Pharmacoscintigraphy: Advancing Nanotheranostic Development Through Radionuclide Imaging
Abstract
1. Introduction
2. Contribution of Radioisotopic Imaging Techniques to Nanomedicine
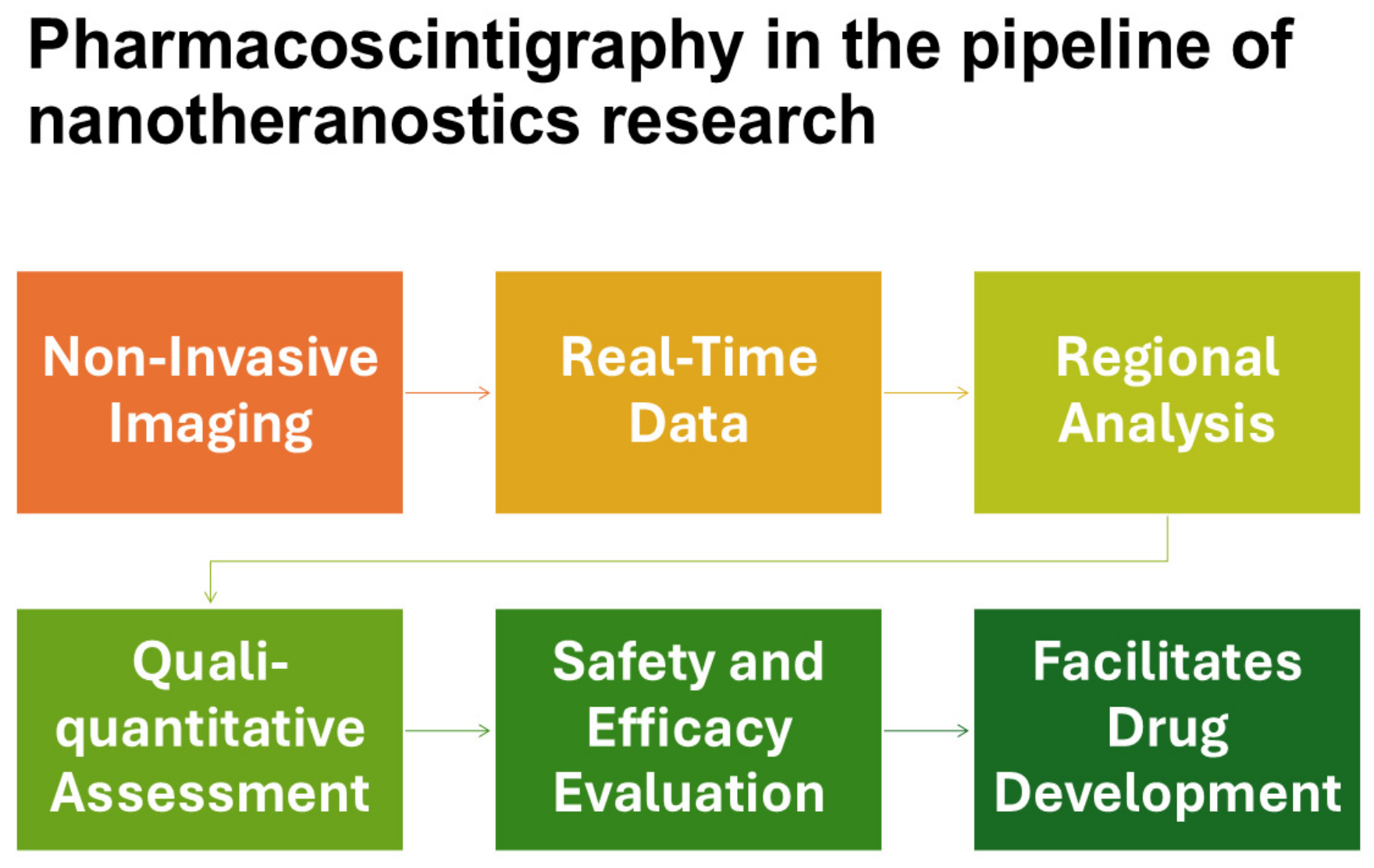
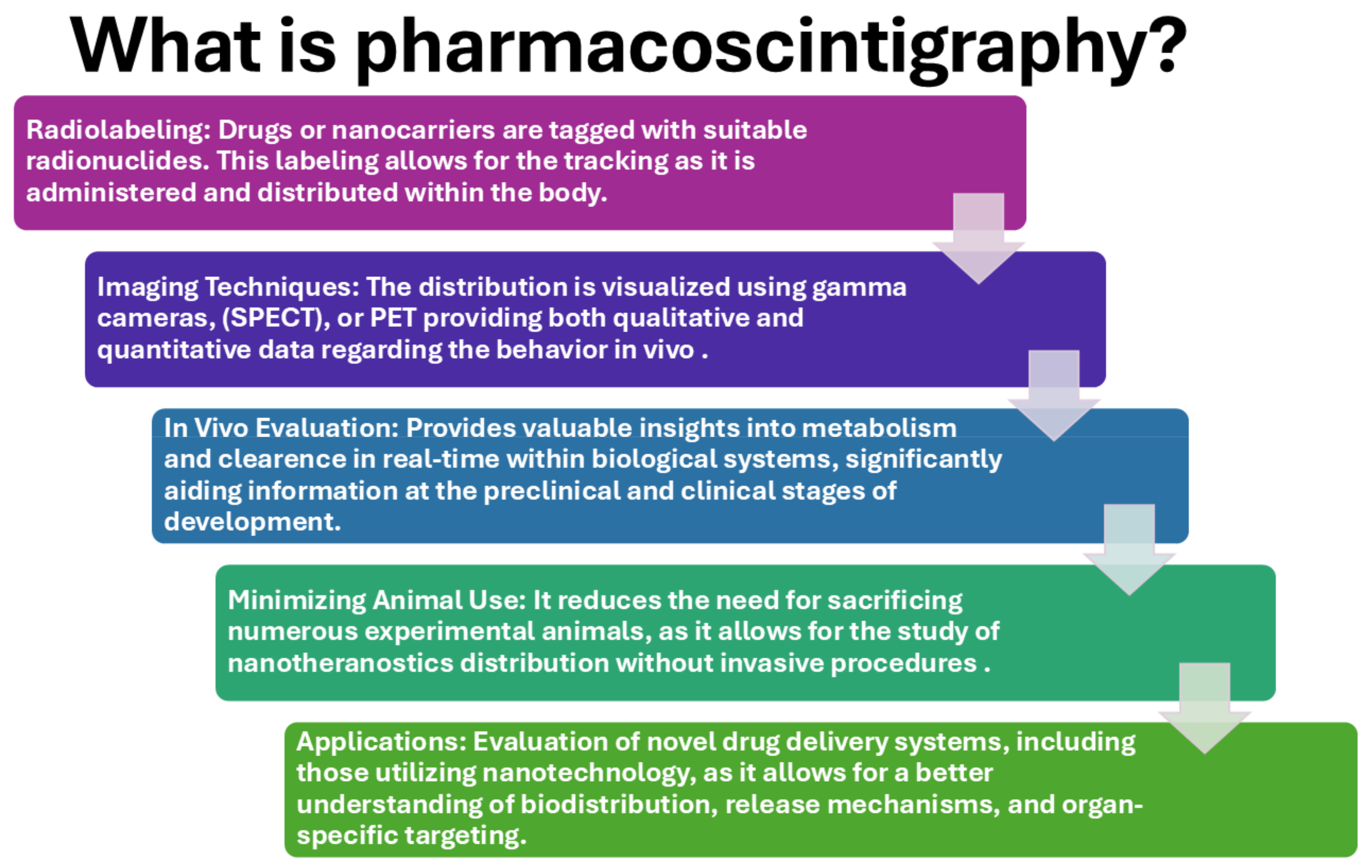
3. Pharmacoscintigraphy in the Development of Nanotheranostics
3.1. Imaging Modalities
3.1.1. Gamma Scintigraphy
3.1.2. Single-Photon Emission Computed Tomography (SPECT)
3.1.3. Positron Emission Tomography (PET)
3.2. Radiolabeling Techniques
3.2.1. Direct Radiolabeling
3.2.2. Chelator-Based Radiolabeling
3.2.3. Covalent Radiolabeling
3.2.4. Encapsulation
3.2.5. Neutron Activation
3.3. Limitations of Imaging Techniques
4. Pharmacoscintigraphy Applications for ADME Studies in Nanomedicine Research
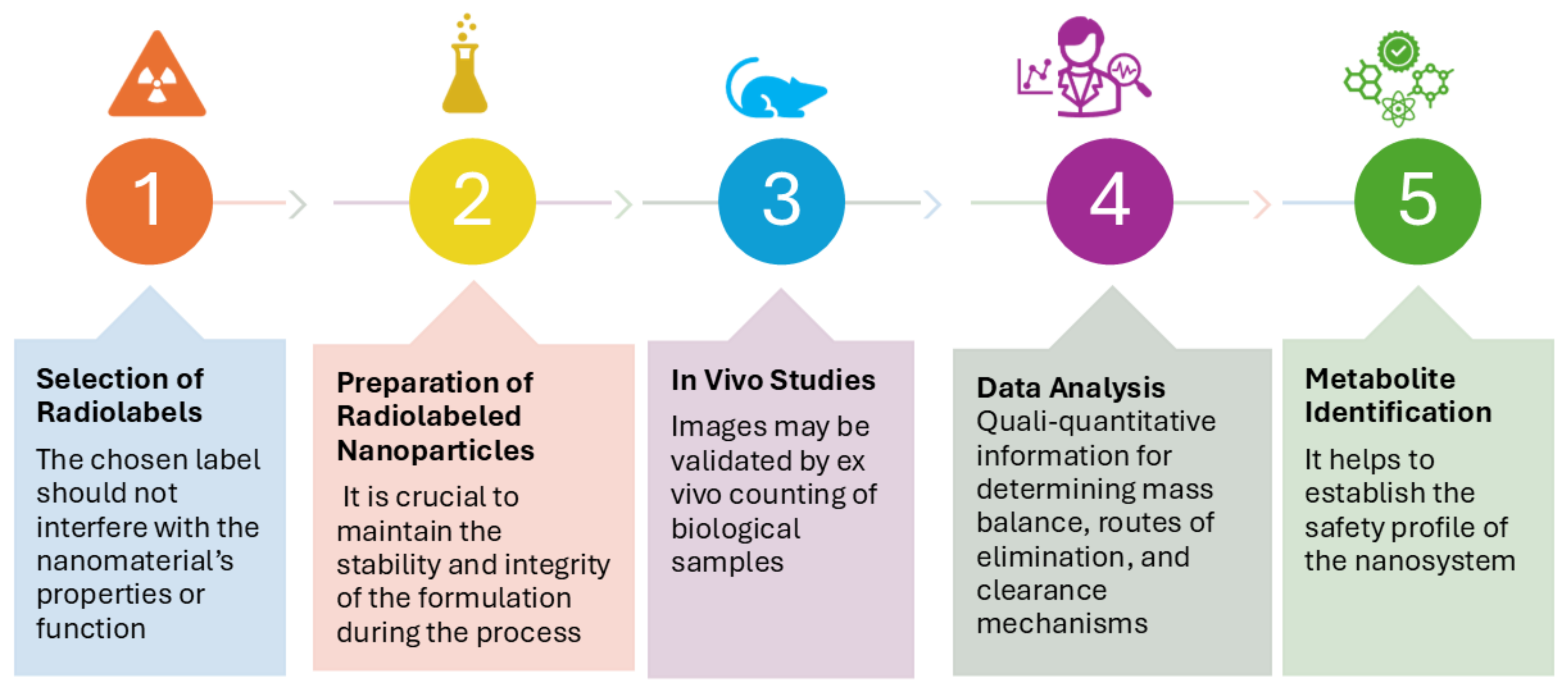
4.1. Objectives
- Determination of Mass Balance: To compare the amount of administered radioactivity to the amount recovered in excreta.
- Routes of Elimination: To identify routes of elimination and evaluate the extent of absorption.
- Metabolite Identification: To identify circulatory and excretory metabolites.
- Clearance Mechanisms: To determine the mechanisms of clearance (renal, biliary, metabolic).
- Distribution Characterization: To characterize the distribution of the compound within tissues and organs.
- Exposure Determination: To ascertain the exposure levels of the parent compound and its metabolites.
- Validation of Animal Models: To help validate the animal species used for toxicological testing.
- Pharmacological/Toxicological Contribution: To explore whether metabolites contribute to the pharmacological or toxicological effects of the drug.
4.2. Why Pharmacoscintigraphic ADME Studies Are Recommended
4.3. How Pharmacoscintigraphic ADME Studies Are Conducted for Nanomedicines
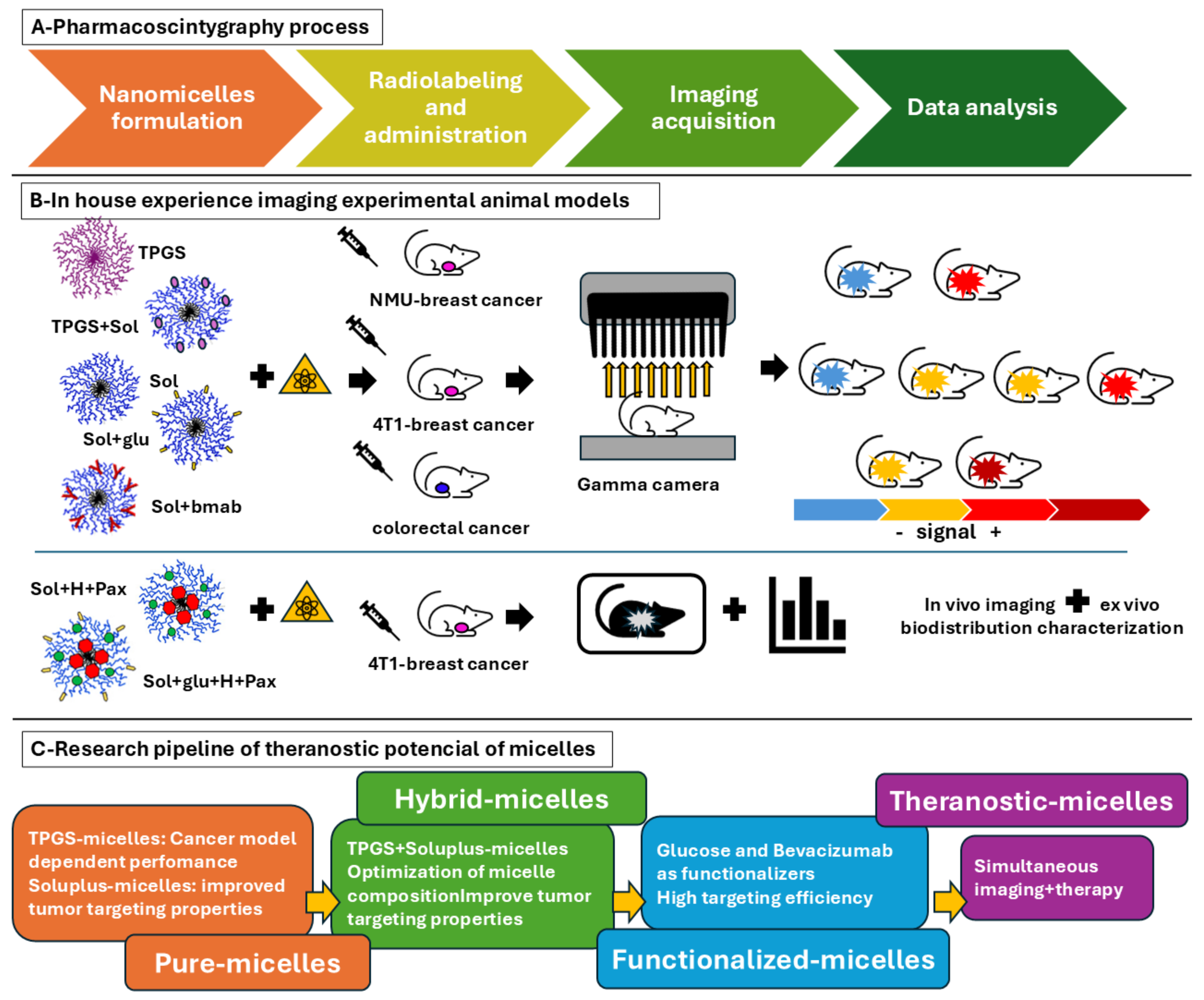
5. In-House Pharmacoscintigraphy Experience: Radiolabeled Nanomicelles in Cancer
6. Discussion and Future Perspectives: Enhancing Nanotheranostics Through Pharmacoscintigraphy
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADME | Absorption, Distribution, Metabolism, Excretion |
| CT | Computed Tomography |
| DNA | Desoxyribonucleic Acid |
| EPR | Enhanced Permeability and Retention |
| GLUT-1 | Glucose Transporter 1 |
| GMP | Good Manufacturing Practices |
| MRI | Magnetic Resonance Imaging |
| NMU | N-nitroso-N-methylurea |
| PET | Positron Emission Tomography |
| PK | Pharmacokinetics |
| Sol | Soluplus® |
| Sol+glu | Soluplus® Micelle Functionalized with Glucose |
| Sol+bmab | Soluplus® Micelle Functionalized with Bevacizumab |
| Sol+H+Pax | Soluplus® Micelle Loaded with Histamine and Paclitaxel |
| Sol+glu+H+Pax | Soluplus® Micelle Functionalized with Glucose Loaded with Histamine and Paclitaxel |
| SPECT | Single Photon Emission Computed Tomography |
| TPGS | D-α-tocopheryl Polyethylene Glycol Succinate |
| TPGS+sol | Hybrid Micelle with Soluplus®+TPGS |
| US | United States |
| VEGF | Vascular Endothelial Growth Factor |
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Kawish, S.M.; Panda, S.K.; Tarique, M.; Malik, A.; Afaq, S.; Al-Samghan, A.S.; Iqbal, J.; Alam, K.; Rahman, M. Nanomedicinal strategies as efficient therapeutic interventions for delivery of cancer vaccines. Semin. Cancer Biol. 2021, 69, 43–51. [Google Scholar] [CrossRef]
- Su, X.; Zhang, X.; Liu, W.; Yang, X.; An, N.; Yang, F.; Sun, J.; Xing, Y.; Shang, H. Advances in the application of nanotechnology in reducing cardiotoxicity induced by cancer chemotherapy. Semin. Cancer Biol. 2022, 86, 929–942. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef]
- Nevins, S.; McLoughlin, C.D.; Oliveros, A.; Stein, J.B.; Rashid, M.A.; Hou, Y.; Jang, M.H.; Lee, K.B. Nanotechnology approaches for prevention and treatment of chemotherapy-induced neurotoxicity, neuropathy, and cardiomyopathy in breast and ovarian cancer survivors. Small 2024, 14, e2300744. [Google Scholar] [CrossRef] [PubMed]
- Bagherifar, R.; Kiaie, S.H.; Hatami, Z.; Ahmadi, A.; Sadeghnejad, A.; Baradaran, B.; Jafari, R.; Javadzadeh, Y. Nanoparticle-mediated synergistic chemoimmunotherapy for tailoring cancer therapy: Recent advances and perspectives. J. Nanobiotechnol. 2021, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Yang, C.; Wu, Y.; Ru, G.; He, X.; Tong, X.; Wang, S. Nanocarriers surface engineered with cell membranes for cancer targeted chemotherapy. J. Nanobiotechnol. 2022, 20, 45. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in cancer: Reviewing the combination of hyperthermia and triggered chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Ahmad, A.; Rashid, S.; Chaudhary, A.A.; Alawam, A.S.; Alghonaim, M.I.; Raza, S.S.; Khan, R. Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin. Cancer Biol. 2023, 89, 38–60. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Desai, A.; Lustberg, M.B.; Lyman, G.H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022, 19, 681–697. [Google Scholar] [CrossRef]
- Jiang, D.; Rosenkrans, Z.T.; Ni, D.; Lin, J.; Huang, P.; Cai, W. Nanomedicines for renal management: From imaging to treatment. Acc. Chem. Res. 2020, 53, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020, 156, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Sofias, A.M.; van der Meel, R.; Schiffelers, R.; Storm, G.; Tacke, F.; Koschmieder, S.; Brümmendorf, T.H.; Kiessling, F.; Metselaar, J.M. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 2020, 15, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Chen, X.; Dobrovolskaia, M.A.; Lammers, T. Cancer nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, D.; Zhao, J.; Yang, G.; Guo, M.; Wang, Y.; Ren, X.; Kim, J.S.; Jin, L.; Tian, Z.; et al. Thermal immuno-nanomedicine in cancer. Nat. Rev. Clin. Oncol. 2023, 20, 116–134. [Google Scholar] [CrossRef]
- Mansoori, G.; Fauzi Soelaiman, T. Nanotechnology—An Introduction for the Standards Community; ASTM International: Chicago, IL, USA, 2005; Volume 2, pp. 1–22. [Google Scholar] [CrossRef]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef] [PubMed]
- National Nanotechnology Initiative (NNI). Available online: www.nano.gov (accessed on 22 July 2019).
- Allhoff, F. On the Autonomy and Justification of Nanoethics. Nanoethics 2007, 1, 185–210. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Taniguchi, N.; Arakawa, C.; Kobayashi, T. On the basic concept of nanotechnology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Kumar, V.; Bayda, S.; Hadla, M.; Caligiuri, I.; Russo Spena, C.; Palazzolo, S.; Kempter, S.; Corona, G.; Toffoli, G.; Rizzolio, F. Enhanced Chemotherapeutic Behavior of Open-Caged DNA@ Doxorubicin Nanostructures for Cancer Cells. J. Cell. Physiol. 2016, 231, 106–110. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 8, 2193. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Haripriyaa, M.; Suthindhiran, K. Pharmacokinetics of nanoparticles: Current knowledge, future directions and its implications in drug delivery. Future J. Pharm. Sci. 2023, 9, 113. [Google Scholar] [CrossRef]
- Tuguntaev, R.G.; Hussain, A.; Fu, C.; Chen, H.; Tao, Y.; Huang, Y.; Liu, L.; Liang, X.J.; Guo, W. Bioimaging guided pharmaceutical evaluations of nanomedicines for clinical translations. J. Nanobiotechnol. 2022, 20, 236. [Google Scholar] [CrossRef]
- Park, S.M.; Baek, S.; Lee, J.H.; Woo, S.K.; Lee, T.S.; Park, H.S.; Lee, J.; Kang, Y.K.; Kang, S.Y.; Yoo, M.Y.; et al. In vivo tissue pharmacokinetics of ERBB2-specific binding oligonucleotide-based drugs by PET imaging. Clin. Transl. Sci. 2023, 16, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Sudha, R.; Gaurav, M.; Krishna, C.; Kumar, S.R. Chapter 6: Nuclear Scintigraphy: A promising tool to evaluate novel and nano-based drug delivery systems. Nanotheranostics 2013, 4, 165–185. [Google Scholar]
- Digenis, G.A.; Sandefer, E.P.; Page, R.C.; Doll, W.J. Gamma scintigraphy: An evolving technology in pharmaceutical formulation development-Part 1. Pharm. Sci. Technol. Today 1998, 1, 100–108. [Google Scholar] [CrossRef]
- Terán, M.; Savio, E.; Paolino, A.; Frier, M. Hydrophilic and lipophilic radiopharmaceuticals as tracers in pharmaceutical development: In vitro–In vivo studies. BMC Nucl. Med. 2005, 5, 5. [Google Scholar] [CrossRef]
- Jain, S.; Dani, P.; Sharma, R.K. Pharmacoscintigraphy: A blazing trail for the evaluation of new drugs and delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 373–426. [Google Scholar] [CrossRef]
- Thombre, A.G.; Shamblin, S.L.; Malhotra, B.K.; Connor, A.L.; Wilding, I.R.; Caldwell, W.B. Pharmacoscintigraphy studies to assess the feasibility of a controlled release formulation of ziprasidone. J. Control. Release 2015, 213, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Khanna, K.; Kumar, N.; Karwasra, R.; Janakiraman, A.K.; Rajagopal, M.S. Illuminating Insights: Clinical Study Harnessing Pharmacoscintigraphy for Permeation Study of Radiolabeled Nimesulide Topical Formulation in Healthy Human Volunteers. ASSAY Drug Dev. Technol. 2023, 21, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Preisig, D.; Varum, F.; Bravo, R.; Hartig, C.; Spleiss, J.; Abbes, S.; Caobelli, F.; Wild, D.; Puchkov, M.; Huwyler, J.; et al. Colonic delivery of metronidazole-loaded capsules for local treatment of bacterial infections: A clinical pharmacoscintigraphy study. Eur. J. Pharm. Biopharm. 2021, 165, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sandal, N.; Mittal, G.; Bhatnagar, A.; Pathak, D.P.; Singh, A.K. Preparation, Characterization, and In Vivo Pharmacoscintigraphy Evaluation of an Intestinal Release Delivery System of Prussian Blue for Decorporation of Cesium and Thallium. J. Drug Deliv. 2017, 2017, 4875784. [Google Scholar] [CrossRef]
- Foppoli, A.; Maroni, A.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Palugan, L.; Cerea, M.; Gazzaniga, A. In vitro and human pharmacoscintigraphic evaluation of an oral 5-ASA delivery system for colonic release. Int. J. Pharm. 2019, 572, 118723. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Sig. Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhardwaj, N.; Bhatnagar, A. Pharmacoscintigraphy: An unexplored modality in India. Indian J. Pharm. Sci. 2004, 66, 18–25. [Google Scholar]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target. 2011, 19, 409–417. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations, and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Mettler, F.A.; Guiberteau, M.J. Essentials of Nuclear Medicine Imaging, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Hess, S.; Blomberg, B.A.; Zhu, H.J.; Høilund-Carlsen, P.F.; Alavi, A. The pivotal role of FDG-PET/CT in modern medicine. Acad. Radiol. 2014, 21, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Long, N.J. Radiopharmaceuticals for imaging and therapy. Dalton Trans. 2011, 21, 6067. [Google Scholar] [CrossRef]
- Polyak, A.; Ross, T.L. Nanoparticles for SPECT and PET Imaging: Towards Personalized Medicine and Theranostics. Curr. Med. Chem. 2018, 25, 4328–4353. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Varani, M.; Bentivoglio, V.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: SPECT Use (Part 1). Biomolecules 2022, 20, 1522. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, J.; Wang, Y.; Jiao, C.; Song, Z.; Ma, Y.; Ding, Y.; Zhang, Z.; He, X. Radiolabeling of Nanomaterials: Advantages and Challenges. Front. Toxicol. 2021, 3, 753316. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Hong, H.; Chen, F.; Valdovinos, H.F.; Hernandez, R.; Barnhart, T.E.; Cai, W. Hollow mesoporous silica nanoparticles for tumor vasculature targeting and PET image-guided drug delivery. Nanomedicine 2015, 10, 1233. [Google Scholar] [CrossRef]
- Lamb, J.; Holland, J.P. Advanced Methods for Radiolabeling Multimodality Nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018, 59, 382–389. [Google Scholar] [CrossRef]
- Cheng, L.; Shen, S.; Jiang, D.; Jin, Q.; Ellison, P.A.; Ehlerding, E.B.; Goel, S.; Song, G.; Huang, P.; Barnhart, T.E.; et al. Chelator-Free Labeling of Metal Oxide Nanostructures with Zirconium-89 for Positron Emission Tomography Imaging. ACS Nano 2017, 11, 12, 12193–12201. [Google Scholar] [CrossRef]
- Munaweera, I.; Shi, Y.; Koneru, B.; Saez, R.; Aliev, A.; Di Pasqua, A.J.; Balkus, K.J., Jr. Chemoradiotherapeutic Magnetic Nanoparticles for Targeted Treatment of Nonsmall Cell Lung Cancer. Mol. Pharm. 2015, 12, 3588–3596. [Google Scholar] [CrossRef]
- Notni, J.; Wester, H.J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Labelled Comp. Radiopharm. 2018, 61, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Garcés, M.; Magnani, N.D.; Pecorelli, A.; Calabró, V.; Marchini, T.; Cáceres, L.; Pambianchi, E.; Galdoporpora, J.; Vico, T.; Salgueiro, J.; et al. Alterations in oxygen metabolism are associated to lung toxicity triggered by silver nanoparticles exposure. Free Radic. Biol. Med. 2021, 166, 324–336. [Google Scholar] [CrossRef]
- Garcés, M.; Marchini, T.; Cáceres, L.; Calabró, V.; Mebert, A.M.; Tuttolomondo, M.V.; Vico, T.; Vanasco, V.; Tesan, F.; Salgueiro, J.; et al. Oxidative metabolism in the cardiorespiratory system after an acute exposure to nickel-doped nanoparticles in mice. Toxicology 2021, 464, 153020. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Bernabeu, E.; Bertera, F.; Garces, M.; Oppezzo, J.; Zubillaga, M.; Evelson, P.; Salgueiro, M.J.; Moretton, M.A.; Höcht, C.; et al. Dual strategy to improve the oral bioavailability of efavirenz employing nanomicelles and curcumin as a bio-enhancer. Int. J. Pharm. 2024, 651, 123734. [Google Scholar] [CrossRef] [PubMed]
- Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin-Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.L.; Salgueiro, J.; Bernabeu, E.; Gonzalez, L.; Manca, M.L.; Amiano, N.O.; Valenti, D.; Manconi, M.; García, V.E.; et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101170. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; Moretton, M.A.; Chiappetta, D.A.; Salgueiro, M.J.; Fornés, M.W.; Manucha, W. Polymeric Nanomicelles Loaded with Anandamide and Their Renal Effects as a Therapeutic Alternative for Hypertension Treatment by Passive Targeting. Pharmaceutics 2023, 15, 176. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Medina, C.; Teunissen, A.J.P.; Kluza, E.; Mulder, W.J.M.; van der Meel, R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliv. Rev. 2020, 154–155, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M.; Scholten, B.; Neumaier, B. New developments in the production of theranostic pairs of radionuclides. J. Radioanal. Nucl. Chem. 2018, 318, 1493–1509. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.J.; de Rosales, R.T. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Quastel, M.; Richter, A.; Levy, J. Tumour scanning with indium-111 dihaematoporphyrin ether. Br. J. Cancer 1990, 62, 885–890. [Google Scholar] [CrossRef]
- Franken, P.R.; Guglielmi, J.; Vanhove, C.; Koulibaly, M.; Defrise, M.; Darcourt, J.; Pourcher, T. Distribution and dynamics of (99m)Tc-pertechnetate uptake in the thyroid and other organs assessed by single-photon emission computed tomography in living mice. Thyroid 2010, 20, 519–526. [Google Scholar] [CrossRef]
- Bottenus, B.N.; Kan, P.; Jenkins, T.; Ballard, B.; Rold, T.L.; Barnes, C.; Cutler, C.; Hoffman, T.J.; Green, M.A.; Jurisson, S.S. Gold(III) bis-thiosemicarbazonato complexes: Synthesis, characterization, radiochemistry and X-ray crystal structure analysis. Nucl. Med. Biol. 2010, 37, 41–49. [Google Scholar] [CrossRef]
- Czernin, J.; Satyamurthy, N.; Schiepers, C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 2010, 51, 1826–1829. [Google Scholar] [CrossRef]
- Sephton, R.G.; Hodgson, G.S.; De Abrew, S.; Harris, A.W. Ga-67 and Fe-59 distributions in mice. J. Nucl. Med. 1978, 19, 930–935. [Google Scholar] [PubMed]
- Chilton, H.M.; Witcofski, R.L.; Watson, N.E., Jr.; Heise, C.M. Alteration of gallium-67 distribution in tumor-bearing mice following treatment with methotrexate: Concise communication. J. Nucl. Med. 1981, 22, 1064–1068. [Google Scholar]
- Spetz, J.; Rudqvist, N.; Forssell-Aronsson, E. Biodistribution and dosimetry of free 211At, 125I- and 131I- in rats. Cancer Biother. Radiopharm. 2013, 28, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Dash, A. 64Cu2+ Ions as PET Probe: An Emerging Paradigm in Molecular Imaging of Cancer. Mol. Pharm. 2016, 13, 3601–3612. [Google Scholar] [CrossRef]
- Piccardo, A.; Paparo, F.; Puntoni, M.; Righi, S.; Bottoni, G.; Bacigalupo, L.; Zanardi, S.; DeCensi, A.; Ferrarazzo, G.; Gambaro, M.; et al. 64CuCl2 PET/CT in Prostate Cancer Relapse. J. Nucl. Med. 2018, 59, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef]
- Graves, S.A.; Hernandez, R.; Fonslet, J.; England, C.G.; Valdovinos, H.F.; Ellison, P.A.; Barnhart, T.E.; Elema, D.R.; Theuer, C.P.; Cai, W.; et al. Novel Preparation Methods of (52)Mn for Immuno PET Imaging. Bioconj. Chem. 2015, 26, 2118–2124. [Google Scholar] [CrossRef]
- Vakili, A.; Jalilian, A.R.; Yavari, K.; Shirvani-Arani, S.; Khanchi, A.; Bahrami-Samani, A.; Salimi, B.; Khorrami-Moghadam, A. Preparation and quality control and biodistribution studies of [90Y]-DOTA-cetuximab for radioimmunotherapy. J. Radioanal. Nucl. Chem. 2013, 296, 1287–1294. [Google Scholar] [CrossRef]
- Breeman, W.A.; van der Wansem, K.; Bernard, B.F.; van Gameren, A.; Erion, J.L.; Visser, T.J.; Krenning, E.P.; de Jong, M. The addition of DTPA to [177Lu-DOTA0,Tyr3] octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur. J. Nucl. Med. 2003, 30, 312–315. [Google Scholar] [CrossRef]
- Dadachova, E.; Bouzahzah, B.; Zuckier, L.S.; Pestell, R.G. Rhenium-188 as an alternative to Iodine-131 for treatment of breast tumors expressing the sodium/iodide symporter (NIS). Nucl. Med. Biol. 2002, 29, 13–18. [Google Scholar] [CrossRef]
- Sukthankar, P.; Avila, A.; Whitaker, S.; Iwamoto, T.; Morgenstern, A.; Apostolidis, C.; Liu, K.; Hanzlik, R.; Dadachova, E.; Tomich, J. Branched amphiphilic peptide capsules: Cellular uptake and retention of encapsulated solutes. Biochim. Biophys. Acta 2014, 1838, 2296–2305. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection. Radiological Protection in Medicine. Annals of the ICRP; SAGE Publications Ltd.: London, UK, 2016; Volume 46, pp. 1–144. [Google Scholar]
- U.S. Food and Drug Administration. Ethical Considerations for Clinical Investigations of Medical Products Involving Children; Food and Drug Administration: Silver Spring, MD, USA, 2022.
- Boschi, S.; Lee, J.T.; Beyer, G. Radiopharmaceuticals for PET and SPECT imaging: A literature review over the last decade. Int. J. Mol. Sci. 2002, 23, 5023. [Google Scholar] [CrossRef]
- Dennahy, I.S.; Han, Z.; MacCuaig, W.M.; Chalfant, H.M.; Condacse, A.; Hagood, J.M.; Claros-Sorto, J.C.; Razaq, W.; Holter-Chakrabarty, J.; Squires, R.; et al. Nanotheranostics for image-guided cancer treatment. Pharmaceutics 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Selvaraj, K. Choice of Nanoparticles for Theranostics Engineering: Surface Coating to Nanovalves Approach. Nanotheranostics 2004, 8, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; van Bruggen, N.; Dinkelborg, L.; Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 591–607. [Google Scholar] [CrossRef]
- Penner, N.; Xu, L.; Prakash, C. Radiolabeled absorption, distribution, metabolism, and excretion studies in drug development: Why, when, and how? Chem. Res. Toxicol. 2012, 25, 513–531. [Google Scholar] [CrossRef]
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: Principles and concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Portillo, M.; Tesán, F.; Nicoud, M.; Medina, V.; Moretton, M.; Chiappetta, D.; Zubillaga, M. Design and development of nanoprobes radiolabelled with 99mTc for the diagnosis and monitoring of therapeutic interventions in oncology preclinical research. EJNMMI Radiopharm. Chem. 2024, 9, 74. [Google Scholar] [CrossRef]
- Tesán, F.C.; Portillo, M.G.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Salgueiro, M.J.; Zubillaga, M.B. Radiolabeling and biological characterization of TPGS-based nanomicelles by means of small animal imaging. Nucl. Med. Biol. 2017, 44, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tesán, F.C.; Nicoud, M.B.; Nuñez, M.; Medina, V.A.; Chiappetta, D.A.; Salgueiro, M.J. 99mTc-Radiolabeled TPGS nanomicelles outperform 99mTc-sestamibi as a breast cancer imaging agent. Contrast Media Mol. Imaging 2019, 2019, 4087895. [Google Scholar] [CrossRef] [PubMed]
- Tesán, F.C.; Portillo, M.; Medina, V.; Nuñez, M.; Zubillaga, M.; Salgueiro, M.J. Tumor imaging in experimental animal models of breast carcinoma with routinely available 99mTc-sestamibi. Int. J. Res. Pharm. Life Sci. 2015, 3, 254–259. [Google Scholar]
- Moretton, M.A.; Bernabeu, E.; Grotz, E.; Gonzalez, L.; Zubillaga, M.; Chiappetta, D.A. A glucose-targeted mixed micellar formulation outperforms Genexol in breast cancer cells. Eur. J. Pharm. Biopharm. 2017, 114, 305–316. [Google Scholar] [CrossRef]
- Nicoud, M.B.; Ospital, I.A.; Táquez Delgado, M.A.; Riedel, J.; Fuentes, P.; Bernabeu, E.; Rubinstein, M.R.; Lauretta, P.; Martínez Vivot, R.; Aguilar, M.; et al. Nanomicellar formulations loaded with histamine and paclitaxel as a new strategy to improve chemotherapy for breast cancer. Int. J. Mol. Sci. 2023, 24, 3546. [Google Scholar] [CrossRef]
| Feature | Gamma Scintigraphy | SPECT | PET |
|---|---|---|---|
| Spatial Resolution | Low | Moderate | High |
| Sensitivity | Moderate | High | Very High |
| Quantification | Limited | Semi-quantitative | Fully Quantitative |
| Cost | Low | Moderate | High |
| Applications |
|
|
|
| Radionuclide | Imaging Modality | Half-Life | Energy (keV) | Primary Applications |
|---|---|---|---|---|
| Fluorine-18 (18F) | PET | 109.8 min | 511 | Oncology, neurology, cardiology |
| Carbon-11 (11C) | PET | 20.4 min | 511 | Neurology, oncology, molecular imaging |
| Zirconium-89 (89Zr) | PET | 78.4 h | 511 | Immuno-PET, antibody labeling |
| Copper-64 (64Cu) | PET | 12.7 h | 511 | Radiotherapy, imaging of hypoxia |
| Gallium-68 (68Ga) | PET | 68 min | 511 | Peptide receptor imaging, neuroendocrine tumors |
| Technetium-99m (99mTc) | SPECT | 6.0 h | 140 | General nuclear medicine imaging |
| Indium-111 (111In) | SPECT | 2.8 d | 171, 245 | Infection imaging, leukocyte labeling |
| Iodine-123 (123I) | SPECT | 13.2 h | 159 | Thyroid imaging, neuroimaging |
| Luthetium-177 (177Lu) * | SPECT-therapy | 6.7 d | 113, 208 | Oncology |
| Chelator | Commonly Used Radionuclides | Application |
|---|---|---|
| DTPA (Diethylenetriaminepentaacetic acid) | 99mTc, 111In | SPECT imaging |
| DOTA (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid) | 177Lu, 68Ga, 64Cu | PET imaging and radiotherapy |
| NOTA (1,4,7-Triazacyclononane-1,4,7-triacetic acid) | 68Ga, 64Cu | PET imaging |
| DFO (Deferoxamine) | 89Zr | Immuno-PET imaging |
| Radiolabeling Method | Nanocarrier Examples | Radionuclides Used | Advantages | Limitations |
|---|---|---|---|---|
| Direct Radiolabeling | Gold, Iron Oxide NPs | 99mTc, 188Re | Simple and fast | Lower in vivo stability |
| Chelator-Based | Liposomes, Micelles | 68Ga, 177Lu, 64Cu | High stability | Requires chemical modification |
| Covalent Binding | Proteins, Peptides | 125I, 131I, 64Cu | Strong attachment | May alter nanocarrier properties |
| Encapsulation | Liposomes, Silica NPs | 111In, 99mTc | Maintains nanoparticle integrity | Risk of leakage |
| Neutron Activation | Holmium Oxide NPs | 166Ho, 89Zr | No chemical modification required | Limited availability |
| Nanotheranostic System | Radionuclide | Labeling Strategy | Application |
|---|---|---|---|
| Polymeric micelles | 99mTc | Direct adsorption | Tumor imaging |
| Liposomes | 111In | Encapsulation | Drug delivery tracking |
| Iron oxide nanoparticles | 89Zr | Chelation (DFO) | Long-term biodistribution studies |
| Mesoporous silica | 177Lu | Lattice incorporation | Radionuclide therapy |
| Nanomicelle Type | Functionalization | Tumor Uptake | Imaging Performance | Theranostic Potential |
|---|---|---|---|---|
| TPGS-Based Micelles | None | Low (4T1 model) High (NMU model) | Poor imaging contrast. Superior to 99mTc-sestamibi. | Limited as standalone therapy. Potential imaging agent. |
| Soluplus® Micelles | None | Moderate (EPR effect) | Good imaging contrast. | Potential for passive drug release. |
| Soluplus®+TPGS Micelles | None | High (4T1 model) | Improved tumor localization. | Synergistic tumor-targeting capability. |
| Soluplus® Micelles | Glucose Bevacizumab | High (GLUT1-mediated) Very High (VEGF-targeting) | Improved tumor imaging. Strong imaging contrast and retention. | Greater drug-targeting capability. Optimal for guided drug delivery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgueiro, M.J.; Moretton, M.A.; Medina, V.; Chiappetta, D.; Zubillaga, M. Pharmacoscintigraphy: Advancing Nanotheranostic Development Through Radionuclide Imaging. J. Nanotheranostics 2025, 6, 12. https://doi.org/10.3390/jnt6020012
Salgueiro MJ, Moretton MA, Medina V, Chiappetta D, Zubillaga M. Pharmacoscintigraphy: Advancing Nanotheranostic Development Through Radionuclide Imaging. Journal of Nanotheranostics. 2025; 6(2):12. https://doi.org/10.3390/jnt6020012
Chicago/Turabian StyleSalgueiro, María Jimena, Marcela Analia Moretton, Vanina Medina, Diego Chiappetta, and Marcela Zubillaga. 2025. "Pharmacoscintigraphy: Advancing Nanotheranostic Development Through Radionuclide Imaging" Journal of Nanotheranostics 6, no. 2: 12. https://doi.org/10.3390/jnt6020012
APA StyleSalgueiro, M. J., Moretton, M. A., Medina, V., Chiappetta, D., & Zubillaga, M. (2025). Pharmacoscintigraphy: Advancing Nanotheranostic Development Through Radionuclide Imaging. Journal of Nanotheranostics, 6(2), 12. https://doi.org/10.3390/jnt6020012






