Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions
Abstract
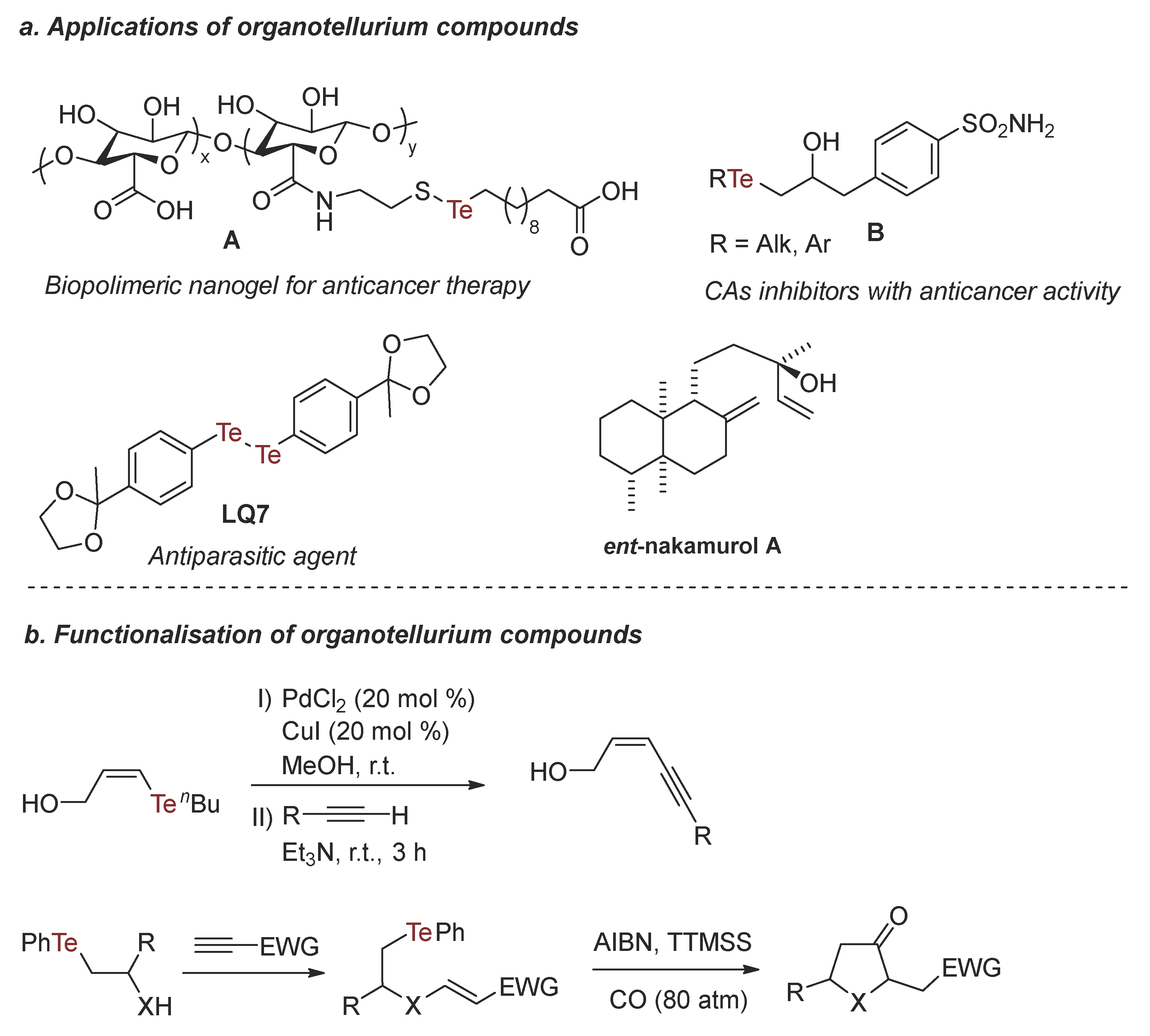
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. General Procedure for the Synthesis of β-Hydroxy-alkyl Ethyl Tellurides 2
2.2.1. Synthesis of 1-(Benzyloxy)-3-(ethyltellanyl)propan-2-ol 2a
2.2.2. Synthesis of 1-(Ethyltellanyl)-3-isopropoxypropan-2-olol 2b
2.2.3. Synthesis of 1-(Ethyltellanyl)hexan-2-ol 2c
2.2.4. Synthesis of 1-(Ethyltellanyl)propan-2-ol 2d
2.2.5. Control Experiment with the Radical Inhibitor
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer: New York, NY, USA, 2018. [Google Scholar]
- Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2012. [Google Scholar]
- Petragnani, N.; Stefani, H.A. Tellurium in Organic Synthesis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Angeli, A.; Carta, F.; Donnini, S.; Capperucci, A.; Ferraroni, M.; Tanini, D.; Supuran, C.T. Selenolesterase enzyme activity of carbonic anhydrases. Chem. Commun. 2020, 56, 4444–4447. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Kumar, S.; Yan, J.; Poon, J.; Singh, V.P.; Lu, X.; Karlsson Ott, M.; Engman, L.; Kumar, S. Multifunctional Antioxidants: Regenerable Radical-Trapping and Hydroperoxide-Decomposing Ebselenols. Angew. Chem. Int. Ed. 2016, 55, 3729–3733. [Google Scholar] [CrossRef]
- Ba, L.A.; Doring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- For a review see: Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Comasseto, J.V.; Gariani, R.A. Biotransformations on Organic Selenides and Tellurides: Synthetic Applications. Tetrahedron 2009, 65, 8447–8459. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Tong, Y.; Lang, M. Self-healing polyurethane based on ditelluride bonds. Appl. Surf. Sci. 2018, 455, 318–325. [Google Scholar] [CrossRef]
- Xia, X.; Xiang, X.; Huang, F.; Zhang, Z.; Han, L. A tellurylsulfide bond-containing redox-responsive superparamagnetic nanogel with acid-responsiveness for efficient anticancer therapy. Chem. Commun. 2017, 53, 13141–13144. [Google Scholar] [CrossRef]
- Engman, L.; Al-Maharik, N.; McNaughton, M.; Birmingham, A.; Powis, G. Thioredoxin reductase and cancer cell growth inhibition by organotellurium antioxidants. Anti-Cancer Drugs 2003, 14, 153–161. [Google Scholar] [CrossRef]
- Rooseboom, M.; Vermeulen, N.P.E.; Durgut, F.; Commandeur, J.N.M. Comparative study on the bioactivation mechanisms and cytotoxicity of Te-phenyl-L-tellurocysteine, Se-Phenyl-L-selenocysteine, and S-Phenyl-L-cysteine. Chem. Res. Toxicol. 2002, 15, 1610–1618. [Google Scholar] [CrossRef]
- Lin, T.; Ding, Z.; Li, N.; Xu, J.; Luo, G.; Liu, J.; Shen, J. 2-Tellurium-bridged β-cyclodextrin, a thioredoxin reductase inhibitor, sensitizes human breast cancer cells to TRAIL-induced apoptosis through DR5 induction and NF-κB suppression. Carcinogenesis 2010, 32, 154–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanini, D.; Ricci, L.; Capperucci, A.; Di Cesare Mannelli, L.; Ghelardini, C.; Peat, T.S.; Carta, F.; Angeli, A.; Supuran, C.T. Synthesis of novel tellurides bearing benzensulfonamide moiety as carbonic anhydrase inhibitors with antitumor activity. Eur. J. Med. Chem. 2019, 181, 111586. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Capperucci, A.; Supuran, C.T.; Angeli, A. Sulfur, selenium and tellurium containing amines act as effective carbonic anhydrase activators. Bioorg. Chem. 2019, 87, 516–522. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. First evaluation of organotellurium derivatives as carbonic anhydrase I, II, IV, VII and IX inhibitors. Bioorg. Chem. 2018, 76, 268–272. [Google Scholar] [CrossRef]
- Rettig, I.D.; Van, J.; Brauer, J.B.; Luo, W.; McCormick, T.M. Tellurorhodamine photocatalyzed aerobic oxidation of organo-silanes and phosphines by visible-light. Dalton Trans. 2019, 48, 5665–5673. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Lupori, B.; Malevolti, G.; Ambrosi, M.; Lo Nostro, P.; Capperucci, A. Direct biocatalysed synthesis of first sulfur-, selenium- and tellurium- containing L-ascorbyl hybrid derivatives with radical trapping and GPx-like properties. Chem. Commun. 2019, 55, 5705–5708. [Google Scholar] [CrossRef] [Green Version]
- Tanini, D.; Grechi, A.; Ricci, L.; Dei, S.; Teodori, E.; Capperucci, A. Novel functionalized organotellurides with enhanced thiol peroxidase catalytic activity. New J. Chem. 2018, 42, 6077–6083. [Google Scholar] [CrossRef] [Green Version]
- Bortoli, M.; Torsello, M.; Bickelhaupt, F.M.; Orian, L. Role of the Chalcogen (S, Se, Te) in the Oxidation Mechanism of the Glutathione Peroxidase Active Site. ChemPhysChem 2017, 18, 2990–2998. [Google Scholar] [CrossRef]
- Singh, V.P.; Poon, J.F.; Engman, L. Catalytic Antioxidants: Regenerable Tellurium Analogues of Vitamin E. Org. Lett. 2013, 15, 6274–6277. [Google Scholar] [CrossRef]
- Braga, A.L.; Alberto, E.E.; Soares, L.C.; Rocha, J.B.T.; Sudati, J.H.; Roos, D.H. Synthesis of telluroamino acid derivatives with remarkable GPx like activity. Org. Biol. Chem. 2009, 7, 43–45. [Google Scholar] [CrossRef]
- Giles, G.I.; Fry, F.H.; Tasker, K.M.; Holme, A.L.; Peers, C.; Green, K.N.; Klotz, L.O.; Sies, H.; Jacob, C. Evaluation of sulfur, selenium and tellurium catalysts with antioxidant potential. Org. Biomol. Chem. 2003, 1, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Tiano, L.; Fedeli, D.; Santroni, A.M.; Villarini, M.; Engman, L.; Falcioni, G. Effect of three diaryl tellurides, and an organoselenium compound in trout erythrocytes exposed to oxidative stress in vitro. Mutat. Res. 2000, 464, 269–277. [Google Scholar] [CrossRef]
- Petragnani, N.; Stefani, H.A. Advances in organic tellurium chemistry. Tetrahedron 2005, 61, 1613–1679. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Barrientos-Astigarraga, R.E. Add a Little Tellurium to Your Synthetic Plans! Aldrichim. Acta 2000, 33, 66–78. [Google Scholar]
- Stefani, H.A.; Pena, J.M.; Manarin, F.; Ando, R.A.; Leal, M.; Petragnani, N. Negishi cross-coupling of organotellurium compounds: Synthesis of biaryls, aryl-, and diaryl acetylenes. Tetrahedron Lett. 2011, 52, 4398–4401. [Google Scholar] [CrossRef]
- Berlin, S.; Ericsson, C.; Engman, L. Radical Carbonylation/Reductive Cyclization for the Construction of Tetrahydrofuran-3-ones and Pyrrolidin-3-ones. J. Org. Chem. 2003, 68, 8386–8396. [Google Scholar] [CrossRef] [PubMed]
- Tucci, F.C.; Chieffi, A.; Comasseto, J.V.; Marino, J.P. Tellurium in Organic Synthesis. Preparation of Z-Vinylic Cuprates from Z-Vinylic Tellurides and Their Reaction with Enones and Epoxides. J. Org. Chem. 1996, 61, 4975–4989. [Google Scholar] [CrossRef]
- Zeni, G.; Comasseto, J.V. Coupling of Z-vinylic tellurides with alkynes catalysed by PdCl2CuI: Synthesis of Z-enynes and Z-enediynes. Tetrahedron Lett. 1999, 40, 4619–4622. [Google Scholar] [CrossRef]
- Hirata, K.; Kotoku, M.; Seki, N.; Maeba, T.; Maeda, K.; Hirashima, S.; Sakai, T.; Obika, S.; Hori, A.; Hase, Y.; et al. SAR Exploration Guided by LE and Fsp3: Discovery of a Selective and Orally Efficacious RORϒ Inhibitor. ACS Med. Chem. Lett. 2016, 7, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Bandeira, P.T.; Souza, J.P.A.; Scariot, D.B.; Garcia, F.P.; Nakamura, C.V.; de Oliveira, A.R.M.; Piovan, L. Diacetal Ditellurides as Highly Active and Selective Antiparasitic Agents toward Leishmania amazonensis. ACS Med. Chem. Lett. 2019, 10, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Cuesta, J.; Gonzàlez, A.; Bonjoch, J. Synthesis of (-)-Nakamurol A and Assignment of Absolute Configuration of Diterpenoid (+)-Nakamurol A. J. Org. Chem. 2003, 68, 7400–7406. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A. Ring opening reactions of heterocycles with selenium and tellurium nucleophiles. New J. Chem. 2019, 43, 11451–11468. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.C.; Borges, E.L.; Lima, D.B.; Jacob, R.G.; Lenardão, E.J.; Perin, G.; Silva, M.S. A simple and non-conventional method for the synthesis of selected β-arylalkylchalcogeno substituted alcohols, amines and carboxylic acids. Arkivoc 2016, 5, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, V.; Chandrasekaran, S. One-Pot Synthesis of β-Amino/β-Hydroxy Selenides and Sulfides from Aziridines and Epoxides. Synthesis 2009, 2009, 3267–3278. [Google Scholar] [CrossRef]
- Braga, A.L.; Schwab, R.S.; Alberto, E.E.; Salman, S.M.; Vargas, J.; Azaredo, J.B. Ring opening of unprotected aziridines by zinc selenolates in a biphasic system. Tetrahedron Lett. 2009, 50, 2309–2311. [Google Scholar] [CrossRef]
- Santi, C.; Santoro, S.; Battistelli, B.; Testaferri, L.; Tiecco, M. Preparation of the First Bench-Stable Phenyl Selenolate: An Interesting “On Water” Nucleophilic Reagent. Eur. J. Org. Chem. 2008, 5387–5390. [Google Scholar] [CrossRef]
- Vargas, F.; Comasseto, J.V. Practical synthesis of chiral β-telluro amines by ring-opening reaction of aziridines. J. Organomet. Chem. 2009, 694, 122–126. [Google Scholar] [CrossRef]
- Leng, T.; Wu, G.; Zhou, Y.-B.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Silver-Catalyzed One-Pot Three-Component Selective Synthesis of β-Hydroxy Selenides. Adv. Synth. Catal. 2018, 360, 4336–4340. [Google Scholar] [CrossRef]
- Alvano Pérez-Bautista, J.; Sosa-Rivadeneyra, M.; Quintero, L.; Hüpfl, H.; Tejeda-Dominguez F., A.; Sartillo-Piscil, F. Highly stereoselective aziridine ring-opening with phenylselenide anion and selective intramolecular aldol closure for the enantiopure synthesis of γ-aminocyclopentene derivatives. Tetrahedron Lett. 2009, 50, 5572–5574. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Wang, J.; Ou, W.; Wang, X.; Huang, S. A New Formal Synthetic Route to Entecavir. Synlett 2019, 30, 748–752. [Google Scholar]
- Braga, A.L.; Paixão, M.W.; Lüdtke, D.S.; Silveira, C.C.; Rodrigues, O.E.D. Synthesis of new chiral aliphatic amino diselenides and their application as catalysts for the enantioselective addition of diethylzinc to aldehydes. Org. Lett. 2003, 5, 2635–2638. [Google Scholar] [CrossRef]
- Tanini, D.; Ricci, L.; Capperucci, A. Rongalite-Promoted on Water Synthesis of Functionalised Tellurides and Ditellurides. Adv. Synth. Catal. 2020, 362, 1323–1332. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A.; Degl’Innocenti, A. Bis-(trimethylsilyl)selenide in the Selective Synthesis of β-Hydroxy, β-Mercapto, and β-Amino Diorganyl Diselenides and Selenides Through Ring Opening of Strained Heterocycles. Eur. J. Org. Chem. 2015, 357–369. [Google Scholar] [CrossRef]
- Detty, M.R.; Seidler, M.D. Bis (trialkylsilyl) chalcogenides. 1. Preparation and reduction of group VIA oxides. J. Org. Chem. 1982, 47, 1354–1356. [Google Scholar] [CrossRef]
- Tanini, D.; Grechi, A.; Dei, S.; Teodori, E.; Capperucci, A. An easy one-step procedure for the synthesis of novel β-functionalised tellurides. Tetrahedron 2017, 73, 5646–5653. [Google Scholar] [CrossRef]
- Tanini, D.; Tiberi, C.; Gellini, C.; Salvi, P.R.; Capperucci, A. A Straightforward access to stable β-functionalized alkyl selenols. Adv. Synth. Catal. 2018, 360, 3367–3375. [Google Scholar] [CrossRef]
- Tanini, D.; Borgogni, C.; Capperucci, A. Mild and selective silicon-mediated access to enantioenriched 1,2-mercaptoamines and β-amino arylchalcogenides. New J. Chem. 2019, 43, 6388–6393. [Google Scholar] [CrossRef]
- Tanini, D.; Scarpelli, S.; Ermini, E.; Capperucci, A. Seleno-Michael reaction of stable functionalised alkyl selenols: A versatile tool for the synthesis of acyclic and cyclic unsymmetrical alkyl and vinyl selenides. Adv. Synth. Catal. 2019, 361, 2337–2346. [Google Scholar] [CrossRef]
- Tanini, D.; D’Esopo, V.; Tatini, D.; Ambrosi, M.; Lo Nostro, P.; Capperucci, A. Selenated and Sulfurated Analogues of triacyl glycerols: Selective synthesis and structural characterization. Chem. Eur. J. 2020, 26, 2719–2725. [Google Scholar] [CrossRef]
- Ollivier, C.; Renaud, P. Organoboranes as a Source of Radicals. Chem. Rev. 2001, 101, 3415–3434. [Google Scholar] [CrossRef] [PubMed]
- Ryohei Uematsu, R.; Saka, C.; Sumiya, Y.; Ichino, T.; Taketsugu, T.; Maeda, S. An autocatalytic cycle in autoxidation of triethylborane. Chem. Commun. 2017, 53, 7302–7305. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.A.; Tashtoush, H. Free-radical chain-substitution reactions of alkylmercury halides. J. Am. Chem. Soc. 1983, 105, 1398–1399. [Google Scholar] [CrossRef]
- Abe, T.; Aso, Y.; Otsuro, T.; Ogura, F. Oxygen-Induced Transmetalation of Organoboranes with Diphenyl Ditelluride. Chem. Lett. 1990, 19, 1671–1674. [Google Scholar] [CrossRef]
- Gladysz, J.A.; Hornby, J.L.; Garbe, J.E. A Convenient One-Flask Synthesis of Dialkyl Selenides and Diselenides via Lithium Triethylborohydride Reduction of Sex. J. Org. Chem. 1978, 43, 1204–1208. [Google Scholar] [CrossRef]
- Krenske, E.H.; Pryor, W.; Houk, K.N. Mechanism of SH2 Reactions of Disulfides: Frontside vs Backside, Stepwise vs Concerted. J. Org. Chem. 2009, 74, 5356–5360. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Huang, S.-H.; Tseng, T.-F.; Yang, J.-F. The Mechanism Study of Free Radical SH2’ Reactions by Leaving Group Effect and Secondary α-Deuterium Kinetic Isotope Effect. J. Chin. Chem. Soc. 2004, 51, 1005–1011. [Google Scholar] [CrossRef]
- If regenerated, ditellurides 3 reasonably undergoes transmetalation with ethyl radicals affording β-hydroxy-alkyl-ethyl-tellurides 2 and radicals 8, following the proposed mechanism reported in the Scheme 3.




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanini, D.; Capperucci, A. Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions. Chemistry 2020, 2, 652-661. https://doi.org/10.3390/chemistry2030041
Tanini D, Capperucci A. Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions. Chemistry. 2020; 2(3):652-661. https://doi.org/10.3390/chemistry2030041
Chicago/Turabian StyleTanini, Damiano, and Antonella Capperucci. 2020. "Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions" Chemistry 2, no. 3: 652-661. https://doi.org/10.3390/chemistry2030041
APA StyleTanini, D., & Capperucci, A. (2020). Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions. Chemistry, 2(3), 652-661. https://doi.org/10.3390/chemistry2030041






