Catalytic Converters for Vehicle Exhaust: Fundamental Aspects and Technology Overview for Newcomers to the Field
Abstract
1. Introduction
2. Working Principles of Three-Way Catalytic Converters
- (1)
- Oxidation of unburned hydrocarbons, where oxygen gas is present in the exhaust gas, has its bonds broken and the oxygen atom reacts with the unburned hydrocarbons to produce CO2 and water vapour as the final products. An example would be the oxidation of benzene (Equation (1)):
- (2)
- Oxidation of CO to form CO2 by using either catalysts platinum or palladium nitrate. Oxygen gas that is present in exhaust gases is adsorbed to the surface of the honeycomb ceramic, and so the oxygen bond is weakened and so the oxygen atom reacts with CO to give CO2 (Equation (2)):
- (3)
- Reduction of N2O to give stable nitrogen and oxygen gas (Equation (3)). Since this is a reduction reaction, rhodium is used instead. Since it is a rare type of noble metal, rhodium is usually alloyed with platinum or palladium.
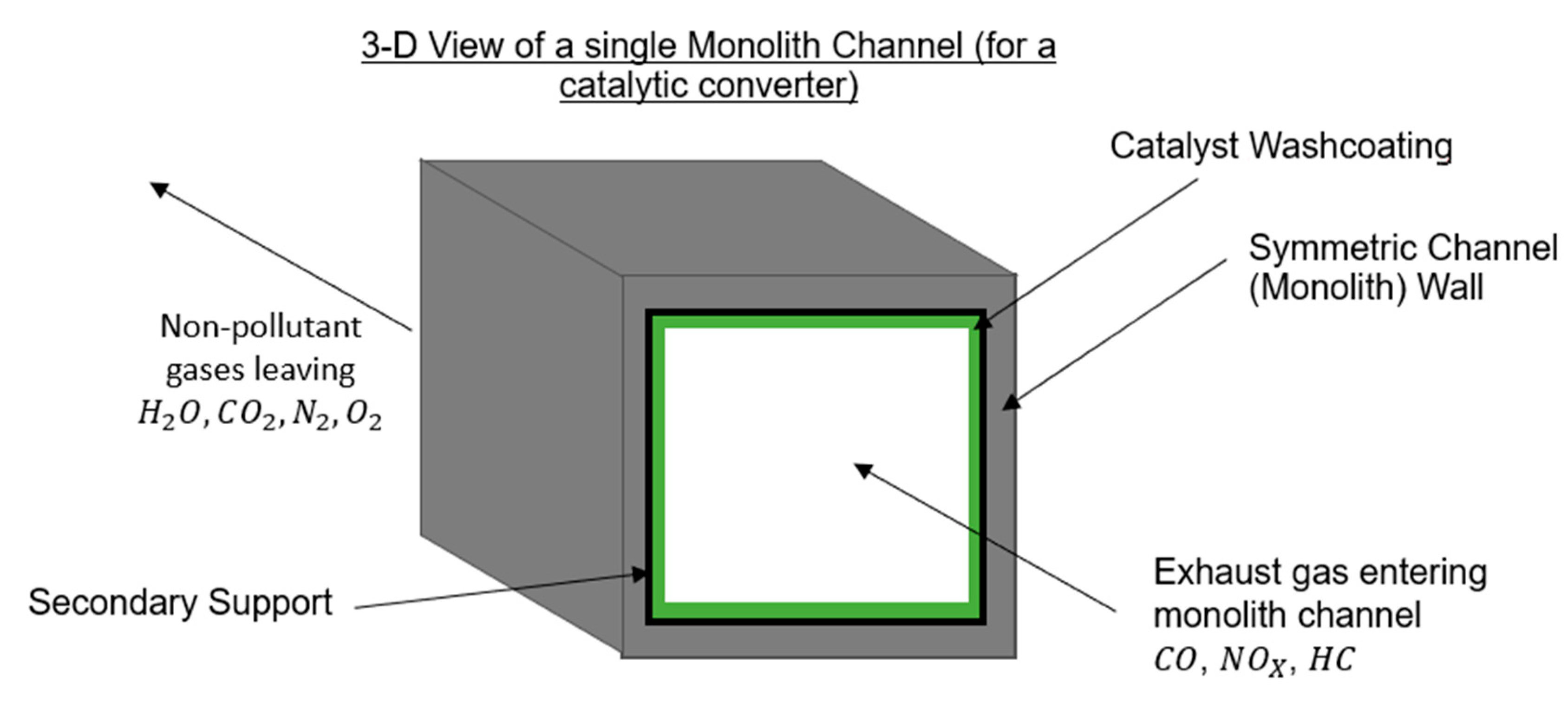
3. Structure of Three-Way Catalytic Converters
- (1)
- Catalyst Core (substrate).
- (2)
- Washcoat
- (3)
- Catalyst Solution
- (4)
- Metal Casing
4. Conditions in Catalytic Converters
4.1. Temperature
4.2. Cold Start Emissions
4.3. Catalyst Conversion Efficiency
4.4. Pressure
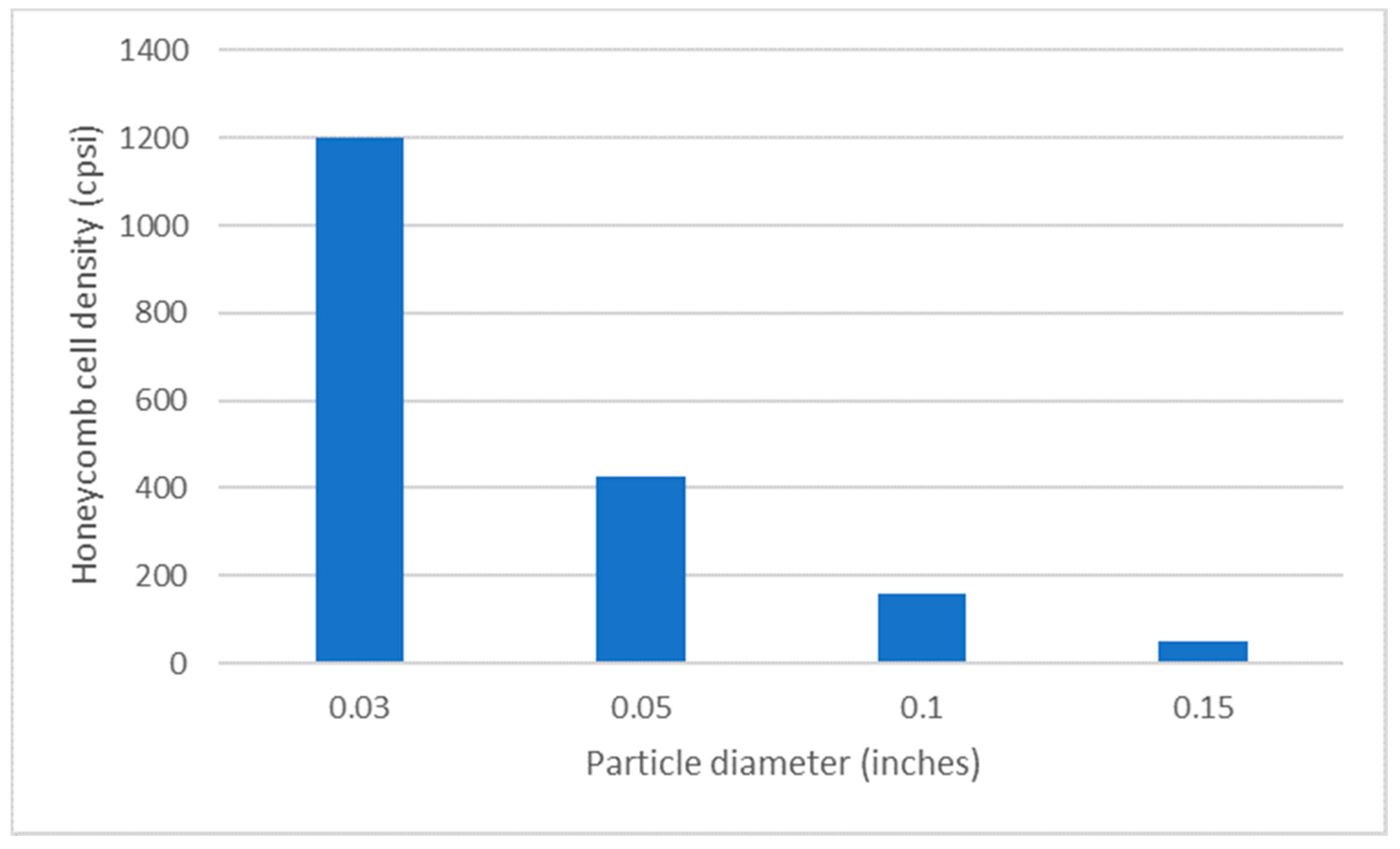
4.5. Surface Areas
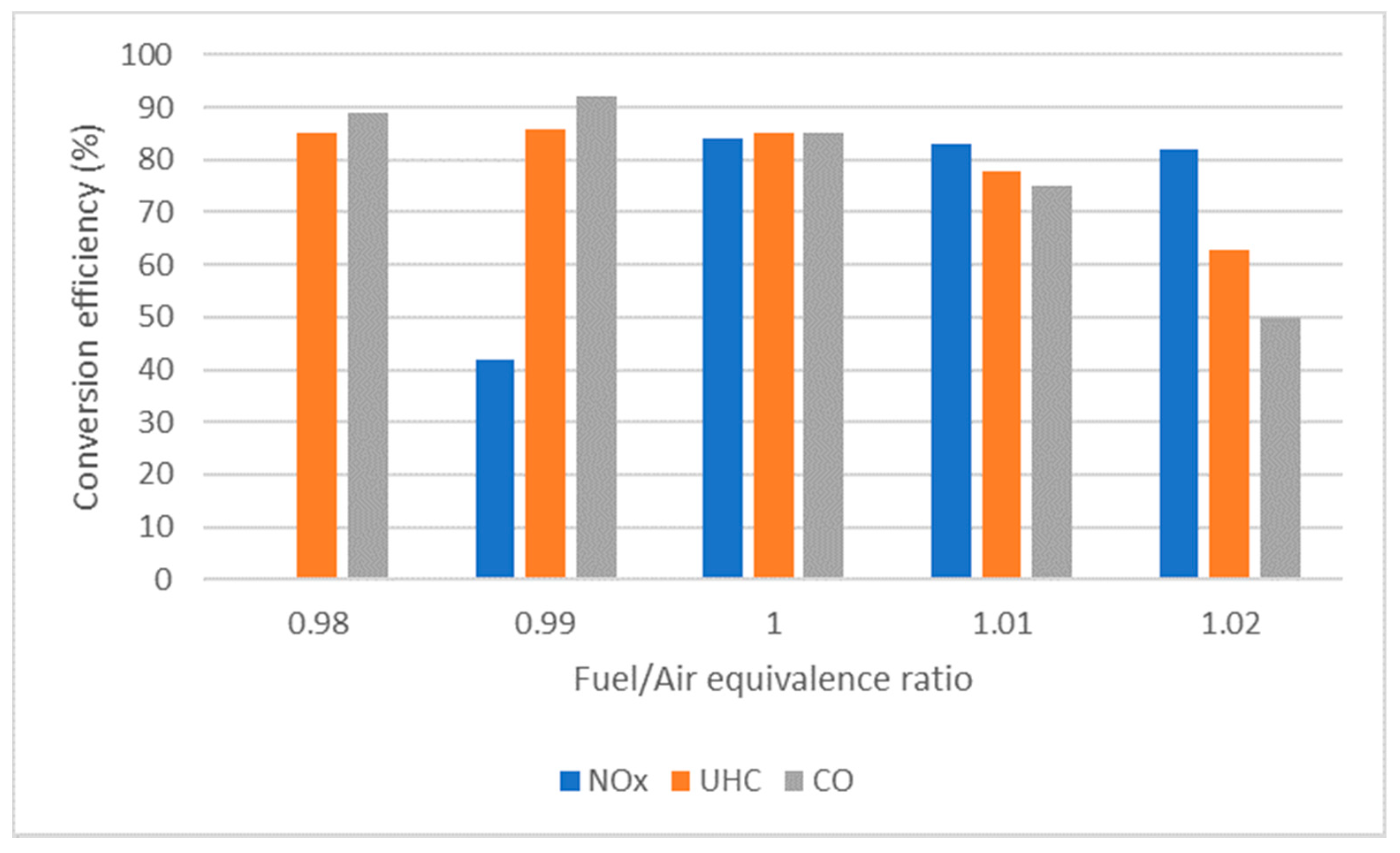
4.6. Air/Fuel Equivalence Ratio
5. Rate of Reaction
6. Pollutant Gases Reduction and Environmental Issues
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- EPA. Carbon Pollution from Transportation 2020. Available online: https://www.epa.gov/transportation-air-pollution-and-climate-change/carbon-pollution-transportation (accessed on 16 May 2021).
- Hannappel, R. The impact of global warming on the automotive industry. AIP Conf. Proc. 2017, 1, 060001. [Google Scholar] [CrossRef]
- Kroeze, C. Nitrous oxide and global warming. Sci. Total Environ. 1994, 143, 193–209. [Google Scholar] [CrossRef]
- European Environment Agency. Greenhouse Gas Emissions from Transport in Europe. European Environment Agency 2019. Available online: https://www.eea.europa.eu/data-and-maps/indicators/transport-emissions-of-greenhouse-gases/transport-emissions-of-greenhouse-gases-12 (accessed on 16 May 2021).
- Baruch, J.J. Combating global warming while enhancing the future. Technol. Soc. 2008, 30, 111–121. [Google Scholar] [CrossRef]
- Hydrogen Council. Hydrogen Scaling Up: A Sustainable Pathway for The Global Energy Transition. Available online: www.hydrogencouncil.com (accessed on 16 May 2021).
- Desantes, J.; Molina, S.; Novella, R.; Lopez-Juarez, M. Comparative global warming impact and NOX emissions of conventional and hydrogen automotive propulsion systems. Energy Convers. Manag. 2020, 221, 113137. [Google Scholar] [CrossRef]
- Datye, A.K.; Votsmeier, M. Opportunities and challenges in the development of advanced materials for emission control catalysts. Nat. Mater. 2020, 1–11. [Google Scholar] [CrossRef]
- Lazkar, A.; Mao-Chang, L. Probing the Operational Temperatures of Vehicular Catalytic Converters Using Clumped Isotopes in Exhaust CO2. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 4–13 April 2018. [Google Scholar]
- Zeng, F.; Finke, J.; Olsen, D.; White, A.; Hohn, K.L. Modeling of three-way catalytic converter performance with exhaust mixtures from dithering natural gas-fueled engines. Chem. Eng. J. 2018, 352, 389–404. [Google Scholar] [CrossRef]
- Avneet Kahlon, T.T. Catalytic Converters. In Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/07%3A_Case_Studies-_Kinetics/7.01%3A_Catalytic_Converters (accessed on 16 May 2021).
- Brown, T.E.; LeMay, H.E.; Bursten, B.E.; Murphy, C.; Woodward, P.; Stoltzfus, M.E. Catalytic Converters. In Chemistry: The Central Science; Pearson: London, UK, 2018. [Google Scholar]
- Clark, J. The Collision Theory of Reaction Rates 2003. Available online: https://www.chemguide.co.uk/physical/basicrates/introduction.html (accessed on 16 May 2021).
- Milton, B.E. Handbook of Air Pollution from Internal Combustion Engines. In Control Technologies in Spark-Ignition Engines; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Kummer, J. Catalysts for automobile emission control. Prog. Energy Combust. Sci. 1980, 6, 177–199. [Google Scholar] [CrossRef]
- Shelef, M.; McCabe, R. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Belton, D.N.; Taylor, K.C. Automobile exhaust emission control by catalysts. Curr. Opin. Solid State Mater. Sci. 1999, 4, 97–102. [Google Scholar] [CrossRef]
- Burch, R. Knowledge and Know-How in Emission Control for Mobile Applications. Catal. Rev. Sci. Eng. 2004, 46, 271–334. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Deutschmann, O.; Grunwaldt, J.-D. Abgasnachbehandlung in mobilen Systemen: Stand der Technik, Herausforderungen und Perspektiven. Chem. Ing. Tech. 2013, 85, 595–617. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Kumar, R.A.; Krishna, R.; Sreenivasan, M. Modeling and fabrication of catalytic converter for emission reduction. Mater. Today: Proc. 2019, 33, 1093–1099. [Google Scholar] [CrossRef]
- Braun, J.; Hauber, T.; Többen, H.; Zacke, P.; Chatterjee, D.; Deutschmann, O.; Warnatz, J. Influence Of Physical And Chemical Parameters On The Conversion Rate Of A Catalytic Converter: A Numerical Simulation Study. In Proceedings of the SAE 2000 World Congress, Cobo Center, Detroit, MI, USA, 6–9 March 2000. [Google Scholar] [CrossRef]
- Haldar, S.K. Exploration Guide. In Platinum-Nickel-Chromium Deposits; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Vanderveer, R.T.; Chandler, J.M. The Development of a Catalytic Converter for the Oxidation of Exhaust Hydrocarbons. SAE Tech. Pap. Ser. 1959. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H.; Redzuan, M.; Mahlia, T.M.I.; Fuad, M.A.; Mohibah, M.; Halim, K.H.; Ishak, A.; Khair, M.; Shahrir, A.; et al. Development and test of a new catalytic converter for natural gas fuelled engine. Sadhana Acad. Proc. Eng. Sci. 2009, 34, 467–481. [Google Scholar] [CrossRef]
- Majewski, W.A. DieselNet Technology Guide 2000. In Emission Control Catalysts; 2002; Available online: https://dieselnet.com/tech/catalysts.php (accessed on 16 May 2021).
- Samuels, G.; Rose, A.; David, G.; Hooker, J.N. Energy Conservation in Transportation. Adv. Energy Syst. Technol. 1982, 187–297. [Google Scholar] [CrossRef]
- Golunski, S.E. Final Analysis. Platin. Met. Rev. 2007, 51, 162. [Google Scholar] [CrossRef]
- USA News Group. Use of Palladium in Catalytic Converters Driving Demand for the Precious Metal 2019. Available online: https://www.prnewswire.com/news-releases/use-of-palladium-in-catalytic-converters-driving-demand-for-the-precious-metal-300937620.html (accessed on 16 May 2021).
- Thakur, P. Advanced Mine Ventilation. Diesel Exhaust Control 2019, 157–187. [Google Scholar] [CrossRef]
- Durilla, M.; Hizny, W.J.; Mack, S. Carbon monoxide oxidizers. In Heat Recover Steam Generator Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Green Chemistry: An Inclusive Approach. Focus Catal. 2018, 2018, 7. [CrossRef]
- Dell, R.M.; Moseley, P.T.; Rand, D.A. Development of Road Vehicles with Internal-Combustion Engines. In Towards Sustainable Road Transport; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catal 2017, 7, 62. [Google Scholar] [CrossRef]
- Mahyon, N.I.; Li, T.; Martinez-Botas, R.; Wu, Z.; Li, K. A new hollow fibre catalytic converter design for sustainable automotive emissions control. Catal. Commun. 2019, 120, 86–90. [Google Scholar] [CrossRef]
- Siemund, S.; Leclerc, J.; Schweich, D.; Prigent, M.; Castagna, F. Three-way monolithic converter: Simulations versus experiments. Chem. Eng. Sci. 1996, 51, 3709–3720. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
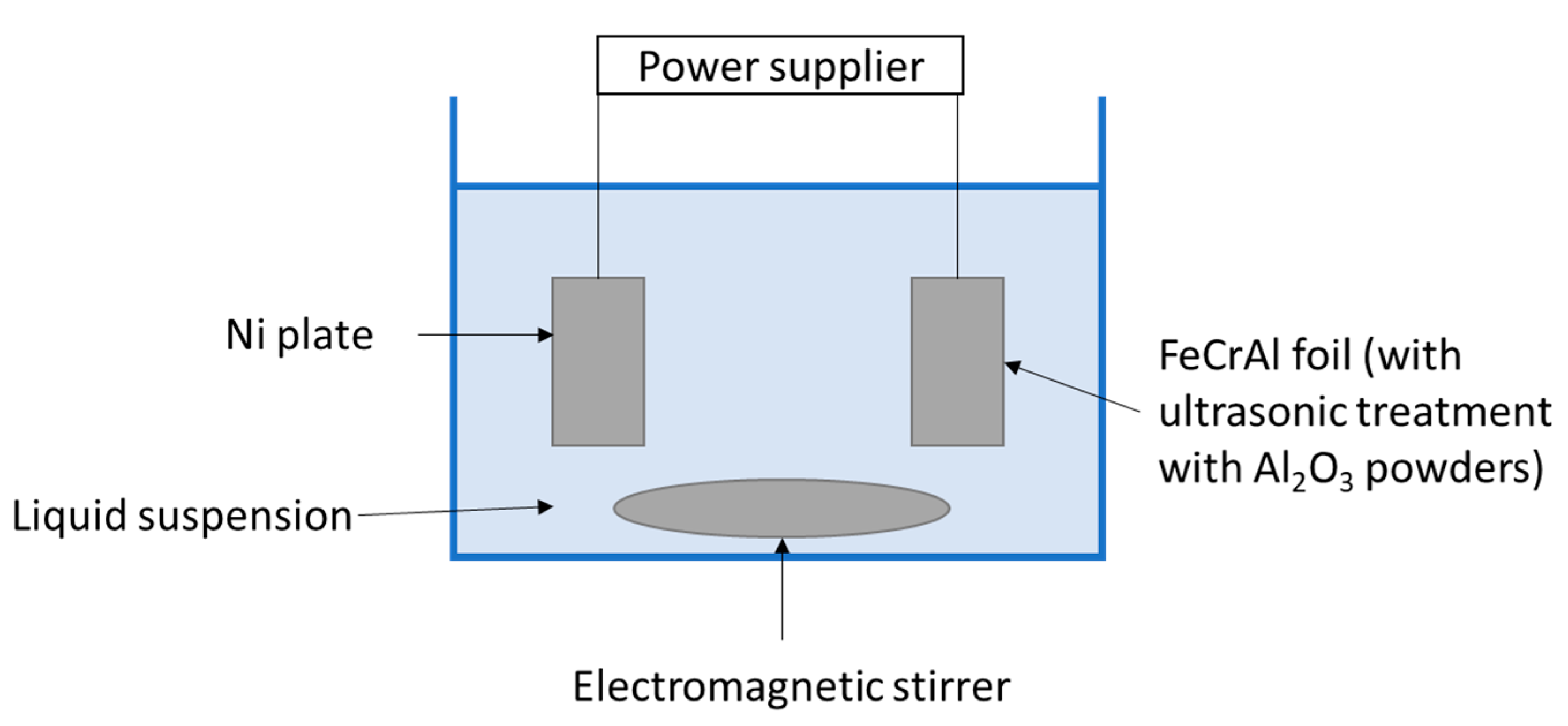
- Leman, A.; Afiqah, J.; Rahman, F.; Feriyanto, D.; Zakaria, S.; Rahmad, R. Catalytic Converter Developed by Washcoat of γ-Alumina on Nickel Oxide (Nio) Catalyst in FeCrAl Substrate for Exhaust Emission Control: A Review. In Proceedings of the MATEC Web Conference 2016; (online). Available online: https://www.matec-conferences.org/articles/matecconf/abs/2016/23/contents/contents.html (accessed on 16 May 2021). [CrossRef]
- Converters, C. Catalytic Converters 2020. Available online: https://www.catalyticconverters.com/construction/ (accessed on 16 May 2021).
- Fornalczyk, A.; Saternus, M. Removal of platinum group metals from the used auto catalytic converter. Metalurgija 2009, 48, 133–136. [Google Scholar]
- Hoffman, J.E. Britannica Encyclopedia: Platinum Group 2018. Available online: https://www.britannica.com/science/platinum-group (accessed on 16 May 2021).
- Johnson, M. Properties of Platinum Group Metals 2020. Available online: http://www.platinum.matthey.com/about-pgm/applications/properties-of-pgm (accessed on 16 May 2021).
- Martinez, N.; Amado, M.; Guerrero, M.P. Modeling the vibrations in a catalytic converter for diesel engines. In Proceedings of the SIMULIA Cust Conference, Providence, RI, USA, 19 May 2011. [Google Scholar]
- Chen, Y.; Bowman, J.; Harris, J. Catalyst Converter Canning Simulation Studies. SAE Tech. Pap. Ser. 2003. [Google Scholar] [CrossRef]
- Fernandes, S.; Martins, S.C.; Olson, J.; Peters, B. The Role of New Catalytic Converter Mounting Materials in Providing Durability and Efficiency. SAE Tech. Pap. Ser. 2002. [Google Scholar] [CrossRef]
- Spreen, K.B.; Heimrich, M.J.; Hornback, L.R.; Montalbano, A.J. Container Deformation Procedure for Ceramic Monolith Catalytic Converters. SAE Tech. Pap. Ser. 2000. [Google Scholar] [CrossRef]
- Taylor, W. CFD Prediction and Experimental Validation of High-Temperature Thermal Behavior in Catalytic Converters. SAE Tech. Pap. Ser. 1999. [Google Scholar] [CrossRef]
- Gao, J.; Tian, G.; Sorniotti, A.; Karci, A.E.; Di Palo, R. Review of thermal management of catalytic converters to decrease engine emissions during cold start and warm up. Appl. Therm. Eng. 2019, 147, 177–1879. [Google Scholar] [CrossRef]
- Lee, S.; Bae, C.; Lee, Y.; Han, T. Effects of Engine Operating Conditions on Catalytic Converter Temperature in an SI Engine. SAE Tech. Pap. Ser. 2002. [Google Scholar] [CrossRef]
- Choice Reviews Online. In Internal Combustion Engine Fundamentals; McGraw-Hill Education: New York, NY, USA, 1988; Volume 26, pp. 26–943. [CrossRef]
- Handbook of Air Pollution from Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 1998. [CrossRef]
- Whelan, I.; Timoney, D.; Smith, W.; Samuel, S. The Effect of a Three-Way Catalytic Converter on Particulate Matter from a Gasoline Direct-Injection Engine During Cold-Start. SAE Int. J. Engines 2013, 6, 1035–1045. [Google Scholar] [CrossRef]
- CITA. CITA Study No 4—Influence of Catalyst Temperature on Effectiveness of In-Service Testing; AEA Technology: Harwell, UK, 1994. [Google Scholar]
- Korin, E.; Reshef, R.; Tshernichovesky, D.; Sher, E. Improving Cold-Start Functioning of Catalytic Converters by Using Phase-Change Materials. SAE Tech. Pap. Ser. 1998. [Google Scholar] [CrossRef]
- Amirnordin, S.H.; Seri, S.M.; Salim, W.S.-I.W.; Rahman, H.A.; Hasnan, K. Pressure Drop Analysis of Square and Hexagonal Cellsand its Effects on the Performance of Catalytic Converters. Int. J. Environ. Sci. Dev. 2011, 239–247. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farrauto, R.J. The application of monoliths for gas phase catalytic reactions. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Effect of substrate geometry and flow condition on the turbulence generation after a monolith. Can. J. Chem. Eng. 2020, 98, 947–956. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdou, S.; Ahmed, W.H. Understanding Flow through Catalytic Converters. In Proceedings of the 5th International Conference of Fluid Flow, Heat and Mass Transfer (FFHMT’18), Orléans, ON, Canada, 7–9 June 2017. [Google Scholar] [CrossRef]
- Heywood, J.B. Pollutant formation and control in spark ignition engines. Symp. (Int.) Combust. 1975, 15, 1191–1211. [Google Scholar] [CrossRef]
- Al-Arkawazi, S.A.F. Analyzing and predicting the relation between air–fuel ratio (AFR), lambda (λ) and the exhaust emissions percentages and values of gasoline-fueled vehicles using versatile and portable emissions measurement system tool. SN Appl. Sci. 2019, 1, 1370. [Google Scholar] [CrossRef]
- Zuo, Q.; Xie, Y.; Jiaqiang, E.; Zhu, X.; Zhang, B.; Tang, Y.; Zhu, G.; Wang, Z.; Zhang, J. Effect of different exhaust parameters on NO conversion efficiency enhancement of a dual-carrier catalytic converter in the gasoline engine. Energy 2020, 191, 116521. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Jie, L. Environmental Effects of Vehicle Exhausts, Global and Local Effects—A Comparison between Gasoline and Diesel. Digit. Vetensk. Ark. 2011, 27. Available online: https://www.diva-portal.org/smash/get/diva2:427347/FULLTEXT01.pdf (accessed on 16 May 2021).




| Chemical Name (Symbol) | Platinum (Pt) | Palladium (Pd) | Rhodium (Rh) |
|---|---|---|---|
| Density (g/cm3) | 21.45 | 12.02 | 12.41 |
| Melting Point (°C) | 1769 | 1554 | 1960 |
| Vickers Hardness No. | 40 | 40 | 101 |
| Thermal Conductivity (W/M/°C) | 73 | 75 | 150 |
| Tensile Strength (kg/mm2) | 14 | 17 | 71 |
| Physical Properties | Soft, ductile and resistant to oxidation and high temperature corrosion | Excellent performance | |
| Conditions | Temperature (°C) |
|---|---|
| Catalyst Light-Off Temperature | 250–300 |
| Operating Temperature | 450–500 |
| Pt sinters | 700 |
| Pt-Rd and Pt-Rh Alloy forms | 700–800 |
| Alumina Sinters | 800–900 |
| Ceramic monolith softens | 1300–1400 |
| Metal Monolith melts | 1500–1600 |
| Pellet Melting | >1900 |
| Thermal Management Methods | Light-off Time Reduction | Cumulative Emission Reduction |
|---|---|---|
| Start of combustion delay | 80% | n.a. |
| Higher idle speed | 90% | n.a. |
| Variable Valve Timing | n.a. | 30% |
| Air/fuel ratio adjustment | n.a. | n.a. |
| After-treatment layout | 26% | n.a. |
| Burner | 40% | n.a. |
| Reformer | 50% | n.a. |
| Thermal energy storage device | 70% | n.a. |
| EHC | 50% | n.a. |
| Coolant and Lubricating Oil Heating | n.a. | 20% |
| Characteristic | 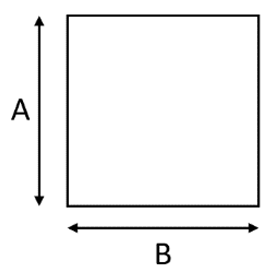 |  |
|---|---|---|
| Cell Shape | Square | Hexagonal |
| A (mm) | 0.95 | 1 |
| B (mm) | 0.95 | 0.575 |
| Cell Length (mm) | 118 | 69.8 |
| Cell density (cpsi) | 600 | 400 |
| Wall thickness (mm) | 0.114 | 0.089 |
| Air velocity for validation of square shaped cell | Experimental pressure drop (Pa) | Simulation pressure drop (Pa) |
| 5 | 417 | 476 |
| 10 | 857 | 987 |
| 15 | 1339 | 1527 |
| 20 | 1857 | 2132 |
| Air velocity for validation of square shaped cell | Experimental pressure drop (Pa) | Simulation pressure drop (Pa) |
| 5 | 152 | 153 |
| 10 | 318 | 364 |
| 15 | 512 | 576 |
| 20 | 747 | 822 |
| Pollutant Name and Symbol | Properties | Effect on Human Health |
|---|---|---|
| Carbon monoxide, CO | Highly poisonous, odourless, colourless and tasteless gas. Flammable. | Great effect on the oxygen delivery to the body’s organs and tissues, which may cause death. CO poisoning can occur. |
| Nitrogen oxides, NOX | Mixture of gases composed of Nitrogen and Oxygen. Nitrogen Oxide, NO is carcinogenic and toxic than Nitrogen Dioxide, NO2. | Linked to a wide range of respiratory problems e.g., cough and sore throat. |
| Unburned hydrocarbons, HC | Produced from incomplete combustion of organic fuels. These compounds contain Carbon and Hydrogen bonds only. | HC, NOX and Sunlight can generate the photochemical smog. Some hydrocarbon compounds caused irritation to the eye and damages lungs e.g., Benzene, C6H6. At high concentrations, it can cause asthma and death. |
| Gasoline Exhaust | No Catalyst | Three-Way Catalytic Converter |
|---|---|---|
| CO | 1800 | 300 |
| NOX | 3.46 | 0.82 |
| HC | 1560 | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritsanaviparkporn, E.; Baena-Moreno, F.M.; Reina, T.R. Catalytic Converters for Vehicle Exhaust: Fundamental Aspects and Technology Overview for Newcomers to the Field. Chemistry 2021, 3, 630-646. https://doi.org/10.3390/chemistry3020044
Kritsanaviparkporn E, Baena-Moreno FM, Reina TR. Catalytic Converters for Vehicle Exhaust: Fundamental Aspects and Technology Overview for Newcomers to the Field. Chemistry. 2021; 3(2):630-646. https://doi.org/10.3390/chemistry3020044
Chicago/Turabian StyleKritsanaviparkporn, Emmy, Francisco M. Baena-Moreno, and T. R. Reina. 2021. "Catalytic Converters for Vehicle Exhaust: Fundamental Aspects and Technology Overview for Newcomers to the Field" Chemistry 3, no. 2: 630-646. https://doi.org/10.3390/chemistry3020044
APA StyleKritsanaviparkporn, E., Baena-Moreno, F. M., & Reina, T. R. (2021). Catalytic Converters for Vehicle Exhaust: Fundamental Aspects and Technology Overview for Newcomers to the Field. Chemistry, 3(2), 630-646. https://doi.org/10.3390/chemistry3020044








