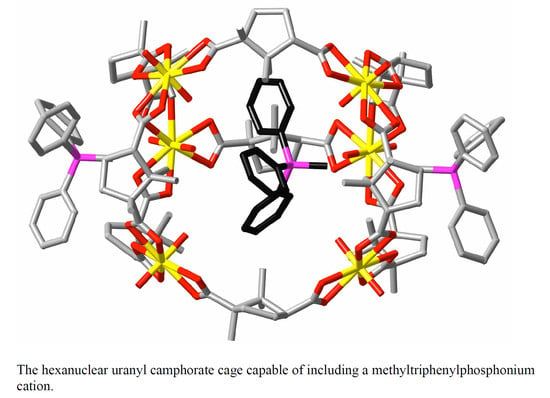
Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities
Abstract
:1. Introduction
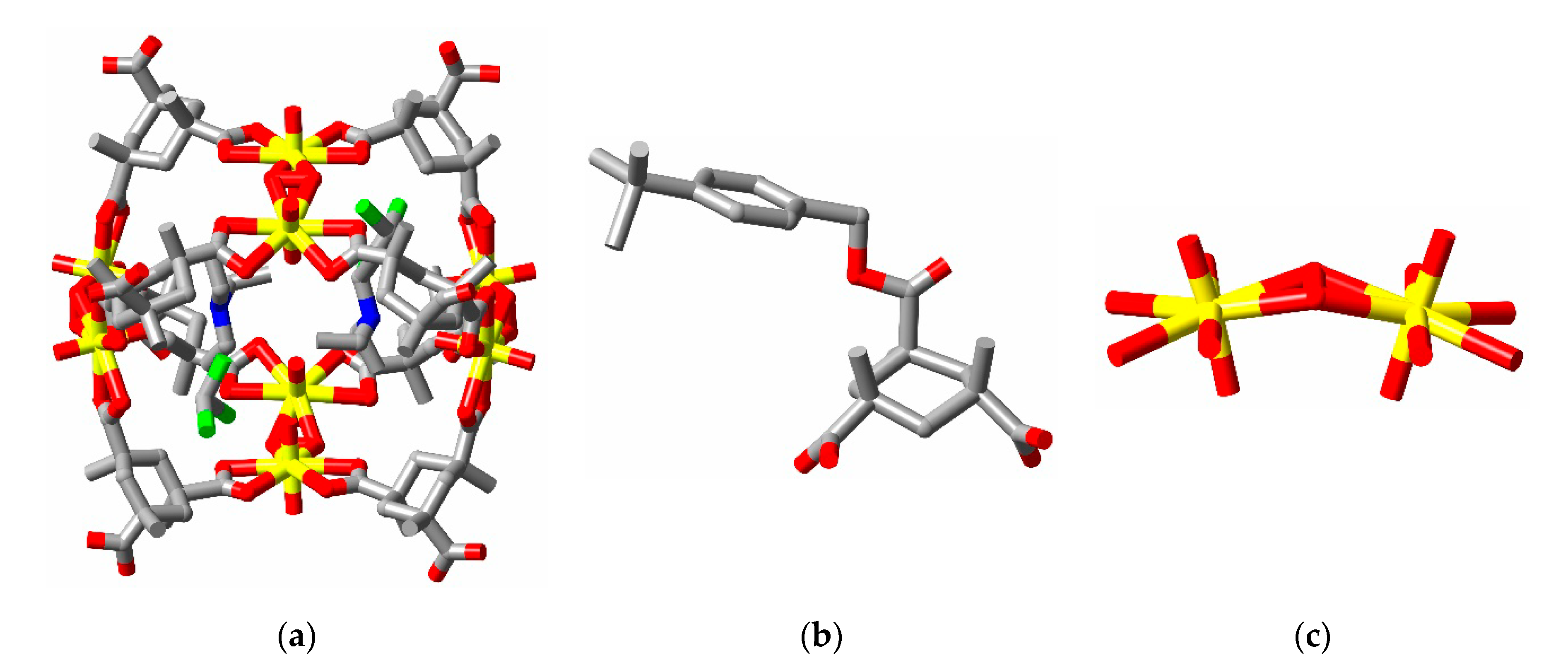
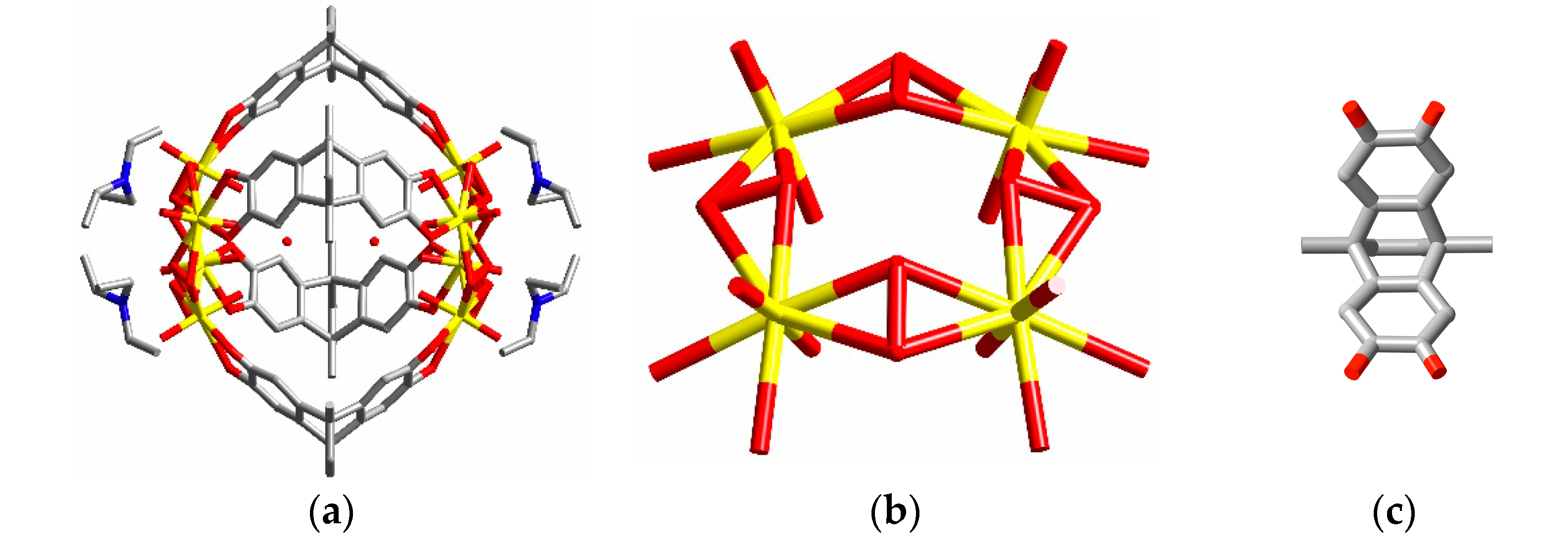
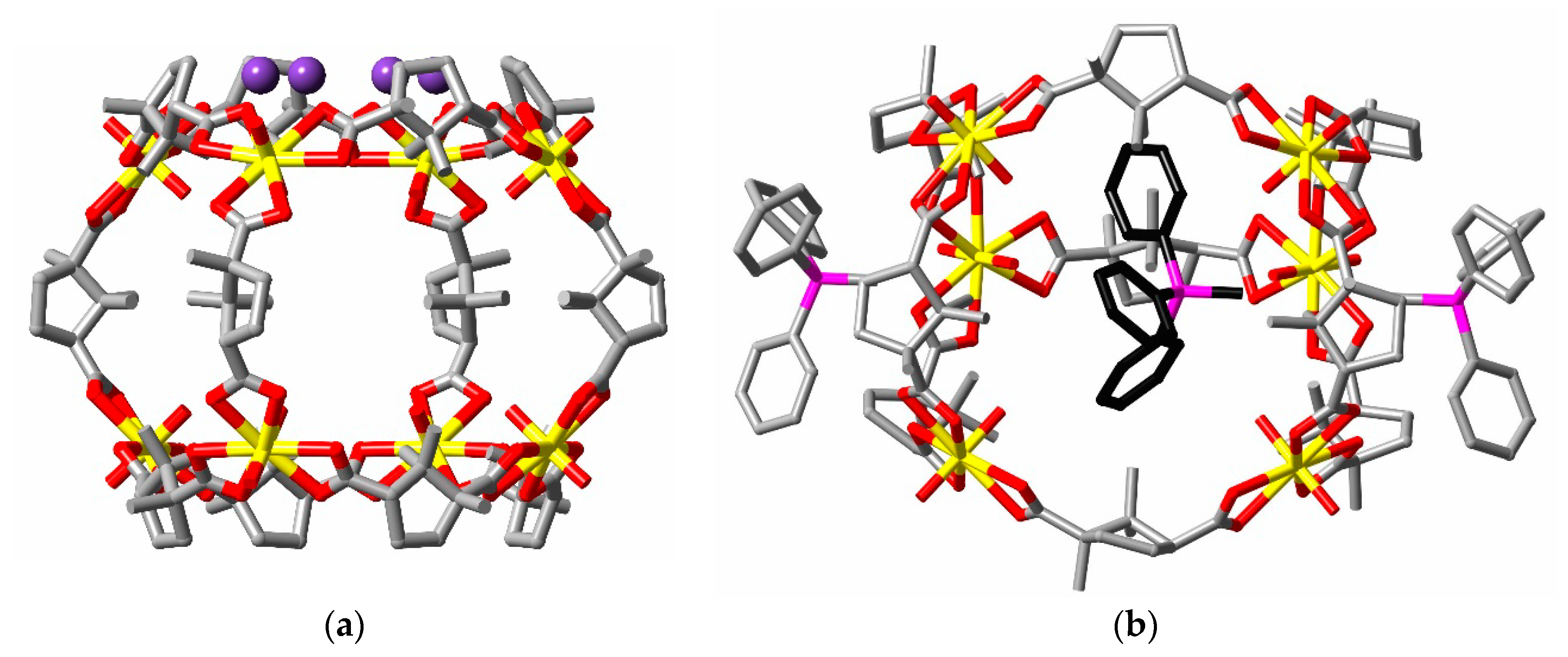
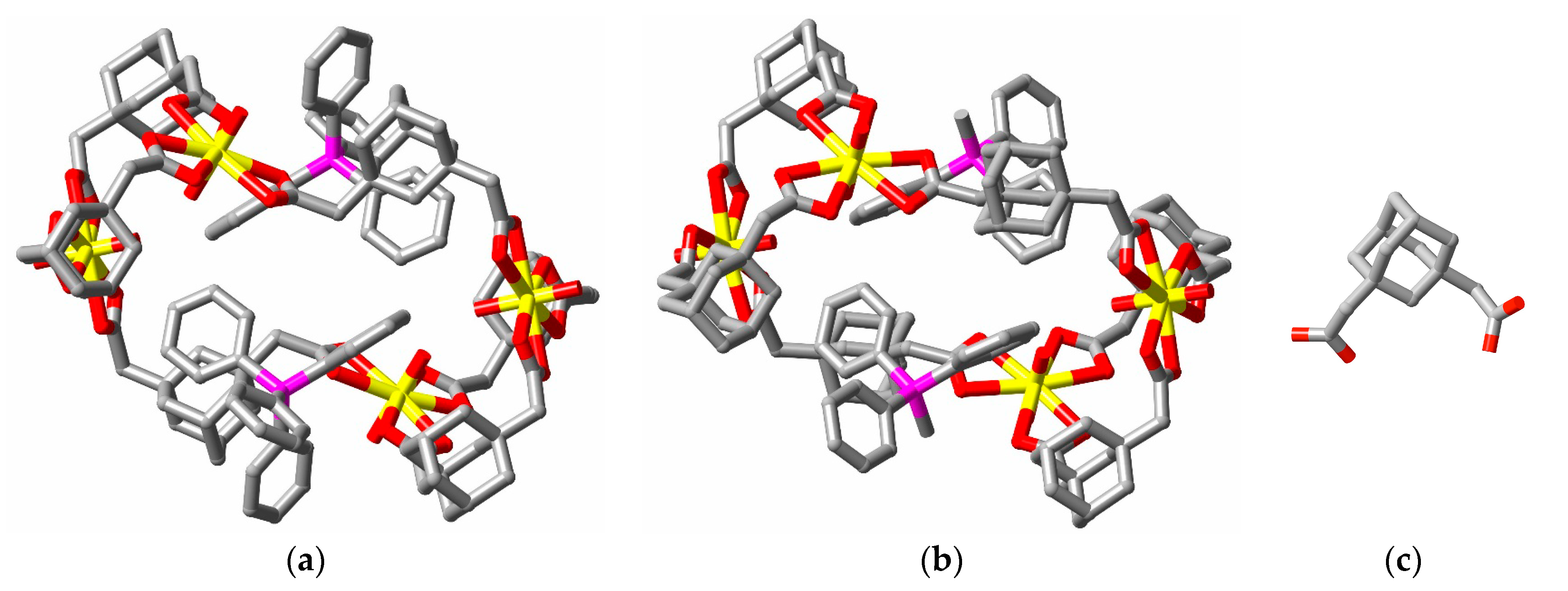
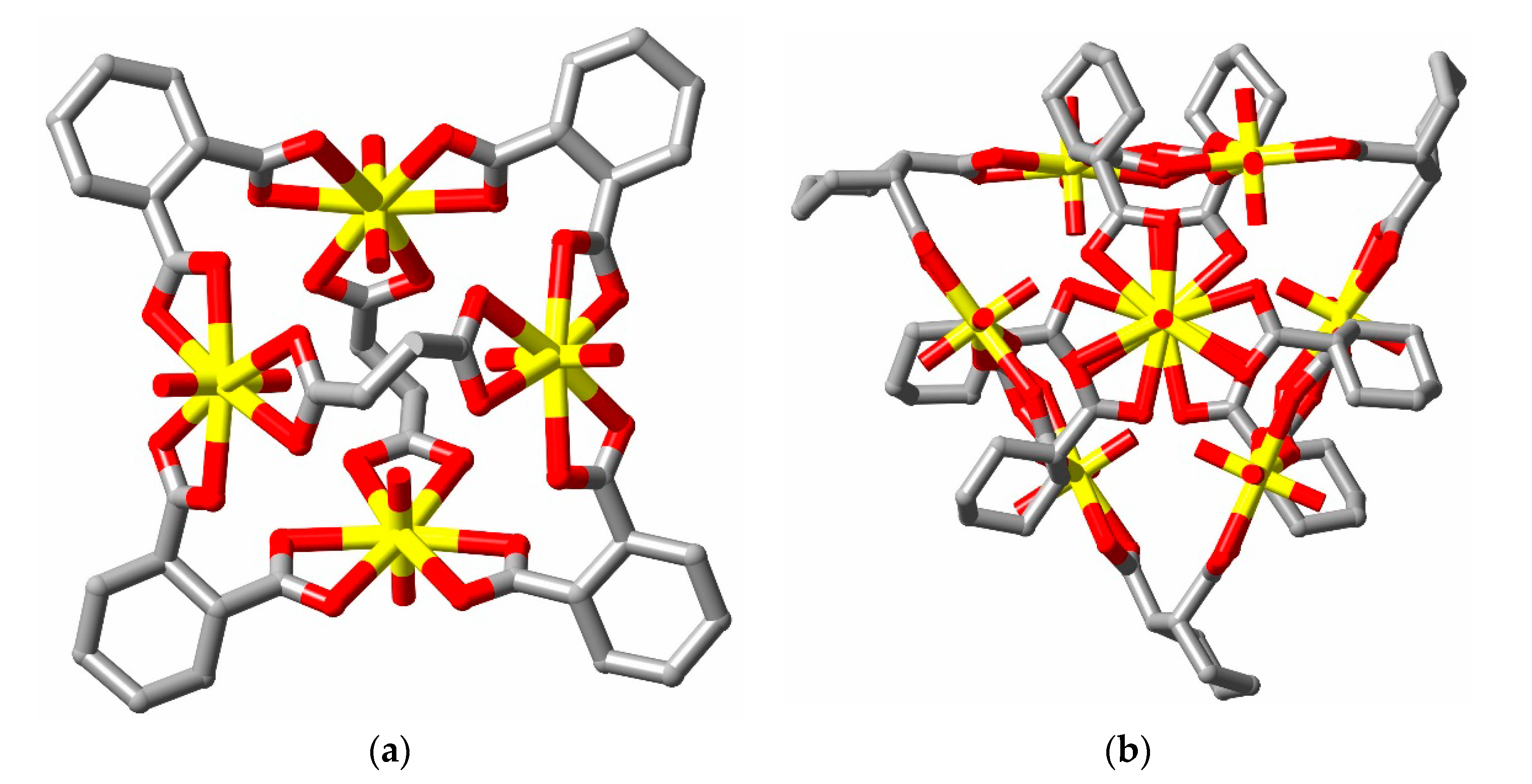
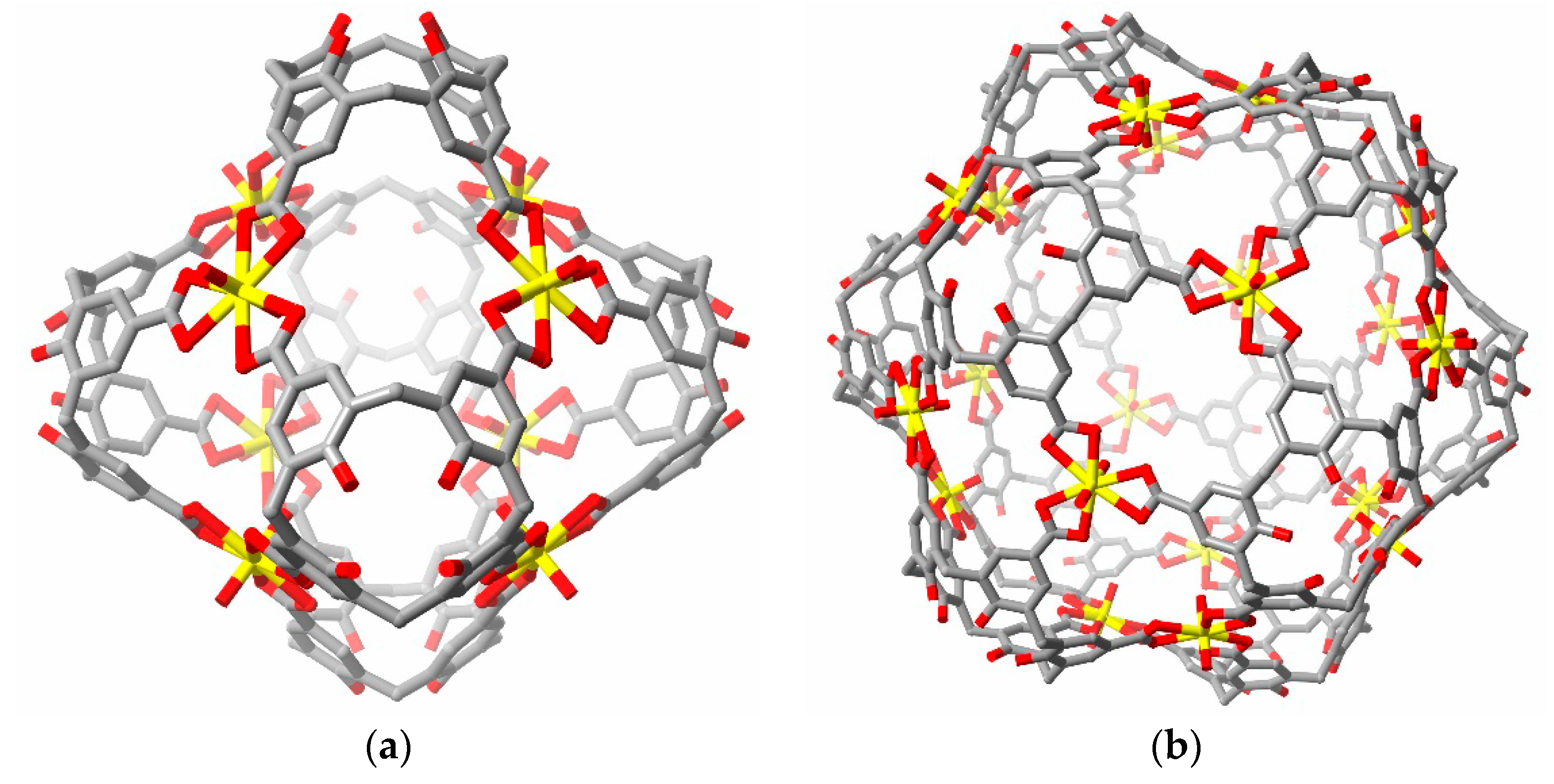
2. Discussion
3. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References and Notes
- Kaneko, K.; Rodríguez-Reinoso, F. (Eds.) Nanoporous Materials for Gas Storage. Green Energy and Technology; Springer: Singapore, 2019. [Google Scholar]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wöll, C. Chemical Reactions at Isolated Single-Sites inside Metal-Organic Frameworks. Catal. Lett. 2018, 48, 2201–2222. [Google Scholar] [CrossRef] [Green Version]
- Hooley, R.J. Rings and Things: The Magic of Building Self-Assembled Cages and Macrocycles. Inorg. Chem. 2018, 57, 3497–3499, and following articles. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Perman, J.A. (Eds.) Elaboration and Applications of Metal-Organic Frameworks; World Scientific: Singapore, 2017. [Google Scholar]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepulveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal-organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, J.; Xu, H.X.; Xiong, Y.J. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Zhuang, J.L.; Terfort, A.; Wöll, C. Formation of oriented and patterned films of metal-organic frameworks by liquid phase epitaxy: A review. Coord. Chem. Rev. 2016, 307, 391–424. [Google Scholar] [CrossRef]
- Liu, J.W.; Chen, L.F.; Cui, H.; Zhang, J.Y.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis and Functionality of Metal-Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, Y.; Kawano, M.; Fujita, M. Crystalline Molecular Flasks. Nat. Chem. 2011, 3, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kapelewski, M.T.; Runčevski, T.; Tarver, J.D.; Jiang, H.Z.H.; Hurst, K.E.; Parilla, P.A.; Ayala, A.; Gennett, T.; Fitzgerald, S.A.; Brown, C.M.; et al. Record High Hydrogen Storage Capacity in the Metal-Organic Framework Ni2(m-dobc) at Near-Ambient Temperature. Chem. Mater. 2018, 30, 8179–8189. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Hoshino, M.; Khutia, A.; Xing, H.; Inokuma, Y.; Fujita, M. The crystalline sponge method updated. IUCr J. 2016, 3, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Gee, W.J. The growing importance of crystalline molecular flasks and the crystalline sponge method. Dalton. Trans. 2017, 46, 15979–15986. [Google Scholar] [CrossRef] [Green Version]
- Sarakha, M.; Bolte, M.; Burrows, H.D. Electron-Transfer Oxidation of Chlorophenols by Uranyl Ion Excited State in Aqueous Solution. Steady-State and Nanosecond Flash Photolysis Studies. J. Phys. Chem. A 2000, 104, 3142–3149. [Google Scholar] [CrossRef] [Green Version]
- Burrows, H.D.; Kemp, T.J. The Photochemistry of the Uranyl Ion. Chem. Soc. Rev. 1974, 3, 139–165. [Google Scholar] [CrossRef]
- Yusov, A.B.; Shilov, V.P. Reduction of the photo-excited uranyl ion by water. Russ. Chem. Bull. 2000, 49, 285–290. [Google Scholar] [CrossRef]
- Deng, X.; Li, Z.; Garcia, H. Visible Light Induced Organic Tranformations over Metal-Organic-Frameworks (MOFs). Chem. Eur. J. 2017, 28, 11189–11209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Q.; El-Anizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Khandan, F.M.; Afzali, D.; Sargazi, G.; Gordan, M. Novel uranyl-curcumin-MOF photocatalysts with high-performance photocatalytic activity toward the degradation of phenol red from aqueous solution: Effective synthesis route, design and a controllable systematic study. J. Mater. Sci. Mater. Electron. 2018, 29, 18600–18613. [Google Scholar]
- Li, H.-H.; Zeng, X.-H.; Wu, H.-Y.; Jie, X.; Zheng, S.-T.; Chen, Z.-R. Incorporating Guest Molecules into Honeycomb Structures Constructed from Uranium(VI) Polycarboxylates: Structural Diversity and Photocatalytic Activity for the Degradation of Organic Dyes. Cryst. Growth Des. 2015, 15, 10–13. [Google Scholar] [CrossRef]
- Yang, W.; Tiang, W.-G.; Liu, X.-X.; Wang, L.; Sun, Z.-M. Syntheses, Structures, Luminescence and Photocatalytic Properties of a Series of Uranyl Coordination Polymers. Cryst. Growth Des. 2014, 14, 5904–5911. [Google Scholar] [CrossRef]
- Hou, Y.-N.; Xu, X.-T.; Xing, N.; Bai, F.-Y.; Duan, S.-B.; Sun, Q.; Wei, S.-Y.; Shi, Z.; Zhang, H.-Z.; Xing, Y.-H. Photocatalytic Application of 4f-5f Inorganic-Organic Frameworks: Influence of the Lanthanide Contraction on the Structure and Functional Properies of Uranyl-Lanthanide Complexes. ChemPlusChem 2014, 79, 1304–1315. [Google Scholar] [CrossRef]
- Kolinko, P.A.; Fillipov, T.N.; Kozlov, D.V.; Parmon, V.N. Ethanol vapour photocatalytic oxidation with uranyl-modified titania under visible light: Comparison with silica and alumina. J. Photochem. Photobiol. A Chem. 2012, 250, 72–77. [Google Scholar] [CrossRef]
- Wang, K.-X.; Chen, J.-S. Extended Structures and Physicochemical Properties of Uranyl-Organic Compounds. Acc. Chem. Res. 2011, 44, 531–540. [Google Scholar] [CrossRef]
- Krishna, V.; Kamble, V.S.; Gupta, N.M.; Selvam, P. Uranyl-Anchored MCM-41 as a Highly Efficient Photocatalyst in the Oxidative Destruction of Short-Chain Linear Alkanes: An in-situ FTIR Study. J. Phys. Chem. C 2008, 112, 15832–15843. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Li, G.-D.; Bi, M.-H.; Chen, J.-S. Preparation, Structures and Photocatalytic Properies of Three New Uranyl-Organic Assembly Compounds. Inorg. Chem. 2008, 47, 4844–4853. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Liao, Z.-L.; Jiang, Y.-S.; Li, G.-H.; Chen, J.-S. Water Insoluble Ag-U-Organic Assemblies with Photocatalytic Activity. Chem. Eur. J. 2005, 11, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Nieweg, J.A.; Lemma, K.; Trewyn, B.G.; Lin, V.S.-Y.; Bakac, A. Mesoporous Silica-Supported Uranyl: Synthesis and Photoreactivity. Inorg. Chem. 2005, 44, 5641–5648, and references therein. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; da G. Miguel, M. Applications and limitations of uranyl ion as a photophysical probe. Adv. Coll. Interf. Sci. 2001, 89–90, 485–496, and references therein. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Tamura, M.; Fujita, M. Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis. Science 2006, 312, 251–254. [Google Scholar] [CrossRef]
- Fiedler, D.; Leung, D.H.; Bergman, R.G.; Raymond, K.N. Selective Molecular Recognition, C–H Bond Activation and Catalysis in Nanocale Reaction Vessels. Acc. Chem. Res. 2005, 38, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Zhai, F.; Wang, Y.; Yue, G.; Li, Y.; Chu, M.; Wang, S. Assembly of porphyrin-based uranium organic frameworks with (3,4)-connected pto and tbo topologies. Dalton. Trans. 2019, 48, 1595–1598. [Google Scholar] [CrossRef]
- Hu, K.-Q.; Huang, Z.-W.; Zhang, Z.-H.; Mei, L.; Qian, B.-B.; Yu, J.-P.; Chai, Z.-F.; Shi, W.-P. Actinide-based Porphyrinic MOF as Dehydrogenation Catalyst. Chem. Eur. J. 2018, 24, 16766–16769. [Google Scholar] [CrossRef]
- Hu, F.; Di, Z.; Lin, P.; Huang, P.; Wu, M.; Jiang, F.; Hong, M. An Anionic Urnaium-Based Metal-Organic Framework with Ultralarge Nanocages for Selective Dye Adsorption. Cryst. Growth Des. 2018, 18, 576–580. [Google Scholar] [CrossRef]
- Hu, K.-Q.; Jiang, X.; Wang, C.-Z.; Mei, L.; Xie, Z.-N.; Tao, W.-Q.; Zhang, X.-L.; Chai, Z.-F.; Shi, W.-Q. Solvent-dependent Synthesis of Porous Anionic Uranyl-organic Frameworks Featuring Highly Symmetrical (3,4)-connected ctn or bor Topology for Selective Dye Adsorption. Chem. Eur. J. 2017, 23, 529–532. [Google Scholar] [CrossRef]
- Li, P.; Vermeulen, N.A.; Malliakas, C.D.; Gomez-Gualdron, D.A.; Howarth, A.J.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; O’Keeffe, M.; Farha, O.A. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 2017, 356, 624–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Vermeulen, N.A.; Gong, X.R.; Malliakas, C.D.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. Design and synthesis of a water-stable anionic uranium-based metal–organic framework (MOF) with ultra large pores. Angew. Chem. Int. Ed. 2016, 128, 10514–10518. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, Y.; Bai, Z.; Liu, W.; Wang, Y.; Xu, X.; Xiao, C.; Sheng, D.; Diwu, J.; et al. Umbellate Distortions of the Uranyl Coordination Environment Result in a Stable and Porous Polycatenated Framework that can Effectively Remove Cesium from Aqueous Solutions. J. Am. Chem. Soc. 2015, 137, 6144–6147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Chugtai, A.H.; Younus, H.A.; Verpoort, F. Discrete metal-carboxylate self-assembled cages: Design, synthesis and applications. Coord. Chem. Rev. 2014, 280, 1–27. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Recent advances in structural studies of heterometallic uranyl-containing coordination polymers and polynuclear closed species. Dalton. Trans. 2017, 46, 13660–13667. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, J.-S. MOFs of Uranium and the Actinides. Struct. Bond. (Berlin) 2015, 163, 265–296. [Google Scholar]
- Loiseau, T.; Mihalcea, I.; Henry, N.; Volkringer, C. The crystal chemistry of uranium carboxylates. Coord. Chem. Rev. 2014, 266–267, 69–109. [Google Scholar] [CrossRef]
- Andrews, M.B.; Cahill, C.L. Uranyl-Bearing Hybrid Materials: Synthesis, Speciation and Solid State Structures. Chem. Rev. 2013, 113, 1121–1136. [Google Scholar] [CrossRef]
- Giesting, P.A.; Burns, P.C. Uranyl-organic complexes: Structure symbols, classification of carboxylates, and uranyl polyhedral geometries. Crystallogr. Rev. 2006, 12, 205–255. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, Q.-Y.; Gao, Y.; Gui, D.; Qiu, S.; Chen, L.; Sheng, D.; Diwu, J.; Shi, W.-Q.; Chai, Z.; et al. Probing the Influence of Phosphonate Bonding Modes to Uranium(VI) on Structural Topology and Stability: A Complementary Experimental and Computational Investigation. Inorg. Chem. 2015, 54, 3864–3874. [Google Scholar] [CrossRef]
- Yang, W.; Yi, F.-Y.; Tian, T.; Tian, W.-G.; Sun, Z.-M. Structural Variation within Heterometallic Uranyl Hybrids based on Flexible Alkyldiphosphonate Ligands. Cryst. Growth Des. 2014, 14, 1366–1374. [Google Scholar] [CrossRef]
- Parker, T.G.; Cross, J.M.; Polinski, M.J.; Liu, J.; Albrecht-Schmitt, T.E. Ionothermal and Hydrothermal Flux Syntheses of Five New Uranyl Phosphonates. Cryst. Growth Des. 2014, 14, 228–235. [Google Scholar] [CrossRef]
- Tian, T.; Yang, W.; Wang, H.; Dang, S.; Sun, Z.-M. Flexible Diphosphonic Acids for the Isolation of Uranyl Hybrids with Heterometallic U(VI)=O–Zn(II) Cation-Cation Interactions. Inorg. Chem. 2013, 52, 8288–8290. [Google Scholar] [CrossRef] [PubMed]
- Thuéry, P.; Nierlich, M.; Baldwin, B.W.; Komatsuzaki, N.; Hirose, T. A metal-organic molecular box obtained from self assembling around uranyl ions. J. Chem. Soc. Dalton. Trans. 1999, 1047–1048. [Google Scholar] [CrossRef]
- McGrail, B.T.; Pianowski, L.S.; Burns, P.C. Photochemical Water Oxidation and Origin of Nonaqueous Uranyl Peroxide Complexes. J. Am. Chem. Soc. 2014, 136, 4797–4800. [Google Scholar] [CrossRef]
- Thangavelu, S.G.; Cahill, C.L. Uranyl-Promoted Peroxide Generation: Synthesis and Characterisation of Three Uranyl Peroxo [(UO2)2(O2)] Complexes. Inorg. Chem. 2015, 54, 4208–4221. [Google Scholar] [CrossRef]
- Qiu, J.; Burns, P.C. Clusters of Actinides with Oxide, Peroxide or Hydroxide Bridges. Chem. Rev. 2013, 113, 1097–1120. [Google Scholar] [CrossRef]
- Qiu, J.; Ling, J.; Jouffret, L.; Thomas, R.; Szymanowski, J.E.S.; Burns, P.C. Water soluble multi-cage super tetrahedral uranyl peroxide phosphate clusters. Chem. Sci. 2014, 5, 303–310. [Google Scholar] [CrossRef]
- Natrajan, L.S. Developments in the Photophysics and Photochemistry of Actinide Ions and their Coordination Compounds. Coord. Chem. Rev. 2012, 256, 1583–1603. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Anchoring Flexible Uranyl Dicarboxylate Chains through Stacking Interactions of Ancillary Ligands on the Chiral U(VI) Centres. CrystEngComm 2016, 18, 3905–3918. [Google Scholar] [CrossRef]
- Thuéry, P.; Masci, B. Self Assembly of an Octa-uranate Cage Complex with a Rigid Bis-Catechol Ligand. Supramol. Chem. 2003, 15, 95–99. [Google Scholar] [CrossRef]
- Thuéry, P.; Nierlich, M.; Harrowfield, J.; Ogden, M. Phenoxide complexes of f-elements. In Calixarenes 2001; Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J., Eds.; Kluwer Academic Publishers: Dordrtecht, The Netherlands, 2001; Chapter 30; pp. 561–582. [Google Scholar]
- Lee, J.; Brewster, J.T., II; Song, B.; Lynch, V.M.; Hwang, I.; Li, X.; Sessler, J.L. Uranyl dication mediated photoswitching of a calix[4]pyrrole-based metal coordination cage. Chem. Commun. 2018, 54, 9422–9425. [Google Scholar] [CrossRef] [PubMed]
- Immirzi, A.; Bombieri, G.; Degetto, S.; Marangoni, G. The crystal and molecular structure of pyridine-2,6-dicarboxylatodioxouranium(VI) monohydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 1023–1028. [Google Scholar] [CrossRef]
- Jayasinghe, A.S.; Unruh, D.K.; Kral, A.; Libo, A.; Forbes, T.Z. Structural Features in Metal−Organic Nanotube Crystals That Influence Stability and Solvent Uptake. Cryst. Growth Des. 2015, 15, 4062–4070. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Tubelike Uranyl–Phenylenediacetate Assemblies from Screening of Ligand Isomers and Structure-Directing Counterions. Inorg. Chem. 2019, 58, 6550–6564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unruh, D.K.; Gojdas, K.; Libo, A.; Forbes, T.Z. Development of Metal-Organic Nanotubes Exhibiting Low Temperature, Reversible Exchange of Confined “Ice Channels”. J. Am. Chem. Soc. 2013, 135, 7398–7401. [Google Scholar] [CrossRef]
- Mihalcea, I.; Henry, N.; Loiseau, T. Revisiting the Uranyl-phthalate System: Isolation and Crystal Structures of Two Types of Uranyl-Organic Frameworks (UOF). Cryst. Growth Des. 2011, 11, 1940–1947. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V.; Tananaev, I.G.; Kaindl, R.; Mersdorf, E.; Myasoedov, B.F. Highly Porous Uranyl Selenate Nanotubes. J. Am. Chem. Soc. 2005, 127, 1072–1073. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V.; Kaindl, R.; Mersdorf, E.; Tananaev, I.G.; Myasoedov, B.F. Nanoscale tubules in uranyl selenates. Angew. Chem. Int. Ed. 2005, 44, 1134–1136. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; Cobeza, A.; Bruque, S.; Poojary, D.M.; Clearfield, A. Polymorphism and Phase Transition in Nanotubular Uranyl Phenylphosphonate: (UO2)3(HO3PC6H5)2(O3PC6H5)2.H2O. Inorg. Chem. 1998, 37, 1827–1832. [Google Scholar] [CrossRef]
- Grohol, D.; Clearfield, A. Alkali-Ion-Catalysed Transformation of Two Linear Uranyl Phosphonates into a Tubular One. J. Am. Chem. Soc. 1997, 119, 9301–9302. [Google Scholar] [CrossRef]
- Poojary, D.M.; Cabeza, A.; Aranda, M.A.G.; Bruque, S.; Clearfield, A. Structure Determination of a Complex Tubular Uranyl Phenylphosphonate, (UO2)3(HO3PC6H5)2(O3PC6H5)2.H2O, from Conventional X-ray Powder Diffraction Data. Inorg. Chem. 1996, 35, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Poojary, D.M.; Grohol, D.; Clearfield, A. Synthesis and X-ray Powder Structure of a Novel Porous Uranyl Phenylphosphonate Containing Unidimensional Channels Flanked by Hydrophobic Regions. Angew. Chem. Int. Ed. 1995, 34, 1508–1510. [Google Scholar] [CrossRef]
- Thuéry, P.; Masci, B. Uranyl-Organic Frameworks with 1,2,3,4-Butanetetracarboxylate and 1,2,3,4-Cyclobutanetetracarboxylate Ligands. Cryst. Growth Des. 2008, 8, 3430–3436. [Google Scholar] [CrossRef]
- Cahill, C.L.; Borkowski, L.A. Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S.V., Burns, P.C., Tananaev, I.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 11. [Google Scholar]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Chiral Discrete and Polymeric Uranyl Ion Complexes with (1R,3S)-(+)-Camphorate Ligands: Counterion-Dependent Formation of a Hexanuclear Cage. Inorg. Chem. 2019, 58, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Harrowfield, J.M. Chiral One- to Three-dimensional Uranyl-organic Assemblies from (1R,3S)-(+)-Camphoric acid. CrystEngComm 2014, 16, 2996–3004. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P. A Nanosized Uranyl Camphorate Cage and its Use as a Building Unit in a Metal–Organic Framework. Cryst. Growth Des. 2009, 9, 4592–4594. [Google Scholar] [CrossRef]
- Thuéry, P. Solvothermal Synthesis and Crystal Structure of Uranyl Complexes with 1,1-Cyclobutanedicarboxylic and (1R,3S)-(+)-Camphoric Acids—Novel Chiral Uranyl–Organic Frameworks. Eur. J. Inorg. Chem. 2006, 3646–3651. [Google Scholar] [CrossRef]
- Thuéry, P.; Villiers, C.; Jaud, J.; Ephritikhine, M.; Masci, B. Uranyl-Based Metallamacrocycles: Tri- and Tetranuclear Complexes with (2R,3R,4S,5S)-Tetrahydrofurantetracarboxylic Acid. J. Am. Chem. Soc. 2004, 126, 6838–6839. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Functionalized Aromatic Dicarboxylate Ligands in Uranyl-Organic Assemblies: The Cases of Carboxycinnamate and 1,2-/1,3-Phenylenedioxydiacetate. Inorg. Chem. 2020, 59. in press, and references therein. [Google Scholar] [CrossRef]
- Adelani, P.O.; Albrecht-Schmitt, T.E. Heterobimetallic Copper(II) Uranyl Carboxyphenylphosphonates. Cryst. Growth Des. 2011, 11, 4676–4683. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. 1,3-Adamantanedicarboxylate and 1,3-Adamantanediacetate as Uranyl Ion Linkers: Effect of Counterions, Solvents and Differences in Flexibility. Eur. J. Inorg. Chem. 2019, 4440–4449. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Closed Uranyl-Dicarboxylate Oligomers: A Tetranuclear Metallatricycle with Uranyl Bridgeheads and 1,3-Adamantanedicarboxylate Linkers. Inorg. Chem. 2018, 57, 7932–7939. [Google Scholar] [CrossRef] [PubMed]
- Thuéry, P.; Harrowfield, J. Solvent effects in solvo-hydrothermal synthesis of uranyl ion complexes with 1,3-adamantanediacetate. CrystEngComm 2015, 17, 4006–4018. [Google Scholar] [CrossRef]
- Thuéry, P.; Rivière, E.; Harrowfield, J. Uranyl- and Uranyl-3d-Block-Cation Complexes with 1,3-adamantanedicarboxylate: Crystal Structures, Luminescence and Magnetic Properties. Inorg. Chem. 2015, 54, 2838–2850. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal-organic frameworks. Chem. Soc. Rev. 2014, 42, 9232–9242. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Zero-, mono- and diperiodic uranyl ion complexes with the diphenate dianion: Influences of transition metal ion coordination and differential UVI chelation. Dalton. Trans. 2020, 49, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Counterion-Controlled Formation of an Octanuclear Uranyl Cage with cis-1,2-Cyclohexanedicarboxylate Ligands. Inorg. Chem. 2018, 57, 6283–6288. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Harrowfield, J. Tetrahedral and cuboidal clusters in Complexes of Uranyl and Alkali or Alkaline-Earth Metal Ions with rac- and (1R,2R)-trans-1,2-Cyclohexanedicarboxylate. Cryst. Growth Des. 2017, 17, 2881–2892. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Harrowfield, J. Coordination Polymers and Cage-Containing Frameworks in Uranyl Ion Complexes with rac- and (1R,2R)-trans-1,2-Cyclohexanedicarboxylates: Consequences of Chirality. Inorg. Chem. 2017, 56, 1455–1469. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. [Ni(cyclam)]2+ and [Ni(R,S-Me6cyclam)]2+ as Linkers or Counterions in Uranyl–Organic Species with cis- and trans-1,2-Cyclohexanedicarboxylate Ligands. Cryst. Growth Des. 2018, 18, 5512–5520. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Crown Ethers and their Alkali Metal Ion Complexes as Assembler Groups in Uranyl–Organic Coordination Polymers with cis-1,3-, cis-1,2- and trans-1,2-Cyclohexanedicarboxylates. Cryst. Growth Des. 2018, 18, 3167–3177. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Uranyl–Organic Coordination Polymers with trans-1,2-, trans-1,4- and cis-1,4-Cyclohexanedicarboxylates: Effects of Bulky PPh4+ and PPh3Me+ Counterions. Cryst. Growth Des. 2018, 18, 2609–2619. [Google Scholar] [CrossRef] [Green Version]
- Borkowski, L.A.; Cahill, C.L. Crystal Engineering with the Uranyl Cation I. Aliphatic Carboxylate Coordination Polymers: Synthesis, Crystal Structures, and Fluorescent Properties. Cryst. Growth Des. 2006, 6, 2241–2247. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. A New Form of Triple-stranded Helicate found in Uranyl Complexes of Aliphatic α,ω-Dicarboxylates. Inorg. Chem. 2015, 54, 10539–10541. [Google Scholar] [CrossRef]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Favoring Framework Formation through Structure-Directing Effects in Uranyl Ion Complexes with 1,2,3,4-(Cyclo)butanetetracarboxylate Ligands. Cryst. Growth Des. 2019, 19, 4109–4120. [Google Scholar] [CrossRef] [Green Version]
- Pasquale, S.; Sattin, S.; Escudero-Adan, E.C.; Martinez-Belmonte, M.; De Mendoza, J. Giant regular polyhedra from calixarene carboxylates and uranyl. Nature Commun. 2012, 3, 785–791. [Google Scholar] [CrossRef]
- Kemp, D.S.; Petrakis, K.S. Synthesis and Conformational Analysis of cis,cis-1,3,5-Trimethylcyclohexane-1,3,5-tricarboxylic Acid. J. Org. Chem. 1981, 46, 5140–5143. [Google Scholar] [CrossRef]
- Thuéry, P. A Highly Adjustable Coordination System: Nanotubular and Molecular Cage Species in Uranyl Ion Complexes with Kemp’s Triacid. Cryst. Growth Des. 2014, 14, 901–904. [Google Scholar] [CrossRef]
- Thuéry, P. Increasing Complexity in the Uranyl Ion–Kemp’s Triacid System: From One- and Two-Dimensional Polymers to Uranyl–Copper(II) Dodeca- and Hexadecanuclear Species. Cryst. Growth Des. 2014, 14, 2665–2676. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Uranyl Ion Complexes with all-cis 1,3,5-Cyclohexanetricarboxylate: Unexpected Framework and Nanotubular Assemblies. Cryst. Growth Des. 2014, 14, 4214–4225. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Two-dimensional assemblies in f-element ion (UO22+, Yb3+) complexes with two cyclohexyl-based polycarboxylates. Polyhedron 2015, 2015 98, 5–11. [Google Scholar] [CrossRef]
- Harrowfield, J.; Thuéry, P. Dipodal, Tripodal, and Discoidal Coordination Modes of Kemp’s Triacid Anions. Eur. J. Inorg. Chem. 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Caulder, D.; Raymond, K.N. Supermolecules by Design. Acc. Chem. Res. 1999, 32, 975–982. [Google Scholar] [CrossRef]
- Jansze, S.M.; Cecot, G.; Wise, M.D.; Zhurov, K.O.; Ronson, T.K.; Castilla, A.M.; Finelli, A.; Pattison, P.; Solari, E.; Scopelliti, R.; et al. Ligand Aspect Ratio as a Decisive Factor for the Self-Assembly of Coordination Cages. J. Am. Chem. Soc. 2016, 138, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C. Expanded ligands—An assembly principle for supramolecular chemistry. Coord. Chem. Rev. 2008, 252, 842–855. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Uranyl Ion-Containing Polymeric Assemblies with cis/trans Isomers of 1,2-, 1,3- and 1,4-Cyclohexanedicarboxylates, Including a Helical Chain and a Sixfold-Interpenetrated Framework. Cryst. Growth Des. 2020, 20, 262–273, and references therein. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Harrowfield, J. Variations on the Honeycomb Topology: From Triangular- and Square-Grooved Networks to Tubular Assemblies in Uranyl Tricarballylate Complexes. Cryst. Growth Des. 2017, 17, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Demazeau, G. Solvothermal Processes: Definition, Key Factors Governing the Involved Chemical Reactions and New Trends. Z. Naturforsch. 2010, 65b, 999–1006. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal reactions: An original route for the synthesis of novel materials. J. Mater. Sci. 2008, 43, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Atoini, Y.; Harrowfield, J. Structure-directing effects of coordinating solvents, ammonium and phosphonium counterions in uranyl ion complexes with 1,2-, 1,3- and 1,4-phenylenediacetate. Inorg. Chem. 2020, 59, 2503–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, K.P.; Kalaj, M.; Kerridge, A.; Cahill, C.L. Probing hydrogen and halogen-oxo interactions in uranyl coordination polymers: A combined crystallographic and computational study. CrystEngComm 2018, 20, 4916–4925. [Google Scholar] [CrossRef] [Green Version]
- Carter, K.P.; Kalaj, M.; Cahill, C.L. Harnessing uranyl oxo atoms via halogen bonding interactions in molecular uranyl materials featuring 2,5-di-iodobenzoic acid and N-donor capping ligands. Inorg. Chem. Front. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Kalaj, M.; Carter, K.P.; Cahill, C.L. Isolating Equatorial and Oxo Based Influences on Uranyl Vibrational Spectroscopy in a Family of Hybrid Materials Featuring Halogen Bonding Interactions with Uranyl Oxo Atoms. Eur. J. Inorg. Chem. 2017, 4702–4713. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrowfield, J.; Thuéry, P. Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities. Chemistry 2020, 2, 63-79. https://doi.org/10.3390/chemistry2010007
Harrowfield J, Thuéry P. Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities. Chemistry. 2020; 2(1):63-79. https://doi.org/10.3390/chemistry2010007
Chicago/Turabian StyleHarrowfield, Jack, and Pierre Thuéry. 2020. "Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities" Chemistry 2, no. 1: 63-79. https://doi.org/10.3390/chemistry2010007
APA StyleHarrowfield, J., & Thuéry, P. (2020). Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities. Chemistry, 2(1), 63-79. https://doi.org/10.3390/chemistry2010007