Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications
Abstract
1. Introduction
2. Pectin: Functions, Structure and Characteristics
3. Methods for the Synthesis of Microspheres
3.1. Emulsion and Coacervation
3.2. Ionotropic Gelation
3.3. Spray Drying
3.4. Hydrothermal Synthesis
3.5. Extrusion
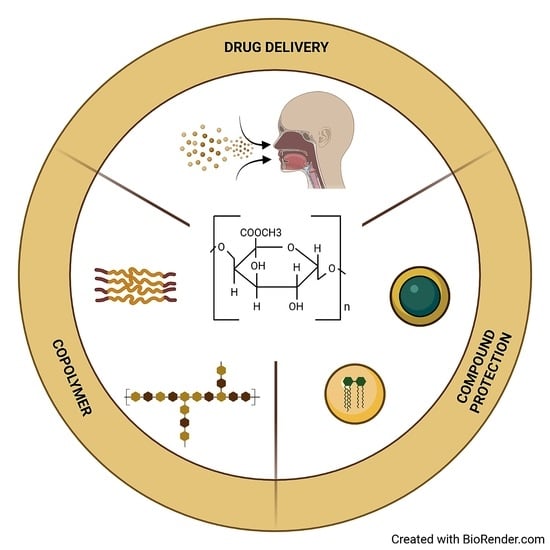
4. Pectin Microsphere Applications
4.1. Drug Delivery
4.2. Multidisciplinary Industrial Applications
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Rampino, A.; Borgogna, M.; Bellich, B.; Blasi, P.; Virgilio, F.; Cesàro, A. Chitosan-pectin hybrid nanoparticles prepared by coating and blending techniques. Eur. J. Pharm. Sci. 2016, 84, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic Biopolymers and Their Composites: Advantages and Limitations—An Overview. Macromol. Rapid Commun. 2021, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Burapapadh, K.; Takeuchi, H.; Sriamornsak, P. Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole. Asian J. Pharm. Sci. 2016, 11, 365–375. [Google Scholar] [CrossRef]
- Peian, Z.; Haifeng, J.; Peijie, G.; Sadeghnezhad, E.; Qianqian, P.; Tianyu, D.; Teng, L.; Huanchun, J.; Jinggui, F. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid. Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Periyasamy, K.G.K.; Zuo, H.; He, S. Flexible printed circuit board magnetic micromirror for laser marking/engraving. J. Micromech. Microeng. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Çaykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Drummond, C. Polymer Surfaces in Motion: Unconventional Patterning Methods; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 1–289. [Google Scholar]
- Vaidya, A.; Jain, S.; Agrawal, R.K.; Jain, S.K. Pectin-metronidazole prodrug bearing microspheres for colon targeting. J. Saudi. Chem. Soc. 2015, 19, 257–264. [Google Scholar] [CrossRef]
- George, S.; Ho, S.S.; Wong, E.S.P.; Tan, T.T.Y.; Verma, N.K.; Aitken, R.J.; Riediker, M.; Cummings, C.; Yu, L.; Wang, Z.M.; et al. The multi-facets of sustainable nanotechnology-Lessons from a nanosafety symposium. Nanotoxicology 2015, 9, 404–406. [Google Scholar] [CrossRef]
- Elkington, J. Triple bottom line.pdf. Environ. Qual. Manag. 1998, 8, 37–51. [Google Scholar] [CrossRef]
- Subramanian, V.; Semenzin, E.; Hristozov, D.; Zondervan-van den Beuken, E.; Linkov, I.; Marcomini, A. Review of decision analytic tools for sustainable nanotechnology. Environ. Syst. Decis. 2015, 35, 29–41. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Yang, X.H.; Wang, J.; Zhang, Q.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. High humidity hot air impingement blanching (HHAIB) enhances drying rate and softens texture of apricot via cell wall pectin polysaccharides degradation and ultrastructure modification. Food Chem. 2018, 261, 292–300. [Google Scholar] [CrossRef]
- Gomez, M.; Lajolo, F.; Cordenunsi, B. Evolution of soluble sugars during ripening of papaya fruit and its relation to sweet taste. J. Food Sci. 2002, 67, 442–447. [Google Scholar] [CrossRef]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef]
- Malik, N.A.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 1–34. [Google Scholar]
- Hael-Conrad, V.; Perato, S.M.; Arias, M.E.; Martínez-Zamora, M.G.; Di Peto, P.D.L.Á.; Martos, G.G.; Castagnaro, J.C.; Díaz-Ricci, J.C.; Chalfoun, N.R. The elicitor protein AsES induces a systemic acquired resistance response accompanied by systemic microbursts and micro-hypersensitive responses in Fragaria ananassa. Mol. Plant-Microbe Interact. 2018, 31, 46–60. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic induction of the defensin and phytoalexin pisatin pathways in pea (Pisum sativum) against aphanomyces euteiches by acetylated and nonacetylated oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Vurmaz, E.; Schaefer, C.; Eberl, F.; Sporer, T.; Haeger, W.; Pauchet, Y. Plants use identical inhibitors to protect their cell wall pectin against microbes and insects. Ecol. Evol. 2020, 10, 3814–3824. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R. Global insights of protein responses to cold stress in plants: Signaling, defence, and degradation. J. Plant Physiol. 2018, 226, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Avci, U.; Peña, M.J.; O’Neill, M.A. Changes in the abundance of cell wall apiogalacturonan and xylogalacturonan and conservation of rhamnogalacturonan II structure during the diversification of the Lemnoideae. Planta 2018, 247, 953–971. [Google Scholar] [CrossRef]
- Roman, L.; Guo, M.; Terekhov, A.; Grossutti, M.; Vidal, N.P.; Reuhs, B.L.; Martinez, M.M. Extraction and isolation of pectin rich in homogalacturonan domains from two cultivars of hawthorn berry (Crataegus pinnatifida). Food Hydrocoll. 2021, 113, 106476. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts. Prog. Drug Res. 2017, 73, 105–123. [Google Scholar]
- Xie, F.; Gu, B.J.; Saunders, S.R.; Ganjyal, G.M. High methoxyl pectin enhances the expansion characteristics of the cornstarch relative to the low methoxyl pectin. Food Hydrocoll. 2021, 110, 106131. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Amsbury, S.; Hunt, L.; Elhaddad, N.; Baillie, A.; Lundgren, M.; Verhertbruggen, Y.; Scheller, H.V.; Knox, J.P.; Fleming, A.J.; Gray, J.E. Stomatal Function Requires Pectin De-methyl-esterification of the Guard Cell Wall. Curr. Biol. 2016, 26, 2899–2906. [Google Scholar] [CrossRef]
- Krivorotova, T.; Staneviciene, R.; Luksa, J.; Serviene, E.; Sereikaite, J. Preparation and characterization of nisin-loaded pectin-inulin particles as antimicrobials. LWT-Food Sci. Technol. 2016, 72, 518–524. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Wang, F.; Zhang, H.; de Vos, P.; Sun, J. Low-methoxyl lemon pectin attenuates inflammatory responses and improves intestinal barrier integrity in caerulein-induced experimental acute pancreatitis. Mol. Nutr. Food Res. 2017, 61, 1–30. [Google Scholar] [CrossRef]
- Rose, P.A.E.; Abilasha, D. Extraction and characterization of pectin from lemon peel. Int. J. Adv. Sci. Res. 2016, 1, 12–15. [Google Scholar]
- Hino, K.; Miyatake, S.; Yamada, F.; Endo, N.; Akiyama, R.; Ebisu, G. Undigested low-methoxy pectin prevents diarrhea and induces colonic contraction during liquid-diet feeding in rats. Nutrition 2020, 78, 110804. [Google Scholar] [CrossRef]
- Wu, C.; Pan, L.L.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Chen, W.; Zhang, H.; et al. Modulation of Gut Microbiota by Low Methoxyl Pectin Attenuates Type 1 Diabetes in Non-obese Diabetic Mice. Front. Immunol. 2019, 10, 1733. [Google Scholar] [CrossRef]
- Liang, R.H.; Wang, L.H.; Chen, J.; Liu, W.; Liu, C.M. Alkylated pectin: Synthesis, characterization, viscosity and emulsifying properties. Food Hydrocoll. 2015, 50, 65–73. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, X.; Yin, X.; Chen, S. Sulfation of citrus pectin by pyridine-sulfurtrioxide complex and its anticoagulant activity. LWT-Food Sci. Technol. 2015, 60, 1162–1167. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Drug loaded composite oxidized pectin and gelatin networks for accelerated wound healing. Int. J. Pharm. 2016, 505, 234–245. [Google Scholar] [CrossRef]
- Chacón-Cerdas, R.; Medaglia-Mata, A.; Flores-Mora, D.; Starbird-Pérez, R. Synthesis of chitosan, pectin, and chitosan/pectin microspheres by two water-in-oil emulsion crosslinking methods. Chem. Pap. 2020, 74, 509–520. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Yao, S.; Liu, H.; Yu, S.; Li, Y.; Wang, X.; Wang, L. Drug-nanoencapsulated PLGA microspheres prepared by emulsion electrospray with controlled release behavior. Regen. Biomater. 2016, 3, 309–317. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef]
- Nernplod, T.; Sriamornsak, P. Effect of solvent on properties of pectin microspheres prepared by emulsion-dehydration technique. Asian J. Pharm. Sci. 2016, 11, 217–218. [Google Scholar] [CrossRef][Green Version]
- Almeida, E.A.M.S.; Bellettini, I.C.; Garcia, F.P.; Farinácio, M.T.; Nakamura, C.V.; Rubira, A.F.; Martins, A.F.; Muniz, E. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017, 171, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Ziemba, R.M.; Shebesta, J.H.; Lipscomb, J.C.; Wang, Y.; Wu, Y.; D O’Connell, K.; Kaltchev, M.G.; van Groningen, A.; Chen, J.; et al. Design of pectin-based bioink containing bioactive agent-loaded microspheres for bioprinting. Biomed. Phys. Eng. Express. 2019, 5, 1–27. [Google Scholar] [CrossRef]
- Fennell, D.; Evans, H.W. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet; Wiley-VCH: Weinheim, Germany, 1999; p. 672. [Google Scholar]
- Pak, C.W.; Kosno, M.; Holehouse, A.S.; Padrick, S.B.; Mittal, A.; Ali, R.; Yunus, A.; Liu, D.R.; Pappu, R.V.; Rosen, M.K. Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Mol. Cell 2016, 63, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Priyadarshini, E.; Rawat, K.; Bohidar, H.B. Complex coacervation in charge complementary biopolymers: Electrostatic versus surface patch binding. Adv. Colloid. Interface Sci. 2017, 250, 40–53. [Google Scholar] [CrossRef]
- Mancer, D.; Allemann, E.; Daoud, K. Metformin hydrochloride microencapsulation by complex coacervation: Study of size distribution and encapsulation yield using response surface methodology. J. Drug Deliv. Sci. Technol. 2018, 45, 184–195. [Google Scholar] [CrossRef]
- Villicaña-Molina, E.; Pacheco-Contreras, E.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Pectin and chitosan microsphere preparation via a water/oil emulsion and solvent evaporation method for drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 467–475. [Google Scholar] [CrossRef]
- Noello, C.; Carvalho, A.G.S.; Silva, V.M.; Hubinger, M.D. Spray dried microparticles of chia oil using emulsion stabilized by whey protein concentrate and pectin by electrostatic deposition. Food Res. Int. 2016, 89, 549–557. [Google Scholar] [CrossRef]
- Aloys, H.; Korma, S.; Alice, T.; Chantal, N.; Ali, A.; Abed, S.; Ildephonse, H. Microencapsulation by Complex Coacervation: Methods, Techniques, Benefits, and Applications. Am. J. Food Sci. Nutr. Res. 2016, 3, 188–192. [Google Scholar]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef]
- Patel, M.A.; AbouGhaly, M.H.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef]
- Boni, F.I.; Prezotti, F.G.; Cury, B.S.F. Gellan gum microspheres crosslinked with trivalent ion: Effect of polymer and crosslinker concentrations on drug release and mucoadhesive properties. Drug Dev. Ind. Pharm. 2016, 42, 1283–1290. [Google Scholar] [CrossRef]
- Khoder, M.; Tsapis, N.; Huguet, H.; Besnard, M.; Gueutin, C.; Fattal, E. Removal of ciprofloxacin in simulated digestive media by activated charcoal entrapped within zinc-pectinate beads. Int. J. Pharm. 2009, 379, 251–259. [Google Scholar] [CrossRef]
- Lascol, M.; Bourgeois, S.; Barratier, C.; Marote, P.; Lantéri, P.; Bordes, C. Development of pectin microparticles by using ionotropic gelation with chlorhexidine as cross-linking agent. Int. J. Pharm. 2018, 542, 205–212. [Google Scholar] [CrossRef]
- Revuelta, M.V.; Villalba, M.E.C.; Navarro, A.S.; Güida, J.A.; Castro, G.R. Development of Crystal Violet encapsulation in pectin-Arabic gum gel microspheres. React. Funct. Polym. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Belščak-Cvitanovic, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Vojvodić, A.; Mršić, G.; Komes, D. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Reynaud, F.; Tsapis, N.; Guterres, S.S.; Pohlmann, A.R.; Fattal, E. Pectin beads loaded with chitosan-iron microspheres for specific colonic adsorption of ciprofloxacin. J. Drug Deliv. Sci. Technol. 2015, 30, 494–500. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Naik, J.B. Optimization of spray-dried diclofenac sodium-loaded microspheres by screening design. Dry Technol. 2016, 34, 1593–1603. [Google Scholar] [CrossRef]
- Zaman, M.; Sajid, N.; Rehman, A.U. Gastrointestinal Mucosa: The Target Site of Mucoadhesive Microspheres, A Review. Adv. Polym. Technol. 2016, 35, 269–276. [Google Scholar] [CrossRef]
- Jurišić Dukovski, B.; Mrak, L.; Winnicka, K.; Szekalska, M.; Juretić, M.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J.; Hafner, A. Spray-dried nanoparticle-loaded pectin microspheres for dexamethasone nasal delivery. Dry Technol. 2019, 37, 1915–1925. [Google Scholar] [CrossRef]
- García, A.; Leonardi, D.; Piccirilli, G.N.; Mamprin, M.E.; Olivieri, A.C.; Lamas, M.C. Spray drying formulation of albendazole microspheres by experimental design. In vitro-In vivo studies. Drug Dev. Ind. Pharm. 2015, 41, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.-M. Spray Drying of Folic Acid within Nano-Emulsions; Optimization by Taguchi Approach. Dry Technol. 1991, 105, 135. [Google Scholar] [CrossRef]
- Nižić, L.; Potaś, J.; Winnicka, K.; Szekalska, M.; Erak, I.; Gretić, M.; Jug, M.; Hafner, A. Development, characterisation and nasal deposition of melatonin-loaded pectin/hypromellose microspheres. Eur. J. Pharm. Sci. 2020, 141, 105115. [Google Scholar] [CrossRef] [PubMed]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, N.K.V.; Bandyopadhyaya, R.; Runkana, V. Thermodynamic analysis of hydrothermal synthesis of nanoparticles. Fluid Phase Equilib. 2018, 456, 33–45. [Google Scholar] [CrossRef]
- Wasly, H.S.; El-Sadek, M.S.A.; Henini, M. Influence of reaction time and synthesis temperature on the physical properties of ZnO nanoparticles synthesized by the hydrothermal method. Appl. Phys. Mater. Sci. Process. 2018, 124, 124–176. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z. Citrus pectin-derived carbon microspheres with superior adsorption ability for methylene blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Gholipourmalekabadi, M.; Mozafari, M. Fabrication of newly developed pectin–GeO2 nanocomposite using extreme biomimetics route and its antibacterial activities. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 655–661. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114417. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Microencapsulation of kenaf seed oil by co-extrusion technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Vallejo-Castillo, V.; Rodríguez-Stouvenel, A.; Martínez, R.; Bernal, C. Development of alginate-pectin microcapsules by the extrusion for encapsulation and controlled release of polyphenols from papaya (Carica papaya L.). J. Food Biochem. 2020, 44, 1–17. [Google Scholar] [CrossRef]
- Leong, M.H.; Tan, C.P.; Nyam, K.L. Effects of Accelerated Storage on the Quality of Kenaf Seed Oil in Chitosan-Coated High Methoxyl Pectin-Alginate Microcapsules. J. Food Sci. 2016, 81, C2367–C2372. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT-Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- Muley, S.; Nandgude, T.; Poddar, S. Extrusion–spheronization a promising pelletization technique: In-depth review. Asian J. Pharm. Sci. 2016, 11, 684–699. [Google Scholar] [CrossRef]
- Martins, A.L.L.; de Oliveira, A.C.; do Nascimento, C.M.O.L.; Silva, L.A.D.; Gaeti, M.P.N.; Lima, E.M.; Taveira, S.; Fernandes, K.; Marreto, R. Mucoadhesive Properties of Thiolated Pectin-Based Pellets Prepared by Extrusion-Spheronization Technique. J. Pharm. Sci. 2017, 106, 1363–1370. [Google Scholar] [CrossRef]
- Pinc, J.; Čapek, J.; Hybášek, V.; Průša, F.; Hosová, K.; Maňák, J.; Vojtech, D. Characterization of newly developed zinc composite with the content of 8 wt. % of hydroxyapatite particles processed by extrusion. Materials 2020, 13, 1716. [Google Scholar] [CrossRef]
- Kiaei Pour, P.; Alemzadeh, I.; Vaziri, A.S.; Beiroti, A. Potential effects of alginate–pectin biocomposite on the release of folic acid and their physicochemical characteristics. J. Food Sci. Technol. 2020, 57, 3363–3370. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Ahirwar, D.; Ahirwar, B.; Anish, C. Microspheres for Targeting an Alkaloidal Anticancer Drug in Colon Cancer. Res. J. Pharm. Technol. 2013, 6, 618–621. [Google Scholar]
- Banerjee, P.; Deb, J.; Roy, A.; Ghosh, A.; Chakraborty, P. Fabrication and Development of Pectin Microsphere of Metformin Hydrochloride. ISRN Pharm. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bayón, B.; Bucalá, V.; Castro, G.R. Development of antimicrobial hybrid mesoporous silver phosphate-pectin microspheres for control release of levofloxacin. Microporous Mesoporous Mater. 2016, 226, 71–78. [Google Scholar] [CrossRef]
- Bourgeois, S.; Laham, A.; Besnard, M.; Andremont, A.; Fattal, E. In vitro and in vivo evaluation of pectin beads for the colon delivery of β-lactamases. J. Drug Target. 2005, 13, 277–284. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Monaco, L.; Cerchiara, T.; Zecchi, V. Pectin-based microspheres for colon-specific delivery of vancomycin. J. Pharm. Pharmacol. 2008, 61, 41–46. [Google Scholar] [CrossRef]
- Chakraborty, S.; Khandai, M.; Sharma, A.; Khanam, N.; Patra, C.; Dinda, S.; Sen, K. Preparation, in vitro and in vivo evaluation of algino-pectinate bioadhesive microspheres: An investigation of the effects of polymers using multiple comparison analysis. Acta Pharm. 2010, 60, 255–266. [Google Scholar] [CrossRef]
- Chaurasia, M.; Chourasia, M.; Jain, N.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S.K. Methotrexate Bearing Calcium Pectinate Microspheres: A Platform to Achieve Colon-Specific Drug Release. Curr. Drug Deliv. 2008, 5, 215–219. [Google Scholar] [CrossRef]
- Da Silva, E.P.; Sitta, D.L.A.; Fragal, V.H.; Cellet, T.S.P.; Mauricio, M.R.; Garcia, F.P.; Nakamura, C.V.; Guilherme, M.R.; Rubira, A.F.; Kunita, M.H. Covalent TiO2/pectin microspheres with Fe3O4 nanoparticles for magnetic field-modulated drug delivery. Int. J. Biol. Macromol. 2014, 67, 43–52. [Google Scholar] [CrossRef]
- Das, S.; Ng, K.Y.; Ho, P.C. Design of a pectin-based microparticle formulation using zinc ions as the cross-linking agent and glutaraldehyde as the hardening agent for colonic-specific delivery of resveratrol: In vitro and in vivo evaluations. J. Drug Target. 2011, 19, 446–457. [Google Scholar] [CrossRef]
- Fullana, S.G.; Ternet, H.; Freche, M.; Lacout, J.L.; Rodriguez, F. Controlled release properties and final macroporosity of a pectin microspheres-calcium phosphate composite bone cement. Acta Biomater. 2010, 6, 2294–2300. [Google Scholar] [CrossRef]
- Islan, G.A.; De Verti, I.P.; Marchetti, S.G.; Castro, G.R. Studies of ciprofloxacin encapsulation on alginate/pectin matrixes and its relationship with biodisponibility. Appl. Biochem. Biotechnol. 2012, 167, 1408–1420. [Google Scholar] [CrossRef]
- Lee, C.M.; Kim, D.W.; Lee, H.C.; Lee, K.Y. Pectin microspheres for oral colon delivery: Preparation using spray drying method and in vitro release of indomethacin. Biotechnol. Bioprocess. Eng. 2004, 9, 191–195. [Google Scholar] [CrossRef]
- Lemos, T.S.A.; Souza, J.F.; De Fajardo, R. Magnetic microspheres based on pectin coated by chitosan towards smart drug release. Carbohydr. Polym. 2021, 265, 1–10. [Google Scholar] [CrossRef]
- Li, T.; Wan, B.; Jog, R.; Costa, A.; Burgess, D.J. Pectin microparticles for peptide delivery: Optimization of spray drying processing. Int. J. Pharm. 2022, 613, 121384. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Tanzi, M.C.; Barbosa, M.A.; Granja, P.L. Biofunctional chemically modified pectin for cell delivery. Soft Matter. 2012, 8, 4731–4739. [Google Scholar] [CrossRef]
- Jain Singhai, N.; Rawal, A.; Maurya, R.; Ramteke, S. Design and Characterization of Dual Drug Loaded Microspheres for Colon. J. Drug Deliv. Ther. 2019, 9, 12–22. [Google Scholar]
- Okunlola, A.; Akindele, O. Application of Response Surface Methodology and Central Composite Design for the Optimization of Metformin Microsphere Formulation using Tangerine (Citrus tangerina) Pectin as Copolymer. Br. J. Pharm. Res. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Orhan, Z.; Cevher, E.; Mülazimoglu, L.; Gürcan, D.; Alper, M.; Araman, A.; Ozsoy, Y. The preparation of ciprofloxacin hydrochloride-loaded chitosan and pectin microspheres. Their evaluation in an animal osteomyelitis model. J. Bone Jt. Surg. Ser. B 2006, 88, 270–275. [Google Scholar] [CrossRef]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-coated pectin microspheres of 5-Fluorouracil for colon targeting. AAPS PharmSciTech 2007, 8, 87–93. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Boni, F.I.; Ferreira, N.N.; de Souza e Silva, D.; Campana-Filho, S.P.; Almeida, A.; Vasconcelos, T.; Daflon, M.P.; Ferreira, B.S.; Sarmento, B. Gellan gum/pectin beads are safe and efficient for the targeted colonic delivery of resveratrol. Polymers 2018, 10, 50. [Google Scholar] [CrossRef]
- Ramana, G.; Krishna Chaitanya, A. Preparation and In-vitro characterization of ethylcellulose coated pectin alginate microspheres of 5-fluorouracil for colon targeting. J. Appl. Pharm. Sci. 2011, 1, 170–176. [Google Scholar]
- Singh, A.; Mandal, U.K.; Narang, R.K. Development and In Vivo Evaluation of Pectin Based Enteric Coated Microparticles Loaded with Mesalamine and Saccharomyces boulardii for Management of Ulcerative Colitis. Assay Drug Dev. Technol. 2022, 20, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.; Moreira, L.; Silva, B.T.; Oliveira, B.P.; Carvalho, A.S.; Silva, P.S.; Verri, W.A., Jr.; Sá-Nakanishi, A.B.; Bracht, L.; Zanoni, J.N.; et al. Low dose of quercetin-loaded pectin/casein microparticles reduces the oxidative stress in arthritic rats. Life Sci. 2021, 284, 119910. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.; Jain, S. Metronidazole Loaded Pectin Microspheres for Colon Targeting. J. Pharm. Sci. 2009, 98, 4229–4236. [Google Scholar] [CrossRef]
- Shukla, S.; Verma, K.; Jain, D.; Verma, S. Pectin-based colon-specific drug delivery. Chronicles Young Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Microencapsulation of peppermint oil in an alginate-pectin matrix using a coaxial electrospray system. Int. J. Food Sci. Technol. 2014, 49, 733–739. [Google Scholar] [CrossRef]
- Chou, W.-M.; Wang, L.-L.; Yu, H.H. Electrophoretic Ink Display Prepared by Jelly Fig Pectin/Gelatin Microspheres. Smart Sci. 2015, 3, 74–79. [Google Scholar] [CrossRef][Green Version]
- Michel, C.R.; Martínez-Preciado, A.H. CO sensing properties of novel nanostructured La2O3 microspheres. Sens. Actuators B Chem. 2015, 208, 355–362. [Google Scholar] [CrossRef]
- Munarin, F.; Giuliano, L.; Bozzini, S.; Tanzi, M.C.; Petrini, P. Mineral phase deposition on pectin microspheres. Mater. Sci. Eng. C 2010, 30, 491–496. [Google Scholar] [CrossRef]
- Li, F.; Xu, Z.; Wen, X.; Li, X.; Bai, Y.; Li, J. Preparation and characterization of Ca(II) cross-linking modified pectin microspheres for Pb(II) adsorption. Water Sci. Technol. 2019, 79, 1484–1493. [Google Scholar] [CrossRef]
- Zamri, N.I.I.; Zulmajdi, S.L.N.; Daud, N.Z.A.; Mahadi, A.H.; Kusrini, E.; Usman, A. Insight into the adsorption kinetics, mechanism, and thermodynamics of methylene blue from aqueous solution onto pectin-alginate-titania composite microparticles. SN Appl. Sci. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Shuai, X.; Liang, R.; Chen, J.; Liu, C. Pectin/Activated Carbon-Based Porous Microsphere for Pb2+ Adsorption: Characterization and Adsorption Behaviour. Polymers 2021, 13, 1–7. [Google Scholar]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and Oxidative Stability of PUFA-Rich Oil Microencapsulated by Spray Drying Using Pea Protein and Pectin. Food Bioprocess. Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; McClements, D.J. Influence of anionic dietary fibers (xanthan gum and pectin) on oxidative stability and lipid digestibility of wheat protein-stabilized fish oil-in-water emulsion. Food Res. Int. 2015, 74, 131–139. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing microencapsulation of α-tocopherol with pectin and sodium alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Long, K.; Nyam, K.L. In-vitro evaluation of kenaf seed oil in chitosan coated-high methoxyl pectin-alginate microcapsules. Ind. Crops Prod. 2015, 76, 230–236. [Google Scholar] [CrossRef]
- Melo, M.; da Silva, A.; Filho, E.S.; Oliveira, R.; Junior, J.S.; Oliveira, J.P.; Vaz, A.; Moura, J.; Pererira, J.; Bezerra, L. Polymeric microparticles of calcium pectinate containing urea for slow release in ruminant diet. Polymers 2021, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.B.; Matos, R.L.N.; de Novelli, A.P.; Couto, R.O.; do Yamashita, F.; Ribeiro, M.A.; dos Santos Ribeiro, M.A.; Meurer, E.C.; Junior, W.A.V.; Casagrande, R.; et al. Spray-drying of casein/pectin bioconjugate microcapsules containing grape (Vitis labrusca) by-product extract. Food Chem. 2022, 368, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Ding, G.; Geng, Q.; Zhu, J.; Guo, M.; Duan, Y.; Wang, B.; Cao, Y. Synthesis, characterization, and application of microbe-triggered controlled-release kasugamycin-pectin conjugate. J. Agric. Food Chem. 2015, 63, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Islan, G.A.; de Urraza, P.J.; Castro, G.R. Novel biopolymer matrices for microencapsulation of phages: Enhanced protection against acidity and protease activity. Macromol. Biosci. 2012, 12, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kaletunç, G. Dissolution kinetics of pH responsive alginate-pectin hydrogel particles. Food Res. Int. 2016, 88, 129–139. [Google Scholar] [CrossRef]
- Bhatia, M.S.; Choudhari, P.; Bhatia, N.M.; Deshmukh, R. Chemical modification of pectins, characterization and evaluation for drug delivery. Sci. Pharm. 2008, 76, 775–784. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Akalin, G.O.; Oztuna Taner, O.; Taner, T. The preparation, characterization and antibacterial properties of chitosan/pectin silver nanoparticle films. Polym. Bull. 2021, 0123456789, 1–18. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Pectin modified metal nanoparticles and their application in property modification of biosensors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100164. [Google Scholar] [CrossRef]
- Dolinska, J.; Holdynski, M.; Pieta, P.; Lisowski, W.; Ratajczyk, T.; Palys, B.; Jablonska, A.; Opallo, M. Noble metal nanoparticles in pectin matrix. Preparation, film formation, property analysis, and application in electrocatalysis. ACS Omega. 2020, 5, 23909–23918. [Google Scholar] [CrossRef]
- Dai, J.; Wu, S.; Jiang, W.; Li, P.; Chen, X.; Liu, L.; Liu, J.; Sun, D.; Chen, W.; Chen, B.; et al. Facile synthesis of pectin coated Fe3O4 nanospheres by the sonochemical method. J. Magn. Magn. Mater. 2013, 331, 62–66. [Google Scholar] [CrossRef]

| Sphere Type | Synthesis Method | Pectin Properties | Characteristics of the Spheres | Drug | Application |
|---|---|---|---|---|---|
| Shellac-coated pectin microspheres | W/O emulsification and linking with CaCl2+ solvent evaporation | N/A | Diameter of 28–35 µm Encapsulation efficiency of 73–82% pH-dependent release; faster with an acidic pH and slower with a basic pH | Vincristine sulfate | Colon cancer treatment by controlled drug release [84]. |
| Pectin microspheres | W/O emulsification + solvent evaporation | N/A | Diameter of 34–71 µm Yield of 78–88% Drug content of 15–33% Encapsulation efficiency of 26–67% 95–98% of drug release in 9 h at a pH of 6.8 | Metformin Hydrochloride | Selective oral drug release for diabetes mellitus type II [85]. |
| Pectin + Ag3PO4 mesoporous hybrid microspheres | Ionotropic gelation | Highly methoxylated (HMP) Esterification degree of 74% Average of 1.60 × 105 Da | Diameter of 1.3–1.5 µm Yield of 90% | Levofloxacin | Antimicrobial particles for controlled drug release [86]. |
| Pectin microspheres | Ionotropic gelation with CaCl2 + linking with polyethyleneimine | Amidated of low methoxylated OG 175C Esterification degree of 22–28% Amidation degree of 19–23% | Average diameter of 1010 µm Encapsulation efficiency of 80% Expansion ratio at 1.5 h of 7.9 Disintegration time of 7 h + 86% of their biological activity remains after 5 h in a basic environment (pH of 6.0) | B-lactamase | Controlled b-lactamase release in colon to reduce bacterial antibiotic resistance [87]. |
| Pectin/chitosan hybrid microspheres | Aerosol drying | Esterification degree of 70–75% 30–100K Mr | Average of diameter of 3 µm 16–32% of drug content Expansion by hydration of 100–180% pH-dependent release 70–80% of drug release in 10 h at a pH of 5.5 | Vancomycin | Controlled antibiotic release for colon infections [88]. |
| Algin/pectinate hybrid microspheres | Ionotropic gelation with CaCl2 | Highly methoxylated (HMP) Average of 1.76 × 106 Da Mr | Diameter of 740–810 µm Yield of 9% Encapsulation efficiency of 84–96% pH-dependent release; faster with a basic pH and slower with an acidic pH 90% of drug release in 12 h at a pH of 6.8 | Aceclofenac | Controlled oral anti-inflammatory drugs [89]. |
| Calcium pectinate microspheres | W/O emulsification and linking with CaCl2 | Low methoxylated Esterification degree of 6% | Diameter of 20–32 µm Drug content of 20% Encapsulation efficiency of 74% Expansion ratio until constant weight of 0.3–1.3% pH-dependent release; more with a basic pH and less with an acidic pH 20% of drug release in 24 h at a pH of 7.0 | Methotrexate | Colon cancer treatment by controlled drug release [90]. |
| TiO2/Fe3O4/pectin hybrid microspheres | W/O emulsification and covalent linking induced by ultrasound | ≥74.0% galacturonic acid | Diameter of 0.68 µm Zeta potential of −4.87 mV 82% of drug content 7% of drug release in 5 h at a pH of 2.0 | Amoxicillin | Slow and maintained, controlled antibiotic release in the stomach [91]. |
| Pectin microspheres | Linking with zinc acetate and glutaraldehyde | Esterification degree of 28% Amidation degree of 20% | Diameter of 898–1053 µm Humidity content of 8–13% Encapsulation efficiency of 93–98% pH-dependent release; faster with a basic pH (6.8) and slower with an acidic pH (1.2) | Resveratrol | Controlled drug release for colon cancer treatment [92]. |
| Dexamethasone/alginate nanoparticles encapsulated by pectin microspheres | Aerosol drying | Amidated of low methyl esterification CF025 Esterification degree of 23–28% Amidation degree of 22–25% | Diameter of 2.76 µm Zeta potential of −36 mV Drug content of 3% Yield of 45–70% | Dexamethasone | Controlled release of mucoadhesive drug in nasal solution [65]. |
| Pectin microspheres | Ionotropic gelation with CaCl2 | Low proportion of amidated methoxylation Molecular weight of 228,000 Da Esterification degree of 30% Amidation degree of 19% | Diameter of 280 µm Polydispersity index of 0.69 Drug content of 4% Encapsulation efficiency of 77% Slow and lengthy release at pH of 7.5, approximately 62% in 45 days | Ibuprofen | Macroporous structuring in bone implant material and controlled drug release [93]. |
| Algin/pectinate hybrid microspheres | Ionotropic gelation with CaCl2 | Highly methoxylated (HMP) Esterification degree of 74% Average MW of 160 kDa | Encapsulation efficiency of 47% Expansion by hydration of 190–300% Slow release at gastric pH of 1.2 and fast release at a basic pH (7.4) | Ciprofloxacin | Controlled antibiotic release in the digestive tract and degradation protection [94]. |
| Pectin microspheres | Aerosol drying and linking with CaCl2 | Low methoxylated amidation Esterification degree of 9% | Encapsulation efficiency of 13% Drug content of 5% Slow release at 1.2 pH in 24 h of 18% Fast release at 7.4 pH | Indomethacin | Controlled drug release for gastrointestinal disease treatment [95]. |
| Pectin/magnetite coated with chitosan microspheres | Ionotropic gelation with CaCl2 | N/A | Diameter of 3.05–3.69 mm Encapsulation efficiency of 88–85% Drug loading of 0.14–0.15% | Metamizole | Smart drug release [96]. |
| Pectin microspheres | Spray drying | Esterification degree of 62–72% | Encapsulation efficiency of 68.4–72.2% Drug loading of 16.6–17.0% Moisture content of 4.37–5.59% | Octreotide acetate | Peptide delivery [97]. |
| Pectin microspheres functionalized with RGD peptide | Ionotropic gelation with CaCl2 | Esterification degree of 14 % Average MW of 29 kDa | Expansion by hydration higher than 5000% Maintenance of viability, proliferation, and cellular differentiation until 30 days 3D structures promotion for cellular growth Higher interaction of pinned cells in the sphere with the medium | N/A | Immobilization substrate and cellular transport for tissue engineering and potential application in regenerative medicine [98,99]. |
| Eugradit-coated pectin microspheres | W/O emulsification and linking with CaCl2 + solvent evaporation | N/A | Diameter of 400–600 µm Yield of 70–80% Drug (prednisolone) content of 75–80% Drug (mesalamine) content of 75% Expansion ratio by hydration of 1.44–1.60 100% of drug release in 14h at a basic pH of 7.4 | Mesalamine + Prednisolone | Controlled drug release for ulcerative colitis treatment [100]. |
| Pectin/hypromellose hybrid microspheres | Aerosol drying | Low methoxylated amidation CF 005 Esterification degree of 35% Amidation degree of 15% | Diameter of 17–22 µm Zeta potential of −21 to −28 mV Yield of 47–65% Encapsulation efficiency of 96–100% Drug content of 25% 2–3% of humidity 80% of fast drug release in 120 min at a pH of 6.8, improving drug solubility | Melatonin | Controlled release of mucoadhesive drug in nasal solution [68]. |
| Pectin/sodium alginate hybrid microspheres | Ionotropic gelation with CaCl2 | Low methoxylated Esterification degree of 18% | Diameter of 1108–653 μm Encapsulation efficiency of 59–95% 90% of fast drug release in 6–10 h at a pH of 6.8 | Metformin Hydrochloride | Controlled drug release for diabetes treatment [101]. |
| Pectin microspheres | Aerosol drying | N/A | Diameter of 4.0–4.5 μm Encapsulation efficiency higher than 98% Drug content of 20–48% Yield of 46–48% 100% of fast drug release in 48 h at a pH of 6.4 | Ciprofloxacin hydrochloride | Controlled antibiotic release for osteomyelitis treatment [102]. |
| Eugradit-coated pectin microspheres | W/O emulsification + solvent evaporation | N/A | Diameter of 24–31 μm Encapsulation efficiency of 64–74% Expansion ratio by hydration of 0.04–0.18 pH-dependent release; faster with a basic pH (7.4) and slower with an acidic pH (1.2) | 5-fluorouracil | Controlled drug release for colon cancer treatment [103]. |
| Pectin/gellan gum hybrid microspheres | Ionotropic gelation with AlCl3 | LM-5206 CS | Average diameter of 914 μm Polydispersity index of 0.29 Encapsulation efficiency of 76% pH-dependent release: slow in an acidic pH (1.2) of 17% in 120 min and gradually controlled in a basic pH (6.8), longer than 48h. | Resveratrol | Controlled antioxidant release for colon disorders treatment [104]. |
| Pectin/sodium alginate hybrid microspheres | Ionotropic gelation with CaCl2 + separation by coacervation | N/A | Diameter of 500–700 μm Encapsulation efficiency of 64–70% Expansion ratio by hydration of 0.11–0.42 pH-dependent release: slow at an acidic pH (1.2) of only 8% in 4 h and maximum release at a basic pH (6.8) in 12 h | 5-fluorouracil | Controlled drug release for colon cancer treatment [105]. |
| Pectin/gellan gum hybrid microspheres | Ionotropic gelation with CaCl2 | Low methoxylated and amidation Esterification degree of 35%–40% Amidation degree of 20% | Average diameter of 250 μm Encapsulation efficiency of 67–88% 30–55% of drug release at pH of 7.4 in 120 min | Methyl violet | Controlled drug release for microorganism and other human parasite treatments [59]. |
| Pectin-based CAP-coated microspheres | Dehydration technique | N/A | Average diameter of 0.8–7.06 and 0.9–10.31 at pH 1.2, whereas at pH 7.4, the particle size was 1.3–9.26 and 0.5–11.64 mm. Polydispersity index of 0.245–0.267 Zeta potential of 26.78–29.36 Mv | Mesalamine | Controlled drug release for ulcerative colitis [106]. |
| Pectin microspheres | Crosslinking with glutaraldehyde + Spray drying | N/A | Sizes between 20 and 500 μm | Quercetin | Stabilization of quercetin with microencapsulation [107]. |
| Eugradit-coated pectin microspheres | W/O emulsification + solvent evaporation | N/A | Diameter of 9–14 μm Encapsulation efficiency of 52–75% 91–99% of maximum drug release at a pH of 7.5 in 8 h | Metronidazole | Controlled antibiotic release for colon disorders treatment [108]. |
| Sphere Type | Synthesis Method | Pectin Properties | Characteristics of the Spheres | Field | Application |
|---|---|---|---|---|---|
| Pectin/alginate hybrid microspheres | Coaxial electrospray system | N/A | Zeta potential of −21–53 mV Diameter of 1.58–3.24 µm Encapsulation efficiency of 26–85% | Cosmetics | Mint essential oil encapsulation for use cosmetics and food [112]. |
| Pectin/jelly fig hybrid microspheres | W/O emulsification and linking + reticulation with formaldehyde | N/A | Diameter of 58–82 µm Image contrast efficiency of 94% | Electronics | Copper phthalocyanine modified by cetylpyridinium chloride to use it in electrophoretic ink display [113]. |
| Pectin-coated lanthanum oxide hybrid microspheres | Precipitation + calcination | N/A | Diameter of 0.6–7 µm | Electronics | Lanthanum oxide sensor to detect CO [114]. |
| Pectin/calcium phosphate hybrid microspheres | Extrusion + linking with calcium chloride | Low methoxylated | Diameter of 400–600 µm | Environmental | Promote biomineralization process with a biomimetic method [115]. |
| Pectin microspheres | pH modification + linking with calcium chloride | Galacturonic acid of >74% Esterification degree of 0.90–47% | Diameter of 2 mm Pb (II) absorption of 69–95% at pH 6 | Environmental | Absorption of Pb(II) by microspheres [116]. |
| Pectin microspheres | Hydrothermal | N/A | Diameter of 1–5 µm Absorption capacity of 905.8 mg/g at t = 0.5 min | Environmental | Absorption of blue methylene by microspheres [72]. |
| Pectin–alginate microspheres | Ionotropic gelation with TiO2 | N/A | Absorption of 51–56% at t = 30 min | Environmental | Removal of methylene blue by microspheres [117]. |
| Pectin/activated carbon microspheres | Ionotropic gelation with CaCl2 | Molecular weight of 786 kDa Degree of methoxylation of 28.3% Degree of amidation of 20.63% | Diameter of 1.30–2.78 mm | Environmental | Absorption of Pb2+ by microspheres [118]. |
| Chitosan-coated pectin microspheres | O/W/O emulsification and linking with CaCl2 | Low methoxylated | Diameter of 100 µm Bioink viscosity of 445 mm2/s Result of assay in cytotoxicity in cells 95.7 ± 1.0 % | Biotechnology | Estradiol encapsulation for use in bioprinting [46]. |
| Pectin/pea protein/maltodextrin hybrid microspheres | W/O emulsification and linking | Esterification degree of 60% | Diameter of 0.3–400 µm Encapsulation efficiency of 77% | Food | A rich oil in polyunsaturated fatty acids encapsulation for use in food [119]. |
| Pectin/xanthan gum/wheat protein hybrid microspheres | W/O emulsification and linking | Esterification degree of >50% Average MW of 200 kDa | Zeta potential of −9.1–23 mV Diameter of 0.23–22 µm | Food | Fish oil encapsulation for use in food [120]. |
| Pectin/sodium alginate hybrid microspheres | W/O emulsification and linking with calcium chloride | Esterification degree of >50% | Diameter of 0.46–0.62 mm Encapsulation efficiency of 52–70% Humidity content of 4.29–4.73% Swelling index of 0.911–0.959 | Food | α-tocopherol encapsulation for use in food [121]. |
| Pectin/maltodextrin/whey protein concentrate hybrid microspheres | W/O/W emulsification + doble layer technique | Esterification degree of 71.1% 65% galacturonic acid | Diameter of 0.536–0.482 µm Encapsulation efficiency of 93–96% | Food | Saffron encapsulation for use in food [122]. |
| Chitosan-coated highly methoxyl pectin alginate hybrid microspheres | Coextrusion + linking with calcium chloride | High methoxylated | Diameter of 475–825 µm Encapsulation efficiency of 33–73% | Food | Kenaf seed oil encapsulation for use in food [123]. |
| Calcium pectinate microspheres | Ionotropic gelation/Extrusion + calcium chloride crosslinking | N/A | Encapsulation efficiency of 25.2–31.1% Yield of 92.2–97.1% | Food | Slow release of urea in the sheep diet [124]. |
| Pectin/casein microspheres | Complex coacervation + Spray drying | N/A | Diameter of 4–8 µm Encapsulation efficiency of 60.09–83.22% Drying yield of 3.49–18.82% | Food | microencapsulation of phytochemicals from Vitis labrusca [125]. |
| Pectin/Kasagumycin hybrid microspheres | Chemical linking with EDC and NHS | Esterification degree of 25% Average MW of 70 kDa | Stable a different pH and temperatures | Agriculture | Kasagumycin encapsulation for use against crop pathogens [126]. |
| Highly methoxylated pectin and guar-gum-coated Low methoxylated pectin/alginate hybrid microspheres | W/O emulsification and linking with calcium chloride | Low and highly methoxylated | Number of bacteriophages per microspheres 4–6 | Agriculture | Bacteriophage encapsulation [127]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Alvarado, K.; Chacón-Cerdas, R.; Starbird-Perez, R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry 2022, 4, 121-136. https://doi.org/10.3390/chemistry4010011
Gutierrez-Alvarado K, Chacón-Cerdas R, Starbird-Perez R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry. 2022; 4(1):121-136. https://doi.org/10.3390/chemistry4010011
Chicago/Turabian StyleGutierrez-Alvarado, Keila, Randall Chacón-Cerdas, and Ricardo Starbird-Perez. 2022. "Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications" Chemistry 4, no. 1: 121-136. https://doi.org/10.3390/chemistry4010011
APA StyleGutierrez-Alvarado, K., Chacón-Cerdas, R., & Starbird-Perez, R. (2022). Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry, 4(1), 121-136. https://doi.org/10.3390/chemistry4010011