | target the abnormal growth of HCT-116 colon cancer cells | [40] |
| Graphene | U251 human glioma cells | 50 nm, | 2.5–10 mg/mL | 24 h | graphene nanoparticles performed significantly better than CNT in inducing photothermal death of U251 cells | [41] |
| Carbon nanotubes | U251 human glioma cells | 60 nm, | 2.5–10 mg/mL | 24 h | graphene nanoparticles performed significantly better than CNT in inducing photothermal death of U251 cells | [41] |
| Carbon nanotube | MCF-7 breast cancer cells | 1.5 nm in diameter and 200 nm in length | 10 mg/ml | 48 h | SWNT-drug showed target specificity in vitro | [42] |
| Nanoparticles | Synthesis Method | Cancer Cells | Drug Delivered | Reference |
|---|---|---|---|---|
| Fe2O3 | Biosynthesis method | MCF7 cancer cells | Doxorubicin | [56] |
| Fe2O3-Au | - | A549 Human lung cancer cells | Doxorubicin | [57] |
| ZnO | Organic precursor method | MCF7 cancer cells | 5-Fluorouracil | [58] |
| ZnO | - | CD44 cancer cells | Doxorubicin | [59] |
| Fe3O4 | Laser pyrolysis | MCF7 cancer cells | Violamycine B1 | [60] |
| Poly(acrylic acid)-calcium phosphate passivated TiO2 | Hydrothermal synthesis | MCF7 cancer cells | Doxorubicin | [61] |
| TiO2 | Green synthesis | HSF and MCF-7 cancer cells | Doxorubicin | [62] |
| CuO-NiO | Co-precipitation method | MCF-7 cancer cells | Paclitaxel | [63] |
| NiO | Hydrothermal synthesis | MDA-MB-231 breast cancer cells | Quercetin | [64] |
| MgO | Sol-gel method | MCF-7 cancer cells | Curcumin/β-cyclodextrin | [65] |
| MgO | Co-precipitation method | MDA-MB-231 cancer cells | 5-fluorouracil | [66] |
| Nanoparticles | Size | MRI Contrast Agent Type | Advantages | Reference |
|---|---|---|---|---|
| Carboxyl decorated-Fe3O4 | ~10 nm | T2 contrast agent | Good colloidal stability, high r2 value, and high-efficiency | [72] |
| dextran-coated Fe3O4 | ~13 nm | T2 contrast agent | Improved MRI images of liver, marrow and lymph | [73] |
| MnO | ~28 nm | T1 contrast agent | High r1 relaxivity, favored infarcted myocardium retention characteristic, and good biocompatibility | [74] |
| Gd2O3 | 18–66 nm | Both T1 and T2 contrast agent | T1- and T2-shortening MRI contrast agents, especially with their large T1 relaxation rate. | [75] |
| Co3O4 | - | T2 contrast agent | High photothermal conversion efficiency, excellent colloidal stability, biocompatibility and multifunctional groups | [76] |
| ZnO | ~20 nm | T2 contrast agent | Directly and dynamically | [77] |
| ZnO/Gd2O3 | ~50 nm | T2 contrast agent | High adsorption and water stability, and high-efficiency | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, N.; Khan, Y.; Yadav, S.; Saini, K.; Maity, D. Application of Metal Oxide Nanoparticles in Different Carcinomas. J. Nanotheranostics 2024, 5, 253-272. https://doi.org/10.3390/jnt5040015
Rani N, Khan Y, Yadav S, Saini K, Maity D. Application of Metal Oxide Nanoparticles in Different Carcinomas. Journal of Nanotheranostics. 2024; 5(4):253-272. https://doi.org/10.3390/jnt5040015
Chicago/Turabian StyleRani, Nutan, Yousuf Khan, Sapna Yadav, Kalawati Saini, and Dipak Maity. 2024. "Application of Metal Oxide Nanoparticles in Different Carcinomas" Journal of Nanotheranostics 5, no. 4: 253-272. https://doi.org/10.3390/jnt5040015
APA StyleRani, N., Khan, Y., Yadav, S., Saini, K., & Maity, D. (2024). Application of Metal Oxide Nanoparticles in Different Carcinomas. Journal of Nanotheranostics, 5(4), 253-272. https://doi.org/10.3390/jnt5040015







