A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors
Abstract
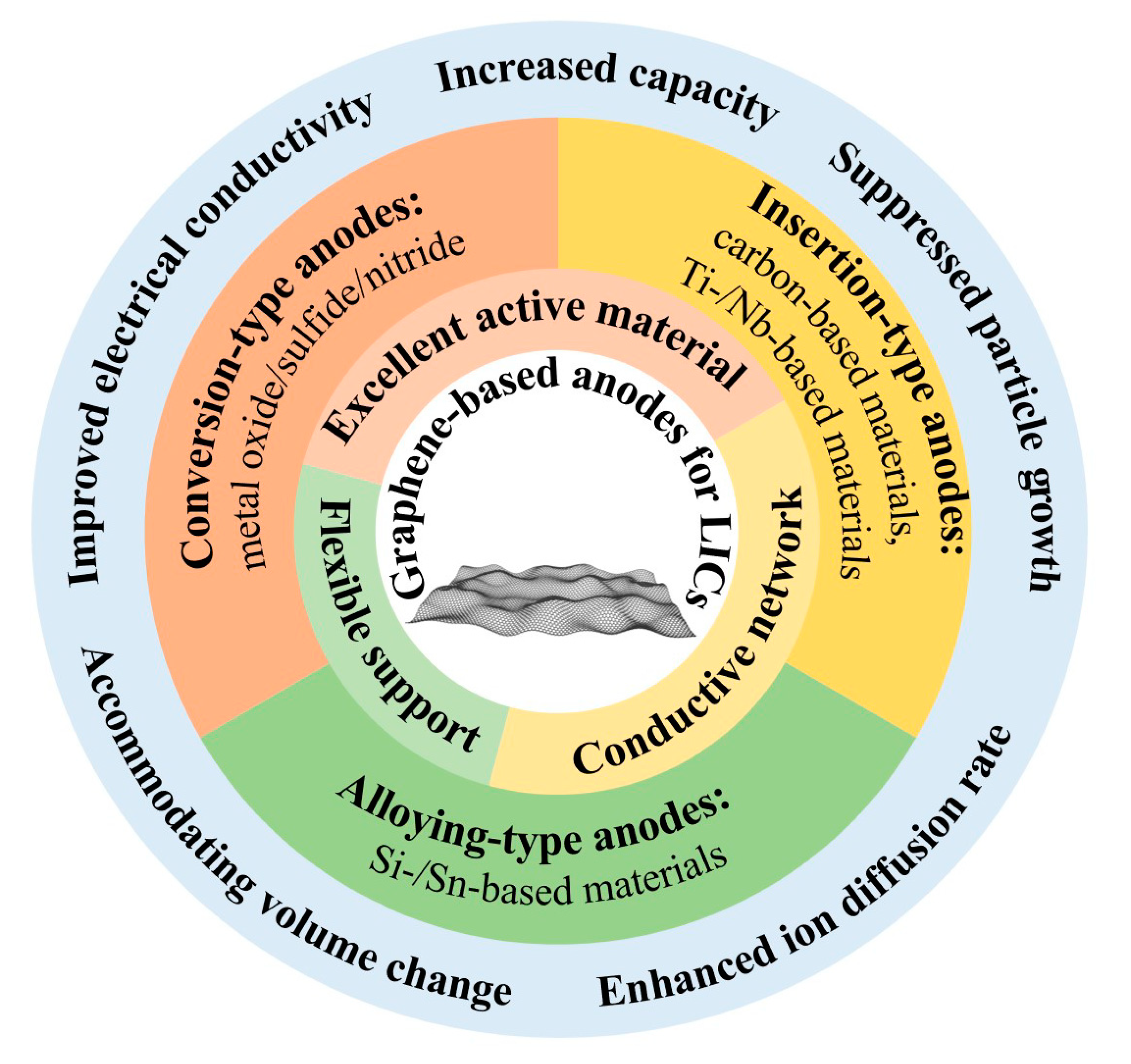
:1. Introduction
2. Graphene-Based Carbonaceous Anode Materials
2.1. Graphene as Anode Material
2.2. Graphene-Modified Carbonaceous Composites as Anode Materials
3. Graphene-Based Hybrid Anode Materials
3.1. Graphene/Intercalation-Type Anode Materials
3.1.1. Graphene/Ti-Based Materials
3.1.2. Graphene/Nb-Based Materials
3.2. Graphene/Conversion-Type Anode Materials
3.2.1. Graphene/Metal Oxides
3.2.2. Graphene/Metal Sulfides or Nitrides
3.3. Graphene/Alloying-Type Anode Materials
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Sun, C.; Wang, K.; Sun, X.; Ma, Y. Recent progress of graphene-based materials in lithium-ion capacitors. J. Phys. D Appl. Phys. 2019, 52, 143001. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Sun, L.; Yuan, M.; Shao, Y.; Ma, J.; Liu, G.; Guo, D.; Liu, X.; He, Y.-B. Hierarchical N-doped graphene coated 1D cobalt oxide microrods for robust and fast lithium storage at elevated temperature. Electrochim. Acta 2019, 310, 70–77. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Z.; Zhang, T.; Qin, B.; Sui, D.; Xie, Y.; Ma, Y.; Chen, Y. Porous asphalt/graphene composite for supercapacitors with high energy density at superior power density without added conducting materials. J. Mater. Chem. A 2017, 5, 21757–21764. [Google Scholar] [CrossRef]
- Tie, D.; Huang, S.; Wang, J.; Ma, J.; Zhang, J.; Zhao, Y. Hybrid energy storage devices: Advanced electrode materials and matching principles. Energy Storage Mater. 2019, 21, 22–40. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.P.; Xu, J.; Yang, S. Lithium ion capacitors (LICs): Development of the materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Du Pasquier, A.; Zheng, T. An Asymmetric Hybrid Nonaqueous Energy Storage Cell. J. Electrochem. Soc. 2001, 148, A930. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Liu, Y.; Li, L.; Wang, J. Non-aqueous dualcarbon lithiumion capacitors: A review. J. Mater. Chem. A 2019, 7, 15541–15563. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Achieving high-energy dual carbon Li-ion capacitors with unique low- and high-temperature performance from spent Li-ion batteries. J. Mater. Chem. A 2020, 8, 4950–4959. [Google Scholar] [CrossRef]
- Lang, J.; Zhang, X.; Liu, B.; Wang, R.; Chen, J.; Yan, X. The roles of graphene in advanced Li-ion hybrid supercapacitors. J. Energy Chem. 2018, 27, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, Z.; Yuan, X.; Mo, J.; Li, C.; Fu, L.; Zhu, Y.; Wu, X.; Wu, Y. A quasi-solid-state Li-ion capacitor with high energy density based on Li3VO4/carbon nanofibers and electrochemically-exfoliated graphene sheets. J. Mater. Chem. A 2017, 5, 14922–14929. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Activated Carbon from Biomass Transfer for High-Energy Density Lithium-Ion Supercapacitors. Adv. Energy Mater. 2016, 6, 1600802. [Google Scholar] [CrossRef]
- Sennu, P.; Arun, N.; Madhavi, S.; Aravindan, V.; Lee, Y.-S. All carbon based high energy lithium-ion capacitors from biomass: The role of crystallinity. J. Power Sources 2019, 414, 96–102. [Google Scholar] [CrossRef]
- Aref, A.R.; Chen, S.-W.; Rajagopalan, R.; Randall, C. Bimodal porous carbon cathode and prelithiated coalesced carbon onion anode for ultrahigh power energy efficient lithium ion capacitors. Carbon 2019, 152, 89–97. [Google Scholar] [CrossRef]
- Cao, W.J.; Zheng, J.P. Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J. Power Sources 2012, 213, 180–185. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, L.; Zhang, C.; Yin, F.; Peng, H.; Wang, G. Heterogeneous nucleation of Li3VO4 regulated in dense graphene aerogel for lithium ion capacitors. J. Power Sources 2020, 468, 228364. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, L.; Peng, J.; Wang, H.; Yan, H. Synthesis of Li4Ti5O12-reduced graphene oxide composite and its application for hybrid supercapacitors. Ionics 2016, 22, 1829–1836. [Google Scholar] [CrossRef]
- Yang, C.; Lan, J.-L.; Ding, C.; Wang, F.; Siyal, S.H.; Yu, Y.; Yang, X. Three-dimensional hierarchical ternary aerogels of ultrafine TiO2 nanoparticles@porous carbon nanofibers-reduced graphene oxide for high-performance lithium-ion capacitors. Electrochim. Acta 2019, 296, 790–798. [Google Scholar] [CrossRef]
- Tian, M.; Liu, C.; Neale, Z.G.; Zheng, J.; Long, D.; Cao, G. Chemically Bonding NiFe-LDH Nanosheets on rGO for Superior Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2019, 11, 35977–35986. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Li, C.; Sun, X.; Liu, J.; Wang, K.; Ma, Y. High-Performance Lithium-Ion Capacitors Based on CoO-Graphene Composite Anode and Holey Carbon Nanolayer Cathode. ACS Sustain. Chem. Eng. 2019, 7, 11275–11283. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhang, X.; Sun, X.; Wang, K.; Ma, Y. High Performance Lithium-Ion Hybrid Capacitors Employing Fe3O4–Graphene Composite Anode and Activated Carbon Cathode. ACS Appl. Mater. Interfaces 2017, 9, 17136–17144. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lei, S.; Liu, J.; Shang, Z.; Zhong, S.; Li, X. Promoting the energy density of lithium-ion capacitor by coupling the pore-size and nitrogen content in capacitive carbon cathode. J. Power Sources 2021, 498, 229912. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.; Zhu, Y.; Pi, X.; Wang, L.; Liu, X.; Qin, Y. A high performance lithium ion capacitor achieved by the integration of a Sn-C anode and a biomass-derived microporous activated carbon cathode. Sci. Rep. 2017, 7, 40990. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Xu, G.; Han, X.; Zhao, J.; Zhou, X.; Cui, G. Lithium Ion Capacitors in Organic Electrolyte System: Scientific Problems, Material Development, and Key Technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Niu, Z. Carbon-based materials for lithium-ion capacitors. Mater. Chem. Front. 2019, 3, 1265–1279. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.-Y.; Cho, M.-Y.; Kim, M.-H.; Hong, J.; Jung, S.-K.; Roh, K.C.; Kang, K. High-Performance Hybrid Supercapacitor Based on Graphene-Wrapped Li4Ti5O12 and Activated Carbon. ChemElectroChem 2014, 1, 125–130. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2014, 2, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Wu, M.; Zhang, M.; Yao, B.; Gao, T.; Wang, H.; Li, C.; Sui, D.; Chen, Y.; et al. Tailoring the oxygenated groups of graphene hydrogels for high-performance supercapacitors with large areal mass loadings. J. Mater. Chem. A 2018, 6, 6587–6594. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Wang, H.; Qian, H.; Yang, Y.; Li, S.; Zhang, Y.; Song, Y. A Brief Review of Catalytic Cathode Materials for Na-CO2 Batteries. Catalysts 2021, 11, 603. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Jia, M.; Yang, D.; Wang, J.; Li, M.; Zhang, J.; Sun, Q.; Jia, Y. Porous graphene for high capacity lithium ion battery anode material. Appl. Surf. Sci. 2016, 363, 318–322. [Google Scholar] [CrossRef]
- Jang, B.Z.; Liu, C.; Neff, D.; Yu, Z.; Wang, M.C.; Xiong, W.; Zhamu, A. Graphene Surface-Enabled Lithium Ion-Exchanging Cells: Next-Generation High-Power Energy Storage Devices. Nano Lett. 2011, 11, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Zhang, F.; Zhang, L.; Zhang, T.; Wu, Y.; Lu, Y.; Huang, Y.; Chen, Y. Graphene-based Li-ion hybrid supercapacitors with ultrahigh performance. Nano Res. 2013, 6, 581–592. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Liu, B.; Ding, Y.; Tang, Y.; Yan, X. One dimensional graphene nanoscroll-wrapped MnO nanoparticles for high-performance lithium ion hybrid capacitors. J. Mater. Chem. A 2021, 9, 6352–6360. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Nerkar, J.Y.; Pandolfo, A.G. Rate capability of graphite materials as negative electrodes in lithium-ion capacitors. Electrochim. Acta 2010, 55, 3330–3335. [Google Scholar] [CrossRef]
- Ren, J.J.; Su, L.W.; Qin, X.; Yang, M.; Wei, J.P.; Zhou, Z.; Shen, P.W. Pre-lithiated graphene nanosheets as negative electrode materials for Li-ion capacitors with high power and energy density. J. Power Sources 2014, 264, 108–113. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhang, H.; Xu, N.; Wang, K.; Ma, Y. High performance lithium-ion hybrid capacitors with pre-lithiated hard carbon anodes and bifunctional cathode electrodes. J. Power Sources 2014, 270, 318–325. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, J.; Shi, J.; Shi, Z. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors. Electrochim. Acta 2016, 187, 134–142. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Chong, C.; Wang, J.; Shi, Z.; Pan, J. Phenolic formaldehyde resin/graphene composites as lithium-ion batteries anode. Mater. Lett. 2016, 170, 217–220. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Graphene: A Two-Dimensional Platform for Lithium Storage. Small 2013, 9, 1173–1187. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Ahn, W.; Lee, D.U.; Li, G.; Feng, K.; Wang, X.; Yu, A.; Lui, G.; Chen, Z. Highly Oriented Graphene Sponge Electrode for Ultra High Energy Density Lithium Ion Hybrid Capacitors. ACS Appl. Mater. Interfaces 2016, 8, 25297–25305. [Google Scholar] [CrossRef]
- Sha, J.; Chu, X.; Xu, T.; Li, Y.; Tang, Y.; Ma, L.; Shi, C.; Liu, E.; Zhao, D.; He, C.; et al. Bi-functional modular graphene network with high rate and cycling performance. J. Power Sources 2021, 504, 230075. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Chen, W.; Huang, Z.; Liu, H. Mesh-Like Carbon Nanosheets with High-Level Nitrogen Doping for High-Energy Dual-Carbon Lithium-Ion Capacitors. Small 2019, 15, 1805173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Zhang, X.; Peng, H.; Xin, G.; Lu, C.; Zhong, Y.; Wang, G.; Zhang, Y. Nitrogen-Doped Defective Graphene Aerogel as Anode for all Graphene-Based Lithium Ion Capacitor. ChemistrySelect 2017, 2, 8436–8445. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Liu, G.; Li, J.; Tian, H.; Li, J.; Ma, Y. Scalable Self-Propagating High-Temperature Synthesis of Graphene for Supercapacitors with Superior Power Density and Cyclic Stability. Adv. Mater. 2017, 29, 1604690. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Ma, Y. High-power and long-life lithium-ion capacitors constructed from N-doped hierarchical carbon nanolayer cathode and mesoporous graphene anode. Carbon 2018, 140, 237–248. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Xu, Y.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. Nitrogen-enriched graphene framework from a large-scale magnesiothermic conversion of CO2 with synergistic kinetics for high-power lithium-ion capacitors. NPG Asia Mater. 2021, 13, 59. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lin, Q.; Ding, B.; Wang, J.; Malgras, V.; Jiang, J.; Li, Z.; Chen, S.; Dou, H.; Alshehri, S.M.; et al. Lithium-ion capacitor based on nanoarchitectured polydopamine/graphene composite anode and porous graphene cathode. Carbon 2020, 167, 627–633. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Liu, Y.; Yang, Y.; Zhang, H.; Ma, Y.; Zhang, L.; Chen, Y. High performance Li-ion capacitor fabricated with dual graphene-based materials. Nanotechnology 2020, 32, 015403. [Google Scholar] [CrossRef] [PubMed]
- Tjandra, R.; Liu, W.; Lim, L.; Yu, A. Melamine based, n-doped carbon/reduced graphene oxide composite foam for Li-ion Hybrid Supercapacitors. Carbon 2018, 129, 152–158. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Q.; Zheng, W.; Ren, Z.; Hu, X.; Li, J.; Lu, L.; Hu, N.; Molenda, J.; Liu, X.; et al. Achieving high energy density in a 4.5 V all nitrogen-doped graphene based lithium-ion capacitor. J. Mater. Chem. A 2019, 7, 19909–19921. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Ajuria, J.; Zarrabeitia, M.; Arnaiz, M.; Urra, O.; Rojo, T.; Goikolea, E. Graphene as Vehicle for Ultrafast Lithium Ion Capacitor Development Based on Recycled Olive Pit Derived Carbons. J. Electrochem. Soc. 2019, 166, A2840–A2848. [Google Scholar] [CrossRef]
- An, Y.; Liu, T.; Li, C.; Zhang, X.; Hu, T.; Sun, X.; Wang, K.; Wang, C.; Ma, Y. A general route for the mass production of graphene-enhanced carbon composites toward practical pouch lithium-ion capacitors. J. Mater. Chem. A 2021, 9, 15654–15664. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing Graphene Sheets from Restacking for High-Capacitance Performance. J. Phys. Chem. C 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Qin, F.; Yuan, J.; Zhang, K.; Li, J.; Zhu, D.-M.; Qin, L.-C. Hybrid lithium-ion capacitors with asymmetric graphene electrodes. J. Mater. Chem. A 2017, 5, 13601–13609. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.R.; Chen, C.; Wang, C. Electrostatically Sprayed Reduced Graphene Oxide-Carbon Nanotubes Electrodes for Lithium-Ion Capacitors. C 2018, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Phattharasupakun, N.; Wutthiprom, J.; Suktha, P.; Ma, N.; Sawangphruk, M. Enhancing the Charge Storage Capacity of Lithium-Ion Capacitors Using Nitrogen-Doped Reduced Graphene Oxide Aerogel as a Negative Electrode: A Hydrodynamic Rotating Disk Electrode Investigation. J. Electrochem. Soc. 2018, 165, A609–A617. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R Rep. 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-G.; Venugopal, N.; Scrosati, B.; Sun, Y.-K. A high energy and power density hybrid supercapacitor based on an advanced carbon-coated Li4Ti5O12 electrode. J. Power Sources 2013, 221, 266–271. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, C.; Peng, H.; Wang, X.; Zhang, Y.; Wang, Z.; Zhong, Y.; Wang, G. Influence of sintering temperature and graphene additives on the electrochemical performance of porous Li4Ti5O12 anode for lithium ion capacitor. Electrochim. Acta 2017, 246, 1237–1247. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Zhang, X.; Peng, H.; Zhang, Y.; Wang, G.; Wang, Z.; Cao, G.; Umirov, N.; Bakenov, Z. Effect of graphene nanosheets on electrochemical performance of Li4Ti5O12 in lithium-ion capacitors. Ceram. Int. 2017, 43, 6554–6562. [Google Scholar] [CrossRef]
- Wang, G.; Lu, C.; Zhang, X.; Wan, B.; Liu, H.; Xia, M.; Gou, H.; Xin, G.; Lian, J.; Zhang, Y. Toward ultrafast lithium ion capacitors: A novel atomic layer deposition seeded preparation of Li4Ti5O12/graphene anode. Nano Energy 2017, 36, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Lee, G.-W.; Kim, Y.H.; Choi, Y.J.; Roh, K.C.; Kim, K.-B. A holey graphene-based hybrid supercapacitor. Chem. Eng. J. 2019, 378, 122126. [Google Scholar] [CrossRef]
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2-x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Chen, J.; Yang, B.; Liu, B.; Lang, J.; Yan, X. Recent advances in anode materials for sodium—And potassium-ion hybrid capacitors. Curr. Opin. Electrochem. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.-E.; Yang, L.; Sheng, J.; Chen, Y.; Gu, H.; Wei, J.; Zhou, Z. Fast Sodium Storage in TiO2@CNT@C Nanorods for High-Performance Na-Ion Capacitors. Adv. Energy Mater. 2017, 7, 1701222. [Google Scholar] [CrossRef]
- Kim, H.-K.; Mhamane, D.; Kim, M.-S.; Roh, H.-K.; Aravindan, V.; Madhavi, S.; Roh, K.C.; Kim, K.-B. TiO2-reduced graphene oxide nanocomposites by microwave-assisted forced hydrolysis as excellent insertion anode for Li-ion battery and capacitor. J. Power Sources 2016, 327, 171–177. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; He, X.; Zhang, X.; Yan, B.; Hou, X.; Du, L.; Placke, T.; Winter, M.; Li, J. A three-dimensional TiO2-Graphene architecture with superior Li ion and Na ion storage performance. J. Power Sources 2020, 461, 228129. [Google Scholar] [CrossRef]
- Auxilia, F.M.; Jang, J.; Jang, K.; Song, H.; Ham, M.-H. Au@TiO2/reduced graphene oxide nanocomposites for lithium-ion capacitors. Chem. Eng. J. 2019, 362, 136–143. [Google Scholar] [CrossRef]
- Zhu, G.; Ma, L.; Lin, H.; Zhao, P.; Wang, L.; Hu, Y.; Chen, R.; Chen, T.; Wang, Y.; Tie, Z.; et al. High-performance Li-ion capacitor based on black-TiO2-x/graphene aerogel anode and biomass-derived microporous carbon cathode. Nano Res. 2019, 12, 1713–1719. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Chao, D.; Yan, Q.; Fan, H.J. Nonaqueous Hybrid Lithium-Ion and Sodium-Ion Capacitors. Adv. Mater. 2017, 29, 1702093. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yang, M.; Zhang, L.; Li, Y.; Wang, J.; Liu, G.; Wu, N.; Kim, J.-K.; Liu, X. Cr2O3 nanosheet/carbon cloth anode with strong interaction and fast charge transfer for pseudocapacitive energy storage in lithium-ion batteries. RSC Adv. 2019, 9, 33446–33453. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xu, L.; Li, Y.; Guo, D.; Wu, N.; Yuan, C.; Qin, A.; Cao, A.; Liu, X. Metal-organic frameworks derived anatase/rutile heterostructures with enhanced reaction kinetics for lithium and sodium storage. Chem. Eng. J. 2021, 430, 132689. [Google Scholar] [CrossRef]
- Li, D.; Shi, J.; Liu, H.; Liu, C.; Dong, G.; Zhang, H.; Yang, Y.; Lu, G.; Wang, H. T-Nb2O5 embedded carbon nanosheets with superior reversibility and rate capability as an anode for high energy Li-ion capacitors. Sustain. Energy Fuels 2019, 3, 1055–1065. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Kim, J.W.; Augustyn, V.; Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X.; Wei, J.; Wang, S.; Xu, B.B.; Long, D.; Chen, F. T-Nb2O5 nanoparticle enabled pseudocapacitance with fast Li-ion intercalation. Nanoscale 2018, 10, 14165–14170. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.P.; Yu, L.; Satish, R.; Zhu, J.; Yan, Q.; Srinivasan, M.; Xu, Z. High-performance hybrid electrochemical capacitor with binder-free Nb2O5@graphene. RSC Adv. 2014, 4, 37389–37394. [Google Scholar] [CrossRef]
- Jiao, X.; Hao, Q.; Xia, X.; Wu, Z.; Lei, W. Metal organic framework derived Nb2O5@C nanoparticles grown on reduced graphene oxide for high-energy lithium ion capacitors. Chem. Commun. 2019, 55, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jian, Z.; Fang, Z.; Gu, L.; Hu, Y.-S.; Chen, W.; Wang, Z.; Chen, L. Atomic-scale investigation on lithium storage mechanism in TiNb2O7. Energy Environ. Sci. 2011, 4, 2638–2644. [Google Scholar] [CrossRef]
- Song, H.; Kim, Y.-T. A Mo-doped TiNb2O7 anode for lithium-ion batteries with high rate capability due to charge redistribution. Chem. Commun. 2015, 51, 9849–9852. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, G. Intercalation pseudo-capacitive TiNb2O7@carbon electrode for high-performance lithium ion hybrid electrochemical supercapacitors with ultrahigh energy density. Nano Energy 2015, 15, 104–115. [Google Scholar] [CrossRef]
- Jiao, X.; Hao, Q.; Xia, X.; Yao, D.; Ouyang, Y.; Lei, W. Boosting long-cycle-life energy storage with holey graphene supported TiNb2O7 network nanostructure for lithium ion hybrid supercapacitors. J. Power Sources 2018, 403, 66–75. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Gong, X.; Wang, J.; Lee, P.S. Holey graphene-wrapped porous TiNb24O62 microparticles as high-performance intercalation pseudocapacitive anode materials for lithium-ion capacitors. NPG Asia Mater. 2018, 10, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Zhang, H.; Xing, C.; Wang, Y.; Zhang, J.; Zhang, X.; Zhang, S. Intensified Energy Storage in High-Voltage Nanohybrid Supercapacitors via the Efficient Coupling between TiNb2O7/Holey-rGO Nanoarchitectures and Ionic Liquid-Based Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 21349–21361. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Liu, A.; Jiao, Y.; Shim, J.-J.; Zhang, S. Fabrication of nanoarchitectured TiO2(B)@C/rGO electrode for 4 V quasi-solid-state nanohybrid supercapacitors. Electrochim. Acta 2017, 258, 343–352. [Google Scholar] [CrossRef]
- Zhu, W.; El-Khodary, S.A.; Li, S.; Zou, B.; Kang, R.; Li, G.; Ng, D.H.L.; Liu, X.; Qiu, J.; Zhao, Y.; et al. Roselle-like Zn2Ti3O8/rGO nanocomposite as anode for lithium ion capacitor. Chem. Eng. J. 2020, 385, 123881. [Google Scholar] [CrossRef]
- Li, S.; Shadike, Z.; Kwon, G.; Yang, X.-Q.; Lee, J.H.; Hwang, S. Asymmetric Reaction Pathways of Conversion-Type Electrodes for Lithium-Ion Batteries. Chem. Mater. 2021, 33, 3515–3523. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Huang, J.-L.; Fan, L.-Q.; Gu, Y.; Geng, C.-L.; Luo, H.; Huang, Y.-F.; Lin, J.-M.; Wu, J.-H. One-step solvothermal synthesis of high-capacity Fe3O4/reduced graphene oxide composite for use in Li-ion capacitor. J. Alloy. Compd. 2019, 788, 1119–1126. [Google Scholar] [CrossRef]
- Kim, H.-K.; Aravindan, V.; Roh, M.H.-K.; Lee, K.; Jung, M.-H.; Madhavi, S.; Roh, K.C.; Kim, K.-B. Exploring High-Energy Li-I(r)on Batteries and Capacitors with Conversion-Type Fe3O4-rGO as the Negative Electrode. ChemElectroChem 2017, 4, 2626–2633. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Q.-Q.; Zhang, S.-W.; Yin, B.-S.; Que, L.-F.; Zhao, L.; Sui, X.-L.; Yu, F.-D.; Li, X.; Gu, D.-M.; et al. High energy and power lithium-ion capacitors based on Mn3O4/3D-graphene as anode and activated polyaniline-derived carbon nanorods as cathode. Chem. Eng. J. 2019, 370, 1485–1492. [Google Scholar] [CrossRef]
- Jiang, S.; Yun, S.; Cao, H.; Zhang, Z.; Feng, H.; Chen, H. Porous carbon matrix-encapsulated MnO in situ derived from metal-organic frameworks as advanced anode materials for Li-ion capacitors. Sci. China Mater. 2021. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Ling, W.C.; Yan, Q.; Madhavi, S. High energy Li-ion capacitors with conversion type Mn3O4 particulates anchored to few layer graphene as the negative electrode. J. Mater. Chem. A 2016, 4, 15134–15139. [Google Scholar] [CrossRef]
- Yang, M.; Zhong, Y.; Ren, J.; Zhou, X.; Wei, J.; Zhou, Z. Fabrication of High-Power Li-Ion Hybrid Supercapacitors by Enhancing the Exterior Surface Charge Storage. Adv. Energy Mater. 2015, 5, 1500550. [Google Scholar] [CrossRef]
- Han, P.; Ma, W.; Pang, S.; Kong, Q.; Yao, J.; Bi, C.; Cui, G. Graphene decorated with molybdenum dioxide nanoparticles for use in high energy lithium ion capacitors with an organic electrolyte. J. Mater. Chem. A 2013, 1, 5949–5954. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Feng, K.; Sy, A.; Liu, Y.; Lim, L.; Lui, G.; Tjandra, R.; Rasenthiram, L.; Chiu, G.; et al. Advanced Li-Ion Hybrid Supercapacitors Based on 3D Graphene–Foam Composites. ACS Appl. Mater. Interfaces 2016, 8, 25941–25953. [Google Scholar] [CrossRef]
- Ock, I.W.; Lee, J.; Kang, J.K. Metal–Organic Framework-Derived Anode and Polyaniline Chain Networked Cathode with Mesoporous and Conductive Pathways for High Energy Density, Ultrafast Rechargeable, and Long-Life Hybrid Capacitors. Adv. Energy Mater. 2020, 10, 2001851. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Zhao, Y.; Yao, H.; Pang, H. MoS2/graphene composites: Fabrication and electrochemical energy storage. Energy Storage Mater. 2020, 33, 470–502. [Google Scholar] [CrossRef]
- Wang, R.; Jin, D.; Zhang, Y.; Wang, S.; Lang, J.; Yan, X.; Zhang, L. Engineering metal organic framework derived 3D nanostructures for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 292–302. [Google Scholar] [CrossRef]
- Benavente, E.; Santa Ana, M.A.; Mendizábal, F.; González, G. Intercalation chemistry of molybdenum disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Fang, X.; Hua, C.; Guo, X.; Hu, Y.; Wang, Z.; Gao, X.; Wu, F.; Wang, J.; Chen, L. Lithium storage in commercial MoS2 in different potential ranges. Electrochim. Acta 2012, 81, 155–160. [Google Scholar] [CrossRef]
- Kong, D.; He, H.; Song, Q.; Wang, B.; Lv, W.; Yang, Q.-H.; Zhi, L. Rational design of MoS2@graphene nanocables: Towards high performance electrode materials for lithium ion batteries. Energy Environ. Sci. 2014, 7, 3320–3325. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Y.; Liu, H.; Ji, H.; Jiang, C.; Zhang, J.; Zhang, X.; Lee, C.-S. Uniform Incorporation of Flocculent Molybdenum Disulfide Nanostructure into Three-Dimensional Porous Graphene as an Anode for High-Performance Lithium Ion Batteries and Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4691–4699. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Jin, D.; Zhang, Y.; Cai, Y.; Ma, J.; Zhang, L. Engineering layer structure of MoS2-graphene composites with robust and fast lithium storage for high-performance Li-ion capacitors. Energy Storage Mater. 2017, 9, 195–205. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, Z.-W. Vanadium nitride as a novel thin film anode material for rechargeable lithium batteries. Electrochim. Acta 2008, 54, 403–409. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Zhang, P.; Lin, Z.; Yan, X. Fast and Large Lithium Storage in 3D Porous VN Nanowires–Graphene Composite as a Superior Anode Toward High-Performance Hybrid Supercapacitors. Adv. Funct. Mater. 2015, 25, 2270–2278. [Google Scholar] [CrossRef]
- Yang, C.; Sun, M.; Zhang, L.; Liu, P.; Wang, P.; Lu, H. ZnFe2O4@Carbon Core–Shell Nanoparticles Encapsulated in Reduced Graphene Oxide for High-Performance Li-Ion Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 14713–14721. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, H.; Park, B.-J.; Han, Y.-H.; Park, J.H.; Cho, J.; Lee, S.-S.; Son, J.G. Etching-Assisted Crumpled Graphene Wrapped Spiky Iron Oxide Particles for High-Performance Li-Ion Hybrid Supercapacitor. Small 2018, 14, 1704209. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.-Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Z.; Zhang, T.; Sui, D.; Ma, Y.; Chen, Y. Excellent cycling stability with high SnO2 loading on a three-dimensional graphene network for lithium ion batteries. Carbon 2016, 102, 32–38. [Google Scholar] [CrossRef]
- Sui, D.; Xie, Y.; Zhao, W.; Zhang, H.; Zhou, Y.; Qin, X.; Ma, Y.; Yang, Y.; Chen, Y. A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell. J. Power Sources 2018, 384, 328–333. [Google Scholar] [CrossRef]
- An, Y.-B.; Chen, S.; Zou, M.-M.; Geng, L.-B.; Sun, X.-Z.; Zhang, X.; Wang, K.; Ma, Y.-W. Improving anode performances of lithium-ion capacitors employing carbon–Si composites. Rare Met. 2019, 38, 1113–1123. [Google Scholar] [CrossRef]
- Shao, R.; Niu, J.; Zhu, F.; Dou, M.; Zhang, Z.; Wang, F. A facile and versatile strategy towards high-performance Si anodes for Li-ion capacitors: Concomitant conductive network construction and dual-interfacial engineering. Nano Energy 2019, 63, 103824. [Google Scholar] [CrossRef]
- Sun, X.; Geng, L.; Yi, S.; Li, C.; An, Y.; Zhang, X.; Zhang, X.; Wang, K.; Ma, Y. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J. Power Sources 2021, 499, 229936. [Google Scholar] [CrossRef]
- Ajuria, J.; Arnaiz, M.; Botas, C.; Carriazo, D.; Mysyk, R.; Rojo, T.; Talyzin, A.V.; Goikolea, E. Graphene-based lithium ion capacitor with high gravimetric energy and power densities. J. Power Sources 2017, 363, 422–427. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, S.; Shao, Y.; Wu, Y.; Miao, S. High-Energy Density Li-Ion Capacitor with Layered SnS2/Reduced Graphene Oxide Anode and BCN Nanosheet Cathode. Adv. Energy Mater. 2020, 10, 1902836. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Shi, D.; Chen, F.; Shao, Y.; Wu, Y.; Hao, X. Lithium-ion capacitor with improved energy density via perfect matching silicon@3D graphene aerogel anode and BCNNTs cathode. J. Mater. Chem. A 2021, 9, 1134–1142. [Google Scholar] [CrossRef]
- Ahn, S.; Haniu, Y.; Nara, H.; Momma, T.; Sugimoto, W.; Osaka, T. Synthesis of Stacked Graphene-Sn Composite as a High-Performance Anode for Lithium-Ion Capacitors. J. Electrochem. Soc. 2020, 167, 040519. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Zhu, S.; Li, Y.; Pan, H.; Meng, X.; Imtiaz, M.; Zhang, D. Construction of SnO2−Graphene Composite with Half-Supported Cluster Structure as Anode toward Superior Lithium Storage Properties. Sci. Rep. 2017, 7, 3276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Y.; Wang, H.-G.; Hou, J.; Li, Y.; Duan, Q. Graphene encapsulated metallic state Ce2Sn2O7 as a novel anode material for superior lithium-ion batteries and capacitors. J. Mater. Chem. A 2020, 8, 5517–5524. [Google Scholar] [CrossRef]
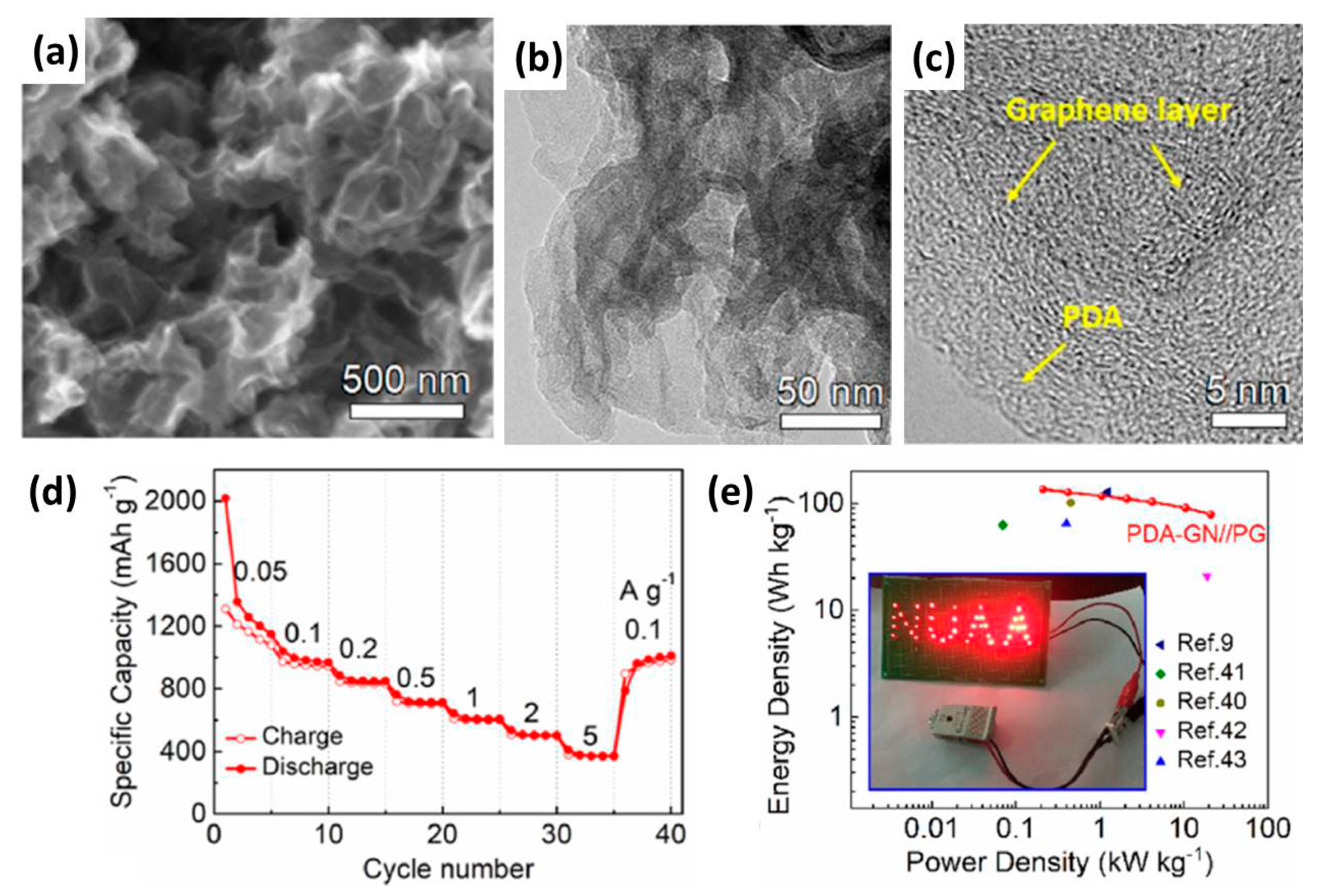
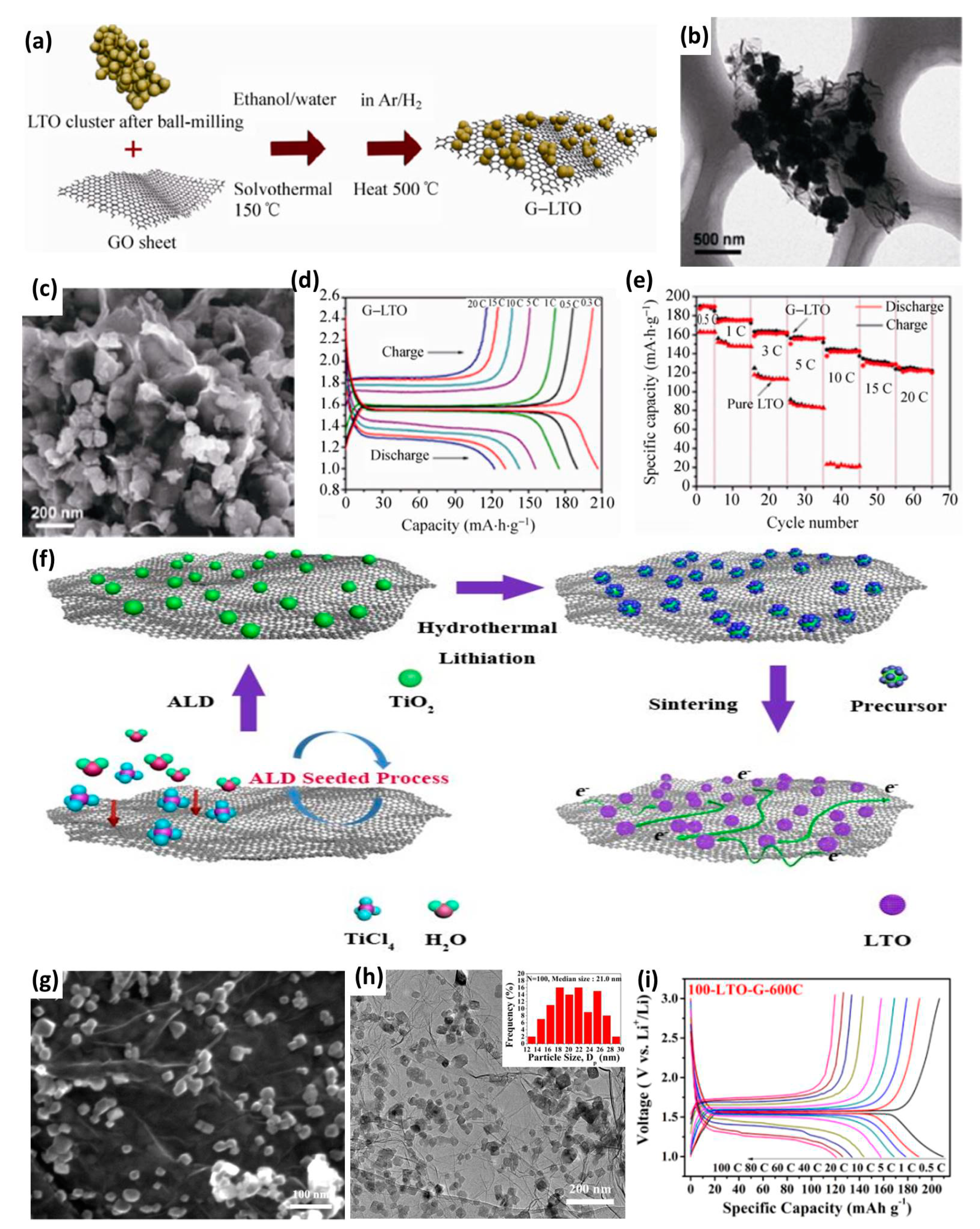

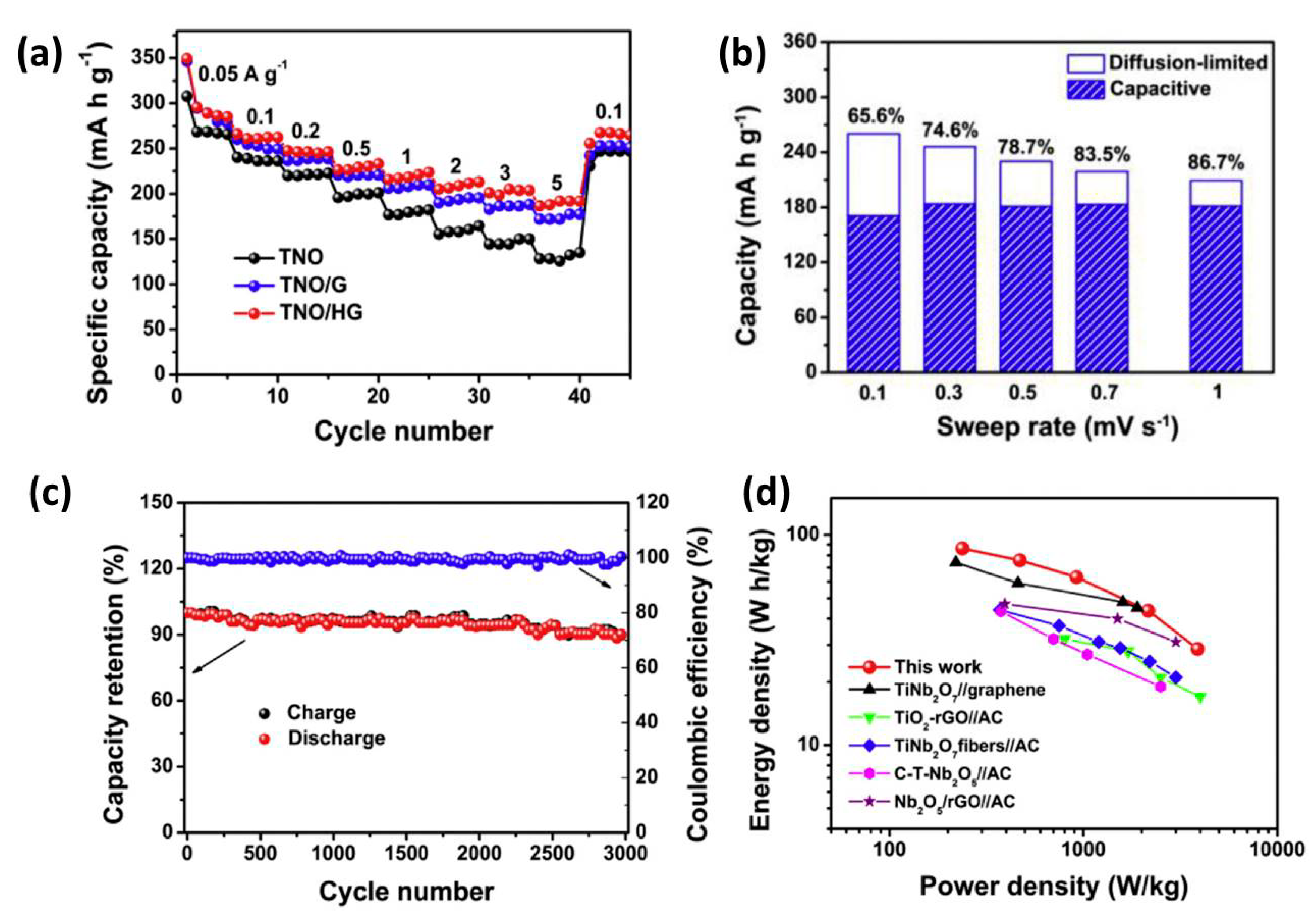
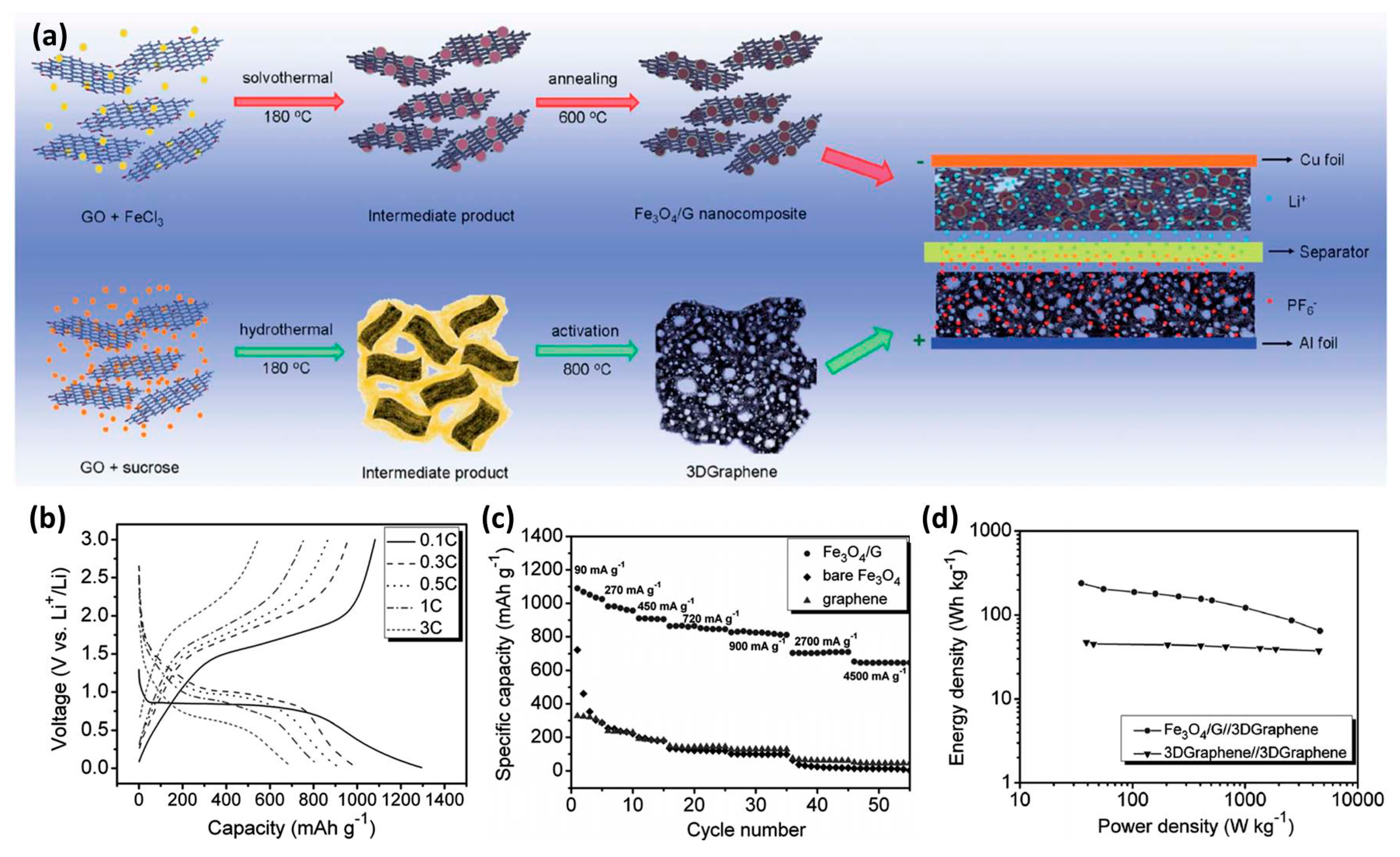
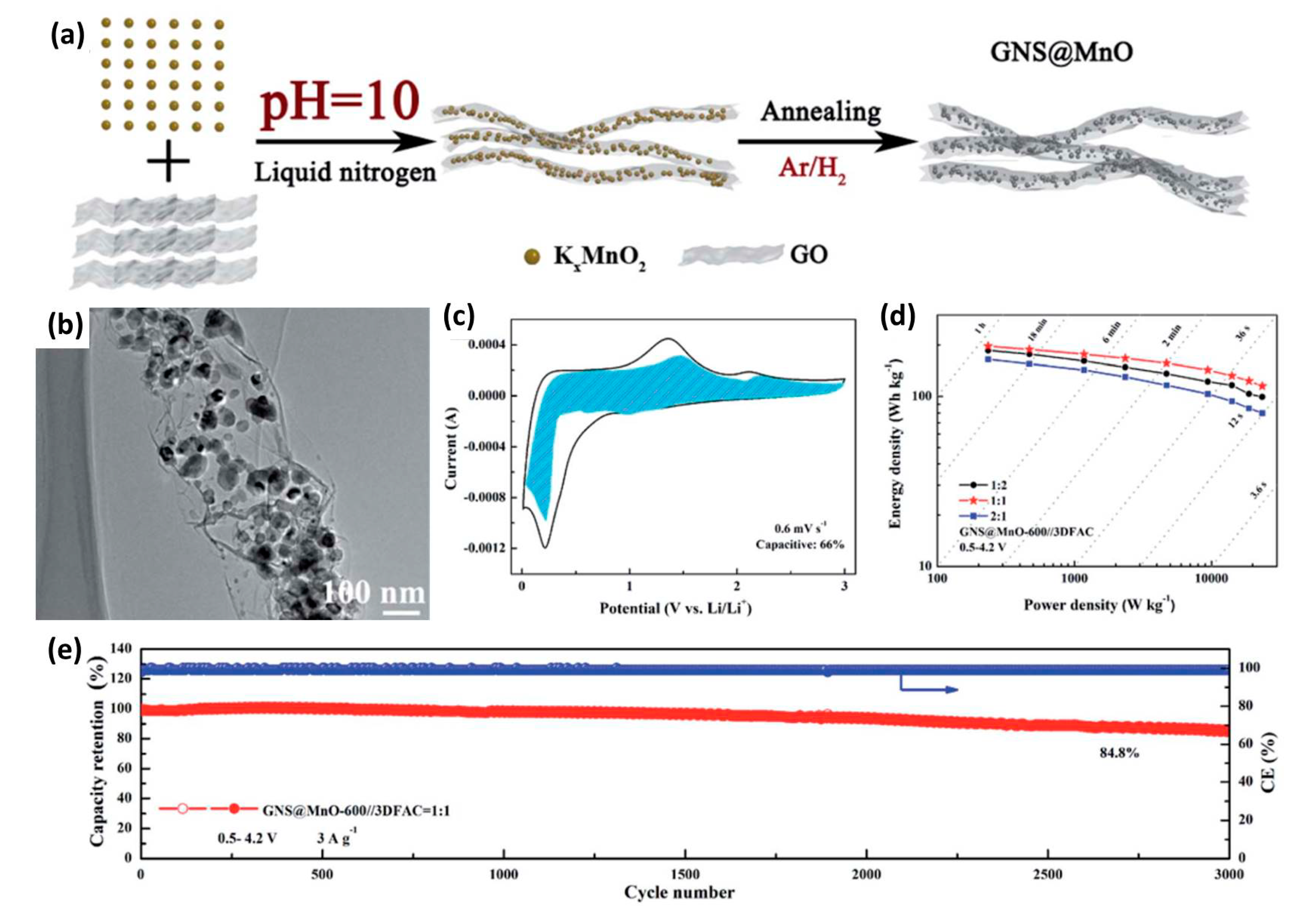
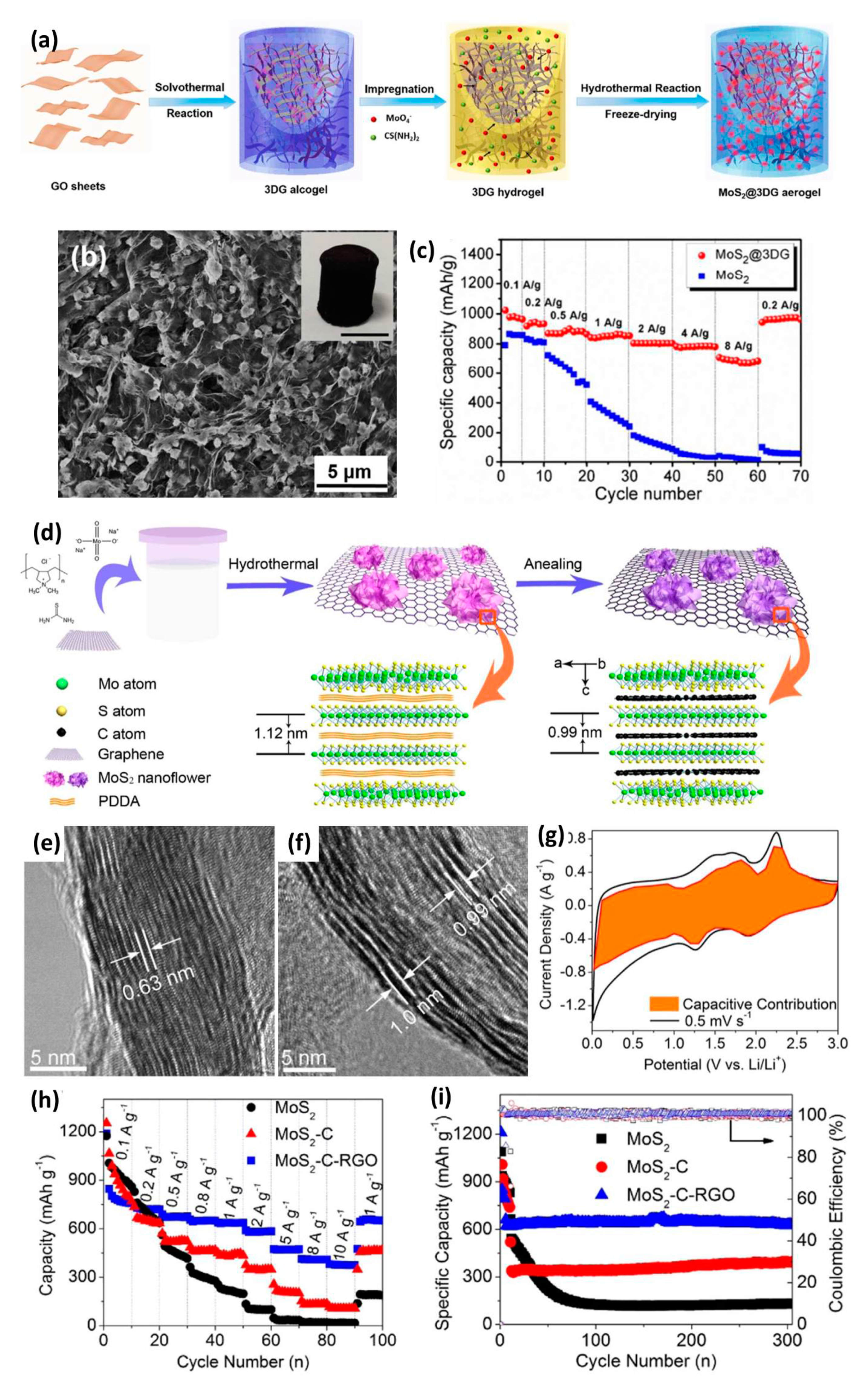
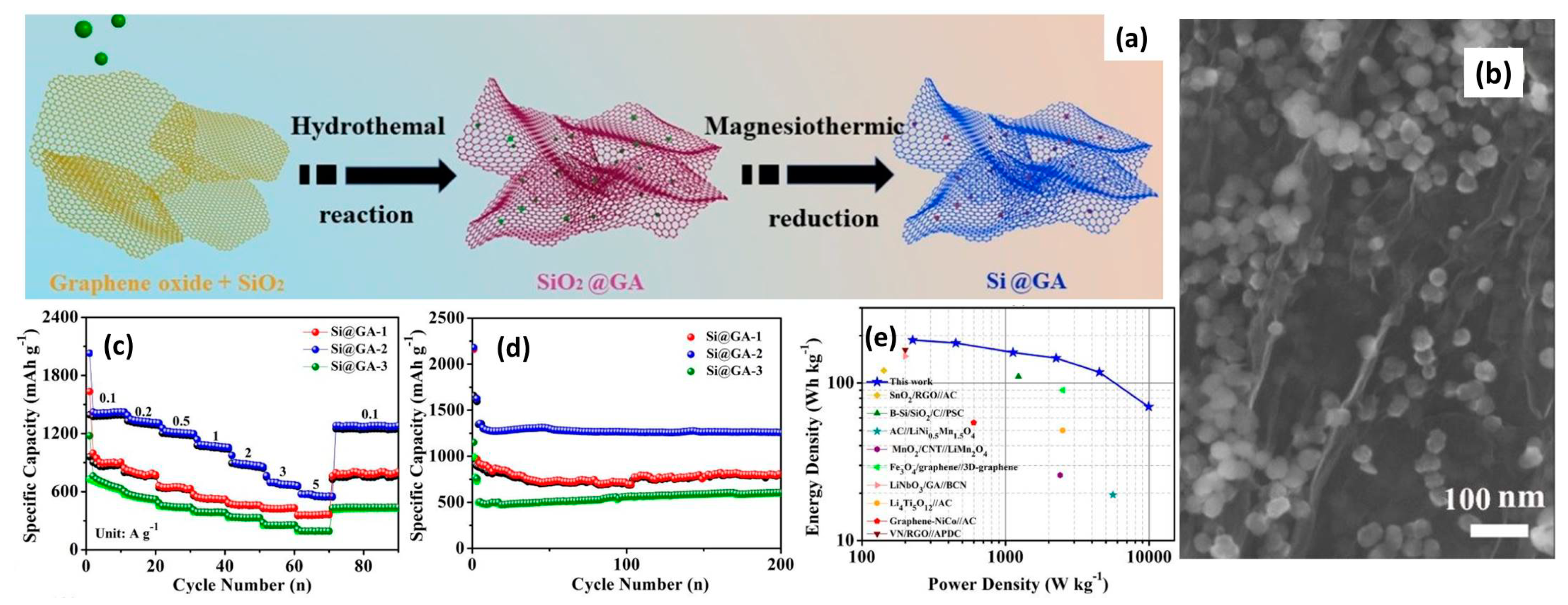
| Anode Materials | Capacity of Anode (mAh g−1) | Area Loading Mass (mg cm−2) | Cell Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | Reference |
|---|---|---|---|---|---|---|---|
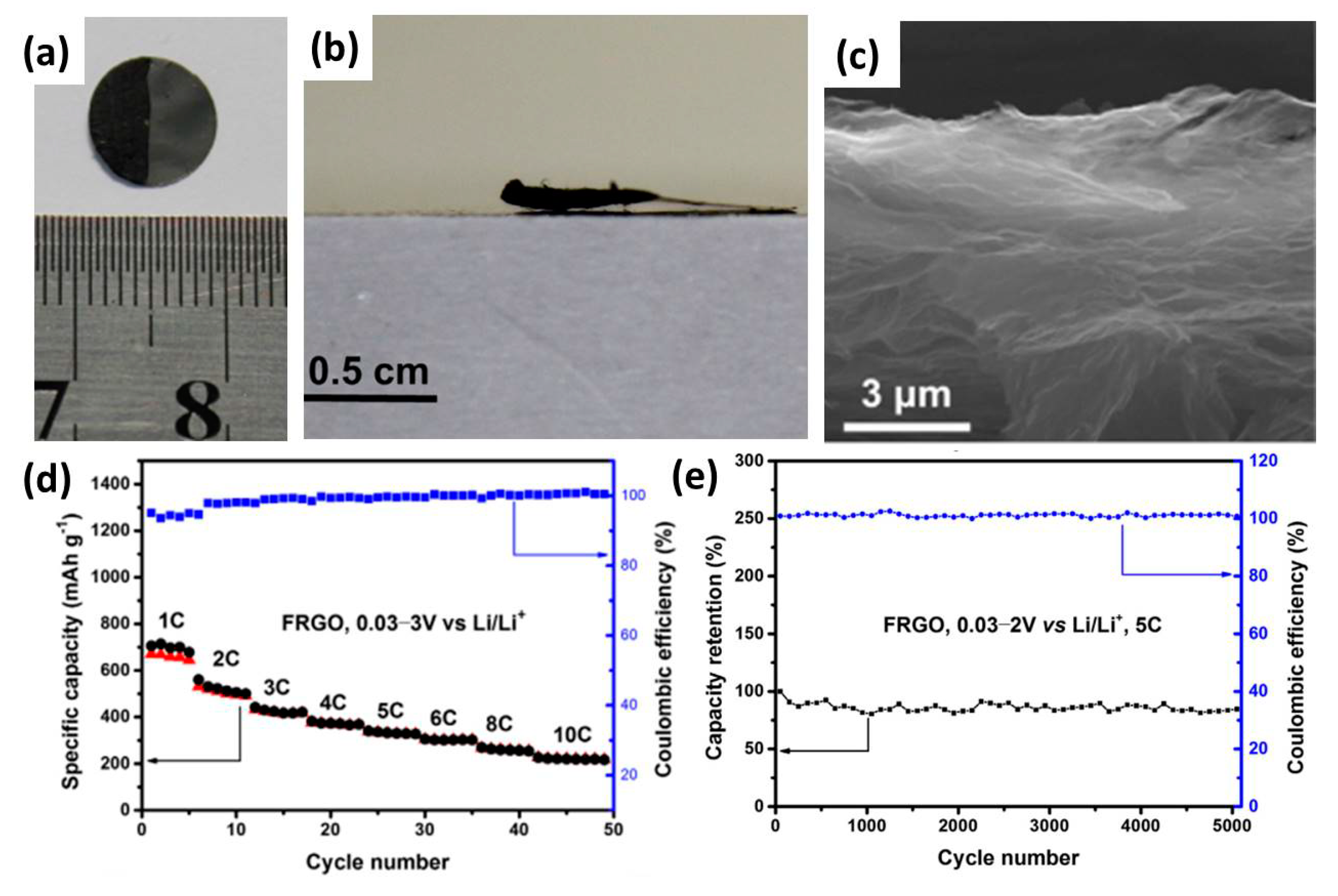
| FRGO | 660 | ~1 | 0–4.2 | 148.3 | 7.8 | ~80% after 3000 | [14] |
| HOG | 1019 | 5 | 2–4.2 | 231.7 | 2.8 | 84.2% after 1000 | [53] |
| SHSG | 854 | 1.5 | 2–4.5 | 146 | 52 | 91% after 40,000 | [58] |
| N-rGO aerogel | 1444.04 | ~0.82 | 2–4 | 170.28 | 25.75 | ~100% after 3000 | [71] |
| N-DGA | 1387 | / | 2–4 | 39 | 1.2 | 71% after 2000 | [56] |
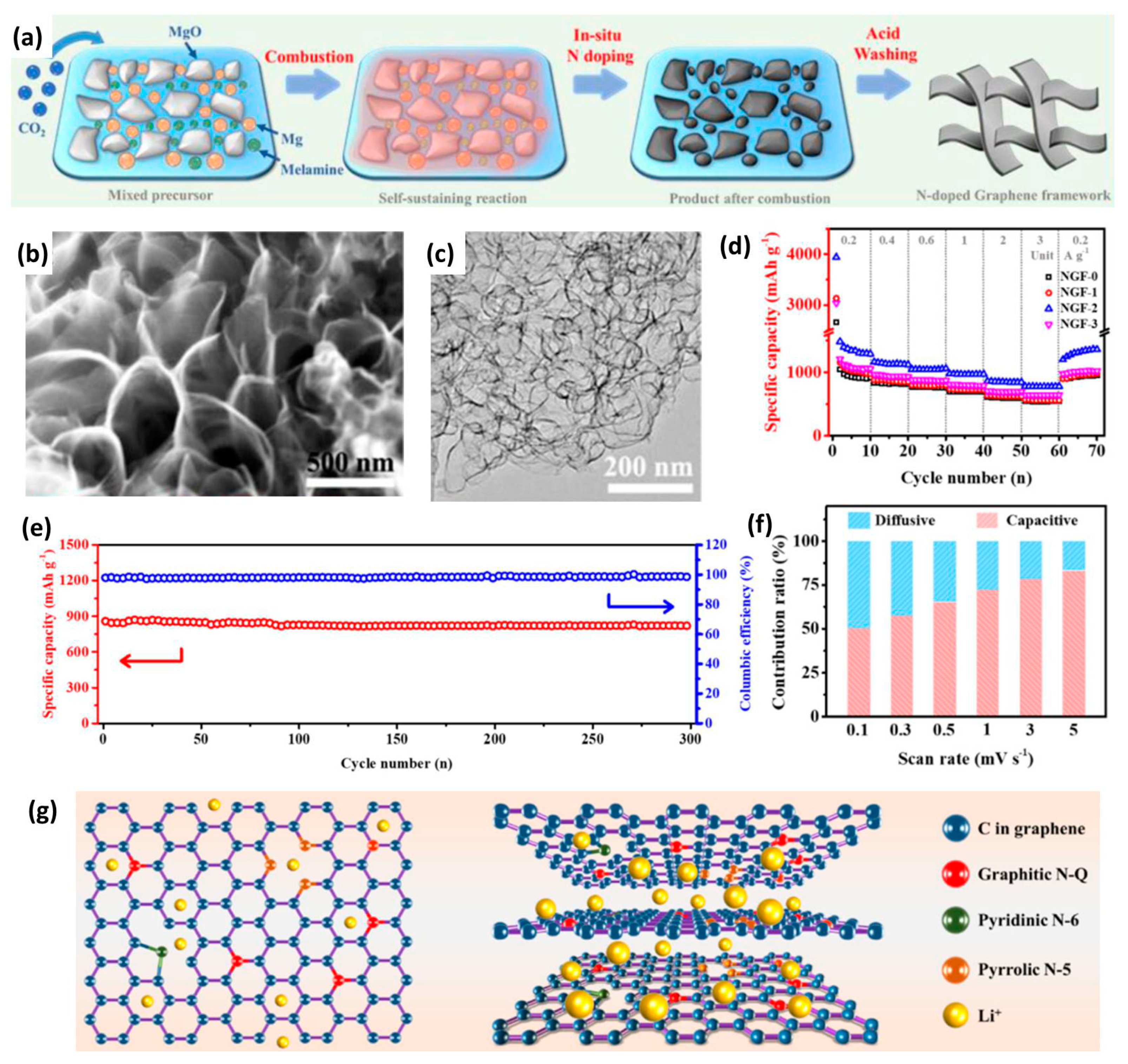
| NGF-2 | 1361 | 2 | 1–4 | 151 | 49 | 87% after 10,000 | [59] |
| N-GS | 395 | 1–1.5 | 0–4.5 | 187.9 | 11.25 | 93.5% after 3000 | [64] |
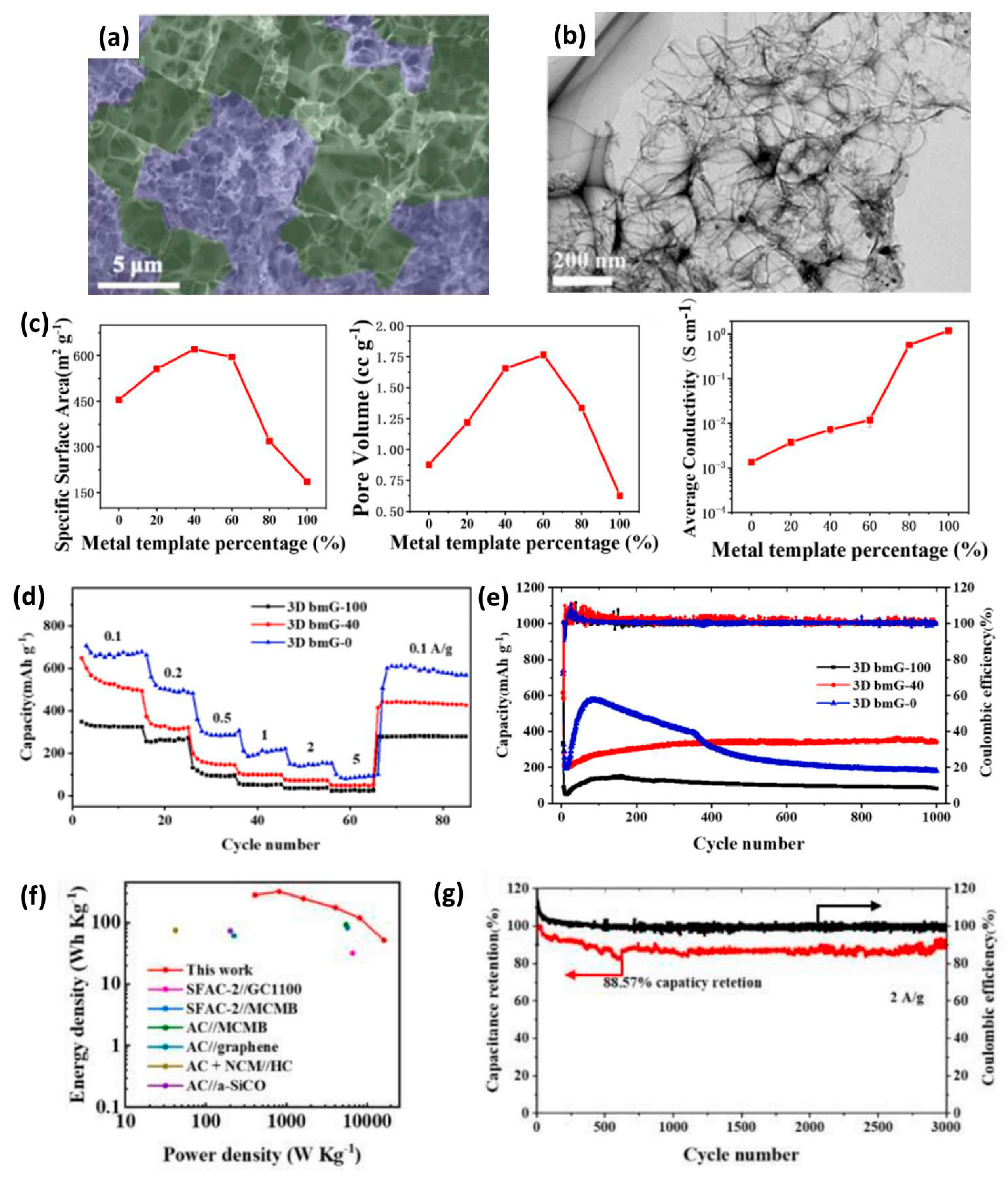
| 3D bmG | ~780 | / | 0–4.5 | 278 | 16.2 | 88.57% after 3000 | [54] |
| GC | 530 | 1–1.5 | 0–4.2 | 142.9 | 12.1 | 88% after 5000 | [62] |
| G/SC | 360 | ~1 | 1–4.1 | 151 | 18.9 | 93.8% after 10,000 | [67] |
| HC-rGO | 450 | ~1 | 1.5–4.2 | 200 | 10 | 88% after 10,000 | [66] |
| Li-SG | 500 | 1 | 0–4.1 | 222 | / | 58% after 5000 | [69] |
| PDA-GN | 1150 | 0.5 | 0.01–4.2 | 135.6 | 21.0 | 98% after 3000 | [61] |
| Anode Materials | Capacity of Anode (mAh g–1) | Area Loading Mass (mg cm–2) | Cell Voltage (V) | Maximum Energy Density (Wh kg–1) | Maximum Power Density (kW kg–1) | Cycling Stability | Reference |
|---|---|---|---|---|---|---|---|
| LTO+G | ~170 | 1.5–2.5 | 1.5–3 | 63 | 2.7 | 97% after 3000 | [76] |
| G–LTO | 207 | / | 0–3 | 95 | 3 | 87% after 500 | [43] |
| LTO/HG | 160.2 | / | 1.5–3 | 117.3 | 19.7 | 81.7% after 2000 | [78] |
| LTO-G-600C | 350.1 | 1.5–2.8 | 1.5–3 | 52 | 57.6 | 97% after 2000 | [77] |
| TiO2@PCNF-GA | 250.1 | ~1.3 | 0–3 | 79.7 | 15 | 93.3% after 10,000 | [27] |
| TiO2-FD | 312 | ~1 | 1–3 | 95% after 5000 | |||
| Au@TiO2/RGO | 905 | ~1 | 1–4 | 110 | 11 | 83% after 1000 | [84] |
| TiO2(B)@C/rGO | 231.7 | / | 0–3 | 59.4 | 17.3 | 70.1% after 5000 | [101] |
| B-TiO2−x/G | 732.7 | / | 1–4 | 166.4 | 7.9 | 87% after 3000 | [85] |
| ZTO/rGO | 560 | / | 0–4.5 | 204 | 67.5 | 76% after 1000 | [102] |
| T-Nb2O5/GCN | ~170 | ~1 | 0–3.5 | 129 | 32 | 80% after 10,000 | [89] |
| HG-TNO | 323 | / | 1–3.5 | 103.9 | 17.9 | 81.8% after 10,000 | [99] |
| TNO/HG | 286.2 | ~1 | 0.8–3.2 | 86.3 | 3.88 | 90.2% after 3000 | [98] |
| TNO/HrGO | 324.7 | ~1.5 | 0–3 | 66.3 | 23.2 | 79.9% after 6000 | [100] |
| Anode Materials | Capacity of Anode (mAh g–1) | Cell Voltage (V) | Maximum Energy Density (Wh kg–1) | Maximum Power Density (kW kg–1) | Cycling Stability | Reference |
|---|---|---|---|---|---|---|
| Fe3O4/G | 1000 | 1–4 | 204 | 4.6 | 70% after 1000 | [104] |
| Fe3O4-G | 820 | 1–4 | 120 | 45.4 | 81.4% after 10,000 | [30] |
| ZnFe2O4@C/RGO | 1011 | 1–4.2 | 174 | 51.4 | 80.5% after 10,000 | [123] |
| CG@SF | 1445 | 1–4 | 121 | 18 | 87% after 2000 | [124] |
| Mn3O4-G | 643 | 1–4 | 97.2 | 6.25 | 76.8% after 3000 | [107] |
| Mn3O4-G | 643 | 1.5–4 | 142 | 6.5 | 80% after 9000 | [109] |
| GNS@MnO | 766 | 0.5–4.2 | 197 | 23.5 | 84.8% after 3000 | [44] |
| MnO@GNS | 1350 | 1–4 | 127 | 25 | 76% after 3000 | [110] |
| MoS2-RGO | 860 | 0–4 | 188 | 40 | 80% after 10,000 | [120] |
| MoS2@3DG | 1022.3 | 0–4 | 156 | 8.31 | 78% after 2000 | [119] |
| MoO2@rGO | 1474.9 | 1.25–4.5 | 242 | 28.75 | 93% after 10,000 | [113] |
| 3DMoO3/GNSs | ~1100 | 0–3.8 | 128.3 | 13.5 | 90% after 3000 | [112] |
| CoO-rGO | 1674 | 1–4 | 132 | 35.8 | 84.7% after 5000 | [29] |
| 3DVN–RGO | ~640 | 0–4 | 162 | 10 | 83% after 1000 | [122] |
| Anode Materials | Capacity of Anode (mAh g−1) | Cell Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | Reference |
|---|---|---|---|---|---|---|
| Si@GA | 1618.8 | 0–4.5 | 197.3 | 11.2 | 82.4% after 10,000 | [134] |
| SrGO-Sn | 292 | 2–3.8 | 30 | 7.4 | 98% after 120,000 | [135] |
| SnO2-rGO | 950 | 1.5–4.2 | 186 | 10 | 70% after 5000 | [132] |
| SnO2@C@half-rGO | 879.6 | 0–4 | 257 | 20 | 78.2% after 2000 | [136] |
| Ce2Sn2O7/RGO | 814.6 | 0.01–3.8 | 122.3 | 9.65 | ~58% after 9000 | [137] |
| SnS2/RGO | 1198 | 0–4.5 | 149.5 | 35 | 90% after 10,000 | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, D.; Si, L.; Li, C.; Yang, Y.; Zhang, Y.; Yan, W. A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry 2021, 3, 1215-1246. https://doi.org/10.3390/chemistry3040089
Sui D, Si L, Li C, Yang Y, Zhang Y, Yan W. A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry. 2021; 3(4):1215-1246. https://doi.org/10.3390/chemistry3040089
Chicago/Turabian StyleSui, Dong, Linqi Si, Changle Li, Yanliang Yang, Yongsheng Zhang, and Weibo Yan. 2021. "A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors" Chemistry 3, no. 4: 1215-1246. https://doi.org/10.3390/chemistry3040089
APA StyleSui, D., Si, L., Li, C., Yang, Y., Zhang, Y., & Yan, W. (2021). A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry, 3(4), 1215-1246. https://doi.org/10.3390/chemistry3040089







