Abstract
Photodynamic and photothermal therapies with IR780 have gained exponential interest, and their photophysical properties have demonstrated promise for use in antitumor and antimicrobial chemotherapy. IR780 and its derivatives are valuable in labeling nanostructures with different chemical compositions for in vitro and in vivo fluorescence monitoring studies in the near-infrared (NIR) spectrum. The current literature is abundant on this topic, particularly with applications in the treatment of different types of cancer using laser illumination to produce photodynamic (PDT), photothermal (PTT), and, more recently, sonodynamic therapy (SDT) approaches for cell death. This review aims to update the state of the art concerning IR780 photosensitizer as a theranostic agent for PDT, PTT, SDT, and photoacoustic (PA) effects, and fluorescence imaging monitoring associated with different types of nanocarriers. The literature update concerns a period from 2017 to 2024, considering, more specifically, the in vivo effects found in preclinical experiments. Some aspects of the labeling stability of nanostructured systems will be discussed based on the evidence of IR780 leakage from the nanocarrier and its consequences for the reliable analysis of biological data.
1. Introduction
The combined therapy and fluorescence-image monitoring mediated by a nanocarrier is called nanotheranostics. It is a valuable tool in preclinical and proof-of-concept studies. It may also be applied in the clinical field where the target site is related to epithelial tissues accessible to light sources. IR780-based nanostructures can be easily tracked using an imaging modality in the near-infrared region of the spectrum. In vivo image monitoring allows one to follow the nanostructure biodistribution, tissue accumulation, and targeted delivery, adding a valuable tool to developing multiple functional nanocarriers [1]. Nanotheranostics are frequently used in preclinical studies, allowing the visual confirmation of the fate of different luminescent and fluorescent drug carriers.
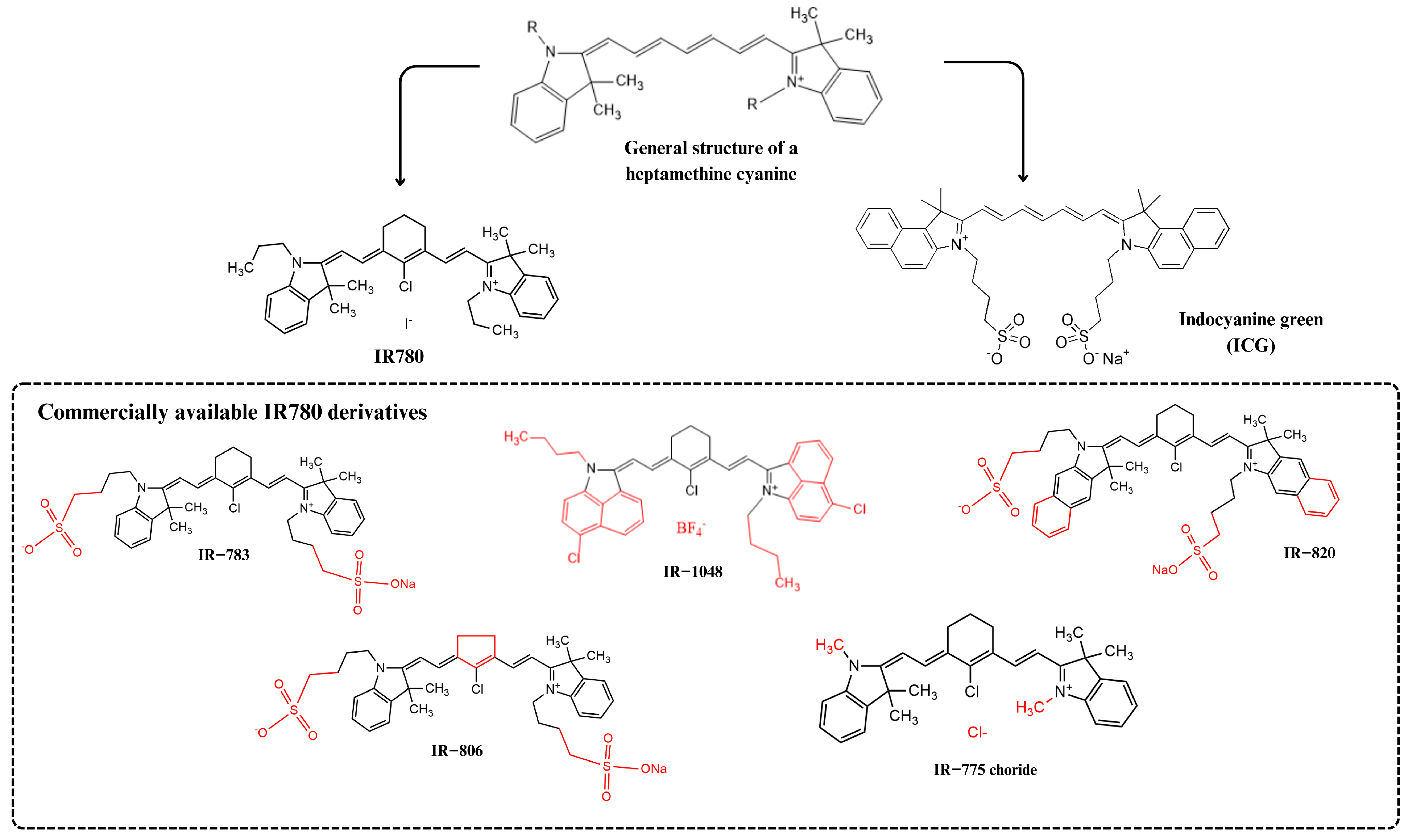
Photosensitizers from the heptamethine cyanine family have been widely studied as preclinical theranostic because of their structural, photophysical, and optical advantages [2]. Based on the publication of an excellent review on the use of IR780 in nanostructured systems in 2018 by Alves et al. [3], this review aims to provide a brief update on the evolution of in vivo studies in this field. Fluorescent dyes belonging to the heptamethine cyanine class have a unique chemical structure containing a rigid heterocyclic ring in the heptamethine chain and a central chlorine atom, which gives these dyes superior optical and photophysical properties when compared to other cyanines, such as indocyanine green (ICG), a water-soluble dye approved by the United States Food and Drug Administration (FDA) (Figure 1).
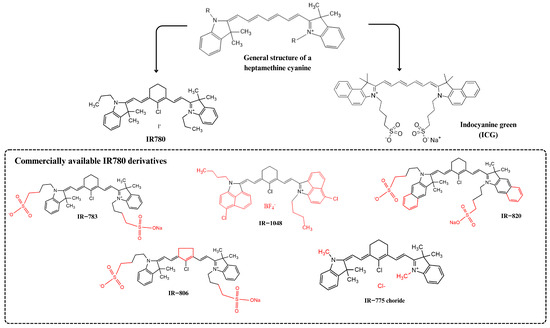
Figure 1.
IR780 photosensitizer and its heptamethine cyanine class of dyes. The chemical modifications in IR780’s original structure are highlighted in red. Indocyanine green (ICG) chemical structure, a more hydrophilic photosensitizer used in clinics, is also shown.
Heptamethine cyanine dyes (Figure 1), especially IR780, have received considerable attention in cancer theranostics because they emit fluorescence in the near-infrared (NIR) region of the electromagnetic spectrum. This facilitates dye distribution monitoring inside biological systems in vivo at a greater tissue penetration depth than using other fluorochromes. In general, the higher the wavelength, the higher the light penetration in the tissue [4,5].
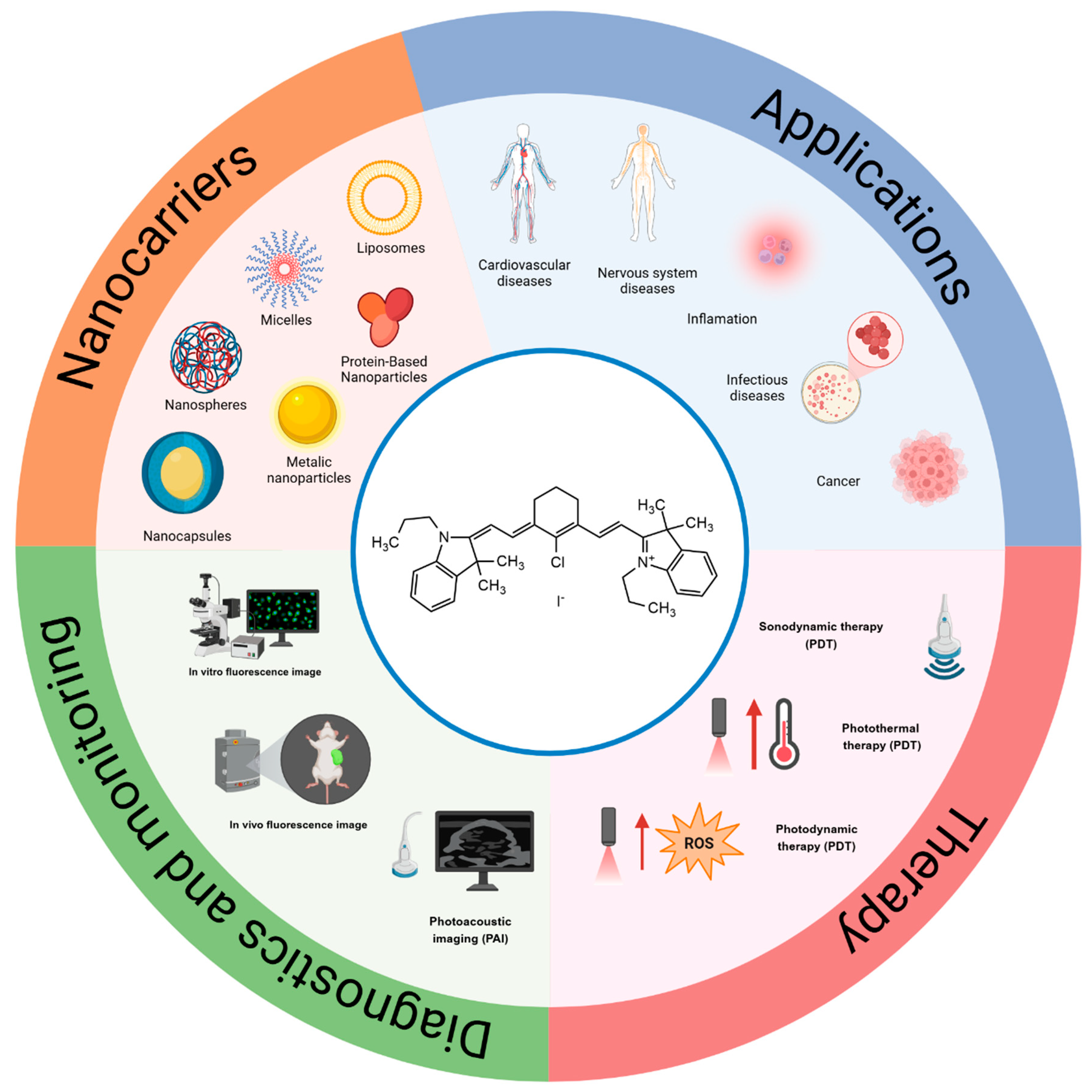
Additionally, several studies have demonstrated the ability of IR780 to act as a photosensitizer, promoting photo-dependent cytotoxicity. When irradiated with laser light at a specific wavelength (usually at 808 nm), IR780 induces a temperature increase in the cell environment, and upon oxygen supply, the formation of reactive oxygen species (ROS), being widely studied for application in photodynamics (PDT) and photothermal therapies (PTT) [6]. The chemotherapy of different dysfunctions can be assessed using this strategy, such as antitumoral, antimicrobial, and many others [5]. Figure 2 shows a schematic representation of IR780’s applications, its mechanisms of action in therapy and image monitoring, and the types of nanocarriers used in association with it.
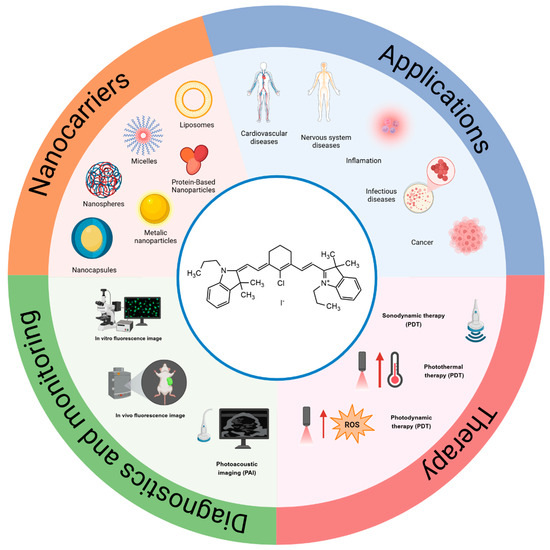
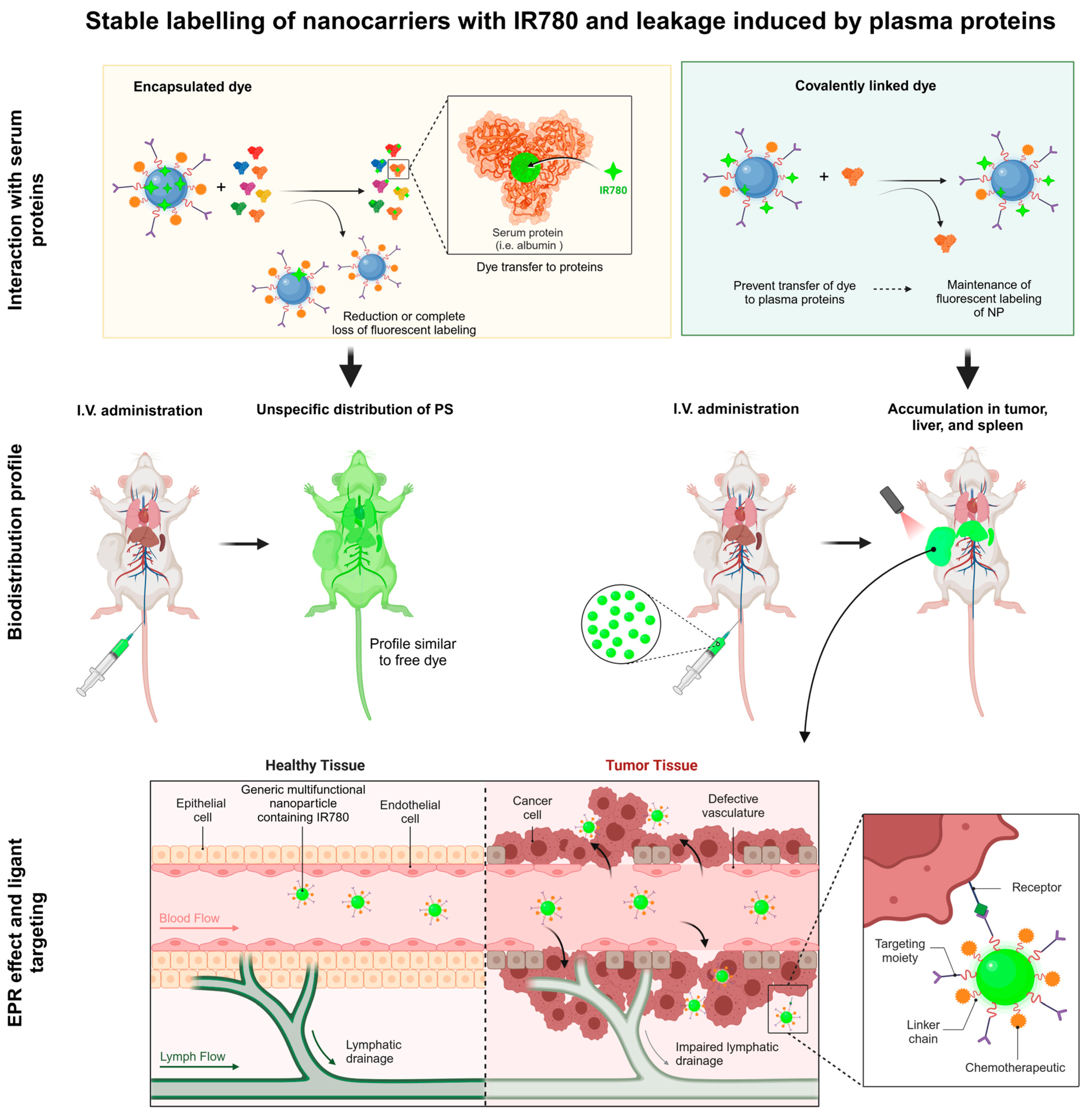
Figure 2.
Schematic representation of IR780 in association with different nanocarriers; therapeutic applications; mechanisms of activation by ultrasound and light; photothermal, photodynamic effects; and applications in fluorescence image monitoring in vitro and in vivo; Created in BioRender De Oliveira, M. (2025). https://BioRender.com/m12s449 (Accessed on 5 March 2025).
Photodynamic therapy (PDT) mediated by light illumination of a target tissue still shows limited success in clinical applications due to solid tumors’ high levels of hypoxia, low tumor targeting and photosensitizer accumulation, and limited light penetration depth [3]. However, the photodynamic process induces transient alterations in the stromal, cellular, and vascular microenvironment called photodynamic priming. This process makes the site more receptive to chemotherapy and immunotherapy, improving the outcome. One of the main issues is the selectivity in the biodistribution of photosensitizer.
Nanotechnology provides an elegant solution to increase the targeting of nanoparticles and associated photosensitizers to tumors and inflamed regions by enhancing the permeation and retention (EPR) effect in infectious, inflamed, and other body sites with altered histological dysfunctions. This review highlights many passive and active targeting strategies used in nanotheranostics to reach the disease sites or improve target cell entry. The focus is directed toward in vivo studies to help readers analyze the main biological effects of the combined chemotherapeutic and image-monitored events provided by IR780-based nanocarriers.
Despite promising optical and photophysical properties for theranostic applications, IR780 has low water solubility (Table 1), a limiting factor for intravenous administration and its widespread use compared to indocyanine green (Figure 1) and other water-soluble photosensitizers. Alongside this review, some studies demonstrated that the apparent “solubility” of IR780 in water has been improved over 1000-fold via association with nanocarriers, such as human serum albumin (HSA) nanocarriers [5,7,8,9,10,11].

Table 1.
Summary of the main physicochemical and photophysical properties of IR780.
Table 1.
Summary of the main physicochemical and photophysical properties of IR780.
| Identification | |||
|---|---|---|---|
| IR780 | 2-[2-[2-Chloro-3-[(1,3-dihydro-3,3-dimethyl-1-propyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-3,3-dimethyl-1-propylindolium iodide | ||
| Empirical Formula | C36H44ClIN2 | ||
| CAS number | 207399-07-3 | ||
| Molecular weight | 667.11 g/mol | ||
| Physicochemical Properties | |||
| Property | Solvent | Value | Ref. |
| Lop P | n-octanol/water | 4.087 | [12] |
| Solubility | Water | <0.4 μg/mL | [9] |
| Water | 5 μg/mL | [13] | |
| PBS pH 7.4 | 9 μg/mL | [13] | |
| PBS with 1% of polysorbate 80 | 101 μg/mL | [14] | |
| Maximum absorption wavelength (nm) | Acetone–water (1:1) | 780 nm | [13] |
| Acetonitrile | 780 nm | [15,16] | |
| Methanol | 780 nm | [17,18] | |
| Ethanol | 780 nm | [19,20] | |
| PBS | 780 nm | [21] | |
| DMSO | 796 nm | [22] | |
| Pyridine | 803 nm | [23] | |
| Molar absorptivity (105 M−1 cm−1) | Methanol (λmax = 780 nm) | 2.65 | [17] |
| 3.3 | [18] | ||
| Ethanol (λmax = 780 nm) | 2.8 | [19] | |
| 2.74 | [20] | ||
| DMSO (λmax = 796 nm) | 2.48 | [22] | |
| Pyridine (λmax = 803 nm) | 2.85 | [23] | |
| Maximal emission wavelength (nm) | Acetonitrile | 790 nm | [16] |
| Methanol | 805 nm | [17] | |
| Methanol | 798 nm | [18] | |
| Ethanol | 799 nm | [20] | |
| DMSO | 826 nm | [22] | |
| Pyridine | 815 nm | [23] | |
| Fluorescence quantum yield (Φf) | Acetonitrile | 0.680 | [16] |
| DMEM | 0.080 | [16] | |
| Encapsulated in PLA nanoparticle/water | 0.483 | [16] | |
| Methanol | 0.07 | [18] | |
| Ethanol | 0.08 | [20] | |
| DMSO | 0.153 | [22] | |
| Pyridine | 0.368 | [23] | |
| Molecular Brightness (M−1·cm−1) | Ethanol | 20,800 | [20] |
| Singlet oxygen quantum yield (Φso)—% | DMSO | 8.1 | [22] |
| 12.7 | [24] | ||
| Photothermal conversion efficiency (η)—% | DMSO | 10.7 | [22] |
| Ethanol | 7.6 | [25] | |
DMEM: Dulbecco’s modified Eagle cell culture medium. DMSO: dimethyl sulfoxide. PLA: polylactide polymer. PBS: phosphate-buffered saline.
2. Photophysical Properties of IR780: Advantages and Challenges
The great interest in using IR780 as a theranostic is directly related to its photophysical properties. IR780 is a dye that absorbs and emits radiation in the near-infrared (NIR) region of the electromagnetic spectrum, whose characteristic maximum absorption wavelength (λmax) is 780 nm, with a high molar absorptivity (Ɛ780nm = 2.65–3.3 × 105 M−1 cm−1), indicating that this dye can strongly absorb light at this wavelength. IR780 also presents an intense fluorescence emission in the 790–826 nm range, with a high-fluorescence quantum yield (Φf) (Table 1). Dyes with absorption and fluorescence emission in the NIR region are ideal for fluorescence imaging since this region is considered a window of biological transparency, with no autofluorescence of tissues being observed.
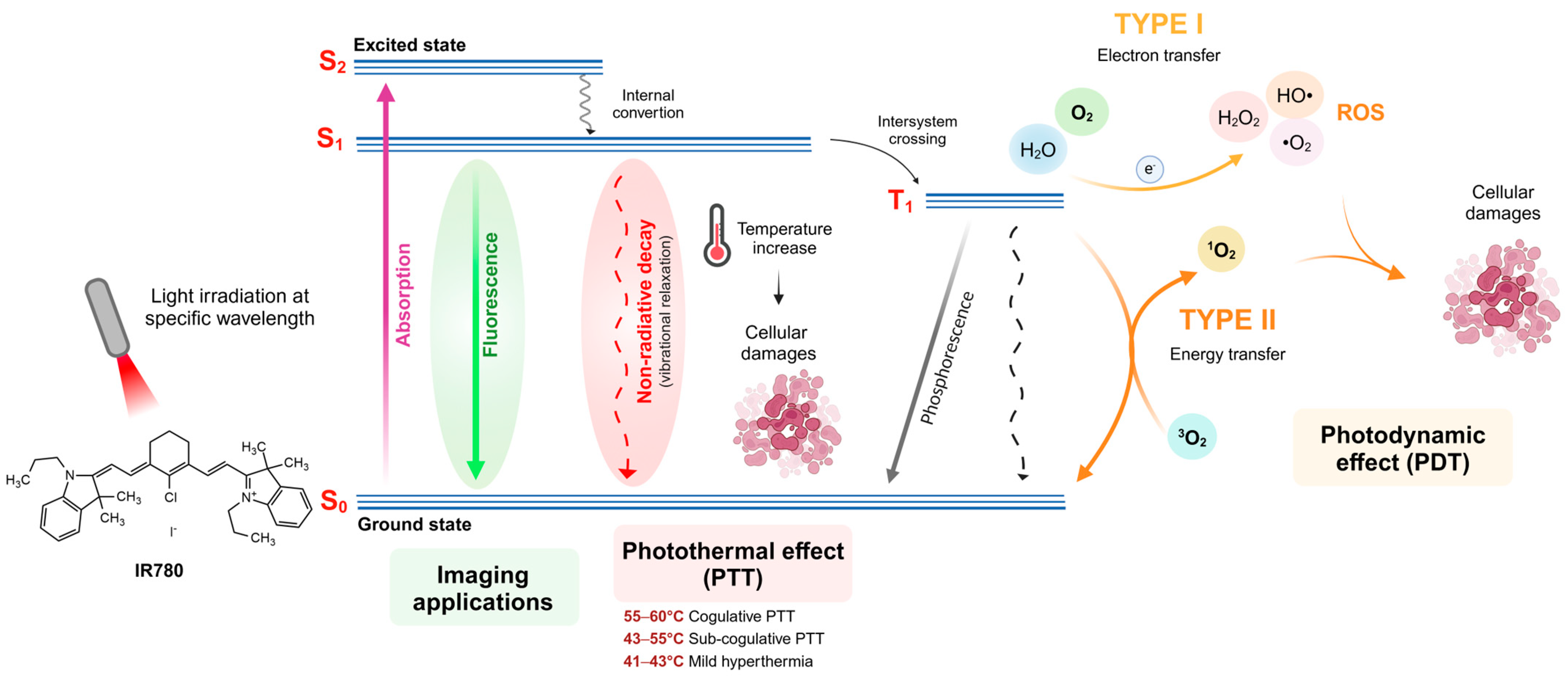
When choosing a fluorophore, molar absorptivity (Ɛ) and fluorescence quantum yield (Φ) are important photophysical properties to be considered. While molar absorptivity indicates the ability of a molecule to absorb light at a specific wavelength, the fluorescence quantum yield expresses the molecule’s ability to convert absorbed light into emitted light. Although these two properties are related, they are independent. Thus, a molecule with a high molar absorptivity may not efficiently convert this absorbed energy into emitted light, presenting a low fluorescence quantum yield. This can occur mainly due to energy loss through non-radiative processes, such as vibrational relaxation of the molecule (Figure 3). Because of that, information about molecular brightness could be more helpful in fluorescence imaging. Molecular brightness represents the number of photons emitted by a fluorophore, and it can be calculated by multiplying the molar absorptivity and fluorescence quantum yield (Ɛ × Φ) or determined empirically. IR780 shows a high molecular brightness (20,800 M−1 cm−1), 11-fold higher than ICG (Figure 1), justifying its widespread use as a fluorescent probe. It is worth noting that these photophysical properties are strictly related to the molecule’s chemical structure. They also depend on the medium where the dye is immersed, and, in the case of fluorescence, the wavelength of maximum emission observed also depends on the wavelength used to excite the molecule (Table 1).
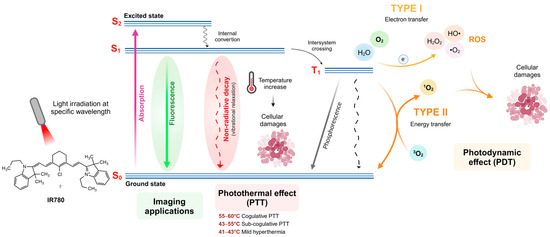
Figure 3.
Adapted Jablonski diagram illustrating possible energy release pathways from excited photosensitizer, including fluorescence emission, non-radiative decay (vibrational relaxation), and intersystem crossing, to promote photodynamic and photothermal effects. Created in BioRender. De Oliveira, M. (2025). https://BioRender.com/r82t021 (Accessed on 5 March 2025).
While the π-conjugated polymethine chain contributes to the high-fluorescence quantum yield of heptamethine cyanine photosensitizers, their molecular planarity induces π-stacking phenomena with a reduction in fluorescence emission. This molecular auto-assemblage occurs mainly in solvent mixtures containing water, where IR780 has poor solubility. In addition to the high hydrophobicity of IR780, a limiting factor for its use is its low stability in aqueous media and the presence of light, evidenced by a reduction in its maximum absorption at 780 nm. The molecular environment has been shown to influence this property considerably (Table 1). For example, IR780’s association with lipids and proteins significantly affects its fluorescence quantum yield, generally increasing it and acting as a biological complex that reduces π-stacking phenomena [16]. The comparison of fluorescence intensity and photoluminescence between IR780 solutions incorporated in synthetic lipid bilayers of liposomes has been investigated. The results indicate that intrinsic proteins in cell-membrane-derived liposomes decrease the IR780 fluorescence. This influences photothermal conversion efficiency and the capability of these liposomes to achieve the therapeutic thermal dose [25]. However, different chemical modification strategies in the parent molecule have reduced this inconvenience, as indicated in Figure 1.
When photodynamic effects are required after illumination with an appropriate wavelength of the laser light, the loss of fluorescence does not mean a loss of biological activity, and this effect is often observed with other photosensitizers such as chloroaluminum phthalocyanine (AlClPC) [26]. This effect can be explained through the Jablonski diagram (Figure 3), which demonstrates that fluorescence emission and photodynamic/photothermal effects occur through distinct pathways to release absorbed energy after laser irradiation. The photodynamic potential of a photosensitizer can be evaluated through its singlet oxygen (1O2) production. IR780 presents a singlet oxygen quantum yield (Φso) of 8.1–12.7% (Table 1). Induced by singlet oxygen (1O2), photodynamic effects are observed due to reactive oxygen species (ROS) generated that induce cell oxidative stress after laser irradiation. ROS generates molecular damage and mediates cell necrosis and apoptosis, depending on the photosensitizer and light doses.
Considering the photosensitizers applied to photothermal therapy (PTT), an important parameter to be evaluated is the photothermal conversion efficiency (η), which is related to the conversion efficiency of absorbed light into heat. IR780 presents photothermal conversion efficiency in the range of 7.6–10.7% (Table 1), indicating that around 10% of the absorbed light after laser irradiation is converted to heat, resulting in a temperature increase in the environment. IR780 exhibited a high molar extinction coefficient, fluorescence quantum yield, and singlet oxygen quantum yield [3,27,28]. Relatively few photosensitizers have, at the same time, a high absorption coefficient, high-fluorescence quantum yield, and high photothermal conversion efficiency, which are the main requirements for efficient photothermal effect [23]. To achieve a good photothermal effect, the light absorbed by the molecule must be released in a non-radiative form, such as vibrational relaxation. In the case of IR780, its rigid structure can be a limiting factor in achieving a higher photothermal conversion efficiency. Despite that, when irradiated with an 808 nm laser, IR780 can promote a temperature increase, reaching temperatures capable of causing cell damage by inducing coagulation of proteins.
IR780 offers advantages over other photosensitizers, standing out due to its high singlet oxygen (1O2) quantum yield (0.127), high photostability, tumor targeting ability, and intense fluorescence (molar extinction coefficient of 265,000–330,000 M−1.cm−1) as described above (Table 1). These photophysical properties confer superiority to IR780 compared to indocyanine green (ICG), concerning ICG values of singlet oxygen (1O2) quantum yield (0.008), molar extinction coefficient (115,000–204,000 M−1.cm−1), and tumor targeting. ICG is the only chromophore approved by the FDA for clinical imaging and diagnosis [21]. Although widely used, ICG exhibits high aqueous instability, leading to aggregation tendency, poor photostability and photobleaching, short plasma half-life, and low thermal stability that limits its efficacy. Moreover, ICG intravenous administration results in non-covalent binding to plasma proteins and rapid elimination, which reduces cellular selectivity and tumor accumulation [29]. These limitations of ICG, a photosensitizer of the same class of IR780, reinforce IR780’s position as a promising alternative for biomedical applications.
In addition to ICG, traditional photosensitizers include hematoporphyrin derivatives, such as Photogem®, a mixture of oligomers with different molecular weights, used in Russia for the treatment of basal cell carcinoma; temoporfin (a chlorin derivative), exemplified by Foscan®, approved in Europe for head and neck cancer; Photofrin®, the sodium porfimer for injection, used in esophageal cancer; and phthalocyanines, such as Photosens®, an aluminum tetrasulfophthalocyanine authorized in Russia and India for the treatment of sarcoma and tumors of the choroid, eye, eyelid, cervix, and bladder. However, one of the challenges faced by these clinically approved photosensitizers is their partial absorption within the visible spectrum (500–750 nm) [29]. IR780 overcomes this limitation by exhibiting an absorption peak in the 780 to 820 nm range, enabling deeper penetration into biological tissues. This characteristic enhances its effectiveness in both image monitoring and therapies, minimizing tissue background interferences [30]. Although phthalocyanines have attracted considerable attention due to their thermal and chemical stability, high extinction coefficients, high-fluorescence quantum yields, and promising redox activity, they exhibit extremely low solubility in various organic solvents and oils, which may hinder their incorporation into controlled release systems [31]. To circumvent phthalocyanine physicochemical issues, different approaches have been investigated using polymeric nanocarriers [26].
Photophysical properties are generally determined using organic solvents capable of solubilizing the dye (Table 1). However, these solvents cannot be administered in vivo. Aqueous media is ideal for biomedical applications, and some co-solvents may be used in limited concentration to allow dye solubilization. IR780 is lipophilic and soluble in more apolar solvents such as DMSO and ethanol (Table 1). This lipophilicity facilitates its incorporation into the core of nanoparticles made of hydrophobic polymers such as PLA, PLGA, and mPEG-PLA. Nanometrical systems have also been applied for IR780 delivery to improve its dispersibility in aqueous media. Considering IR780 limitations, because it is a hydrophobic molecule showing low stability in aqueous media, the association of IR780 with nanostructures improves its dispersibility in aqueous media. It reduces -stacking and photobleaching, thus ensuring the maintenance of its photophysical properties. Consequently, IR780 loading in nanocarriers improved its biological effect compared to free IR780 (in aqueous media). Furthermore, the interaction of IR780 with the nanostructures’s components can modify its biological effect. Nanoparticles influence the IR780 release and can exhibit better control of biodistribution and targeting, making it a versatile option for administration by different routes and widening its applications as nanotheranostics in PDT and PTT.
3. Development of IR780-Based Nanocarriers
Nanocarriers have been used as an essential tool to overcome the limitations of different poorly soluble photosensitizers. IR780 has already been encapsulated in a plethora of types of nanostructured systems, including transferrin-decorated nanoparticles [32], albumin nanoparticles [8,9], micelles [6,33], liposomes [34], nanostructured lipid carriers [35], poly(lactic acid) (PLA), poly(lactic-co-glycolic) acid (PLGA), and poly-ɛ-caprolactone (PCL) nanospheres and nanocapsules [13,14,36,37].
The association of IR780 with different nanostructures can alter some of their photophysical properties, which directly influences their biological effects, as discussed below. One of the main photophysical properties that is altered by the modification of the IR780 microenvironment is the wavelength of maximum absorption. In general, the incorporation of IR780 into nanostructured systems of different compositions results in a small red shift in its maximum absorption wavelength, which may be caused by hydrophobic interactions between IR780 and the components of these particles.
The influence of the microenvironment on IR780 photophysical properties can be observed in the work carried out by Barcelos and co-workers (2023) [25]. The authors observed that the optical properties of IR780 varied according to the nanostructure used to encapsulate the dye (synthetic liposomes, hybrid liposomes, and cell membrane vesicles), with its association with cell membrane vesicles being the one that presented the most significant deviation in the maximum absorption wavelength (λmax = 804 nm) and the highest reduction in the intensity of light absorption. This red shift in the absorption spectrum also resulted in changes in the fluorescence emission of the dye in the different nanostructures. The authors observed that these changes were related to the incorporation of IR780 into the cell membrane used to prepare the vesicles and depended on the dye concentration, cholesterol content, and amount of protein present in the membrane. In the case of liposomes, the presence of cholesterol causes an increase in the rigidity of the lipid bilayer, which hinders the lateral molecular diffusion of IR780, preventing its aggregation. In addition, this rigidity can reduce the quenching of the dye by lipids and/or by the solvent infiltrated into the lipid bilayer. These effects can favor the absorption and emission of fluorescence by IR780, thus improving its biological effects applied to imaging and PDT. On the other hand, the authors observed that fluorescence emission was lower for IR780 associated with cell membrane vesicles. This effect may be associated with the interaction of IR780 with proteins present in the vesicle membrane, which may result in a dye quenching. The composition of the nanocarrier also influenced the photobleaching of IR780, which may be associated with the energy transfer from the dye to the surrounding oxygen and consequent generation of ROS, with synthetic liposomes containing cholesterol being the formulation that presented the lowest photobleaching. The inhibition of photobleaching may favor the release of energy through heat generation in the medium improving PTT [25].
Wang and collaborators evaluated the photothermal conversion efficiency of IR780 in different solvents and in transferrin nanoparticles [32]. The authors observed that IR780 solubilized in ethanol presented a high heat conversion efficiency, reaching temperatures above 50 °C, while IR780 in aqueous media presented a low thermal conversion capacity (temperature below 30 °C), which may be due to the formation of aggregates in aqueous media. However, when associated with transferrin nanoparticles, IR780 presented a high thermal conversion capacity, allowing it to reach temperatures high enough to be used in PTT, indicating that the interaction of the dye with transferrin can prevent the formation of aggregates to allow the maintenance of photothermal effects even in an aqueous environment [32]. Similar effects were observed after the association of IR780 with other nanostructures of different compositions [32].
The biological activities of different combinations of drugs with lipid-based nanocarriers are shown in Table 2, with polymeric-based nanocarriers in Table 3, and mixed nanocarriers in Table 4, all representing nanostructures where IR780 was not covalently linked to the carrier. In these examples, IR780 was physically entrapped in the nanoparticle. In Table 5, IR780 is attached covalently to the nanocarriers in the examples summarized. Different ligands used to increase the accumulation of bioactive and IR780 in targeted cells were used and are exemplified in all tables.
3.1. Lipid-Based Nanocarriers
Lipid-based nanostructures have been prepared to load IR780, including liposomes, self-emulsifying drug delivery systems (SEDDSs), cell membrane-derived lipid vesicles, and nanostructured lipid carriers (NLCs), as shown in Table 2. IR780 has been integrated into the lipid bilayers to form liposomes. Immunoliposomes have been designed to target activated platelets, enabling fluorescence-guided thrombolysis and photothermal therapy
Table 2 highlights different approaches and examples of lipid formulations employed for IR780 delivery. Among these, N-acetyl glucosamine-modified PEG-coated and 68Ga-labeled liposomes were designed for multimodal imaging and therapy, exhibiting superior tumor accumulation and minimal off-target effects in glioblastoma-bearing mice. The integration of IR780 into these liposomes allowed for enhanced fluorescence and PET/CT imaging, as well as improved PTT and PDT efficacy [38]. Similarly, IR780-loaded hybrid SMEDDS curcumin-phospholipid complexes demonstrated significant tumor suppression and enhanced oral bioavailability in breast cancer models, further supporting the advantage of lipid-based systems in improving systemic drug absorption [39]. In addition to conventional liposomes, novel lipid formulations incorporating targeting ligands have been developed to enhance selectivity toward tumor cells. For instance, targeted SLNs functionalized with c(RGDyK) effectively eradicated glioblastoma tumors in a xenograft model through PTT-induced hyperthermia, demonstrating the benefits of integrin αvβ3 targeting [40]. Moreover, pH-responsive lipid membranes encapsulating perfluorooctyl bromide, IR780, and mTHPC exhibited enhanced tumor oxygenation and phototherapy efficacy under laser irradiation, highlighting the role of lipid-based carriers in modulating the tumor microenvironment [41]. The liposomes hybridized with M1-like macrophage exosomes and conjugated with the AS1411 aptamer further demonstrated potential for tumor microenvironment remodeling, overcoming hypoxia and immunosuppression while significantly improving PDT efficacy [42].
IR780 and docetaxel (DTX) were encapsulated in multifunctional lipid-shelled nanobubbles prepared from DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[polyethylene glycol-2000], [DSPE-PEG(2000)]. The aim was to develop ultrasound contrast agents (UCAs) and combined therapeutic systems for the diagnosis and treatment of pancreatic cancer. These nanobubbles resulted in a selective, stable, and promising formulation for integrated diagnostic and therapeutic applications in pancreatic cancer [43]. Lipid droplets of DSPE-PEG enriched with perfluorohexane (PFH) and loading IR780 were prepared. PFH dissolves oxygen, and this system improved the singlet oxygen quantum yield of IR780, reducing hypoxia and inhibiting tumor growth in mice [44].

Table 2.
Lipid-based nanocarriers containing IR780 encapsulated (not covalently linked) and main outcomes in vitro and in vivo.
Table 2.
Lipid-based nanocarriers containing IR780 encapsulated (not covalently linked) and main outcomes in vitro and in vivo.
| Lipid-Based Nanocarriers | API Co-Loaded | Targeting Approach/Moiety | Application/ Approach | In Vitro/In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|
| Liposome | Sunitinib IR780-loaded | No | PDT | Inhibit angiogenesis in vitro in HUVEC in Matrigel and 4T1 tumor in BALB/c mouse model. | [34] |
| Nanostructured lipid carrier (NLC) CXCR4 targeted | IR780-loaded | AMD3100 coating (CXCR4 antagonist) | PTT | Photothermal efficiency: IR780 < IR780-NLCs < IR780-AMD-NLCs; stronger fluorescence intensity (2.18-fold) in tumors than free IR780 in Balb/C mice carrying 4T1-luc tumor. | [35] |
| N-acetyl glucosamine- modified PEG-coated and 68Ga-labeled liposomes | 68Ga IR780-loaded | N-acetyl glucosamine (NAG)- modified | PDT, PTT, FI, PET/CT, and NIR imaging | Targeted formulations reduced glioblastoma cells (U87 and RG2) viability more effectively with higher intracellular uptake; free IR780 was more cytotoxic than encapsulated formulations; targeted liposomes displayed superior tumor accumulation and targeting in U87 glioblastoma in cd1 nude mice; higher tumor uptake; reduced off-target effects; reduced glioblastoma growth; minimal toxicity to healthy tissues. | [38] |
| Hybrid SMEDDS curcumin–phospholipid complex | Curcumin IR780-loaded | No | PTT, PDT, bioavailability | CUR/IR780@SMEDDS by PTT and PDT suppresses lung metastasis; inhibited tumor progression in orthotopic 4T1 tumor-bearing nude mice model of breast cancer upon oral administration; SMEDDS enhanced oral bioavailability of curcumin and IR780 in rats; inhibited migration and invasion in vitro. | [39] |
| Targeted solid lipid nanoparticle (SLN) | IR780-loaded | c(RGDyK) | PTT, PDT | Target cell lines overexpressing αvβ3 integrin; reduces U87MG (glioblastoma) cells viability under laser irradiation; PTT of tumor induced by U87MG transplantation was eradicated by applying cRGD-IR-780 SLNs + laser in nude mice bearing xenograft U87 MG tumor. | [40] |
| Liposomes | Perfluorooctyl bromide IR780-loaded | IR780-mediated targeting | PAI, FI-guided pre/intraoperative surgery, PTT, computed tomography | improved survival of mice through image-guided tumor resection and PTT in orthotopic breast cancer mouse models; NP-IR780 served as a tumor indicator for precise resection of lesions during surgery as an image guide. | [41] |
| Liposomes termed (M1E/AALs) hybridized with exosomes of M1-like macrophage conjugated with AS1411 aptamer | Perfluorotributylamine (PFTBA) IR780-loaded | AS1411 aptamer-conjugated; exosomes of M1-like macrophage | PDT, immunotherapy | Overcomes hypoxic and immunosuppressive TME by targeted TAM reprogramming and enhanced tumor photodynamic immunotherapy; suppresses tumor growth and prolongs the survival of 4T1 tumor-bearing mice. | [42] |
| Lipid shells of nanobubbles | Docetaxel IR780-loaded | No | PDT, PTT, NIRF, CEUI | IR780-NBs-DTX targeted pancreatic cancer cells; precisely detected pancreatic xenotransplant (Mia-Paca2) through NIRF imaging and CEUI pancreatic cancer in vivo; tumor almost disappeared 18 days after combined treatment; NIRF signals were detected mainly in the liver, lungs, and kidneys. | [43] |
| Perfluorohexane lipid particles with lecithin DSPE-PEG2000 | IR780-loaded | Oxygen self-enriching PDT (Oxy-PDT) | Oxygen self-enriching PDT (Oxy-PDT) | Improved singlet oxygen quantum yield of IR780 and direct injection into tumors inhibited tumor growth in mice; intravenous injection reduces tumor hypoxia and enhances tumor accumulation by passive targeting. | [44] |
| Liposomes | Baicalein IR780-loaded | No | PTT, PDT, antifungal | Liposomes exhibit potent anticancer activity in two-dimensional (2D) cell cultures and three-dimensional (3D) tumor spheroids of TNBC cells, inhibiting cancer cell proliferation and migration; exhibited biocompatibility in zebrafish embryos and inhibited fungal growth. | [45] |
| IR780/SB-505124-based nanoliposomes | Immunomodulator (TGF-β inhibitor, SB-505124) IR780-loaded | No | PTT immunotherapy | PTT-induced immunogenic cell death and dual mitigation of immunosuppression strategy (TGF-β inhibition/PD-1/PD-L1 blockade); TGF-β pathway is inhibited by SB to drive effector T cells into a responsive state and reduce the infiltration of Treg cells; immunosuppressive “protection” of tumor cells is also neutralized by blocking PD-1/PD-L1 immune checkpoint; selectively accumulate, penetrate deeply in tumor tissues of 4T1 tumor-bearing mice. | [46] |
| Nanoliposomes | IR780-loaded chlorophyll-rich fraction of Anthocephalus cadamba (CfAc) | No | PDT | Bioactive phyto fraction-mediated autophagic cancer cell death triggered by NIR light; anti-tumor potential through a combined effect (via heat and CfAc). | [47] |
| mPEG 2000-DSPE liposomes loaded with gambogic acid | Gambogic acid (Hsp90 inhibitor) IR780-loaded | No | PTT, PDT, CDT | PTT after 808 nm laser irradiation (2.0 W/cm2) induces 89.7% tumor ablation in xenograft 4T1-bearing mice model; produced ROS proving PDT effect; exhibited tumor accumulation; no systemic toxicity indicated by unaltered histological profiles of major organs. | [48] |
| pH-responsive liposome | Metformin IR780-loaded | No | PAI, FI, SDT, PDT | Long blood circulation half-life and a high tumor absorption rate in breast xenograft tumor model, combined with US irradiation, inhibited breast tumors by ROS production; metformin reduced tumor oxygen consumption. | [49] |
| Liposomes DSPE-PEG/CHOL/DPPC loading perfluoro pentane | IR780-loaded | IR780 as a mitochondria-targeting moiety | SDT, FI, PAI, US imaging | The generated bubbles enhanced US imaging. In the presence of US, the bubbles increase the acoustic droplet vaporization (ADV) effect and assist the conveyance of IR780-NDs from the circulatory system to tumor regions; the acoustic wave force increases the penetration depth within tumor tissues. | [50] |
| Thermosensitive liposomes (CAP-TSL) targeted with peptide | Paclitaxel–albumin (HSA-PTX) IR780-loaded | FAP-α-responsive cleavable amphiphilic peptide (CAP) | PTT, CDT | PTT is effective in a luciferase-labeled orthotopic tumor model (Pan 02-luc cell) in the pancreas of C57BL/6 mice (pancreatic ductal adenocarcinoma); IR780 induces hyperthermia and expands the tumor interstitial space; and it promotes the HSA-PTX release in deep tumors. | [51] |
| Nanostructured lipid carrier (NLC) | Erlotinib IR780-loaded | No | NIR imaging | After oral administration, the free IR780 solution exhibited high ROI throughout the body; IR780-loaded NLC has low radiance; ERL suspension distributes better across the body; NLCs avoided first-pass metabolism by adopting the intestinal lymphatic pathway; and oral bioavailability of ERL was enhanced. | [52] |
| Thermos-sensitive lipid nanostructures | Tirapazamine IR780-loaded | IR780 as mitochondria targeting | PDT, PTT, PCT, FI, PAI, image guidance | Anti-tumor efficacy under PAI and FL imaging guidance and monitoring; improved anti-tumor effectiveness; fatty acids that undergo a solid–liquid phase transition at 39 °C. | [53] |
| Lipid mixed—artificial synaptic vesicles aptamer-functionalized | IR780-loaded | Functionalized 5-Hydroxytryptophan (5-HTP) aptamer | PTT, FI | Improved BBB permeability (RSC-96, bEnd.3 and HUVEC cells); PTT-triggered 5-HTP release; enhanced cerebral drug enrichment; reduced depressive-like behaviors in chronic, unpredictable, moderate stress model mice. | [54] |
| RBC membrane-based vesicles | Doxorubicin IR780-loaded | No | PTT | Increased the accumulation into the Dox-resistant prostate cancer cells (PC-3/Dox); enhanced anticancer performance and accumulation in vivo. | [55] |
| pH-responsive lipid membrane-enclosed perfluorooctyl bromide oil droplet nanoparticles | Co-delivering oxygen, IR780, and mTHPC-loaded | Surface-modified with N-acetyl histidine-modified D-α-tocopheryl polyethylene glycol 1000 succinate | PDT, PTT, pH sensitivity | After 808 nm laser (1.0 W/cm2) irradiation for 5 min of TRAMP-C1 cells in SC tissue of C57BL/6 mice, NPs induced inhibition of tumor growth, exhibited tumor targeting, and relieved tumor hypoxia. | [56] |
API: active pharmaceutical ingredient; PDT: photodynamic therapy; PTT: photothermal therapy; PAI: photoacoustic imaging; NIRF: near-infrared fluorescence; FI: fluorescence imaging; SDT: sonodynamic therapy; CEUI: contrast-enhanced ultrasound imaging; CT: computed tomography, CDT: chemodynamic therapy; PCT: photochemodynamic therapy.
3.2. Polymeric-Based Nanocarriers
Polymeric nanocarriers have emerged as versatile and effective platforms for the transport and controlled release of photosensitizers and dyes. Table 3 shows the polymeric-based nanocarriers loading IR780 via physical encapsulation. Albumin nanoparticles, polyester nanoparticles, and polymeric micelles are the most employed systems. Self-assembled multifunctional micelles, prepared with the amphiphilic copolymer folic acid-hyaluronic acid-SS-vitamin E succinate (FHSV), were developed to combine chemotherapy and phototherapy, encapsulating the chemotherapeutic agent paclitaxel (PTX) and the photosensitizer IR780. These polymeric micelles enable the gradual and controlled release of IR780 in specific microenvironments, such as glutathione (GSH)-rich tumors [57]. Moreover, modifying the particles with surface ligands, such as folic acid and hyaluronic acid, preferentially directs IR780 to tumor cells [57]. Doxorubicin is an antitumoral drug frequently co-loaded with IR780 to obtain a chemotherapeutic effect [58,59,60,61,62,63,64].
In addition to cancer therapy, polymeric nanocarriers have been employed in multimodal imaging and immune modulation. Polydopamine-based nanoparticles co-loaded with camptothecin and IR780 exhibited robust photothermal conversion efficiency, increasing tumor temperatures and enhancing apoptosis in lung cancer models [65]. In another approach, platelet membrane-coated PLGA nanoparticles containing doxorubicin and IR780 actively targeted 4T1 breast cancer cells while evading immune clearance, demonstrating long circulation times and effective photothermal ablation [62]. These findings highlight the synergistic potential of polymeric carriers in combining phototherapy with chemotherapy and immune modulation.
Using the interfacial polymer deposition method via solvent displacement, polymeric nanocapsules (NCs) containing IR780 were developed, exhibiting high encapsulation efficiency (~99%). In this study, two distinct types of NCs were prepared, differing in how the photosensitizer IR780 was associated with the particles. On the one hand, IR780 was physically encapsulated in the oily core during the nanoprecipitation process, and on the other hand, IR780 was covalently linked to the poly-D,L-lactide (PLA) polymer through a chemical reaction, enabling the preparation of NCs from the IR780-PLA-conjugated polymer [14]. The study demonstrated that IR780, when covalently bound to PLA (IR-PLA), ensured greater stability and colocalization with the nanoparticles, unlike physically encapsulated IR780, which was released very fast from NCs in the presence of serum proteins. NCs containing the modified IR-PLA polymer proved promising for biodistribution studies and image-based therapies, ensuring the dye remained colocalized with the nanoparticles [14].
Polymeric nanocarriers have also facilitated stimuli-responsive drug release, improving the specificity and efficacy of IR780-based therapies. For example, pH-sensitive PLGA nanoparticles modified with zwitterionic diblock copolymers exhibited prolonged tumor retention and increased NIR-triggered hyperthermia, demonstrating a more effective photothermal response in prostate cancer models [66]. These results underscore the advantages of polymeric nanocarriers in achieving precise tumor targeting and controlled drug release.
M2/IR780@PLGA nanoparticles have been developed as multifunctional therapeutic agents for antimicrobial applications and imaging monitoring. The physical association of IR780 within the PLGA matrix in nanoparticles enabled an efficient generation of reactive oxygen species (ROS) under ultrasonic irradiation (1 MHz, 2 W/cm2), enhancing antimicrobial effects. M2/IR780@PLGA nanoparticles retained their photothermal and photodynamic properties for extended periods, effectively eradicating antibiotic-resistant bacterial biofilms and showcasing their potential in combating multidrug-resistant infections. Furthermore, their colloidal stability highlights their potential as promising sonodynamic agents for in vivo studies [67].

Table 3.
Polymeric nanostructures containing IR780 encapsulated (not covalently), and the main outcomes in vitro and in vivo.
Table 3.
Polymeric nanostructures containing IR780 encapsulated (not covalently), and the main outcomes in vitro and in vivo.
| Polymeric Nanoparticles | API Co-Loaded | Targeting Approach/Moiety | Application/ Approach | In Vitro/In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|
| Albumin-loaded nanoparticles | Tanshinone IIA IR780-loaded | No | PDT | Enhanced the antibacterial activity both in vitro and in vivo; enhanced antibacterial activity under near-infrared irradiation in wounds. | [5] |
| Human serum albumin nanoparticles | IR780-loaded | No | PDT | HSA-IR780 NPs exhibited tumor inhibition by intratumoral injection CT26 (colon adenocarcinoma) tumor-bearing mice MCF-7 cells. | [9] |
| Albumin-coated trimethyl chitosan NPs | Bufalin IR780-loaded | Bufalin | PTT, PDT | TBH NPs inhibited cell proliferation and mitochondrion activity of metastatic 4T1 breast cancer cells; albumin camouflage resulted in tumor accumulation and penetration within tumor mass; potent inhibition of tumor growth with laser irradiation; efficient prevention of lung metastasis. | [10] |
| Albumin Nanocarrier | IR 780-loaded | Magnetic core | PDT, PTT | Safety studies (acute oral toxicity, cardiovascular evaluation, and histopathological analysis) demonstrated an increase in tumoral necrosis areas 24 and 72 h after treatment, indicating tumor regression; viability determined in Ehrlich ascites carcinoma cells. | [11] |
| PEG-PLA nanocapsules and nanospheres | IR780-loaded | No | PDT | Improved uptake, cell death, and reduced migration in human breast cancer cells (MDA-MB231 and MCF-7) and reduced cytotoxicity in normal breast cells (MCF-10A). | [14] |
| Transferrin-protein-based nanoparticles | IR780-loaded | Transferrin | PTT, PDT | Treatment with Tf-IR780 NPs resulted in significant tumor suppression in CT26 tumor-bearing mice, enhanced generation of ROS under laser illumination, and increased tumor-to-background ratio in CT26 tumor-bearing mice. | [32] |
| Micelles of synthetic amphiphilic hyaluronic acid derivative (FHSV) micelles | Paclitaxel IR780-loaded | Hyaluronic acid targeting GSH-rich tumors | PTT, PDT | IR780/PTX/FHSV micelles accumulate 1.9-fold in tumor tissues compared to free IR780/PTX; upon NIR illumination showed more substantial tumor suppression, with 1.4-times-higher tumor inhibition than that of the IR780/PTX group in Kunming mice bearing S18 tumor; fast release of PTX and IR780 under GSH-rich tumor microenvironment; produced local hyperthermia and sufficient reactive oxygen species inducing apoptosis and necrosis. | [57] |
| PEG-PCL nanospheres with internal SS cross-link | Doxorubicin IR780-loaded | No | PTT, NIR illumination, NIR-imaging | After 21 days, the DOX&IR780@PEG-PCL-SS NPs with NIR irradiation reduce tumor size and inhibit their growth in the orthotopic bladder cancer model in C57BL/6 mice. | [58] |
| PEG-PCL paramagnetic nanoparticle | Doxorubicin IR780-loaded Manganese | No | PTT, CDT dual mode imaging-guided | PCL-block-PIEt-Mn could be accumulated effectively at the tumor sites. Upon the NIR laser irradiation, tumor growth was inhibited by PTT-enhanced chemotherapy. | [59] |
| Polymersomes of PEG-block-poly(β-amino acrylate)-block-PEG copolymers | Doxorubicin IR780-loaded | No | PDT/PTT | Target-specific drug release through destruction of carrier structure via 1O2-mediated photocleavage of the membrane upon NIR light irradiation; excellent antitumor effect in Balb/c mice bearing 4T1 tumor. | [60] |
| P-DOX-(P(FPMA-co-DEA)-block-POEGMA)-conjugated micelles | Doxorubicin IR780-loaded | No | PTT, PDT, CDT | IR780-PDMs show remarkably long blood circulation; intravenous micelles showed high delivery efficiency and exhibited 97.6% tumor growth inhibition in A549 tumor-bearing mice. | [61] |
| Platelet membrane-coated PLGA-nanoparticles | Doxorubicin IR780-loaded | No | PTT, PDT | PM-NPs actively targeted 4T1 cells via platelet-mimicking; low uptake by Raw 264.7 cells; circulated longer in the blood; accumulated more at the tumor site; with NIR irradiation, 4T1 tumor eliminated without recurrence in 18 days. | [62] |
| Micelles IR780-CSOSA/DOX | Doxorubicin IR780-loaded | No | CDT, PDT, FI | Significant reduction in tumor volume in Balb/C mice carrying MCF-7/4T1/H22 tumors; 85.3% tumor growth inhibition by chemo-photothermal therapy; targeting. | [63] |
| DOX-SS-DOX self-assemble nanoparticles stabilized with hydroxyethyl starch-folic acid | Doxorubicin IR780-loaded | Folic acid | PDT TME modulation | FDINs showed high tumor accumulation in SC and orthotopic 4T1 tumor-bearing mice model; modulated the tumor mechanical microenvironment; depleted cancer stem cells; suppressed tumor growth in both tumor models. | [64] |
| Polymeric nanoparticles of polydopamine | Camptothecin (CPT) IR780-loaded | No | CDT PTT PDT | CPT-PDA-IR780 exhibited a higher PTT effect on A549 human lung cancer cells and LLC murine lung cancer cells under NIR irradiation; induced apoptosis; increased cytotoxic effect of CPT-PDA-IR780 + NIR for A549 cell 2.5-fold; increased tumor temperatures with NIR irradiation in BALB/c nude mice bearing tumor of A549 cells; exhibited superior tumor a with accumulation and retention of NPs; induced potent tumor growth inhibition. | [65] |
| pH-sensitive PLGA NPs coated with zwitterionic diblock copolymers, mPEG-block-poly(methacrylic acid-co-histamine methacrylamide) | IR780-loaded | No | PDT, PTT NIR imaging | mPEG-block-P(MAA-co-HMA acidity-elicited structural transformation of NPs with increased uptake by TRAMP-C1 cells (mouse prostate cancer); prolonged tumor retention time; increase in NIR-elicited hyperthermia by intratumoral injection of IR780-loaded PMHPN in TRAMP-C1 tumor-bearing mice; prolong tumor retention; reduce nanoparticle/drug elimination caused by high interstitial fluid pressure of tumor extracellular matrix. | [66] |
| PLGA nanoparticles | IR780-loaded | No | SDT, PDT, PTT | Effectively combat resistant bacterial infections. | [67] |
| PLGA nanoparticles | Polyphyllin II (PPII) IR780-loaded | Aptamer AS1411 | CDT, PTT, ICD | Apt/PPII/IR780-NPs significantly improved the Anti-PD-1 efficacy; aptamer AS1411 was modified on the surface of nanoparticles to construct the targeting HCC. | [68] |
| Albumin nanoplatform co-delivering | MnO2, NLG919 paclitaxel dimer IR780-loaded | MnO2 | PDT, PTT, CDT | Improved the effect in Female BALB/c mice bearing 4T1 tumor. | [69] |
| Human serum albumin-modified multifunctional persistent luminescence nanoplatform | IR780-loaded | Fe3+ Human serum albumin | Photo-enhanced CDT, PDT, and PTT, MRI, PLI, PAI, reduce hypoxia | Inhibit tumor growth in 4T1 tumor model mice; theranostic platform for PLI/PAI/MRI multimodal imaging and efficient CDT/PTT/PDT combination cancer therapy; tumor imaging; exhibits high T1 contrast effect; photo-enhanced Fenton-like activity of Fe3+. | [70] |
| Self-assembled albumin nanoparticles (IGM) combined with MnO2 | IR780 and gambogic acid | MnO2 | PTT, PDT | GA inhibits Hsp90 and increases sensitivity to PTT in 4T1 tumor-bearing mice and HUVEC cells; increases temperature and releases O2 to reduce hypoxia; increases the PDT effect and tumor accumulation. | [71] |
| PLGA core/shell nanoparticle with hematoporphyrin monomethyl ether core (HMME) | Glucose oxidase IR780 in the shell | Hematoporphyrin monomethyl ether | SDT starvation therapy PAI FL | Accumulation in cancer cells/sites; mitochondrial targeting for synergistic SDT and starvation therapy; improved outcome in treatment (4.7-fold lower tumor growth); excellent PAI/FL imaging contrast agents to simultaneously monitor and guide cancer therapy. | [72] |
| PLGA nanospheres | IR780-loaded glucose oxidase | No | PDT, PAI, NIR imaging, PDT, FI, starvation | The therapeutic process is guided/monitored by photoacoustic (PAI) and fluorescence (FL) dual imaging. PTT- and PDT-induced tumor deep penetration guided by IR780 mitochondria targeting. | [73] |
| Poly(lactic-co-glycolic acid) nanoparticles | 3-bromopyruvate (oxygen regulator) IR780-loaded | IR780 as a targeting moiety for mitochondria | PDT, PAI, dual mode FI | Alleviate tumor hypoxia by decreasing physiological oxygen consumption in 4T1 tumors Balb/c nude mice; 3BP@PLGA-IR780 interrupted the energy metabolism of tumor cells; increased the internalization 3BP@ PLGA-IR780 in vitro in 3D tumor spheroid models; diffused throughout the tumor spheroid; NPs enriched in tumor tissues. | [74] |
| Mesoporous polydopamine nanoparticles | IR780-loaded | No | PDT, PTT, PAI, FI, ICD | Intravenous IR-780@MPDA triggered immunogenic cell death (ICD) in 4T1 breast tumor model and PTT conversion ability; cellular accumulation; 1.6-fold-higher accumulation than free IR-780; significant suppression of tumor growth. | [75] |
| Methoxy-PEG-block-poly(2-hexoxy-2-oxo-1,3,2-dioxaphospholane micelles (mPEG-block-PHEP) nanocomposite | Zinc manganese sulfide IR780-loaded | PTT-responsive; thermally sensitive flowable core | PTT, CDT, ICD, TME reprogram, NIR light-triggered release | TME reprogramming; PPIR780-ZMS evokes an immune response and protects male C57BL/6 mice from pulmonary metastasis melanoma model (B16F10); NIR triggered active release and suppressed B16F10 cell invasion and migration in vitro with high ROS production. | [76] |
| Micelles of mercaptopropionic acid grafted PEG-block-poly(ε-caprolactone)-block-poly(allyl-glycidyl ether) copolymer | IR780-loaded | No | PTT, PDT, FI | IR-780@TBMPA accumulates in tumor with hyperthermia to kill tumor cells; intratumoral injection in U14 cervical cancer in mice model; mPEG5K-PCL10K-PAGE6 induces hyperthermia and ROS to Hela cell inducing apoptosis and tumor necrosis without relapse. | [77] |
| Chitosan nanoparticles | 5-aminolevulinic acid IR780-loaded | No | PTT/PDT | Chitosan NP improved colon cancer management and oral 5-ALA absorption, and local accumulation in SC mouse colon tumors (CT-26 cells) model with no overt adverse effects. | [78] |
| Maltodextrin nanoparticles | Cinnamaldehyde IR780-loaded | Oxidative stress inducer through acid-labile acetal linkage | PTT, PDT | IV-administered NPs combined with NIR laser eradicated tumors in mouse xenograft model; hyperthermia- and oxidative stress-inducing. | [79] |
| Multifunctional heparin-folic acid-conjugated nanoparticles | IR780-loaded | Folic acid (FA) conjugated with heparin | PDT, PTT, FI | Increased tumor temperature in MCF-7 tumor-bearing nude mice; induced necrosis. | [80] |
| Hyaluronic acid conjugated with C18 chain micelles | IR780-loaded | Hyaluronic acid conjugated with micelles targeting CD44 | PDT, PTT | HA-IR780 selectively accumulated in tumors within 24 h and caused photothermal ablation in the tumor region in TC-1 xenografts mice model; exhibited CD44- and EPR-based tumor accumulation; temperature reached 49.9° in CTC-1 cells. | [81] |
| Poly-ϵ-caprolactone targeted nanoparticles with bovine albumin as a stabilizer | Paclitaxel IR780-loaded | Peptide-recognizing luteinizing hormone receptors on ovarian cancer cells | PTT, CDT, PDT | PCL-LHRH/IR780-PTX efficiently hinders the growth of drug-resistant xenografts with an 808 nm NIR laser with selective tumor targeting. | [82] |
| PEG-PCL nanoparticles | Sorafenib IR780-loaded | Decorated with legumain-activable melittin (LM) | PDT, CDT, FI | LPN improves the oral delivery of water-insoluble sorafenib, which accumulates at the tumor site with deep penetrating capacity. NIR laser irradiation inhibited tumor growth. Oral bioavailability of sorafenib was remarkably increased (75.9-fold), and uptake increased in BGC-823 (gastric cancer cells). | [83] |
| iRGD peptide as ligand-mediated polymeric micelles conjugated with disulfide bond prodrug polymer | Camptothecin IR780-loaded | Ligand-iRGD peptide conjugated with PEG | PTT, PDT | CPD@IR780 showed favorable ability to cross the BBB and target glioma cells via αv β integrin and neuropilin-1-mediated ligand transportation in vitro (BEnd3 cell U87 cells) and in vivo; enhanced the antitumor effect with NIR laser irradiation. | [84] |
| Polymeric hybrid micelles PCL-PEI/PCL-PEG/lumbrokinase | IR-780-loaded | FXIII peptide conjugated | PAI, MSOT, FM | FXIII-conjugated micelles designed for obstructive thrombosis rapidly target FXIIIa-rich clots after IV administration undergoing thrombogenesis; increased the imaged embolized vessel by MSOT; dredge the vessel; dissolved the fibrin framework of the thrombus in a FeCl3-induced carotid thrombosis model. | [85] |
| Lycium barum polysaccharide nanoparticles | Cardamonin IR780-loaded | No | PTT, PDT | CRD-IR780-LBP induces a 1.24-fold increase in tumor suppression compared to NPs containing only CRD in 4T1-bearing mice model; PTT effects upon NIR irradiation increased anti-tumor efficacy. | [86] |
| Self-Assembled Nanoparticles | Berberine Hydrochloride IR780-loaded | No | PDT, PP, FI | Accumulation in the tumor of nude mice bearing subcutaneous HepG2 xenograft tumors; 96% reduction in tumor growth; increased tumor temperature; exhibited efficient cellular uptake; induced 3.5-times-higher ROS production after laser irradiation in (HepG2 and Huh7) cell lines; FI confirmed targeting. | [87] |
API: active pharmaceutical ingredient; PDT: photodynamic therapy; PTT: photothermal therapy; PAI: photoacoustic imaging; FI: fluorescence imaging; SDT: sonodynamic therapy; PLGA: poly(lactide-co-glycolide) polymer; PLA: polylactide polymer; PCL-PEI: polycaprolactone-polyethylenimine; PCL-PEG: polycaprolactone-polyethyleneglycol; MSOT: multispectral optoacoustic tomography; FM: fluorescence microscopy; CDT: chemodynamic therapy; CT: computed tomography.
3.3. Mixed Composition-Based Nanocarriers
Innovative systems that combine organic and inorganic components enhanced the effects of IR780 as a nanotheranostic. Table 4 shows IR780 associated with mixed-composition nanocarriers. Most of them associated IR780 with other molecules to improve the image-monitoring performance of the nanosystem or their targeting abilities. For instance, endogenous functional magnetic lipid droplets have been developed to overcome multidrug resistance (MDR) in breast cancer treatment [88]. This system integrates IR780, magnetic iron oxide nanoparticles coated with oleic acid (Fe3O4/OA), and lipid droplets derived from adipocytes (IR780@LDs-Fe3O4/OA), targeting the lysosome and mitochondria to reverse MDR in breast cancer and promote advancements in sonodynamic therapy (SDT) [88]. The development of an inorganic system called MMFn combined manganese ferrite nanoparticles (MnFe2O4) with the photosensitizer IR780 in magneto-fluorescent nanocarrier biomimetically coated with erythrocyte membranes. MMFn prolongs blood circulation time and enhances tumor delivery efficiency [89]. A similar strategy has been employed to design hybrid polymer–lipid nanoparticles loaded with zinc-doped copper oxides (ZCNP), IR780-loaded in a PLGA matrix coated by triphenylphosphonium (TPP-ZC-IR-PNPs) engineered to target the mitochondria of cancer cells [90].

Table 4.
Metallic and mixed nanocarriers containing IR780 encapsulated and main outcomes in vitro and in vivo.
Table 4.
Metallic and mixed nanocarriers containing IR780 encapsulated and main outcomes in vitro and in vivo.
| Mixed Nanocarriers | API Co-Loaded | Targeting Approach/Moiety | Application/ Approach | In Vitro/In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|
| Three-dimensional multi-FUS-TSE (Focused Ultrasound Temporal Sequential Excitation) | IR780-loaded | No | FI imaging | Three-dimensional FUS therapy increased 66.4% IR-780 accumulation in tumor of BALB/c mice bearing subcutaneous 4T1 breast cancer xenografts; 2.5-times-greater efficiency in drug delivery compared to the 2D FUS system. | [48] |
| Magnetic endogenous functional lipid droplets: IR780@LDs-Fe3O4/OA | Doxorubicin IR780-loaded Fe3O4 magnetic | Magnetic field | SDT, PDT, CDT, Reverse MDR | IR780@LDs-Fe3O4/OA targeting the lysosome and mitochondria to reverse MDR in breast cancer; promoted advancements in SDT; enriched within tumor sites in a static magnetic field; inhibited Pgp efflux; Mediated Cascade-Targeted Sonodynamic Therapy for MDR breast cancer (MCF-7/ADR cells). | [88] |
| Magneto-fluorescent nanocarrier with erythrocyte membrane-camouflaged | IR780-loaded manganese ferrite (MnFe2O4) | Erythrocyte membrane-derived camouflage | FI, biodistribution studies | IR-780 accumulates in fat tissues right after the distribution phase and subsequently undergoes a slower elimination during the post-distributive phase; the elimination half-life of free IR780 was 170 h and NPs was 92 h, both distribution half-life was short (~1–2 h); ex vivo 2D-FMT images indicated a preferential accumulation of MMFns within the tumor 72 h after the administration. | [89] |
| TPP-conjugated Zn Cu-loaded polymer-lipid hybrid nanoparticles (PLGA/Lecithin/ DSPE-PEG-5000/TPP-PEG) | IR780-loaded Zn-doped Cu | Mitochondria-targeting TPP-PEG triphenylphosphonium | PTT, PDT, FI | NP enhanced efficacy in tumor model in vivo; induced ROS generation upon irradiation; enhanced accumulation in tumor tissue; prolonged circulation in blood; TPP provides lipophilic cationic mitochondria targeting and dysfunction. | [90] |
| Targeted nanoparticle with H-40 polymer core coated with red blood cells + WSU-HN6 oral squamous cell hybrid membrane | Tirapazamine IR780-loaded | Asp8–aspartic acid; RBC membrane promotes immune evasion | PTT, PDT, Hypoxia, CDT | Asp8[H40-TPZ/IR780@(RBC-H)] increased 4-fold cytotoxicity against human WSU-HN6, HeLa, RAW 264.7, and HUVEC cells lines; hypoxia + laser irradiation reduced 85% viability; induced homotypic targeting and 2-fold-higher uptake in BALB/c nude mice with WSU-HN6-induced mandibular bone invasion; immune evasion and 90% reduction in macrophage uptake; dual targeting increased 2-fold accumulation; 3-fold reduction in tumor weight; increased tumor temperatures to 57 °C. | [91] |
| lncRNA nanoparticles NONHSAT159592.1 | si-lncRNA IR780-loaded | Silencing RNA | PDT | Enhanced effect against U87 and U251 glioblastoma cell lines; silencing lncRNA could significantly inhibit proliferation in orthotopic xenograft nude mice tumor model; prolonged the survival time. | [92] |
| Lysolipid-based thermosensitive liposome decorated with cRGD peptide conjugated on the surface of an IR780-loaded microbubble | Doxorubicin IR780-loaded | cRGD peptide | US, PAI, CDT, thermo-responsive, FI, SDT | US-mediated drug release upon laser irradiation enhances DOX efficacy in breast cancer cell MCF-7 xenograft nude mice; a combination of RTSL-IMBs and US shows a 2.8-fold increase in tumor accumulation. | [93] |
| PLGA nanoparticles and fused breast cancer cell and bacterial outer membranes in a hybrid membrane | IR780-loaded | Hybrid cell membranes (HMs) | SDT, PDT | NP targeted to 4T1 tumors promoted macrophage type I polarization and DC activation, strengthened anti-tumor inflammatory factors expression, and presented the ability to kill tumors both in vitro and in vivo; reduced breast cancer bone metastasis. | [94] |
| Platelet-mimicking nanoparticles | Metformin (Met) IR780-loaded | Platelet mimicking membrane | PDT, Immune activation | Reversed tumor hypoxia; 4T1 tumor bearing BALB/c mice; immunogenic activation; immunosuppressive reversion by mitochondrial-respiration-inhibited platelet-mimicking NPs. | [95] |
| Hydrophobin-based nanoparticle | IR780-loaded | Tumor-penetrating peptide tLyP-1 | PTT | Reduced lung and liver metastasis, primary tumor growth, and recurrence; enhanced tumor targeting and photothermal therapeutic efficacy. | [96] |
| Iron tetroxide core coated with cetuximab | IR780-loaded | DSPE-PEG-cetuximab | SDT, TME, FI, RMI | Biodistribution of IR780@INPs-CTX using fluorescence image indicates the accumulation in the tumor and a small amount in the liver; MRI displays fast enrichment into the tumor tissue, and after ultrasound irradiation, the complete disappearance of the tumor or a continued decrease in tumor volume. | [97] |
| Specific nanoreactor utilizing EGCG and Ce3+ | IR780-loaded EGCG Ce3+ | AS1411 aptamer | PTT, PDT, CDT | Inhibited tumor growth and prolonged the survival of 4 T1 tumor-bearing mice; exhibited prolonged accumulation at the tumor tissues; improved the tumor immunosuppressive microenvironment; activated the systemic immune system, and generated long-term immune memory via the combined effects of ferroptosis and PTT. | [98] |
| Oxidation-sensitive nanoparticles of mesoporous silica with CeO2 | IR780 and Metformin loaded | CeO2 as gatekeeper | PDT, oxidation sensitivity, biodistribution | The plasma half-life of NPs was much longer than that of free IR780 in B16F10 (melanoma) tumor-bearing mouse models; two-times-higher accumulation in tumor than free IR780; higher antitumor and antimetastatic effects; NPs were etched by overexpressed endogenous H2O2 in tumor tissues. | [99] |
API: active pharmaceutical ingredient; PDT: photodynamic therapy; PTT: photothermal therapy; PAI: photoacoustic imaging; RMI: resonance magnetic image; FI: fluorescence imaging; SDT: sonodynamic therapy; PLGA: poly(lactide-co-glycolide) polymer; CDT: chemodynamic therapy; MDR: multidrug resistance; RBC: red blood cells.
4. Mechanisms of IR780 Action Inside Cells and Tissues
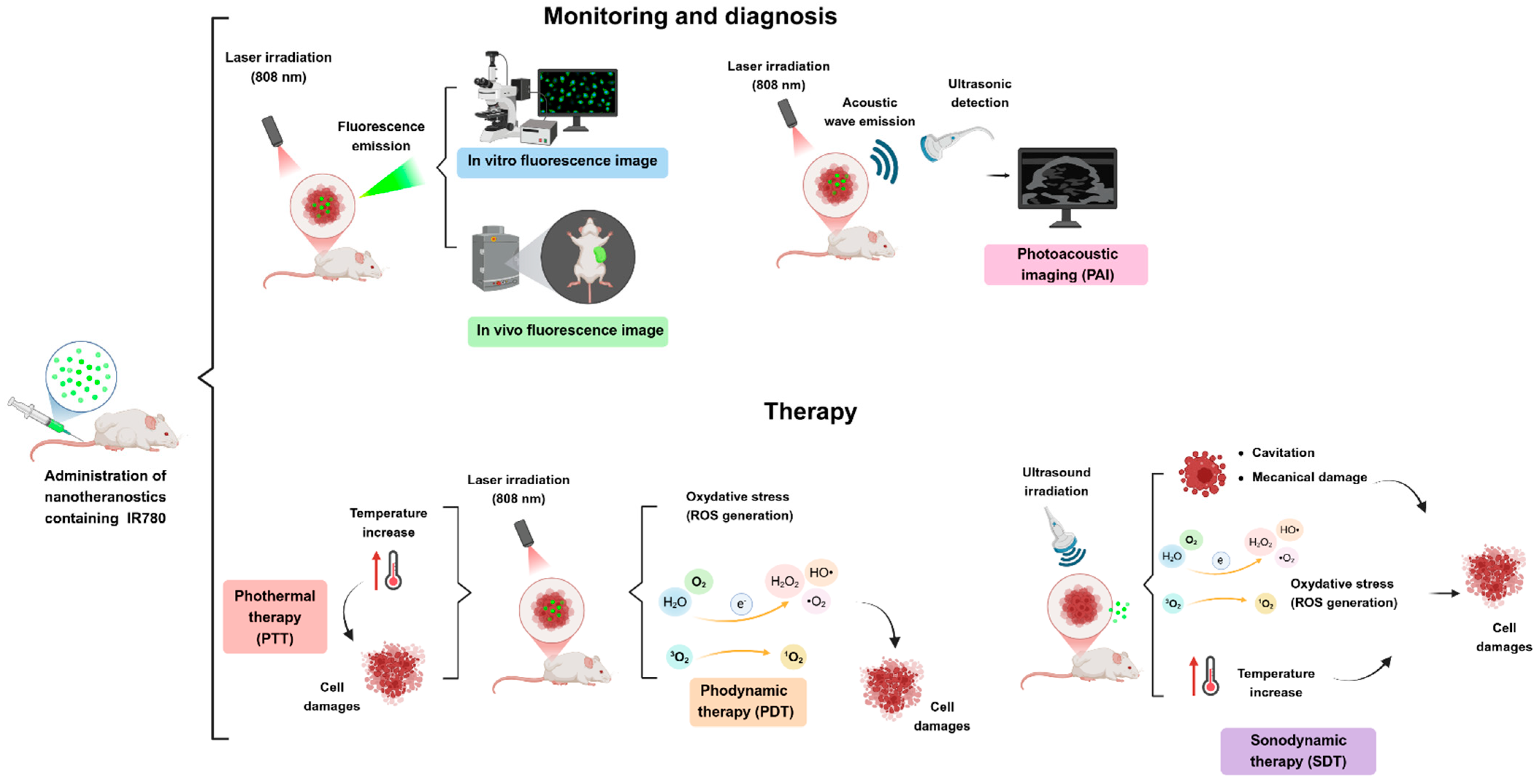
IR780 is a multifunctional molecule. In the literature presented in this review, the applicability of IR780 as a photosensitizer (PS) in PDT and PTT is predominant. Its use in sonodynamic therapy (SDT) using ultrasound has also been reported. Another important applicability is its use as a fluorescent probe/dye for obtaining images for tumor monitoring using near-infrared fluorescence (NIRF) in vivo images, producing photoacoustic images (PAI), or combining them with ultrasound to induce drug release. Obtaining images after the administration of nanotheranostics is essential for the in vivo monitoring of the biodistribution of the active molecule and its ability to reach the biological target/receptor at an ideal time for its activation by external laser light illumination [100]. Activating the therapeutic molecule in the specific target tissue improves the chemotherapeutic efficacy of photosensitizer (PS) and the other active pharmaceutical ingredients (APIs) associated with PS. This strategy drastically reduces toxicity and adverse effects on healthy tissues (Figure 2).
The mechanisms by which this molecule acts as a photodynamic, photothermal, or sonodynamic agent are shown in Figure 4. These mechanisms are based on photochemical reactions involving IR780 photosensitizer, as represented in the Jablonski diagram (Figure 3). Light-activated IR780 inside cells in the presence of molecular oxygen mediate the cascade of radical reactions. The oxygen singlet generation yield, a photophysical parameter for a photosensitizer, is a direct measure of damage in the cellular environment (Table 1).
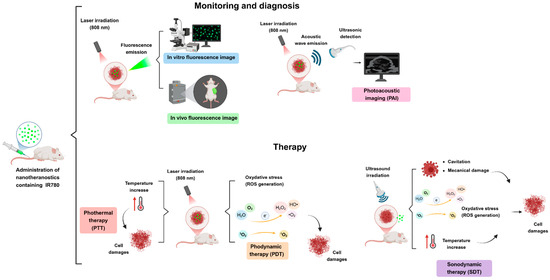
Figure 4.
Schematic representation of the main mechanisms and methods to obtain fluorescence images (FI), photoacoustic imaging (PAI), and photodynamic (PDT), photothermal (PTT), and sonodynamic (SDT) effects in selected body sites upon laser or ultrasound irradiation. Created in BioRender. De oliveira, M. (2025). https://BioRender.com/u31x919 (Accessed on 5 March 2025).
4.1. IR780 Targeting Mitochondria in Tumoral Cells
IR780 (iodide salt) is a lipophilic dye with preferential tumor mitochondria-specific accumulation mediated by the lipophilic cationic character of the molecule and by the active transport of organic-anion-transporting polypeptides (OATPs), which is significantly overexpressed in various types of tumor cells [88,89,90,100,101,102]. The selectivity of IR780 for tumor cells is a key factor that underpins its use in oncology applications. Thus, IR780 stands out among other photosensitizers for its ability to accumulate preferentially in the mitochondria of tumor cells. This was first demonstrated by Zhang and colleagues [103]. The authors investigated IR780 cellular trafficking using tumor and non-tumor cellular models and staining by Mito-tracker Red (mitochondria staining). The cellular internalization of IR780 was found to be significantly greater in tumor cells than in non-tumor cells, probably mediated by OATP1B3, a subtype of organic-anion-transporting polypeptides (OATP) overexpressed in tumor cells. A competitive inhibitor of OATP was used to achieve this result, which reduced the cellular internalization of IR780 by tumor cells by approximately 70 to 80%. Furthermore, the IR780 and Mito-tracker Red markers had a high degree of co-location, confirming their targeting of tumor cell mitochondria [103].
Many studies have widely explored IR780’s ability to target tumor cells (Table 2, Table 3, Table 4 and Table 5). Interestingly, IR780 has also been used as a targeting moiety, demonstrating the ability of this molecule to direct nanocarriers to tumors through interaction with the overexpressed receptor OATP. This is a promising strategy in cancer treatment [41,74].
4.2. Near-Infrared Fluorescence (NIRF) Imaging
Photosensitizing probes with excitation and fluorescence emission in the near-infrared region (NIR, 750–1000 nm) of the light spectrum are of great interest in developing new imaging and diagnostic techniques. At wavelengths encompassing the NIR spectral window, the absorption and autofluorescence of living tissues are low, so this region of the electromagnetic spectrum is called the “window of biological transparency”. Furthermore, light penetration into tissue at these wavelengths is much deeper than that of ultraviolet or visible wavelengths, which facilitates detection using imaging techniques in live animals and avoids interference with external agents [4,104,105,106]. The light penetration depth for NIR fluorophores is around 0.1–1.5 cm, depending on the wavelength of light [105,106,107,108] Thus, IR780 can be adequately detected by non-invasive imaging technique using NIR fluorescence. NIRF is an option for detecting tumors due to their sensitivity and can be used in experimental preclinical studies. IR780 has been used to visualize breast, cervical, lung, and osteosarcoma tumors in mice, as the fluorescence signals observed in the tumor after intravenous administration were much higher when compared to normal tissues [109]. This technique allows the real-time monitoring of biological samples in vitro, in vivo, and ex vivo [110,111]. This is a technique of choice for small animals, imaging inflammation and tumor areas, the pharmacokinetics monitoring of drug distribution, and anti-tumor outcomes along with treatment time (Table 3, Table 4 and Table 5).
Even to activate singlet oxygen production (PDT) or a temperature increase (PTT) at the desired site, a simultaneous image-guided study is needed to determine the most suitable time of highest accumulation in the target tissue to perform illumination. Subsequently, upon irradiation with a laser, photothermal or photodynamic effects may be achieved more precisely (Figure 4). Furthermore, small tumors and sentinel lymph nodes can be detected by endoscopy and intraoperative imaging [41,112].
4.3. Photodynamic Therapy (PDT)
Photodynamic therapy relies on the use of a light-activatable photosensitizer, non-toxic, which accumulates in tissues and cells. The irradiation with an appropriate wavelength of light mediates a photochemistry reaction with molecular oxygen generating reactive molecular species inducing cell toxicity. Photodynamic therapy (PDT) kills cells by converting oxygen in the cell environment into reactive singlet oxygen, such as hydroxyl radical (OH•), singlet oxygen (1O2), and superoxide radical (O2•−), which are produced by the reaction of the excited photosensitizer and the molecular oxygen (O2). The preferential accumulation is not universal, and depending on the photosensitizer and site of administration, the ratio of the target tissue to normal tissue may be reversed in favor of normal tissue. To overcome this condition, fine-tuning light energy and the right illumination time can provide a pathway to improve selectivity [113]. IR780 has the advantage of accumulation in tumor cells, particularly in mitochondria. PDT occurs with IR780 administration and laser irradiation after a specific time post-administration. After the intravenous administration of formulations containing the photosensitizer, it is biodistributed, and the light is precisely applied to the site of interest. This irradiation causes the photosensitizer molecule to transfer energy to the oxygen present in the tumor environment, leading to the formation of singlet oxygen (1O2), which is cytotoxic and leads to cell death at the site and surrounding areas, generating molecular damage in proteins, lipids, and DNA [114]. This mechanism is shown in Figure 3 and Figure 4. The generation of reactive oxygen species (ROS) in the target tissue causes damage to tumor cells and blood vessels, triggering cell death and photodynamic priming of tumor microenvironment. However, pre-existing hypoxia in the cell or tissue environment and PDT-mediated oxygen consumption reduce PDT efficacy. To best overcome PDT, an oxygen supply is needed during this process. PDT can produce necrotic, apoptotic, and other pathways of cell death. Necrotic-mediated cell death is preferentially promoted if there is severe damage to the cell membrane disrupting cell structure. Damage to cell organelles generate a cascade of cell events that start preferentially apoptosis-mediated pathways. In addition, the activation of innate and adaptive immunity may also occur, and necrosis may stimulate non-specific immune reactions, thus contributing to an antitumor immune response [108,113].
For effective PDT, sufficient oxygen must be available in the tumor environment. The generation of ROS by IR780 with albumin nanoparticles after 808 nm laser irradiation in human prostate tumor cells has been demonstrated [115]. It is known that the tumor microenvironment (TME) is often marked by hypoxia, especially in solid tumors. Therefore, many studies have sought to insert, together with IR780 and the nanocarrier, an active molecule that increases the amount of oxygen available in the tumor microenvironment to intensify the production of ROS after laser irradiation, acting synergistically as oxygen suppliers. For this, an oxygen delivery system, or in situ oxygen generator systems, were proposed, such as delivering O2 by perfluorocarbon molecules [116], generation of oxygen by hydrogen peroxide (H2O2), and using MnO2 [117]. Another interesting approach is provided by lipid nanodroplets with the oxygen self-enriching properties of perfluorohexane (PFH) and IR780 co-loaded (Table 2). PFH has a high capacity for oxygen solubilization and synergistically increases singlet oxygen quantum yield, improving the ability of IR780 to inhibit tumor growth by PDT [44]
4.4. Photothermal Therapy (PTT)
When a photothermal agent absorbs light of a specific wavelength, it is excited and dissipates the absorbed energy in the form of heat, increasing local temperature. Generally, a non-toxic photothermal agent accumulates within tumor tissue after intravenous administration, and after irradiation by NIR light, the PTT effect induces tumor cell death (Figure 4). In this way, it can cause irreversible cellular damage through mild hyperthermia, sub-coagulative and coagulative processes, and thermal ablation [108] (Figure 3). PTT has a more straightforward mechanism of action than PDT and does not depend on oxygen to have a cytotoxic effect on tumor cells [118]. Thus, even if the molecule exhibited a low-fluorescence quantum yield and low efficiency in the production of ROS after laser irradiation, it may still be capable of generating hyperthermia [108]. Wang and collaborators studied the effect of IR780 in simultaneous PDT/PTT since they have a synergistic effect. The incidence of the laser leads to the photothermal effect (PTT), in which the increase in temperature can cause irreversible damage to cells, resulting in necrosis [32,119]. The mechanism of cell death depends on the conditions in which PTT is carried out, and one of the main challenges is to reduce disruptive pathways of death such as necrosis. The temperature variation is frequently measured with thermal cameras, and this increase varies mainly with the IR780 type of nanocarrier and its ability to absorb light, as well as with the other associated molecules. The main parameters that determine the efficacy of PTT include the surface features of the nanoparticles, morphology, size, and NIR light characteristics, such as wavelength and power density.
4.5. Immunogenic Cell Death (ICD)
Moreover, it was demonstrated that IR780-induced PTT can cause immunogenic cell death (ICD) and release tumor-associated antigens (TAAs) in vivo. ICD is a specific form of cancer cell death induced by certain chemotherapeutic agents that can enhance antitumor T-cell responses. During ICD, tumor cells upregulate calreticulin (CRT) on their surfaces, signaling dendritic cells (DCs) to engulf tumor cell debris and present tumor-associated antigens (TAAs). Additionally, the release of ATP and HMGB1 by ICD induces tumor cell antigens to activate dendritic cells (DCs) and triggers antigen-specific T-cell responses. While ICD-inducing agents can kill cancer cells and promote antitumor responses, their effectiveness depends on tumor targeting, TAA exposure, and the immune response level. However, conventional ICD inducers face limitations due to weak ICD induction and poor tumor targeting [113,119,120].
4.6. Sonodynamic Therapy (SDT)
Sonodynamic therapy (SDT), a promising alternative to cancer therapy, utilizes a sonosensitizer molecule combined with ultrasound (US) irradiation to damage tumor cells/tissues, designed for therapeutic purposes. This association may improve contrast-enhanced ultrasound imaging. The ability of sonosensitizers to specifically accumulate in tumor cells/tissues could greatly influence their therapeutic efficiency. SDT is an emerging technique involving low-intensity ultrasound (US) to activate photosensitive compounds. This activation enhances their ability to permeate tissues and target and destroy cancer cells, showing potential as an innovative cancer treatment approach. However, the nonselective enrichment and unsatisfactory penetration depth of sonosensitizers in tumor tissues limit their application [72].
IR780 has also been investigated as a sonosensitizer in different studies, as shown in Table 2, Table 3, Table 4 and Table 5 [49,67,88,93,94,121]. Zhang and co-workers (2019) reported a strategy used to increase selectivity using SDT [50]. Liposomal nanodroplets containing IR780 were activated by ultrasound, and the acoustic droplet vaporization effect increased the conveyance of the nanocarrier from the circulatory system to tumor regions and increased the penetration depth within tumor tissues (Table 2). In this context, TAT-IR780 was associated with a nanocarrier, which enhances the properties of the sonosensitizer IR780, facilitating its cellular internalization, more efficient distribution, and increased therapeutic activity [122].
In another approach, a nanotherapeutic platform of an iron tetroxide core loading sound-sensitive IR780 was coated with cetuximab (CTX) to target epidermal growth factor (EGFR) sensitive to inhibiting non-small-cell lung cancer (NSCLC) proliferation and differentiation (Table 4). Using IR780 fluorescence imaging, the biodistribution of IR780@INPs-CTX indicates the accumulation in the tumor and a small amount in the liver. MRI displays fast enrichment into the tumor tissue, and after ultrasound irradiation, the complete disappearance of the tumoral tissue or a continued decrease in tumor volume was observed. Thus, a successful nanoplatform for dual-mode imaging diagnosis combining targeting and sonodynamic therapy was achieved to reshape TME [97].
4.7. Photoacoustic Imaging (PAI)
Among fluorescent markers, some have the ability to improve photoacoustic images. PAI is a noninvasive imaging modality for the accurate diagnosis of the cardiovascular system. It is a useful technique to detect thrombi with excellent spatial resolution and high optical contrast [85]. IR780 associated with nanocarriers has demonstrated the ability to produce precise photoacoustic images in different studies [41,43,50,53,70,72,73,74,75,85,93]. Liposomes encapsulating doxorubicin [93], metformin [53], tirapazamine [49], and glucose oxidase [72] demonstrated increased efficacy in tumor growth inhibition and image precision. IR780-loaded and antitumoral platinum derivatives complexed with cyclodextrin resulted in photoacoustic images and high efficacy (Table 2, Table 3, Table 4 and Table 5).
4.8. Chemodynamic Therapy (CDT)
Chemodynamic therapy (CDT) is used in tumor-specific therapy and consists of disrupting the redox balance of cancer cells, reducing antioxidant capacity and increasing tumor sensitivity to conventional chemotherapeutic drugs. In general, it is a combination of conventional drugs used in chemotherapy with redox-active agents. Among nanocarriers with associated IR780 and antitumoral drugs, many examples can be found, such as doxorubicin [58,59,60,61,62,63,64,88,93,123,124], paclitaxel [57,69,82], camptothecin [65,84], sorafenib [83] (Table 2, Table 3, Table 4 and Table 5). This effect is generally obtained with transition metal ions that can react with hydrogen peroxide in the tumor microenvironment (TME) via Fenton-like reactions, creating toxic hydroxyl radicals that induce apoptosis in tumor cells. Furthermore, CDT may elicit immunogenic cell death (ICD) for the activation of the antitumor immune response because ICD can trigger the release of damage-associated molecular patterns (DAMPs) to maturate dendritic cells (DCs). This subsequently activates cytotoxic T lymphocytes. In this sense, a combination of conventional cancer chemotherapy and CDT may enhance ICD, boosting the antitumor response to tumors. In Table 2, Table 3, Table 4 and Table 5, some examples of this strategy are shown.
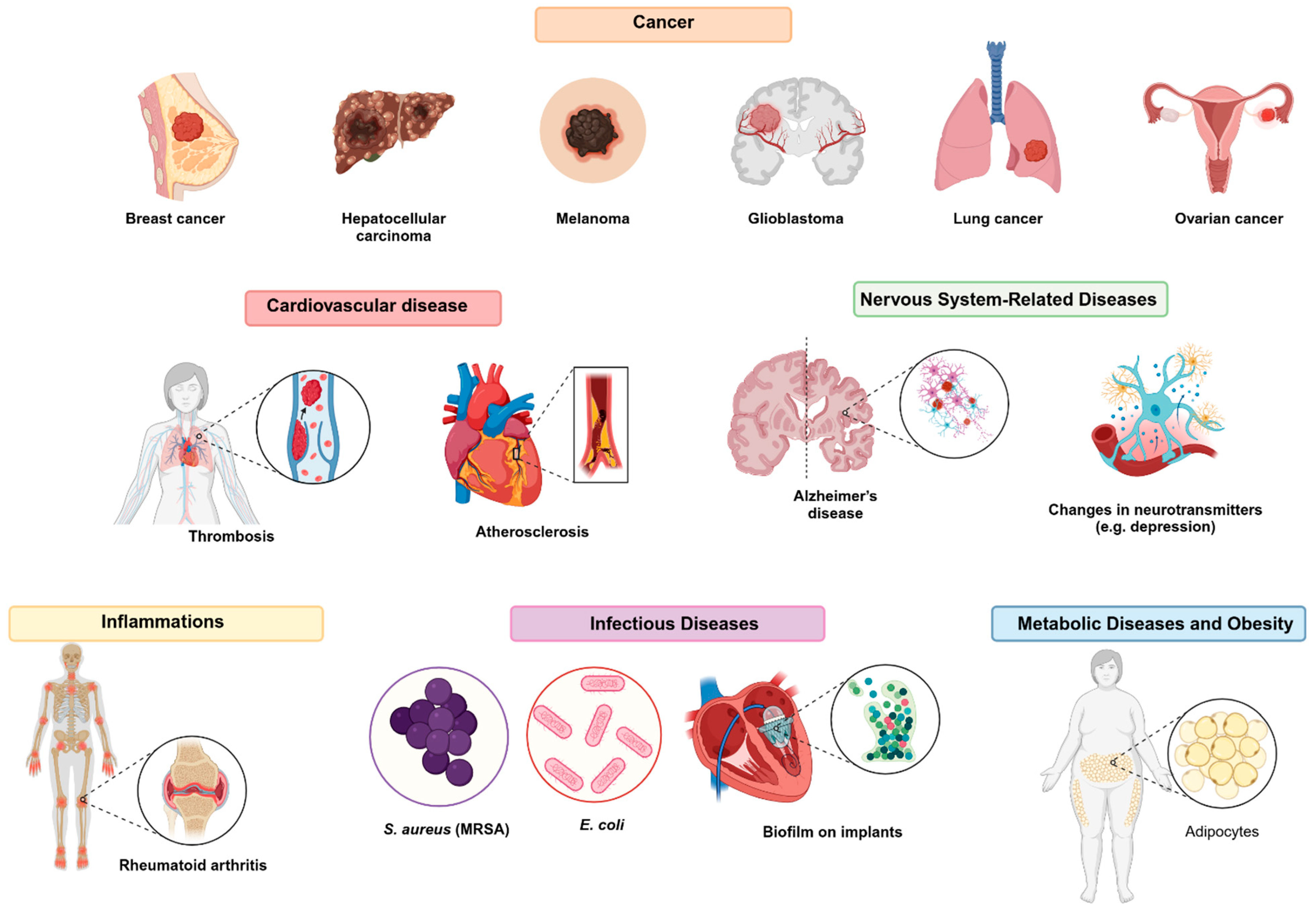
5. Biomedical Applications
IR780 has been extensively studied as an imaging probe and a photosensitizer for biomedical applications. It stands out in photodynamic therapy (PDT) and photothermal therapy (PTT), particularly in combination with nanostructures designed for cancer treatment. Moreover, recent preclinical research has explored its use to treat or diagnose various clinical conditions, expanding the scope of its applications. The following sections detail the experimental treatment of different diseases and applications in image monitoring in which IR780 has been employed, with an emphasis on its role in advanced imaging and therapies. The main applications are summarized in Figure 5.
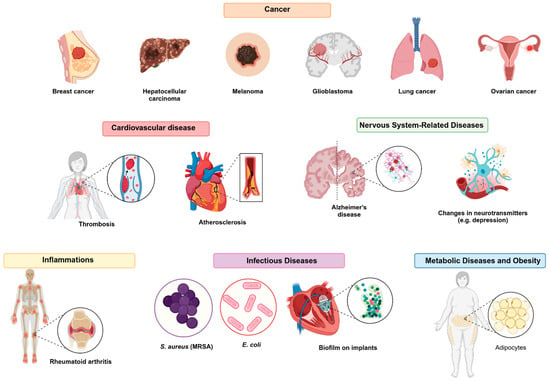
Figure 5.
Summary of the main applications of IR780-based nanocarriers in the diagnosis and treatment of different diseases. Created in BioRender. De Oliveira, M. (2025) https://BioRender.com/l24x664 (Accessed on 5 March 2025).
5.1. Imaging Probe
The fluorophore IR780 with emission in the NIR spectrum (750–900 nm) has emerged as a versatile agent for in vivo imaging applications. This allows the deeper tissue penetration of light and reduced interference from biological autofluorescence. These characteristics make it ideal for non-invasive imaging in preclinical studies, particularly for tumor visualization, lymph node mapping, and the monitoring of nanostructure biodistribution. IR780 has been successfully employed as an imaging agent for fluorescence-guided surgery, enabling precise tumor resection by clearly delineating tumor margins [41]. Additionally, its application in photoacoustic imaging (PAI) has further enhanced its utility by providing high-resolution images through sound wave generation upon laser excitation. This dual functionality ensures precise tumor localization and can be essential for improving therapeutic interventions.
When incorporated into nanostructured delivery systems, IR780 enhances stability and apparent solubility/dispersibility in aqueous media. These nanostructured formulations can enhance their fluorescence quantum yield [16]. Nanocarriers, such as liposomes, polymeric micelles, and albumin nanoparticles labeled with IR780, have demonstrated increased tumor accumulation and prolonged retention at the target site (Table 2, Table 3, Table 4 and Table 5). The fluorescent properties of IR780-loaded nanoparticles facilitate the real-time imaging of nanocarrier biodistribution and tumor microenvironment interactions. Moreover, combining IR780 with diagnostic agents like radionuclides or other imaging probes has further amplified its potential for multimodal imaging applications.
5.2. Cancer
The therapeutic applications of IR780 as an antitumor agent are highly diverse and significant. Its photophysical properties facilitate its use in photodynamic therapy (PDT) [42,54,121,125,126], photothermal therapy (PTT) [41,55,56,61,63,99], sonodynamic therapy (SDT) and photoacoustic imaging (PAI) [42,49,75,115,116]. When exposed to NIR light, IR780 generates reactive oxygen species (ROS) for PDT or converts absorbed light into localized heat for PTT, leading to tumor cell death [41,124,127,128]. This dual modality provides a powerful and targeted approach to cancer treatment, minimizing damage to surrounding healthy tissues. As a single-agent therapy, IR780 has demonstrated impressive efficacy in preclinical animal models as can be observed in outcomes summarized in Table 2, Table 3, Table 4 and Table 5 corresponding to lipid-based nanocarriers, polymer-based nanocarriers, hybrid nanostructures, and IR780 covalently linked to nanocarriers, respectively. Studies highlighted IR780’s ability to accumulate selectively in tumor mitochondria, which improves phototherapeutic effectiveness. NIR laser irradiation achieves significant tumor ablation through ROS-mediated apoptosis and hyperthermia-induced necrosis [53,125,129,130]. The ability of IR780 to target hypoxic tumor regions, a common feature of solid and aggressive cancers, further enhances its therapeutic relevance [46,47,69,131]. Studies demonstrate that IR780 preferentially accumulates in tumor tissues due to its lipophilicity and high affinity for organic-anion-transporting polypeptide (OATP), which are overexpressed in cancer cells [103]. IR780 has been shown to exert synergistic effects in combination with chemotherapeutic agents. The co-encapsulation of IR780 with drugs, like doxorubicin, paclitaxel, and cisplatin, among others, in nanoparticles has improved drug delivery efficiency, enhanced tumor penetration, and provided controlled release profiles. The synergistic action of PTT/PDT with chemotherapy amplifies antitumor efficacy, as demonstrated by increased tumor inhibition rates and reduced systemic toxicity [35,99,126,132]. For instance, IR780-loaded nanoparticles co-delivering doxorubicin have significantly accumulated within the tumor by the enhanced permeability and retention (EPR) effect, improving both the photothermal and chemotherapeutic responses [37,93,99,126,132], as exemplified in Figure 6.
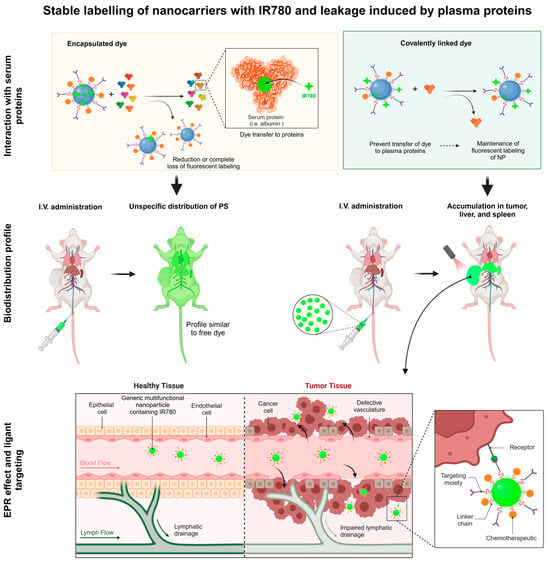
Figure 6.
Schematic representation of the different passive and active strategies to deliver IR780 to tumoral sites and the effect of leakage of the dye mediated by nanocarrier exposure to serum proteins acting as IR780 acceptors. IR780 covalently linked to the nanocarrier structure provides more stable labeling and reliable tracking of biodistribution of nanocarrier inside the body. Created in BioRender. De oliveira, M. (2025) https://BioRender.com/g55v926 (Accessed on 5 March 2025).
The versatility of IR780 extends to its integration with immune modulation strategies. Studies indicate that IR780-mediated PTT or PDT can trigger immunogenic cell death (ICD), releasing tumor-associated antigens that enhance antitumor immunity [60,72,127,130,133]. In combination with immune checkpoint inhibitors or cytokine therapies, IR780-based formulations have shown promise in converting “cold” tumors into “hot” ones, thereby sensitizing them to immune responses [130,134]. This ability to integrate phototherapeutic and immunotherapeutic approaches underscores the transformative potential of IR780 in modern cancer treatment.
The abnormal physiology of solid tumors is a barrier to anticancer drug delivery, and the development of effective therapeutic strategies for cancer treatment remain highly challenging (Figure 6). Tumor masses are three-dimensional (3D). Furthermore, IR780 interacts with light in the near-infrared (NIR) region, triggering the production of reactive oxygen species (ROS) [109]. These ROS selectively damage biomolecules in the tumor region, induce localized cytotoxicity, and enhance therapeutic efficacy, as previously reported. Altogether, these features position IR780 as a promising tool for both phototherapy and diagnostic applications [55,56,124]. The recent literature highlights a wide range of applications for IR780 as theranostics of various types of cancer, including breast cancer [20,33,127,128,129,131,135], hepatocellular carcinoma [136,137,138,139], glioblastoma [140,141], lung cancer [65,142], ovarian cancer, melanoma, and many others (Figure 5).
Polymeric micelles loaded with IR780 and zinc and manganese sulfide (ZMS) nanoparticles in a thermosensitive amphiphilic copolymer (mPEG-b-PHEP) (PP IR780-ZMS) have been developed. This approach aimed to combine three therapeutic modalities, namely photothermal, chemodynamic, and immunological therapies, creating a versatile platform for treating melanoma and metastases related to disease progression [76]. The primary mechanism involving IR780 in this study is its ability to accumulate in the mitochondria of tumor cells, promoting photothermal ablation. Simultaneously, the ZMS nanoparticles generated hydroxyl radicals (HO•), enhancing the efficacy of chemodynamic therapy. The multifunctional platform proved effective, significantly increasing the survival rate of B16F10 tumor-bearing mice [76].
A responsive nanoplatform (NP-IR780) for theranostic applications in breast cancer treatment was developed [41]. The platform integrates advanced technologies, including multimodal imaging such as computed tomography (CT), photoacoustic imaging (PA), and near-infrared fluorescence (NIR-FL), as well as intraoperative photothermal therapy (PTT). The study demonstrated the efficiency of the application of IR780 as a theranostic agent, owing to its ability to target the drug directly to the tumor, serve as a contrast agent for simultaneous PA, CT, and NIR-FL imaging, and support preoperative planning for precise tumor removal. Furthermore, residual lesions that cannot be surgically removed have been effectively treated using PTT mediated by IR780-NPs, reducing the risk of tumor recurrence [41]. A nanotheranostic based on IR780-loaded N-acetyl glucosamine (NAG)-modified liposome, PEG-coated and 68Ga-labeled, was specifically designed to target the GLUT1 receptor, which is overexpressed in glioblastoma cells [38]. This system enabled dual-mode imaging (NIR fluorescence and PET/CT) and combined PTT and PDT for synergistic treatment. The results showed precision targeting of glioblastoma cells, enhancing PET/CT imaging and treatment efficiency [38].
Jia and co-workers (2024) incorporated IR780 into carrier-free NPs alongside berberine hydrochloride for hepatocellular carcinoma (HCC) treatment [87]. These nanoparticles combined PDT and PTT under laser irradiation, resulting in 96% tumor inhibition and effective fluorescence-guided imaging. The NPs targeted tumors via the enhanced permeability and retention (EPR) effect, enabling precise localization and enhanced therapeutic outcomes [87]. IR780 played a central role in camptothecin nanoparticles, combining chemotherapy with PTT and PDT in lung cancer models. The nanoparticles achieved a 2.5-fold increase in cytotoxicity over free drugs and demonstrated enhanced fluorescence-guided therapy, allowing precise tumor localization and ablation [63]. IR780 embedded in Asp8[H40-TPZ/IR780(RBC-H)] nanoparticles, which leveraged hybrid membranes and hypoxia-activated drugs for treating oral squamous cell carcinoma have been reported [91]. These nanoparticles combined PDT and PTT, achieving a 4-fold increase in cytotoxicity under NIR laser irradiation and significantly reducing tumor weight while preserving bone integrity. The system showed IR780’s ability to enhance targeting precision and improve outcomes for this particularly difficult oncologic treatment.
5.3. Cardiovascular Disease
The potential of IR780 in nanotheranostics has been demonstrated for cardiovascular conditions, particularly in addressing thrombosis and atherosclerosis [85,126,143]. Wang et al. (2021) developed self-assembled hybrid polymeric micelles (NPs IR780/FPHM/LK) functionalized with the FXIIIa peptide, aiming for increased selectivity toward FXIIIa-rich thrombi [85]. The designed system successfully carried IR780 and lumbrokinase (LK), a fibrinolytic agent. A potent thrombolytic effect was observed, while normal hemostasis was preserved in a carotid thrombosis model in mice (Table 3). IR780 was also employed as a near-infrared (NIR) imaging probe in the development of aspirin-polyconjugated particles targeted to thrombi (T-APP). This innovative system integrates H2O2-activatable aspirin and fibrin-specific peptides for precise targeting. IR780 proved indispensable for detailed thrombus visualization. Furthermore, T-APP particles exhibited rapid localization in fibrin-rich thrombi, promoting H2O2 elimination, reducing inflammatory cytokines (sCD40L, TNF-α), and demonstrating highly specific antithrombotic actions [143].
Furthermore, IR780 has been applied in treating atherosclerosis, targeting foam cells, and stabilizing plaques [126]. Nanomicelles with IR780 and gadolinium-based MRI contrast agents covalently bound to PEG for dual-mode imaging and phototherapy have been developed [126]. These micelles targeted foam cells in atherosclerotic plaques through osteopontin (OPN) ligands, achieving 2.5-fold-higher plaque accumulation and significantly reducing plaque size and lipid content. Furthermore, combining PTT and PDT improved plaque stability by increasing collagen content and reducing macrophage-driven inflammation. Polybutylcyanoacrylate nanoparticles (CDNPs) loaded with cyclodextrin and IR780 have been prepared to treat and diagnose atherosclerosis [144]. The studies demonstrated improved cellular uptake and a significant reduction in the size of atherosclerotic plaques in ApoE−/− mice fed a high-fat diet. The photosensitizer IR780 could provide multimodal imaging (ultrasound and near-infrared), enabling the precise diagnosis and treatment monitoring of atherosclerotic plates [145]. CDNPs proved to be a promising platform for nanotheranostics in cholesterol-related conditions [144]
5.4. Nervous System-Related Diseases
Wang et al. (2024) developed a multifunctional nanoparticle aimed at treating Alzheimer’s disease [146]. The main goal was to inhibit β-amyloid (Aβ) aggregation and reduce oxidative stress caused by reactive oxygen species (ROS). The designed nanoparticle synergistically combines the antioxidant action of curcumin, the ROS-eliminating capacity of cerium oxide nanoparticles (CeO2), and a near-infrared (NIR) laser-activated controlled release system. When activated by an 808 nm laser, IR780 facilitates the targeted release of curcumin in the brain, enhancing its antioxidant effect and ability to prevent Aβ aggregation. Moreover, IR780 significantly increases the efficiency of curcumin transport and release in the brain due to its affinity for hydrophobic environments. In studies conducted in mice, the nanocarrier showed promising results, reducing Aβ deposits, alleviating oxidative stress, and demonstrating potential for treating Alzheimer’s disease in its early stages [146]. Zhang et al. developed ultrasound-responsive nanoparticles (AUT NPs) loaded with astaxanthin (ATX) and IR780, encapsulated within a polydopamine (PDA) layer [147]. The main objective was to enhance the stability, bioavailability, and therapeutic efficacy of ATX in treating subarachnoid hemorrhage (SAH), targeting brain-specific sites with drug release. IR780 enabled real-time monitoring of the NP’s distribution into the brain. The AUT NPs protected ATX from degradation, released their contents under ultrasound stimuli, crossed the blood–brain barrier, and exhibited excellent antioxidant and anti-apoptotic effects in vivo, with high biosafety [147].
Chang et al. developed nanoparticles composed of artificial synaptic vesicles (ASVs) responsive to NIR light, loading IR780 and functionalized with 5-hydroxytryptophan (5-HTP) aptamer (H&I@ASV-A), aiming to treat depression [54]. The system employs photothermal therapy to enhance the permeability of the blood–brain barrier (BBB), enabling the targeted release of 5-HTP in the brain. High efficiency in BBB penetration and rapid antidepressant action were observed in mice subjected to the chronic unpredictable stress (CUMS) model. Treatment with H&I@ASV-A reduced depressive behaviors in just 4 weeks, overcoming the limitations of conventional treatments [54].
5.5. Inflammations
A responsive multifunctional nanoplatform aimed at enabling the early diagnosis of rheumatoid arthritis has been developed. This platform integrates active targeting functions, activatable magnetic resonance imaging (MRI), and near-infrared (NIR) fluorescent imaging, overcoming the limitations associated with the low selectivity of conventional techniques. IR780 was used as a fluorophore, capable of being activated in mildly acidic environments related to rheumatoid arthritis. The nanoplatform demonstrated significant potential for achieving sensitive molecular detection, enabling the acquisition of NIR images that provide molecular insights into the inflammatory environment characteristic of rheumatoid arthritis [148].
5.6. Infectious Diseases
Polymeric nanoparticles containing IR780 for photothermal therapy (PTT) have been designed to treat bacterial infections, particularly against methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli. IR780 was loaded in an N-octyl-D-gluconamide (GA) polymer, forming nanoparticles (IR780-GA NPs) with a photothermal effect. These nanoparticles demonstrated high efficacy in inactivating MRSA, primarily attributed to IR780’s ability to act as a photothermal agent, converting light energy into heat to promote bacterial destruction [149].
Furthermore, IR780 has been investigated as an antimicrobial agent without association with nanocarriers. A study investigated the potential of IR780 as a photosensitizer capable of generating singlet oxygen (1O2) in combination with red phosphorus (RP) and RGDC peptides to eliminate biofilms on bone implants using photothermal and photodynamic therapy with near-infrared light (808 nm). IR780, as a photosensitizer, generated singlet oxygen (1O2), increasing bacterial membrane permeability and sensitizing Staphylococcus aureus biofilms to heat. These infections pose a significant clinical challenge due to the resistance of bacterial biofilms to conventional treatments. The proposed strategy demonstrated a promising approach for eradicating biofilms on bone implants, achieving an approximately 96% eradication of S. aureus biofilms without damaging surrounding tissues while promoting cell adhesion, proliferation, and osteogenic differentiation [150].
Albumin nanoparticles loaded with tanshinone IIA and IR780 were designed to improve antimicrobial effects on wound healing [5]. The PTT properties of IR780 exposed to NIR radiation in vivo significantly improve wound closure rates, better manage chronic and infected wounds, and promote tissue repair and infection control.
5.7. Metabolic Diseases and Obesity
An innovative therapeutic approach for the treatment of diet-induced obesity combines localized photothermal therapy (PTT) with the administration of rosiglitazone (RSG) using cationic albumin nanoparticles (cNPs). These nanoparticles were designed to specifically target RSG to white adipocytes, inducing browning and promoting increased energy expenditure. A thermosensitive hydrogel loaded with IR780 was employed to create a subcutaneous reservoir that sustains the controlled release of cNPs and enables localized PTT application. The cNPs significantly enhanced internalization efficiency in adipocytes, prolonged therapeutic retention in adipose tissue, and effectively reduced fat accumulation [151].
6. Strategies for Improvement of IR780 as a Biological Tracer in Nanocarrier
As previously discussed, IR780, despite being a promising near-infrared photosensitizer for biomedical applications, faces challenges that limit its clinical efficacy, including low solubility, poor stability upon light exposure, aggregation, potential toxic effects at high doses, and, more importantly, the precocious leakage of the IR780 from the nanocarrier [16]. In this sense, different approaches have been developed to overcome these limitations.
6.1. IR780 Chemical Conjugation
The typical bottleneck in NIRF monitoring and PDT/PTT/CDT is the premature release of the NIR/photosensitizer from the nanocarrier (Figure 6). This causes many issues and erroneous interpretations of data analysis because of the dissemination of photosensitizers in the whole body with undesired off-target effects. One of the main concerns about using lipophilic probes and photosensitizers is their ability to accumulate or to transpose cell membrane in its free form and distribute inside the cell following the probe’s physicochemical characteristics. If the nanocarrier is not able to retain the dye inside the structure of the carrier, the analyst will track the free dye and not the nanocarrier. This fact may induce enormous bias in the biological tracking of nanocarriers, reducing the reliability of the study conducted in vitro (cellular localization and trafficking) or in vivo (wrong biodistribution data) using NIRF images. To circumvent this issue, strong interaction with the carrier is essential; in most cases, a covalent bond is required with nanocarrier components that are not prone to destabilization and leakage during the journey inside the body. Some of these approaches concerning IR780 covalently linked to nanocarrier structure are summarized in Table 5. Thus, polymers or lipids conjugated with IR780 are more suitable to produce reliable biological data. Examples of this type of study are available in the literature (Table 5).
One of them compared free IR780 with IR780 conjugated with PLA-polymer used in PEG-PLA in vitro nanocapsules (IR-PLA NC) and with IR780 physically loaded inside these nanocarriers (IR780-NC), and compared the main outcomes [14,15,16]. To improve the stability of IR780 in nanocarriers, De Oliveira et al. (2019) developed an innovative approach by chemically coupling IR780 to polylactide (PLA), through a “click chemistry” reaction. The researchers synthesized a conjugate IR780-PLA [15]. This covalent modification was designed to ensure a more stable association between the fluorophore and the nanocarrier, whether in the form of nanocapsules or polymeric nanospheres. The stability of the conjugate IR780-PLA (IR-PLA) was evaluated using a fractionation analysis in an asymmetric flow field in the presence of biological medium and serum proteins [16]. The results showed that nanospheres containing IR-PLA maintained 96% of their fluorescence after exposure to serum proteins. In contrast, nanospheres containing only IR780 physically associated (loaded) (NS-IR780) showed fluorescence levels reduced to 30%, suggesting that the free IR780 interacts with proteins. The proteins and serum components act as dye acceptors in the biological environment and promote dye/PS dissociation from the nanocarrier before interacting with the target cell [16]. This process is very fast and compromises nanocarrier tracking using different lipophilic dyes [16]. IR780 conjugated to PLA minimized the transfer of fluorescence to serum proteins, maintaining a stable association with the nanocarrier even in the complexity of the biological media [15]. The fast release of IR780 from nanocarriers is a critical limitation for their application in nanotheranostics. This issue considerably reduces its application in image monitoring and may also reduce the targeting ability and therapeutic efficacy. Its hydrophobic nature causes early dissociation from nanocarriers to lipoproteins and proteins of plasma in biological environments, impairing the targeting of tumor regions [14,16] (Figure 6).
The above-mentioned studies gave insights into the dramatic differences in distribution inside cells and inside the animal body, and the ability of IR780 to interact with serum proteins. The covalent bond between IR780 and different types of macromolecules and biomolecules can improve the studies of the biodistribution of nanocarriers, and these examples are summarized in Table 5. Furthermore, depending on the types of chemical bonds with IR780 molecules, the red shift may be achieved, as well as improved photodynamic and photothermal properties. In some cases, IR780 conjugation can reduce the fluorescence quantum yield due to the electronic shift caused [16].

Table 5.
Types of nanostructures containing IR780 conjugated (covalently linked) to nanocarrier used as nanotheranostics and main outcomes in vitro and in vivo.
Table 5.
Types of nanostructures containing IR780 conjugated (covalently linked) to nanocarrier used as nanotheranostics and main outcomes in vitro and in vivo.
| Nanostructure | API Co-Loaded | Targeting Approach | Application/ Approach | In Vitro/In Vivo Main Outcomes | Ref. |
|---|---|---|---|---|---|
| PEG-PLA nanocapsules and nanospheres IR780-conjugated | IR-PLA (IR780 conjugated to PLA polymer) | No | PDT | Improved uptake, cell death, and reduced migration in human breast cancer cells (MDA-MB231 and MCF-7) and reduced cytotoxicity in normal breast cells (MCF-10A). Conjugated IR780-PLA improved tumor distribution. | [14,15] |
| Thermos-sensitive lipid nanostructures | Tirapazamine (TPZ) | Fatty acids that undergo a solid–liquid phase transition at 39 °C; IR780 as mitochondria targeting | PDT, PTT, PCT, FI, PAI, image guidance | Anti-tumor efficacy under PAI and FL imaging guidance and monitoring; improved anti-tumor effectiveness. | [53] |
| PLGA core/shell nanoparticle with hematoporphyrin monomethyl ether | Glucose oxidase IR780 in the shell | Hematoporphyrin monomethyl ether core | SDT Starvation therapy PAI FL | Accumulation in cancer cells/sites; mitochondrial targeting for synergistic SDT and starvation therapy; improved outcome in treatment (4.7-fold lower tumor growth); excellent photoacoustic (PA)/fluorescent (FL) imaging contrast agents to simultaneously monitor and guide cancer therapy. | [72] |
| PLGA nanoparticles with Lithocholic acid/IR780 nanoconjugate | Lithocholic acid and IR780 iodide | Lithocholic acid (LA)/IR780 conjugate | PDT, PTT, SDT, PAI | Accumulation in breast cancer cell lines in a time-dependent manner, a synergistic anticancer effect in the presence of the NIR light compared to the free conjugate; NIR light-activated ROS mediated apoptosis; phantom experiments revealed a significant photoacoustic signal intensity in the novel bioconjugate. | [121] |
| Solid lipid nanoparticles (SLN) with biomimetic osteosarcoma membrane | Doxorubicin, IR780-conjugated (DSPE-PEG- IR780) | Osteosarcoma membrane, mitochondria targeting with IR780-conjugated PEG-lipid | PDT, CDT | SLN accumulated in subcutaneous murine osteosarcoma (K7M2) in male BABL/c mice model; achieved intracellular drug release upon NIR stimulation in mitochondria; triggered apoptosis pathway; increased expression of cytochrome C, Bax, C-Caspase 3, Caspase 9, and decreased expression of Bcl-2; exhibited photo-cytotoxicity under NIR laser irradiation, 14-fold-increased suppression of cell migration. | [123] |
| TAT peptide-conjugated IR780 nanoparticle | Doxorubicin | TAT peptide-conjugated IR780 | PDT, PTT, CDT | Improved cellular internalization and nuclear localization; achieved significant synergistic effects on breast tumor ablation and recurrence after laser irradiation. | [124] |
| Natural protein ferritin (FRT) and nanoscale graphene oxide (NGO) as dual-carriers | Resveratrol | IR780-conjugated for mitochondrion targeted | PTT, PDT, CDT | Synergistic photothermal chemotherapy induces tumor suppression in ovarian cancer models, causes cell apoptosis, and increases animal survival. | [125] |
| IR780-Gd-OPN polymeric micelles | Osteopontin (OPN) conjugated IR780-PEG-OPN | IR780-PEG-Gd-DOTA IR780-conjugated | PTT, PDT and dual-mode imaging NIR-FI, MRI, macrophage modulation | Dual-mode imaging enabled precise localization of atherosclerotic plaques; strong signals in NIRFI and T1-weighted MRI; IR780-Gd-OPN micelles exhibited 2.5-fold-higher uptake by foam cells (RAW 264.7-derived treated with oxidized LDL); 4-times-reduced foam cell lipid content; 2.3-fold upregulated HSP27 protein, protecting from apoptosis and reducing inflammation; IR780-Gd-OPN showed 3-fold-greater accumulation in aortic plaques compared to IR780-Gd; decreased by ~50%, lipid content in plates. | [126] |
| Lipid nanoparticles | IR-780-oleyl (lipophilic derivative) | Cyclic RGD peptides | PDT, PTT, FI, biodistribution | In vitro targeting of the receptor with cRGD-LNPs in HEK293(β3), HEK293(β3)-αvRFP, DU145, and PC3 cell lines; cRGD-LNPs bind to αvβ3, interfere with cell adhesion to vitronectin and co-internalize with αvβ3; biodistribution and tumor targeting in mice bearing DU145 or M21 tumors indicate no difference in accumulation/retention in tumors. | [127] |
| Hyaluronic acid-IR780-conjugated self-assembled nanoparticles | - | Hyaluronic acid-conjugated with IR780 | PTT, PDT | Bladder cancer cells (MB-49) overexpressing CD44 in an orthotopic mouse model treated with NPs showed high tumor selectivity, efficacy, good bioavailability, and biocompatibility, and reduced tumor growth, preserving the bladder. | [128] |
| Cationic thermosensitive lipid nanoparticles | IR780-PEG-ODC (octadecyl alcohol) BMS202 (PD-1/PD-L1 inhibitors) | IR780-conjugated with PEG and octadecyl alcohol | PDT, PTT, FI, ICD | Orthotopic 4T1 tumors exhibited a pronounced PTT with induced hyperthermia (55.4 °C) with NIR-laser irradiation; higher fluorescence intensity and cell uptake efficiency against 4T1 tumor compared to 293T normal cells; reduced cancer-associated fibroblasts and remodeled the spatial distribution of TILs in TME; enhanced the antigen-presenting ability of DCs to activate cytotoxic T lymphocytes; tumor tissues showed the highest fluorescence intensity. | [131] |
| Micelles of PEG-IR-780-C13 Conjugated self-assembled polymer | PEG-IR-C13 | No | PDT, PTT | Tumors were ablated by combining PEG-IR-780-C13 micelles with 808 nm laser irradiation in Balb/c mice bearing CT26 (colon carcinoma) xenograft; no toxicity was observed after intravenous injection. | [152] |
| Self-assembled cyclodextrin functionalized with IR780 loading biotin-Pt(IV)-derivative | IR780 linked to cyclodextrin; biotin-labeled Pt(IV) prodrug derivative | IR780 as a ligand for mitochondria targeting | PTT, PDT, CDT, NIRF, PAI | Overcome cisplatin resistance and eliminate A549R tumors completely; increase the Pt accumulation, reduce GSH levels; avoid DNA repair machinery in cisplatin-resistant cancer cells (A549R); inhibit A549R tumor growth on animal models. | [153] |
PDT: photodynamic therapy; PTT: photothermal therapy; PAI: photoacoustic imaging; NIRF: near-infrared fluorescence; FI: fluorescence imaging; SDT: sonodynamic therapy; PLGA: Poly(lactide-co-glycolide) polymer; PLA: polylactide polymer; TME: Tumor microenvironment; DC: dendritic cells; ICD: immunogenic cell death.
Table 6 summarizes the main strategies developed to overcome IR780 limitations, such as the use of nanocarriers to increase the dispersibility of IR780 in the medium and to alter its bioavailability [6]; the integration of image monitoring and therapy in multimodal platforms; the development of hybrid systems that incorporate additional anticancer properties [117] for CDT; and the surface modifications to improve targeting [154]. Some of these strategies are also highlighted in the previously reviewed studies concerning IR780 and other photosensitizers [105,106].
For example, the insertion of a chlorine atom in the IR780 chemical structure makes this marker the only commercially available reactive cyanine that can be obtained on a large scale (Figure 2). It allows chemical modifications to be made to the structure of the IR780 for conjugation with other molecules. This is a convenient route for obtaining IR780-conjugated derivatives as raw materials to produce nanotheranostic [155]. Different modifications in the IR780 chemical structure were reported, as shown in Figure 2. The chlorine atom reactions in the IR780 molecule enabled the insertion of a PEG 2000 chain and a modified alkyl chain containing thirteen carbon atoms (C13) [152]. In addition to improving the dispersibility of the IR780, the authors obtained a polymeric derivative (PEG-IR780-C13) capable of self-organizing in micelles, which was used for photothermal therapy and in vivo imaging [152]. In another study, IR780 was conjugated with a block copolymer of polyethylene glycol and a methacrylate derivative of α-tocopheryl succinate (PEG-b-polyMTOS), aiming to obtain a polymeric system that had photothermal, photodynamic, and tumor-targeting properties. In this nanosystem, the IR780 is present on the surface of the nanoparticles, making it possible to encapsulate an additional amount of the marker [156]. IR780 has been conjugated to hyaluronic acid to obtain a self-organizing system applied in photothermal therapy in bladder cancer with the overexpression of CD44 (hyaluronic acid receptor) [128]. These studies demonstrated that covalent conjugations can be carried out with IR780 without compromising its photophysical properties as a photosensitizer in PDT/PTT. Additionally, poly(2-ethyl-2-oxazoline)–IR780 conjugate nanoparticles have been developed aimed at photothermal cancer therapy [138]. This system exhibited higher colloidal stability compared to free IR780, a critical aspect for biological applications. Additionally, the conjugate demonstrated biocompatibility and low cytotoxicity in healthy cells within the therapeutic dose range. The stabilization of IR780’s photophysical properties is essential to protect them from photodegradation, ensuring prolonged stability and sustained efficacy. Structures such as homodimers, which can self-assemble into nanoparticles, improve the photostability of IR780 and enhance reactive oxygen species (ROS) generation compared to free IR780 [157]. Furthermore, self-organized nanotubes derived from peptide conjugates with IR780 (PepIR) significantly reduce IR780’s self-quenching, improving its photodynamic and photothermal efficiency [158].

Table 6.
Summary of comprehensive strategies to optimize IR780 performance and enhance the biomedical applications of IR780.
Table 6.
Summary of comprehensive strategies to optimize IR780 performance and enhance the biomedical applications of IR780.
| Strategies | Description | Examples/Applications | Ref. |
|---|---|---|---|
| IR780 physical association with nanocarriers (loaded) (Table 2, Table 3 and Table 4) |
| Amphiphilic micelles loaded with IR780 controlled release in tumor microenvironment (TME). | [6] |
| PEG-PLA nanocapsules enhanced IR780 stability and promote its controlled release. | [37] | ||
| Greater photostability and reactive oxygen species (ROS) generation capacity compared to free IR780, attributed to assembly into homodimers. | [157] | ||
| The organization of IR780 photosensitizers into crystalline nanotubes significantly reduces molecular self-quenching, enhancing photodynamic and photothermal efficiency. | [158] | ||
| Targeting moieties conjugated with nanocarriers loading IR780 |
| cRGD peptides as ligands in polymeric micelles: solid lipid nanoparticles; lipid nanoparticles; nanoparticles. | [40,84,93,127] |
| Tumor-penetrating peptides (tLyP-1). | [96] | ||
| As1411 aptamer associated with different nanocarriers. | [9,42,98] | ||
| 5-HTP aptamer in lipid vesicles. | [54] | ||
| N-acetyl-glucosamine (NAG) linked to liposomes. | [38] | ||
| Folic acid conjugated with nanocarriers. | [64,80] | ||
| Transferrin conjugated with nanoparticles. | [32] | ||
| Hyaluronic acid as a ligand for active targeting of U87 cancer cells (IR780-rGOG-HA/DOX); hyaluronic acid ligand in micelles; and hyaluronic acid in polymeric nanoparticles. | [57,81,128,154] | ||
| IR780 covalent conjugation with biocompatible polymers |
| IR780 conjugated-PLA minimizes dye degradation and prevents leakage from nanospheres and nanocapsules, ensuring reliable NIR fluorescence for in vitro and in vivo imaging. | [15] |
| Poly(2-Ethyl-2-Oxazoline)–IR780 conjugate nanoparticles reduce photodegradation, and increase chemical stability and PDT efficiency in biological environments. | [138] | ||
| Prodrug of IR780 design for pH and temperature sensitivity |
| Acidic TME-activated nanoparticle release actives. | [112] |
| NIR laser irradiation induces thermosensitive lipid nanoparticles with responsive and controlled release of IR780 and other active ingredients. | [131] | ||
| Hybrid systems: activation of hyperthermia, pyroptosis, apoptosis Multi-modal theranostic platforms |
| IR780 nanoplatforms—enabling PDT, PTT, and CDT therapeutic synergy through pyroptosis, ferroptosis, and hyperthermia. | [159] |
| MnO2/IR780 nanospheres—improves photothermal properties combined with MnO2 catalytic activity as multifunctional nanozyme, and enhances the sensitivity and specificity of exosome detection. | [117] | ||
| Iron nanoparticles—combines imaging diagnostics (IR780 fluorescence + magnetic resonance imaging) plus SDT-lung cancer treatment. | [88,89] | ||
| NIR-responsive nanoplatforms—integrate PTT, PDT, mitochondrial pathway-mediated apoptosis for laryngeal cancer treatment. | [160] | ||
| Genomic or proteomic approaches |
| Micelles inside microneedles platform: Integrates CRISPR/Cas9 with the IR780 photosensitizer for localized and efficient delivery with synergic effect; genomic editing of the FBXO44 gene by CRISPR/Cas9 inhibits tumor cell migration and invasion. | [136] |
| Encapsulating silencing RNA. | [92] | ||
| TAT-IR780—Targeted photothermal and photodynamic therapy directed at the nucleus directly destroys genetic material (DNA/RNA), disrupting replication and transcription in cancer cells and inducing apoptosis. | [124] |
6.2. Targeting Moieties at Nanocarrier Surface
Some photosensitizers have inherent selectivity toward tumor cells, such as IR780, and light irradiation of target sites post-administration enhances the control of the PDT response. Cell and tissue selectivity can be improved by targeting moieties linked to nanocarrier surfaces, which carry bioactive payloads, leading to accumulation within target cells. Surface modifiers with suitable ligands have been demonstrated to enhance the selectivity and the potential of IR780 in therapeutics, enabling active and specific targeting of biological sites (Table 6). Different tumor markers have been exploited as receptors, including growth factors (VEGFRs, EGFR), transferrin receptors (TfRs), folate receptors, low-density lipoprotein (LDL) receptors, CD44 receptors, glucose transporters, and integrin receptors, as one of the strategies to improve IR780 effects shown in Table 6. Furthermore, aptamers, monoclonal antibodies, and hyaluronic acid-decorated nanocarriers have also been used as ligands.
The use of targeting moieties covalently linked to nanocarriers not only increases therapeutic efficacy but also significantly reduces side effects, making treatments safer and more precise. For example, Tar-scuPA-IR-L immunoliposomes were designed with antibodies as targeting agents, which bind to specific receptors present in thrombi. This strategy allows IR780 to be delivered directly to activated platelets, promoting a localized photothermal effect and minimizing the exposure of healthy tissues [145]. Similarly, the reduced graphene oxide-based system IR780-rGO-HA/DOX was developed with hyaluronic acid (HA) as a surface modifier, targeting cancer cells such as U87 glioblastoma cells. HA, known for its high affinity for CD44 receptors, often overexpressed in tumors, ensures efficient targeting and increased intracellular uptake [154]. Some examples of ligands, such as aptamers, cell-penetrating peptides, HA, folic acid, and others, used to target specific cells in PDT/PTT treatments with IR780 are summarized in Table 6.
6.3. Combination with Other Antitumoral Agents
IR780 association with other antitumoral agents and other active molecules designed to be delivered in tumoral sites has been frequently reported, and in general, this strategy enhances chemodynamic therapy (CDT). For example, hybrid systems that integrate IR780 with other materials have demonstrated great potential to achieve synergistic effects in diagnosis and therapy. An example is the use of IR780 combined with sodium persulfate (Na2S2O8) in Na2S2O8-IR780 NPs, developed for colorectal cancer treatment. The integration of these agents into a single nanoparticle enhances the solubility of IR780 and the stability of Na2S2O8, while also enabling controlled and selective release in the tumor microenvironment. IR780, by inducing hyperthermia, accelerates the degradation of Na2S2O8, releasing sodium ions and sulfate radicals that cause lethal osmotic and oxidative alterations in tumor cells [159]. Multifunctional manganese dioxide/IR780 nanosheets (MnO2/IR780) combine the photothermal properties of IR780 with the catalytic activity of MnO2, creating a hybrid multifunctional nanoenzyme that improves the sensitivity and specificity of exosome detection [117]. In this same direction, mPEG-poly(2-hexoxy-2-oxo-1,3,2-dioxaphospholane) copolymer with a thermally sensitive core, loading IR780 and manganese zinc sulfide particles, form micelles that precisely controlled Mn2+ release upon NIR illumination. This nanocomposite evokes an immune response and protects male C57BL/6 mice from pulmonary metastasis of melanoma B16F10 cells [76].
6.4. pH- and Temperature-Sensitive Nanocarriers and Prodrugs
The design of IR780 prodrugs enables controlled release at the target site, optimizing its therapeutic efficacy. An innovative strategy involves the use of a prodrug system based on folic acid-conjugated IR780 nanoparticles, which are activated in acidic tumor microenvironments, ensuring higher selectivity and reduced adverse effects. IR780 remains “silenced” in normal tissues due to the fluorescence self-quenching effect, but its fluorescence is recovered in the tumor’s acidic environment, significantly increasing the tumor-to-background ratio (TBR) and system specificity [112]. Heat-triggered doxorubicin release mediated by the IR780 thermal effect has been reported in nanostructured liposomes prepared from synthetic lipid bilayers [25]. The author highlights the effect of cholesterol and proteins on the IR780 photobleaching.
Another promising approach involves IR780-loaded thermosensitive lipid nanoparticles, where the combination of the thermosensitive lipid with the photosensitizer IR780 induces localized hyperthermia under laser irradiation. This hyperthermia not only disrupts the extracellular matrix (ECM) but also facilitates the penetration of tumor-infiltrating lymphocytes (TILs) into deeper regions of the tumor microenvironment (TME), promoting a more efficient and targeted immune response against cancer [131].
6.5. Genomic and Proteomics-Based Approaches
Genomic or proteomic modifications leverage genetic engineering and protein-based strategies to enhance therapeutic specificity. One example is the combination of gene editing and phototherapy for the treatment of triple-negative breast cancer using dissolvable microneedles incorporated with nanoparticles. This nanosystem integrates CRISPR/Cas9 with the IR780 photosensitizer in a microneedle platform, enabling localized and efficient delivery. CRISPR/Cas9 plasmids targeting the FBXO44 gene (pFBXO44) disrupt tumor invasion pathways, while IR780 enhances phototherapy under light activation [136]. Another innovative strategy involves TAT-IR780 nanoparticles developed for breast cancer treatment. This approach combines gene therapy with PTT/PDT and chemotherapy. The system directly destroys genetic material (DNA/RNA), disrupts replication and transcription processes, and induces apoptosis in cancer cells, further improving therapeutic efficacy [124]. Approaches combining the photophysical stabilization of IR780 [158] with targeting properties of nanocarriers represent significant advances in improving their safety and efficiency, paving a promising path for future clinical applications.
7. Concluding Remarks and Future Perspectives
Although IR780 is often studied in association with nanocarriers, there are alternative strategies for its application, including free use in solution, incorporation into films and polymeric membranes, conjugation to biomolecules, and formulations in hydrogels. These approaches expand the use of IR780 for optical diagnostics, photodynamic therapy, photothermal therapy, and biomedical sensors, without relying on nanoparticulate systems. Yang et al. incorporated IR780 into attenuated Salmonella enterica, using it as a biological vector to target the compound to the tumor microenvironment, exploiting its affinity for hypoxic regions [161]. In addition to enabling photothermal therapy, this strategy increased the stability of IR780, enhanced its targeting to the tumor, and extended its action time, resulting in more effective tumor ablation [161]. An infrared-responsive (NIR) hydrogel was developed to optimize the immune response and promote tissue regeneration. The IR780 dye was used as a photo-responsive agent, capable of absorbing NIR irradiation and generating localized heat. This process was essential for inducing changes in the hydrogel’s rigidity, providing a controlled physical stimulus that modulated the immune response non-invasively through macrophage phenotype modulation, without requiring additional biochemical mediators [162]. These examples highlight that alternative solutions can be found to improve IR780 biological activity apart from nanocarriers. However, these approaches are much rarer in the literature than the IR780-associated nanostructures.
The chemical nature of the nanocarrier has been shown to successfully modulate the photophysical and photothermal properties of IR780 introducing photophysical changes in its performance. The chemical conjugation of IR780 (covalent bonding) with polymers and lipids to produce nanostructures has significantly improved the reliability of biological imaging in vitro and in vivo. However, the conjugation of IR780 may compromise its fluorescence quantum yield. This is an important concern, considering the existence of biases in the interpretation of biodistribution results of IR780-labeled nanocarriers in tumors and in their applications in other diseases. Furthermore, IR780 iodide is subject to differences in quantum fluorescence yield depending on the microenvironment in which IR780 is inserted. The photophysical behavior of IR780 in organic solutions and in water is markedly different from IR780 in a medium with serum proteins or immersed in lipids of the cell membrane bilayers. These differences should be considered in studies using IR780. The photophysical properties and the biological environment also interfere with photothermal conversion, thermal dose, photodynamic effect, and oxygen consumption.
In addition to conventional nanoparticles, IR780 has been investigated in various chemical conjugation strategies. For example, modification with the TAT peptide, forming the TAT-IR780 compound, conferred nuclear-targeting properties, efficiently inducing cell death. Combining IR780 with a multifunctional nanoplatform, including well-known drugs, also proves to be advantageous in treating certain diseases and exploring synergistic interactions. Though IR780 has a targeting capacity to tumor cell mitochondria, nanocarriers direct them to a mononuclear phagocytic system (MPS) and still end up in the liver and spleen as shown in Figure 6. This fact, unfortunately, limits their clinical value. The EPR effect can enhance the PS concentration in tumoral and inflamed tissues. Although challenging, well-designed surface modification combined with conjugated targeting moieties are needed to improve targeting efficiency. To improve the efficacy of IR780, it is necessary to increase active targeting to the sites of interest, such as infections and tumors, in order to also reduce toxicity.
Another weakness of PDT with IR780 is the dependence on O2 for effective PDT since PDT also consumes O2 in hypoxic tissue. This fact may increase cell resistance to PDT, and synergistic strategies to supply molecular oxygen were successfully used to improve antitumoral therapy in this review. In the previous section (Section 6), strategies applied to enhance the properties of IR780 were described in detail with an emphasis on IR780’s reliable association with nanocarriers.
Simpler nanosystems with biodegradable and safer raw materials associated with well-designed nanocarriers to maintain the best photophysical properties of IR780 are welcome in this regard. Therefore, the performance of IR780 depends on the adjustment of its environment inside a nanocarrier. The choice of nanocarrier type to minimize photobleaching and improve IR780 dispersibility in the system is critical to obtain reliable, enhanced image monitoring and achieve the maximum PTT and PDT effect. Highly complex nanocarriers with many preparation steps make it difficult to scale up. The long-term toxicity of PS in nanocarriers must be continuously studied in the future. Thus, simple systems made from highly biocompatible raw materials with well-defined metabolic pathways should be used to improve safety in future clinical studies.
Another challenge in advancing to clinical trials is the penetration depth limitations of NIR light, limiting IR780 applicability to superficial tumors [163,164]. The complexity of the structures associated with IR780 dye may compromise its transposition to clinical trials. Although a large number of recent studies have been focused on new structures and functional designs, including chemical modifications of IR780 molecules and the use of different nanocarriers to improve its therapeutic and imaging properties, some potential obstacles to its clinical application still persist.
Furthermore, most studies involving nanomaterials containing heptamethine cyanines, such as IR780, designed for the treatment of breast and melanoma tumors and their metastases were evaluated in mice [165]. However, in humans, tumors tend to be located in deeper regions, compared to those found in mice. To overcome this limitation, a promising approach is the use of fiber-type laser-coupled endoscopes to irradiate deeper primary tumors [166]. The efficacy of IR780 associated with nanostructures as nanotheranostics has not yet been validated in larger animal models [165]. Further long-term biosafety and biocompatibility testing are needed. Despite the obstacles still encountered in the clinical application of IR780, this marker has great potential for clinical translation.
8. Conclusions
In this review, from results reported in the literature, it is evident that without the aid of nanocarriers, IR780 may be rapidly eliminated from the body or fail to efficiently reach specific sites of action. A detailed review of in vivo studies published with IR780-based nanocarriers over the past 9 years is presented. The vast list of advantages in this review points out IR780’s beneficial properties that can be applied to a plethora of diseases. The association of IR780 with lipid- and polymeric-based nanocarriers has significantly increased their bioavailability, stability, and therapeutic potential. Future research should focus on refining nanocarrier design to achieve better tumor specificity and controlled drug release while minimizing systemic toxicity. Additionally, more in-depth investigations to understand the role of IR780 in immune modulation, its effects on the microenvironment, its potential for drug combination, and its activity upon associations with other treatment modalities will further contribute to the advancement of IR780 as a nanotheranostic.
Although only ICG from the heptamethine cyanine family has been approved by the FDA for clinical applications, another molecule from the same class of IR780, IRDye800CW, is in the process of clinical approval worldwide [167]. This is proof that this family of photosensitizers exhibited excellent biocompatibility and biosafety, being promising as theranostics. IR780 is a fluorescent photosensitizer intensively investigated over the last 15 years. However, it has limited solubility and dispersity in aqueous media, which hampers its use. In this sense, this review provides innovative approaches to overcome this limitation using nanocarriers. Taken together, these preclinical studies support the use of IR780 as a photosensitizer for PDT and PTT, as an NIR probe for imaging, as an adjuvant to improve accuracy in photoacoustic imaging, and more recently, as a sonosensitizer.
Author Contributions
Conceptualization, M.A.d.O., M.C.P.F., M.G.C.M. and V.C.F.M.; resources, V.C.F.M.; data curation, M.A.d.O., M.C.P.F., M.G.C.M., C.M.L. and V.C.F.M.; writing—original draft preparation, M.A.d.O., M.C.P.F., M.G.C.M., C.M.L. and V.C.F.M.; writing—review and editing, M.A.d.O., M.C.P.F., C.M.L. and V.C.F.M.; supervision and funding acquisition, V.C.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
The corresponding author, V. Mosqueira, received a technological productivity grant (#313602/2019-0) and a project grant [436460/2018-1] from the National Council for Scientific and Technological Development (CNPq/Brazil); and a partial support from the FAPEMIG project grant (APQ-02576-18).
Acknowledgments
We thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES/MEC) for PhD scholarship (code 001) to the first four authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Qiu, Y.; Yuan, B.; Cao, Y.; He, X.; Akakuru, O.U.; Lu, L.; Chen, N.; Xu, M.; Wu, A.; Li, J. Recent progress on near-infrared fluorescence heptamethine cyanine dye-based molecules and nanoparticles for tumor imaging and treatment. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1910. [Google Scholar] [CrossRef]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Chen, D.; Fang, Y.; Gong, X.; Wang, K.; Ma, C. Photothermally enhanced antibacterial wound healing using albumin-loaded tanshinone IIA and IR780 nanoparticles. Front. Bioeng. Biotechnol. 2024, 12, 1487660. [Google Scholar] [CrossRef]
- Pais-Silva, C.; de Melo-Diogo, D.; Correia, I.J. IR780-loaded TPGS-TOS micelles for breast cancer photodynamic therapy. Eur. J. Pharm. Biopharm. 2017, 113, 108–117. [Google Scholar] [CrossRef]
- Rajendrakumar, S.K.; Cherukula, K.; Park, H.J.; Uthaman, S.; Jeong, Y.Y.; Lee, B.-I.; Park, I.-K. Dual-stimuli-responsive albumin-polyplex nanoassembly for spatially controlled gene release in metastatic breast cancer. J. Control. Release 2018, 276, 72–83. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Correia, I.J. IR780 loaded sulfobetaine methacrylate-functionalized albumin nanoparticles aimed for enhanced breast cancer phototherapy. Int. J. Pharm. 2020, 582, 119346. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, H.; Yuan, A.; Tang, X.; Wu, J.; Hu, Y. Hydrophobic IR780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomater. 2015, 14, 61–69. [Google Scholar] [CrossRef]
- Hu, H.; Qi, Q.; Dong, Z.; Yu, X.; Mo, Y.; Luo, J.; Wang, Y.; Du, S.; Lu, Y. Albumin coated trimethyl chitosan-based targeting delivery platform for photothermal/chemo-synergistic cancer therapy. Carbohydr. Polym. 2020, 241, 116335. [Google Scholar] [CrossRef]
- Capistrano, G.; Sousa-Junior, A.A.; Silva, R.A.; Mello-Andrade, F.; Cintra, E.R.; Santos, S.; Nunes, A.D.; Lima, R.M.; Zufelato, N.; Oliveira, A.L.S.; et al. IR-780-Albumin-Based Nanocarriers Promote Tumor Regression Not Only from Phototherapy but Also by a Nonirradiation Mechanism. ACS Biomater. Sci. Eng. 2020, 6, 4523–4538. [Google Scholar] [CrossRef]
- Bazylińska, U.; Skrzela, R.; Szczepanowicz, K.; Warszyński, P.; Wilk, K.A. Novel approach to long sustained multilayer nanocapsules: Influence of surfactant head groups and polyelectrolyte layer number on the release of hydrophobic compounds. Soft Matter 2011, 7, 6113–6124. [Google Scholar] [CrossRef]
- Bazylińska, U.; Lewińska, A.; Lamch, Ł.; Wilk, K.A. Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 42–49. [Google Scholar] [CrossRef]
- Machado, M.G.C.; Pound-Lana, G.; de Oliveira, M.A.; Lanna, E.G.; Fialho, M.C.P.; de Brito, A.C.F.; Barboza, A.P.M.; Aguiar-Soares, R.D.d.O.; Mosqueira, V.C.F. Labeling PLA-PEG nanocarriers with IR780: Physical entrapment versus covalent attachment to polylactide. Drug Deliv. Transl. Res. 2020, 10, 1626–1643. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Machado, M.G.C.; Silva, S.E.D.; Nascimento, T.L.; Lima, E.M.; Pound-Lana, G.; Mosqueira, V.C.F. IR780-polymer conjugates for stable near-infrared labeling of biodegradable polyester-based nanocarriers. Eur. Polym. J. 2019, 120, 109255. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Pound-Lana, G.; Capelari-Oliveira, P.; Pontífice, T.G.; Silva, S.E.D.; Machado, M.G.C.; Postacchini, B.B.; Mosqueira, V.C.F. Release, transfer and partition of fluorescent dyes from polymeric nanocarriers to serum proteins monitored by asymmetric flow field-flow fractionation. J. Chromatogr. A 2021, 1641, 461959. [Google Scholar] [CrossRef]
- Li, Q.; Tan, J.; Peng, B.-X. Synthesis and Characterization of Heptamethine Cyanine Dyes. Molecules 1997, 2, 91–98. [Google Scholar] [CrossRef]
- Kiyose, K.; Aizawa, S.; Sasaki, E.; Kojima, H.; Hanaoka, K.; Terai, T.; Urano, Y.; Nagano, T. Molecular Design Strategies for Near-Infrared Ratiometric Fluorescent Probes Based on the Unique Spectral Properties of Aminocyanines. Chem. Eur. J. 2009, 15, 9191–9200. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Harada, Y.; Sakakura, M.; Minamikawa, T.; Nishino, S.; Maehara, S.; Hamano, S.; Tanaka, H.; Takamatsu, T. Photoacoustic microscopy using ultrashort pulses with two different pulse durations. Opt. Express 2014, 22, 17063–17072. [Google Scholar] [CrossRef]
- Levitz, A.; Marmarchi, F.; Henary, M. Synthesis and Optical Properties of Near-Infrared meso-Phenyl-Substituted Symmetric Heptamethine Cyanine Dyes. Molecules 2018, 23, 226. [Google Scholar] [CrossRef]
- Lu, Y.-J.; T. S., A.; Chuang, C.-C.; Chen, J.-P. Liposomal IR-780 as a Highly Stable Nanotheranostic Agent for Improved Photothermal/Photodynamic Therapy of Brain Tumors by Convection-Enhanced Delivery. Cancers 2021, 13, 3690. [Google Scholar] [CrossRef]
- Shen, D.; Ding, S.; Lu, Q.; Chen, Z.; Chen, L.; Lv, J.; Gao, J.; Yuan, Z. Nitroreductase-Responsive Fluorescent “Off-On” Photosensitizer for Hypoxic Tumor Imaging and Dual-Modal Therapy. ACS Omega 2024, 9, 30685–30697. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Rybkin, A.Y.; Belik, A.Y.; Kostina, E.A.; Goryachev, N.S.; Sulimenkov, I.V.; Kozlovskiy, V.I.; Istakova, O.I.; Konev, D.V.; Kotelnikov, A.I. Synthesis and photophysical properties of heptamethine cyanine–fullerene C60 dyads with non-quenched fluorescence. Mendeleev Commun. 2021, 31, 807–809. [Google Scholar] [CrossRef]
- Bian, H.; Ma, D.; Zhang, X.; Xin, K.; Yang, Y.; Peng, X.; Xiao, Y. Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 nm Laser toward Deep-Seated Tumors. Small 2021, 17, 2100398. [Google Scholar] [CrossRef]
- Barcelos, J.M.; Hayasaki, T.G.; de Santana, R.C.; Lima, E.M.; Mendanha, S.A.; Bakuzis, A.F. Photothermal Properties of IR-780-Based Nanoparticles Depend on Nanocarrier Design: A Comparative Study on Synthetic Liposomes and Cell Membrane and Hybrid Biomimetic Vesicles. Pharmaceutics 2023, 15, 444. [Google Scholar] [CrossRef]
- de Paula, C.S.; Tedesco, A.C.; Primo, F.L.; Vilela, J.M.C.; Andrade, M.S.; Mosqueira, V.C.F. Chloroaluminium phthalocyanine polymeric nanoparticles as photosensitisers: Photophysical and physicochemical characterisation, release and phototoxicity in vitro. Eur. J. Pharm. Sci. 2013, 49, 371–381. [Google Scholar] [CrossRef]
- Leitão, M.M.; de Melo-Diogo, D.; Alves, C.G.; Lima-Sousa, R.; Correia, I.J. Prototypic Heptamethine Cyanine Incorporating Nanomaterials for Cancer Phototheragnostic. Adv. Healthc. Mater. 2020, 9, e1901665. [Google Scholar] [CrossRef]
- Leitão, M.M.; Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Moreira, A.F.; Correia, I.J. Sulfobetaine methacrylate-functionalized graphene oxide-IR780 nanohybrids aimed at improving breast cancer phototherapy. RSC Adv. 2020, 10, 38621–38630. [Google Scholar] [CrossRef]
- Xu, P.-Y.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Development of highly stable ICG-polymeric nanoparticles with ultra-high entrapment efficiency using supercritical antisolvent (SAS)-combined solution casting process. Int. J. Pharm. 2022, 629, 122348. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]
- Yahya, M.; Nural, Y.; Seferoğlu, Z. Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: A review. Dye. Pigment. 2022, 198, 109960. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef]
- Han, X.; Dong, X.; Li, J.; Wang, M.; Luo, L.; Li, Z.; Lu, X.; He, R.; Xu, R.; Gong, M. Free paclitaxel-loaded E-selectin binding peptide modified micelle self-assembled from hyaluronic acid-paclitaxel conjugate inhibit breast cancer metastasis in a murine model. Int. J. Pharm. 2017, 528, 33–46. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Qian, C.; Guo, Y.; Li, C.; Gao, F.; Yang, Y.; Wang, K.; Oupicky, D.; Sun, M. Near-infrared light-activated IR780-loaded liposomes for anti-tumor angiogenesis and Photothermal therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2283–2294. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef]
- Pietkiewicz, J.; Wilk, K.A.; Bazylińska, U. In vitro studies of serum albumin interaction with poly (D, L-lactide) nanospheres loaded by hydrophobic cargo. J. Pharm. Biomed. Anal. 2016, 117, 426–435. [Google Scholar] [CrossRef]
- Machado, M.G.C.; de Oliveira, M.A.; Lanna, E.G.; Siqueira, R.P.; Pound-Lana, G.; Branquinho, R.T.; Mosqueira, V.C.F. Photodynamic therapy with the dual-mode association of IR780 to PEG-PLA nanocapsules and the effects on human breast cancer cells. Biomed. Pharmacother. 2022, 145, 112464. [Google Scholar] [CrossRef]
- Onaral, F.G.; Silindir-Gunay, M.; Uluturk, S.; Ozturk, S.C.; Cakir-Aktas, C.; Esendagli, G. Development and In Vitro and In Vivo Efficacy Investigation of Multifunctional, Targeted, Theranostic Liposomes for Imaging and Photodynamic Therapy of Glioblastoma. J. Drug Deliv. Sci. Technol. 2024, 101, 106236. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Hou, X.; Yan, F.; Jiang, Z.; Shi, J.; Xie, X.; Shen, J.; Fan, Q.; Wang, Z.; et al. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int. J. Nanomed. 2019, 14, 3311–3330. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 Dye Encapsulated in cRGD-Conjugated Solid Lipid Nanoparticles for NIR Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interface 2017, 9, 12217–12226. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Yi, H.; Huang, J.; Zhang, N.; Zhong, Y.; Hao, L.; Yang, K.; Wang, Z.; Wang, D.; et al. NIR-responsive nanoplatform for pre/intraoperative image-guided carcinoma surgery and photothermal ablation of residual tumor tissue. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102020. [Google Scholar] [CrossRef]
- Zhen, X.; Li, Y.; Yuan, W.; Zhang, T.; Li, M.; Huang, J.; Kong, N.; Xie, X.; Wang, S.; Tao, W. Biointerface-Engineered Hybrid Nanovesicles for Targeted Reprogramming of Tumor Microenvironment. Adv. Mater. 2024, 36, e2401495. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, P.; Zhou, Y.; Li, Q.; Cai, W.; Zhao, Z.; Shen, J.; Yao, K.; Duan, Y. Preparation of multifunctional nanobubbles and their application in bimodal imaging and targeted combination therapy of early pancreatic cancer. Sci. Rep. 2021, 11, 6254. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef]
- Sharma, M.; Bhattacharya, S.; Maheshwari, R. EpCAM aptamer-engineered, azadiradione-loaded, chemo-photo-responsive nano-erythroliposomes for the treatment of triple-negative breast cancer. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135176. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Zhou, W.; Wang, J.; Zhang, R.; Wang, Z.; Ran, H.; Li, P.; Li, R. Dual mitigation of immunosuppression combined with photothermal inhibition for highly effective primary tumor and metastases therapy. Biomaterials 2021, 274, 120856. [Google Scholar] [CrossRef]
- Appidi, T.; Pemmaraju, D.B.; Khan, R.A.; Alvi, S.B.; Srivastava, R.; Pal, M.; Khan, N.; Rengan, A.K. Light-triggered selective ROS-dependent autophagy by bioactive nanoliposomes for efficient cancer theranostics. Nanoscale 2020, 12, 2028–2039. [Google Scholar] [CrossRef]
- Margolis, R.; Basavarajappa, L.; Li, J.; Obaid, G.; Hoyt, K. Image-guided focused ultrasound-mediated molecular delivery to breast cancer in an animal model. Phys. Med. Biol. 2023, 68, 155012. [Google Scholar] [CrossRef]
- Zhang, N.; Tan, Y.; Yan, L.; Zhang, C.; Xu, M.; Guo, H.; Zhuang, B.; Zhou, L.; Xie, X. Modulation of Tumor Hypoxia by pH-Responsive Liposomes to Inhibit Mitochondrial Respiration for Enhancing Sonodynamic Therapy. Int. J. Nanomed. 2020, 15, 5687–5700. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H.; Song, J.; Huang, J.; Yang, K.; Tan, B.; Wang, D.; Yang, N.; Wang, Z.-G.; Li, X. Mitochondria-Targeted and Ultrasound-Activated Nanodroplets for Enhanced Deep-Penetration Sonodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 9355–9366. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Li, J.; Tang, X.; Wang, X.; Cun, X.; Xu, S.; Liu, Y.; Li, M.; Zhang, Z.; et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J. Control. Release 2020, 321, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mangla, B.; Imam, S.A.; Aggarwal, G. Oral in vivo biodistribution of fluorescent labelled nano lipid carrier system of erlotinib using real time optical imaging technique. Int. J. Pharm. 2024, 664, 124588. [Google Scholar] [CrossRef]
- Qu, J.; Teng, D.; Sui, G.; Guan, S.; Wang, Y.; Wang, Q.; Lin, Y.; Ran, H.; Wang, Z.; Wang, H. A photothermal-hypoxia sequentially activatable phase-change nanoagent for mitochondria-targeting tumor synergistic therapy. Biomater. Sci. 2020, 8, 3116–3129. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Ma, J.; Li, K.; Wang, W.; Chen, D.; Liu, Z.; Zhan, W.; Zeng, Y.; Zhan, Y. 5-Hydroxytryptophan artificial synaptic vesicles across the blood-brain barrier for the rapid-acting treatment of depressive disorder. Mater. Today Bio 2024, 29, 101357. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.; Liu, F.; Ding, T.; Wang, Z. Red blood cells membrane vehicle co-delivering DOX and IR780 for effective prostate cancer therapy. J. Mater. Res. 2020, 35, 3116–3123. [Google Scholar] [CrossRef]
- Kv, R.; Liu, T.-I.; Lu, I.-L.; Liu, C.-C.; Lu, T.-Y.; Chiang, W.-H.; Chiu, H.-C. Tumor microenvironment-responsive and oxygen self-sufficient oil droplet nanoparticles for enhanced photothermal/photodynamic combination therapy against hypoxic tumors. J. Control. Release 2020, 328, 87–99. [Google Scholar] [CrossRef]
- Yang, Y.; Yun, K.; Li, Y.; Zhang, L.; Zhao, W.; Zhu, Z.; Tian, B.; Chen, F.; Pan, W. Self-assembled multifunctional polymeric micelles for tumor-specific bioimaging and synergistic chemo-phototherapy of cancer. Int. J. Pharm. 2021, 602, 120651. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, K.; Qin, H.; Zhao, X.; Chen, W.; Xu, L.; Cao, W.; Guo, H. Internal cross-linked polymeric nanoparticles with dual sensitivity for combination therapy of muscle-invasive bladder cancer. J. Nanobiotechnol. 2020, 18, 124. [Google Scholar] [CrossRef]
- Gao, Z.; Mu, W.; Tian, Y.; Su, Y.; Sun, H.; Zhang, G.; Li, A.; Yu, D.; Zhang, N.; Hao, J.; et al. Self-assembly of paramagnetic amphiphilic copolymers for synergistic therapy. J. Mater. Chem. B 2020, 8, 6866–6876. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Park, H.; Kim, J.; Park, D.; Lim, J.; Lee, J.; Kim, W.J. Polymersomes with singlet oxygen-labile poly (β-aminoacrylate) membrane for NIR light-controlled combined chemo-phototherapy. J. Control. Release 2020, 327, 627–640. [Google Scholar] [CrossRef]
- Song, C.; Li, Y.; Li, T.; Yang, Y.; Huang, Z.; de la Fuente, J.M.; Ni, J.; Cui, D. Long-Circulating Drug-Dye-Based Micelles with Ultrahigh pH-Sensitivity for Deep Tumor Penetration and Superior Chemo-Photothermal Therapy. Adv. Funct. Mater. 2020, 30, 1906309. [Google Scholar] [CrossRef]
- Pei, W.; Huang, B.; Chen, S.; Wang, L.; Xu, Y.; Niu, C. Platelet-Mimicking Drug Delivery Nanoparticles for Enhanced Chemo-Photothermal Therapy of Breast Cancer. Int. J. Nanomed. 2020, 15, 10151–10167. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhu, Y.; Wen, L.; Yang, X.; Liu, X.; Meng, T.; Dai, S.; Ping, Y.; Yuan, H.; Hu, F. Mitochondria-Responsive Drug Release along with Heat Shock Mediated by Multifunctional Glycolipid Micelles for Precise Cancer Chemo-Phototherapy. Theranostics 2019, 9, 691–707. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Wang, H.; Li, Z.; Chen, J.; Zhang, Z.; Zeng, H.; Yu, X.; Yang, X.; Yang, X.; et al. Hydroxyethyl starch-folic acid conjugates stabilized theranostic nanoparticles for cancer therapy. J. Control. Release 2023, 353, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhang, Y.; Yang, L.; Li, H.; Li, X.; Feng, J.; Jia, L.; Zhang, Z. Camptothecin-loaded and IR780-doped polydopamine nanomedicine used for multifunctional chemo-photothermal-photodynamic therapy for lung cancer. J. Drug Deliv. Sci. Technol. 2024, 97, 105657. [Google Scholar] [CrossRef]
- Lu, I.-L.; Liu, T.-I.; Lin, H.-C.; Chang, S.-H.; Lo, C.-L.; Chiang, W.-H.; Chiu, H.-C. IR780-loaded zwitterionic polymeric nanoparticles with acidity-induced agglomeration for enhanced tumor retention. Eur. Polym. J. 2020, 122, 109400. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Tang, K.; Liao, H.; Xu, Y.; Wang, L.; Niu, C. Multi-Modal Imaging Monitored M2 Macrophage Targeting Sono-Responsive Nanoparticles to Combat MRSA Deep Infections. Int. J. Nanomed. 2022, 17, 4525–4546. [Google Scholar] [CrossRef]
- Huang, H.; Fu, J.; Peng, H.; He, Y.; Chang, A.; Zhang, H.; Hao, Y.; Xu, X.; Li, S.; Zhao, J.; et al. Co-delivery of polyphyllin II and IR780 PLGA nanoparticles induced pyroptosis combined with photothermal to enhance hepatocellular carcinoma immunotherapy. J. Nanobiotechnol. 2024, 22, 647. [Google Scholar] [CrossRef]
- He, M.; Zhang, M.; Xu, T.; Xue, S.; Li, D.; Zhao, Y.; Zhi, F.; Ding, D. Enhancing photodynamic immunotherapy by reprograming the immunosuppressive tumor microenvironment with hypoxia relief. J. Control. Release 2024, 368, 233–250. [Google Scholar] [CrossRef]
- Wu, S.; Qiao, Z.; Li, Y.; Hu, S.; Ma, Y.; Wei, S.; Zhang, L. Persistent Luminescence Nanoplatform with Fenton-like Catalytic Activity for Tumor Multimodal Imaging and Photoenhanced Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 25572–25580. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, W.; Du, L.; Xu, C.; Li, J. Synergy of hypoxia relief and heat shock protein inhibition for phototherapy enhancement. J. Nanobiotechnol. 2021, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Ran, H.; Li, P.; Huang, J.; Tan, M.; Yang, Y.; Wang, Z. A mitochondria-targeted anticancer nanoplatform with deep penetration for enhanced synergistic sonodynamic and starvation therapy. Biomater. Sci. 2020, 8, 4581–4594. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Zhang, L.; Huang, J.; Li, P.; Zhao, Y.; Zhou, C.; Liu, M.; Li, W.; He, J. Mitochondria-targeted nanospheres with deep tumor penetration for photo/starvation therapy. J. Mater. Chem. B 2020, 8, 7740–7754. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Luo, Y.; Gao, H.; Zhang, L.; Wang, X.; Huang, J.; Shang, T.; Zhou, D.; Wang, D.; Wang, Z.; et al. Mitochondria-targeted nanoplatforms for enhanced photodynamic therapy against hypoxia tumor. J. Nanobiotechnol. 2021, 19, 440. [Google Scholar] [CrossRef]
- Tian, Y.; Younis, M.R.; Tang, Y.; Liao, X.; He, G.; Wang, S.; Teng, Z.; Huang, P.; Zhang, L.; Lu, G. Dye-loaded mesoporous polydopamine nanoparticles for multimodal tumor theranostics with enhanced immunogenic cell death. J. Nanobiotechnol. 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, Z.; Yang, J.; Qian, H.; Xu, J.; Chen, B.; Tian, T.; Chen, H.; Xu, Y.; Wang, F. Immunogenic Cell Death Augmented by Manganese Zinc Sulfide Nanoparticles for Metastatic Melanoma Immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef]
- Shao, N.; Qi, Y.; Lu, H.; He, D.; Li, B.; Huang, Y. Photostability Highly Improved Nanoparticles Based on IR-780 and Negative Charged Copolymer for Enhanced Photothermal Therapy. ACS Biomater. Sci. Eng. 2019, 5, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef]
- Hong, E.; Hyun, H.; Lee, H.; Jung, E.; Lee, D. Acid-sensitive oxidative stress inducing and photoabsorbing polysaccharide nanoparticles for combinational anticancer therapy. Int. J. Pharm. 2020, 574, 118893. [Google Scholar] [CrossRef]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef]
- Uthaman, S.; Mathew, A.P.; Park, H.J.; Lee, B.-I.; Kim, H.-S.; Huh, K.M.; Park, I.-K. IR 780-loaded hyaluronic acid micelles for enhanced tumor-targeted photothermal therapy. Carbohydr. Polym. 2018, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Tian, J.; Zhu, H.; Hong, L.; Mao, Z.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-Targeting Polycaprolactone Nanoparticles with Codelivery of Paclitaxel and IR780 for Combinational Therapy of Drug-Resistant Ovarian Cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Rong, R.; Wang, D.; Wang, D.; Yu, Y.; Wu, P.; Li, Y.; Zhang, Z. Enhancing the oral bioavailability of poorly water-soluble amisupiride with solid nanodispersion. J. Drug Deliv. Sci. Technol. 2023, 86, 104635. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Zhao, X.; Yin, Z. Functionalized polymeric hybrid micelles as an efficient nanotheranostic agent for thrombus imaging and thrombolysis. Acta Biomater. 2021, 122, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Li, Y.; Cheng, W.; Zhou, L.; Shen, Y.; Wang, Y. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol. Med. Rep. 2013, 7, 1977–1981. [Google Scholar] [CrossRef]
- Jia, W.; Yang, M.; Zhang, W.; Xu, W.; Zhang, Y. Carrier-Free Self-Assembled Nanomedicines for Promoting Apoptosis and Inhibiting Proliferation in Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2024, 10, 4347–4358. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zeng, Y.; Luo, J.; Wang, X.; Ma, G.; Zhang, T.; Huang, P. Endogenous Magnetic Lipid Droplet-Mediated Cascade-Targeted Sonodynamic Therapy as an Approach to Reversing Breast Cancer Multidrug Resistance. ACS Nano 2024, 18, 28659–28674. [Google Scholar] [CrossRef]
- Sousa-Junior, A.A.; Mendanha, S.A.; Carrião, M.S.; Capistrano, G.; Próspero, A.G.; Soares, G.A.; Cintra, E.R.; Santos, S.F.O.; Zufelato, N.; Alonso, A.; et al. Predictive Model for Delivery Efficiency: Erythrocyte Membrane-Camouflaged Magnetofluorescent Nanocarriers Study. Mol. Pharm. 2020, 17, 837–851. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Ruttala, R.R.T.; Tran, T.H.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Mitochondria-targeting multi-metallic ZnCuO nanoparticles and IR780 for efficient photodynamic and photothermal cancer treatments. J. Mater. Sci. Technol. 2021, 86, 139–150. [Google Scholar] [CrossRef]
- Chen, H.; Deng, J.; Yao, X.; He, Y.; Li, H.; Jian, Z.; Tang, Y.; Zhang, X.; Zhang, J.; Dai, H. Bone-targeted erythrocyte-cancer hybrid membrane-camouflaged nanoparticles for enhancing photothermal and hypoxia-activated chemotherapy of bone invasion by OSCC. J. Nanobiotechnol. 2021, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Q.; Chu, X.; Li, N.; Liang, H.; He, F. Nanoparticles (NPs)-meditated si-lncRNA NONHSAT159592.1 inhibits glioblastoma progression and invasion through targeting the ITGA3/FAK/PI3K/AKT pathway. Metab. Brain Dis. 2024, 40, 31. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Chen, H.; Liang, Y.; Zhao, B.; Luo, W.; Xiao, Q.; Li, J.; Zhu, J.; Peng, C.; et al. An acoustic/thermo-responsive hybrid system for advanced doxorubicin delivery in tumor treatment. Biomater. Sci. 2020, 8, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, S.; Chen, S.; Ma, T.; Chen, M.; Niu, C.; Leng, Y.; Wang, L. Bacterial outer membrane vesicle-cancer cell hybrid membrane-coated nanoparticles for sonodynamic therapy in the treatment of breast cancer bone metastasis. J. Nanobiotechnol. 2024, 22, 328. [Google Scholar] [CrossRef]
- Mai, X.; Zhang, Y.; Fan, H.; Song, W.; Chang, Y.; Chen, B.; Shi, J.; Xin, X.; Teng, Z.; Sun, J.; et al. Integration of immunogenic activation and immunosuppressive reversion using mitochondrial-respiration-inhibited platelet-mimicking nanoparticles. Biomaterials 2020, 232, 119699. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Huang, S.; Guo, D.; Yu, L.; Qiao, W.; Zhang, X.; Han, Z.; Song, B.; Xu, X.; et al. Production, Characterization, and Application of Hydrophobin-Based IR780 Nanoparticles for Targeted Photothermal Cancer Therapy and Advanced Near-Infrared Imaging. Adv. Healthc. Mater. 2024, 14, e2402311. [Google Scholar] [CrossRef]
- Qiu, G.; Xue, L.; Zhu, X.; Lu, X.; Liu, L.; Wang, Z.; Li, X.; Huang, C.; Liu, J. Cetuximab Combined with Sonodynamic Therapy Achieves Dual-Modal Image Monitoring for the Treatment of EGFR-Sensitive Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 756489. [Google Scholar] [CrossRef]
- Mu, M.; Chen, B.; Li, H.; Fan, R.; Yang, Y.; Zhou, L.; Han, B.; Zou, B.; Chen, N.; Guo, G. Augmented the sensitivity of photothermal-ferroptosis therapy in triple-negative breast cancer through mitochondria-targeted nanoreactor. J. Control. Release 2024, 375, 733–744. [Google Scholar] [CrossRef]
- Zuo, H.; Hou, Y.; Yu, Y.; Li, Z.; Liu, H.; Liu, C.; He, J.; Miao, L. Circumventing Myeloid-Derived Suppressor Cell-Mediated Immunosuppression Using an Oxygen-Generated and -Economized Nanoplatform. ACS Appl. Mater. Interfaces 2020, 12, 55723–55736. [Google Scholar] [CrossRef]
- Choi, S.K. Activation Strategies in Image-Guided Nanotherapeutic Delivery. J. Nanotheranostics 2020, 1, 78–104. [Google Scholar] [CrossRef]
- Peng, C.-L.; Shih, Y.-H.; Lee, P.-C.; Hsieh, T.M.-H.; Luo, T.-Y.; Shieh, M.-J. Multimodal Image-Guided Photothermal Therapy Mediated by 188Re-Labeled Micelles Containing a Cyanine-Type Photosensitizer. ACS Nano 2011, 5, 5594–5607. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Huang, X.; Wu, Z.; Gao, M.; Li, R.; Luo, S. A Mitochondria-Targeted Heptamethine Indocyanine Small Molecular Chelator for Attenuating Uranium Nephrotoxicity. Pharmaceuticals 2024, 17, 995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Machado, M.G.C.; de Oliveira, M.A. Polymeric Nanocarriers in Cancer Theranostics. In Cancer Nanotechnology; Springer International Publishing: Cham, Germany, 2023; pp. 45–70. [Google Scholar] [CrossRef]
- Machado, M.G.C.; de Oliveira, M.A.; Araújo, R.S.; Mosqueira, V.C.F. Theragnostic applications. In Smart Nanomaterials for Bioencapsulation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 197–213. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Su, Y.; Luo, S.; Zhu, Y.; Tan, X.; Fan, S.; Zhang, L.; Zhou, Y.; Cheng, T.; et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials 2010, 31, 6612–6617. [Google Scholar] [CrossRef]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Song, J.; Ye, H.; Jiang, S.; Yang, Y.; Li, X. An Acid Response IR780-Based Targeted Nanoparticle for Intraoperative Near-Infrared Fluorescence Imaging of Ovarian Cancer. Int. J. Nanomed. 2022, 17, 4961–4974. [Google Scholar] [CrossRef]
- De Silva, P.; Saad, M.A.; Thomsen, H.C.; Bano, S.; Ashraf, S.; Hasan, T. Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization—A Thomas Dougherty Award for Excellence in PDT paper. J. Porphyr. Phthalocyanines 2020, 24, 1320–1360. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Wu, J.; Hu, Y.; Guo, H. Self-assembled albumin nanoparticles for combination therapy in prostate cancer. Int. J. Nanomed. 2017, 12, 7777–7787. [Google Scholar] [CrossRef]
- Li, N.; Xu, F.; Cheng, J.; Zhang, Y.; Huang, G.; Zhu, J.; Shen, X.; He, D. Perfluorocarbon Nanocapsules Improve Hypoxic Microenvironment for the Tumor Ultrasound Diagnosis and Photodynamic Therapy. J. Biomed. Nanotechnol. 2018, 14, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, H.; Hou, T.; An, B.; Li, F. Portable multi-amplified temperature sensing for tumor exosomes based on MnO2/IR780 nanozyme with high photothermal effect and oxidase-like activity. Chin. Chem. Lett. 2023, 34, 107607. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Inoue, H.; Tani, K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014, 21, 39–49. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Ghosh, A.; Rajdev, B.; Parihar, N.; Ponneganti, S.; Das, P.; Naidu, V.; Krishnanand, P.R.; Murty, U.; Kumar, J.; Pemmaraju, D.B. Bio-nanoconjugates of lithocholic acid/IR 780 for ROS-mediated apoptosis and optoacoustic imaging applications in breast cancer. Colloids Surf. B Biointerfaces 2023, 221, 113023. [Google Scholar] [CrossRef]
- Wan, G.; Chen, X.; Wang, H.; Hou, S.; Wang, Q.; Cheng, Y.; Chen, Q.; Lv, Y.; Chen, H.; Zhang, Q. Gene augmented nuclear-targeting sonodynamic therapy via Nrf2 pathway-based redox balance adjustment boosts peptide-based anti-PD-L1 therapy on colorectal cancer. J. Nanobiotechnol. 2021, 19, 347. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, J.; Luo, W.; Wen, C.; Mao, W.; Yang, Y.; Liu, C.; Xu, Y.; Chen, W.; Wen, L. Mitochondria targeted biomimetic platform for chemo/photodynamic combination therapy against osteosarcoma. Int. J. Pharm. 2024, 652, 123865. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Cheng, Y.; Song, J.; Chen, Q.; Chen, B.; Liu, Y.; Ji, S.; Chen, H.; Wang, Y. Nucleus-targeting near-infrared nanoparticles based on TAT peptide-conjugated IR780 for photo-chemotherapy of breast cancer. Chem. Eng. J. 2020, 380, 122458. [Google Scholar] [CrossRef]
- Guo, X.; Mei, J.; Zhang, C. Development of Drug Dual-Carriers Delivery System with Mitochondria-Targeted and pH/Heat Responsive Capacity for Synergistic Photothermal-Chemotherapy of Ovarian Cancer. Int. J. Nanomed. 2020, 15, 301–313. [Google Scholar] [CrossRef]
- He, W.; Tu, S.; Han, J.; Cui, H.; Lai, L.; Ye, Y.; Dai, T.; Yuan, Y.; Ji, L.; Luo, J.; et al. Mild phototherapy mediated by IR780-Gd-OPN nanomicelles suppresses atherosclerotic plaque progression through the activation of the HSP27-regulated NF-κB pathway. Acta Biomater. 2024, 182, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Rustique, E.; Henry, M.; Guidetti, M.; Josserand, V.; Sancey, L.; Boutet, J.; Coll, J.-L. Targeting tumors with cyclic RGD-conjugated lipid nanoparticles loaded with an IR780 NIR dye: In vitro and in vivo evaluation. Int. J. Pharm. 2017, 532, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yuan, A.; Zhao, X.; Lian, H.; Zhuang, J.; Chen, W.; Zhang, Q.; Liu, G.; Zhang, S.; Cao, W.; et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017, 53, 427–438. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, J.; Zhang, H.; Jiang, Y.; Song, A.; Luan, Y. Low side-effect and heat-shock protein-inhibited chemo-phototherapy nanoplatform via co-assembling strategy of biotin-tailored IR780 and quercetin. Chem. Eng. J. 2020, 382, 123043. [Google Scholar] [CrossRef]
- Cheng, Y.; Hou, Y.; Ye, Z.; Qiu, C.; Li, S.; Li, L.; Lin, Y.; Chen, N.; Yao, Y.; Jiang, Z.; et al. Photothermal nanocomposite reactivate “immune-hot” for triple-negative breast cancer treatment via glutamine metabolism reprograming. Colloids Surf. B Biointerfaces 2025, 245, 114268. [Google Scholar] [CrossRef]
- Tan, Y.-N.; Li, Y.-P.; Huang, J.-D.; Luo, M.; Li, S.-S.; Lee, A.W.-M.; Hu, F.-Q.; Guan, X.-Y. Thermal-sensitive lipid nanoparticles potentiate anti-PD therapy through enhancing drug penetration and T lymphocytes infiltration in metastatic tumor. Cancer Lett. 2021, 522, 238–254. [Google Scholar] [CrossRef]
- Zhang, B.; Ding, Z.; Wen, X.; Song, G.; Luo, Q. Salinomycin and IR780-loaded upconversion nanoparticles influence biological behavior of liver cancer stem cells by persistently activating the MAPK signaling pathway. Exp. Cell Res. 2024, 434, 113865. [Google Scholar] [CrossRef]
- Far, H.R.; Amiri, M.; Elyasigorji, Z.; Heydari, E.; Saviz, M.; Faraji-Dana, R.; Solouk, A.; Moeini, M. Photothermal Oxygen-Releasing IR780/CaO2 Nanoplatform for Enhanced and On-Demand Photodynamic Therapy. ACS Appl. Nano Mater. 2024, 7, 23627–23640. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Liu, S.; Yan, H.; Ji, J.; Xi, Y.; Yang, X.; Zhai, G. NIR-triggerable ROS-responsive cluster-bomb-like nanoplatform for enhanced tumor penetration, phototherapy efficiency and antitumor immunity. Biomaterials 2021, 278, 121135. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Xu, Y.; Ren, X.; Zhou, S.; Shang, Q.; Jiang, Y.; Luan, Y. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem. Eng. J. 2020, 400, 125995. [Google Scholar] [CrossRef]
- Wang, T.; Chen, G.; Zhang, S.; Li, D.; Wei, G.; Zhao, X.; Liu, Y.; Ding, D.; Zhang, X. Steerable Microneedles Enabling Deep Delivery of Photosensitizers and CRISPR/Cas9 Systems for Effective Combination Cancer Therapy. Nano Lett. 2023, 23, 7990–7999. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.L.; Lima-Sousa, R.; Alves, C.G.; Ferreira, P.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Nanomedicine: Sulfobetaine Methacrylate-Albumin-Coated Graphene Oxide Incorporating IR780 for Enhanced Breast Cancer Phototherapy. Nanomedicine 2021, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.G.; Lima-Sousa, R.; Melo, B.L.; Ferreira, P.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Poly (2-Ethyl-2-Oxazoline)–IR780 Conjugate Nanoparticles for Breast Cancer Phototherapy. Nanomedicine 2022, 17, 2057–2072. [Google Scholar] [CrossRef]
- Tan, H.; Tian, Y.; Yang, H.; Liu, Z.; Liang, X.; Li, B.; Cheng, W. Oxygen-sufficient lipid nanobubbles combined with UTMD for enhanced sonodynamic therapy of Hep-G2 cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1796–1806. [Google Scholar] [CrossRef]
- Wei, M.; Wang, X.; Mo, Y.; Kong, C.; Zhang, M.; Qiu, G.; Tang, Z.; Chen, J.; Wu, F. Combined Effects of Anti-PD-L1 and Nanosonodynamic Therapy on HCC Immune Activation in Mice: An Investigation. Int. J. Nanomed. 2024, 19, 7215–7236. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, H.; Guo, Y.; Yu, S.; Sun, J.; Wang, H.; Zhao, Y.; Chen, X.; Shen, H.; Geng, J.; et al. A Glucose Metabolic Intervention Nanoplatform for Enhanced Chemodynamic Therapy and Sensitized Photothermal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2023, 15, 25437–25451. [Google Scholar] [CrossRef]
- Zhang, A.; Gao, A.; Zhou, C.; Xue, C.; Zhang, Q.; De La Fuente, J.M.; Cui, D. Confining Prepared Ultrasmall Nanozymes Loading ATO for Lung Cancer Catalytic Therapy/Immunotherapy. Adv. Mater. 2023, 35, e2303722. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, L.; Jung, E.; Ko, C.; Seon, S.; Noh, J.; Lee, D. Thrombus targeting aspirin particles for near infrared imaging and on-demand therapy of thrombotic vascular diseases. J. Control. Release 2019, 304, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Bongcaron, V.; Nguyen, T.K.; Jirwanka, Y.; Maluenda, A.; Walsh, A.P.G.; Palasubramaniam, J.; Hulett, M.D.; Srivastava, R.; Bobik, A.; et al. An Ultrasound-Responsive Theranostic Cyclodextrin-Loaded Nanoparticle for Multimodal Imaging and Therapy for Atherosclerosis. Small 2022, 18, e2200967. [Google Scholar] [CrossRef]
- Refaat, A.; del Rosal, B.; Bongcaron, V.; Walsh, A.P.G.; Pietersz, G.; Peter, K.; Moulton, S.E.; Wang, X. Activated Platelet-Targeted IR780 Immunoliposomes for Photothermal Thrombolysis. Adv. Funct. Mater. 2023, 33, 2209019. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Li, P.; Sun, S.; Su, J.; Liu, S.; Wei, W. Multifunctional Nanocarrier for Synergistic Treatment of Alzheimer’s Disease by Inhibiting β-Amyloid Aggregation and Scavenging Reactive Oxygen Species. ACS Appl. Mater. Interfaces 2024, 16, 27127–27138. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, J.; Chen, W.; Yang, W.; Tan, X.; Fan, X.; Shen, G.; Qu, L.; Chen, Z.; Shi, C. Mitochondrial-targeting fluorescent small molecule IR-780 alleviates radiation-induced brain injury. Brain Res. 2023, 1805, 148285. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.; Yan, J.; Lu, Z.; Shi, L.; Zhang, Y.; Lin, J.; Cao, Y.; Pei, R. A responsive nanoplatform with molecular and structural imaging capacity for assisting accurate diagnosis of early rheumatoid arthritis. Int. J. Biol. Macromol. 2024, 271, 132514. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, W.; Li, Z.; Wang, B.; Chitrakar, B.; Xiong, W.; Liu, Y.; Zeng, Z. Photothermal and selective microbial inactivation behaviors of gluconamide-coated IR780 nanoparticles. Colloids Surf. B Biointerfaces 2023, 222, 113126. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Jia, Y.; Gao, T.; Deng, L.; Gong, T.; Zhang, Z.; Cao, X.; Fu, Y. Adipocyte-targeted delivery of rosiglitazone with localized photothermal therapy for the treatment of diet-induced obesity in mice. Acta Biomater. 2024, 181, 317–332. [Google Scholar] [CrossRef]
- Yuan, A.; Qiu, X.; Tang, X.; Liu, W.; Wu, J.; Hu, Y. Self-assembled PEG-IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials 2015, 51, 184–193. [Google Scholar] [CrossRef]
- Yang, G.-G.; Pan, Z.-Y.; Zhang, D.-Y.; Cao, Q.; Ji, L.-N.; Mao, Z.-W. Precisely Assembled Nanoparticles against Cisplatin Resistance via Cancer-Specific Targeting of Mitochondria and Imaging-Guided Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 43444–43455. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.S.; Lu, Y.-J.; Pejrprim, P.; Lan, Y.-H.; Chen, J.-P. Hyaluronic acid-modified, IR780-conjugated and doxorubicin-loaded reduced graphene oxide for targeted cancer chemo/photothermal/photodynamic therapy. Biomater. Adv. 2022, 136, 212764. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhang, J.; Yan, F.; Lu, Z.; Huang, J.; Pan, C.; Yuan, J.; Zheng, W.; Zhang, K.; Wei, D.; et al. Synthesis of IR-780 dye-conjugated abiraterone for prostate cancer imaging and therapy. Int. J. Oncol. 2016, 49, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Martín-Saavedra, F.M.; Aguilar, M.R.; Escudero-Duch, C.; Martín-Saldaña, S.; Parra-Ruiz, F.J.; Rohner, N.A.; Thomas, S.N.; Vilaboa, N.; Román, J.S. Photothermal and photodynamic activity of polymeric nanoparticles based on α-tocopheryl succinate-RAFT block copolymers conjugated to IR-780. Acta Biomater. 2017, 57, 70–84. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, K.; Chen, Y.; Liu, Y.; Tang, J.; Zhang, X.; Liu, J. Engineering Diselenide-IR780 Homodimeric Nanoassemblies with Enhanced Photodynamic and Immunotherapeutic Effects for Triple-Negative Breast Cancer Treatment. ACS Nano 2023, 17, 22553–22570. [Google Scholar] [CrossRef]
- Cai, X.; Wang, M.; Mu, P.; Jian, T.; Liu, D.; Ding, S.; Luo, Y.; Du, D.; Song, Y.; Chen, C.-L.; et al. Sequence-Defined Nanotubes Assembled from IR780-Conjugated Peptoids for Chemophototherapy of Malignant Glioma. Research 2021, 2021, 9861384. [Google Scholar] [CrossRef]
- Shi, J.; Tian, H.; Peng, L.; Huang, C.; Nice, E.C.; Zou, B.; Zhang, H. A nanoplatform reshaping intracellular osmolarity and redox homeostasis against colorectal cancer. J. Control. Release 2022, 352, 766–775. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Zhao, X.; Chang, L.; Hu, Y.; Chen, Z.; Mei, X.; Chen, D. Preparation of NIR-sensitive, photothermal and photodynamic multi-functional Mxene nanosheets for laryngeal cancer therapy by regulating mitochondrial apoptosis. Mater. Des. 2022, 220, 110887. [Google Scholar] [CrossRef]
- Yang, J.; Yang, M.; Wang, Q.; Luo, Q.; Wang, Y.; Sun, J.; Liu, J.; Chen, J.; Mao, J.; Yin, H.; et al. A photosensitive modification of recombinant Salmonella carrying shRNA-PD-L1 promotes host immunity against poorly immunogenic tumors. Chem. Eng. J. 2024, 500, 157034. [Google Scholar] [CrossRef]
- Yuan, P.; Luo, Y.; Ma, L. NIR-triggered hydrogel with dynamic stiffness via ion chelation to modulate macrophage phenotypes. J. Polym. Sci. 2022, 60, 3176–3185. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; An, Y.; Li, Y.; Liu, Y.; Wang, L.; Guo, J.; Wang, J.; Zhou, J. Thermoresponsive Nanogel-Encapsulated PEDOT and HSP70 Inhibitor for Improving the Depth of the Photothermal Therapeutic Effect. Adv. Funct. Mater. 2016, 26, 4749–4759. [Google Scholar] [CrossRef]
- Shramova, E.I.; Kotlyar, A.B.; Lebedenko, E.N.; Deyev, S.M.; Proshkina, G.M. Near-infrared activated cyanine dyes as agents for photothermal therapy and diagnosis of tumors. Acta Naturae 2020, 12, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.G.; Lima-Sousa, R.; Melo, B.L.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Heptamethine Cyanine-Loaded Nanomaterials for Cancer Immuno-Photothermal/Photodynamic Therapy: A Review. Pharmaceutics 2022, 14, 1015. [Google Scholar] [CrossRef] [PubMed]
- Gournaris, E.; Park, W.; Cho, S.; Bentrem, D.J.; Larson, A.C.; Kim, D.-H. Near-Infrared Fluorescent Endoscopic Image-Guided Photothermal Ablation Therapy of Colorectal Cancer Using Dual-Modal Gold Nanorods Targeting Tumor-Infiltrating Innate Immune Cells in a Transgenic TS4 CRE/APCloxΔ468 Mouse Model. ACS Appl. Mater. Interfaces 2019, 11, 21353–21359. [Google Scholar] [CrossRef]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).