U(VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]
Abstract
:1. Introduction
2. Materials and Methods
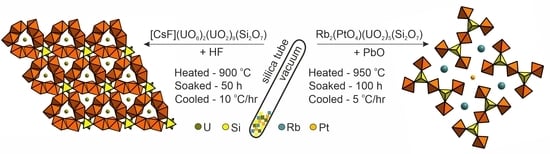
2.1. Synthesis
2.2. Single-Crystal X-Ray Experiments
2.3. Elemental Analysis
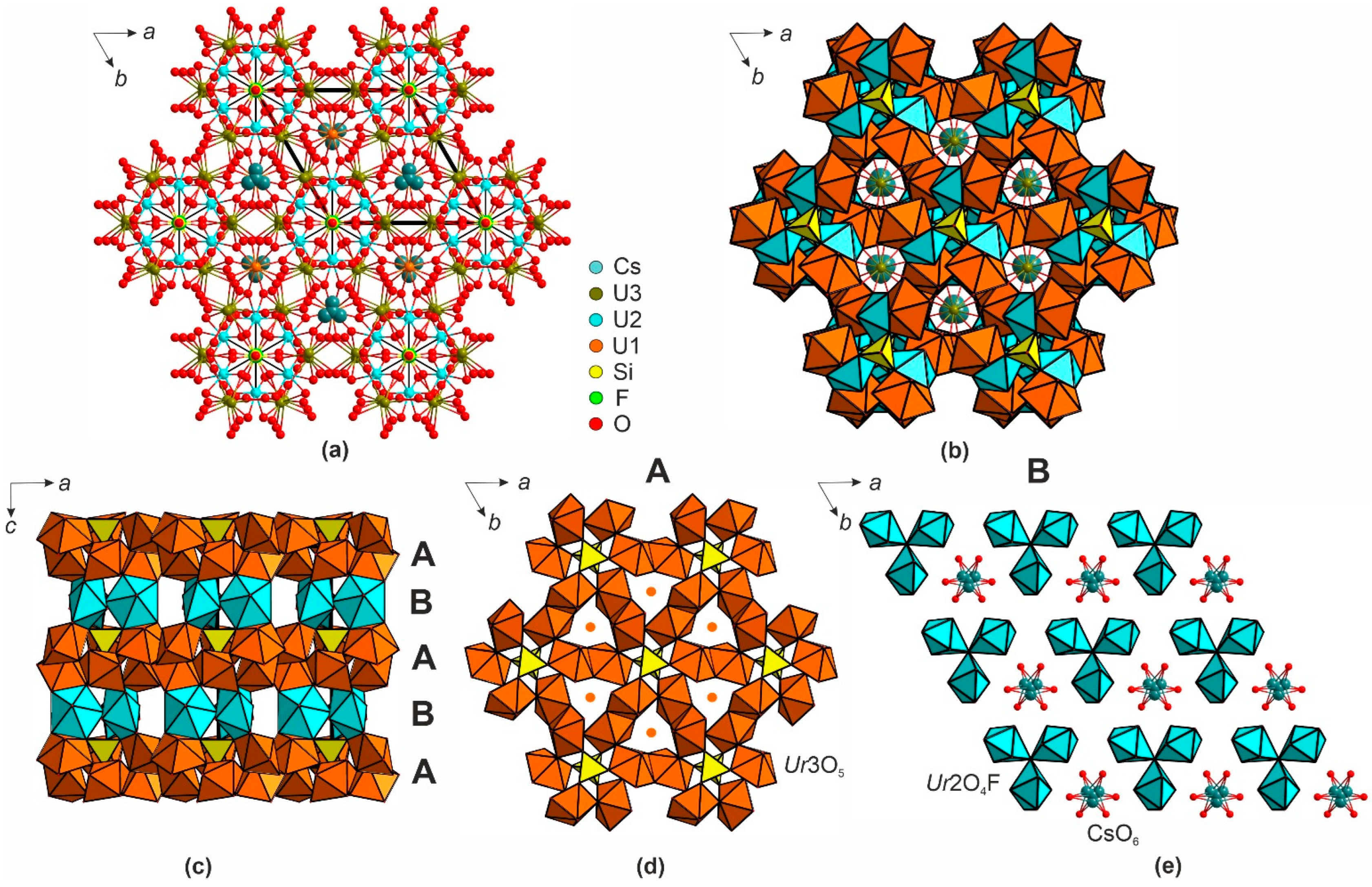
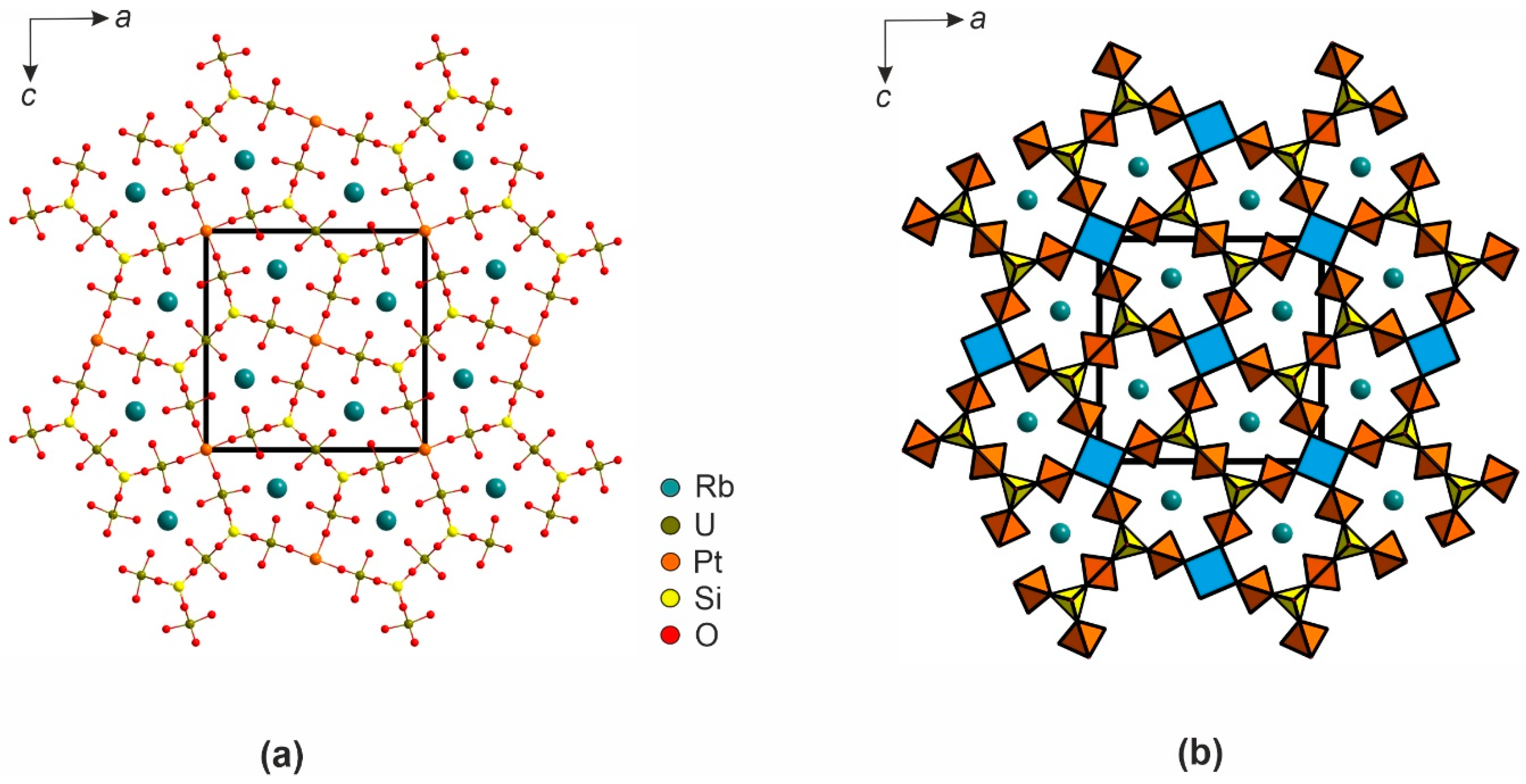
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belova, L.N.; Doynikova, O.A. Conditions of formation of uranium minerals in the oxidation zone of uranium deposits. Geol. Ore Deposit 2003, 45, 130–132. [Google Scholar]
- Baik, M.H.; Cho, H.R. Roles of uranyl silicate minerals in the long-term mobility of uranium in fractured granite. J. Radioanal. Nucl. Chem. 2022, 331, 451–459. [Google Scholar] [CrossRef]
- Plášil, J. Mineralogy, crystallography and structural complexity of natural uranyl silicates. Minerals 2018, 8, 551. [Google Scholar] [CrossRef] [Green Version]
- Utsunomiya, S.; Ewing, R.C. Radiation-induced decomposition of U(VI) alteration phases of UO2. Mater. Res. Soc. Symp. Proceed. 2006, 932, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Burns, P.; Olson, R.; Finch, R.; Hanchar, J.; Thibault, Y. KNa3(UO2)2(Si4O10)2(H2O)4, a new compound formed during vapor hydration of an actinide-bearing borosilicate Waste Glass. J. Nucl. Mater. 2000, 278, 290–300. [Google Scholar] [CrossRef]
- Burns, P.C.; Ewing, R.C.; Miller, M.L. Incorporation mechanisms of actinide elements into the structures of U6+ phases formed during the oxidation of spent nuclear fuel. J. Nucl. Mater. 1997, 245, 1–9. [Google Scholar] [CrossRef]
- Burns, P.C. Cs boltwoodite obtained by ion exchange from single crystals: Implications for radionuclide release in a nuclear repository. J. Nucl. Mater. 1999, 265, 218–223. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Avdontseva, M.S. Cs-exchanged cuprosklodowskite. In Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2012; Volume 2, pp. 163–166. [Google Scholar]
- Morrison, G.; Smith, M.D.; Tran, T.T.; Halasyamani, P.S.; Zur Loye, H.C. Synthesis and structure of the new pentanary uranium(vi) silicate, K4CaUSi4O14, a member of a structural family related to fresnoite. Cryst. Eng. Comm. 2015, 17, 4218–4224. [Google Scholar] [CrossRef]
- Morrison, G.; Zur Loye, H.C. Flux growth of [NaK6F][(UO2)3(Si2O7)2] and [KK6Cl][(UO2)3(Si2O7)2]: The effect of surface area to volume ratios on reaction products. Cryst. Growth Des. 2016, 16, 1294–1299. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Liu, L.; Jacobson, A.J. [(CH3)4N][(C5H5NH)0.8((CH3)3NH)0.2]U2Si9O23F4 (USH-8): An organically templated open-framework uranium silicate. J. Mater. Chem. 2002, 12, 406–410. [Google Scholar] [CrossRef]
- Liu, H.-K.; Lii, K.-H. Cs2USi6O15: A tetravalent uranium silicate. Inorg. Chem. 2011, 50, 5870–5872. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Chiang, R.K.; Kao, H.M.; Lii, K.H. High-temperature, high-pressure hydrothermal synthesis, crystal structure, and solid-state NMR spectroscopy of Cs2(UO2)(Si2O6) and variable-temperature powder X-ray diffraction study of the hydrate phase Cs2(UO2)(Si2O6)·0.5H2O. Inorg. Chem. 2005, 44, 3914–3918. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Jacobson, A.J. Hydrothermal synthesis and structures of the new open-framework uranyl silicates Rb4(UO2)2(Si8O20)(USH-2Rb), Rb2(UO2)(Si2O6)H2O (USH-4Rb) and A2(UO2)(Si2O6)0.5H2O (USH-5A; A = Rb, Cs). J. Mater. Chem. 2003, 13, 191–196. [Google Scholar] [CrossRef]
- Liu, C.-L.; Liu, H.-K.; Chang, W.-J.; Lii, K.-H. K2Ca4[(UO2)(Si2O7)2]: A uranyl silicate with a one-dimensional chain structure. Inorg. Chem. 2015, 54, 8165–8167. [Google Scholar] [CrossRef] [PubMed]
- Juillerat, C.A.; Klepov, V.V.; Morrison, G.; Pace, K.A.; Zur Loye, H.C. Flux crystal growth: A versatile technique to reveal the crystal chemistry of complex uranium oxides. Dalton Trans. 2019, 48, 3162–3181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Langer, E.M.; Kegler, P.; Alekseev, E.V. Structural and spectroscopic investigation of novel 2D and 3D uranium oxo-silicates/germanates and some statistical aspects of uranyl coordination in oxo-salts. Inorg. Chem. 2019, 58, 10333–10345. [Google Scholar] [CrossRef]
- Fejfarová, K.; Plášil, J.; Yang, H.; Čejka, J.; Dušek, M.; Downs, R.T.; Barkley, M.C.; Škoda, R. Revision of the crystal structure and chemical formula of weeksite, K2(UO2)2(Si5O13) 4H2O. Am. Mineral. 2012, 97, 750–754. [Google Scholar] [CrossRef]
- Morrison, J.M.; Moore-Shay, L.J.; Burns, P.C. U(VI) uranyl cation-cation interactions in framework germanates. Inorg. Chem. 2011, 50, 2272–2277. [Google Scholar] [CrossRef]
- Zadoya, A.I.; Siidra, O.I.; Bubnova, R.S.; Nazarchuk, E.V.; Bocharov, S.N. Tellurites of hexavalent uranium: First observation of polymerized (UO4)2– tetraoxido cores. Eur. J. Inorg. Chem. 2016, 2016, 4083–4089. [Google Scholar] [CrossRef]
- Krot, N.N.; Grigor’ev, M.S. Cation-cation interaction in crystalline actinide compounds. Uspekhi Khimii. 2004, 73, 94–107. [Google Scholar]
- Alekseev, E.V.; Krivovichev, S.V.; Malcherek, T.; Depmeier, W. One-dimensional array of two- and three-center cation-cation bonds in the structure of Li4[(UO2)10O10(Mo2O8)]. Inorg. Chem. 2007, 46, 8442–8444. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagne, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Alkali and alkaline-earth metals. Acta Crystallogr. Sect B Struct. Sci. 2016, 72, 602–625. [Google Scholar] [CrossRef] [Green Version]
- Attfield, M.P.; Catlow, C.R.A.; Sokol, A.A. True structure of trigonal bipyramidal SiO4F- species in siliceous zeolites. Chem. Mater. 2001, 13, 4708–4713. [Google Scholar] [CrossRef]
- Maynard, B.A.; Sykora, R.E.; Mague, J.T.; Gorden, A.E.V. Actinide tetracyanoplatinates: Synthesis and structural characterization with uncharacteristic Th-N C coordination and thorium fluorescence. Chem. Commun. 2010, 46, 4944–4946. [Google Scholar] [CrossRef] [PubMed]
- Maynard, B.A.; Lynn, K.S.; Sykora, R.E.; Gorden, A.E.V. Emission, raman spectroscopy, and structural characterization of actinide tetracyanometallates. Inorg. Chem. 2013, 52, 4880–4889. [Google Scholar] [CrossRef]
- Siidra, O.I.; Gogolin, M.; Lukina, E.A.; Kabbour, H.; Bubnova, R.S.; Mentré, O.; Agakhanov, A.A.; Krivovichev, S.V.; Colmont, M.; Gesing, T. Structural evolution from 0D units to 3D frameworks in Pb Oxyhalides: Unexpected strongly corrugated layers in Pb7O6Br2. Inorg. Chem. 2015, 54, 11550–11556. [Google Scholar] [CrossRef]
- Hao, Y.; He, L.; Ge, G.; Zhang, Q.; Luo, N.; Huang, S.; Li, H.; Alekseev, E.V. Mg3Pt(BO3)2O2: The first platinum borate from the flux technique. J. Solid State Chem. 2020, 281, 121046. [Google Scholar] [CrossRef]



| Compound | 1 | 2 |
|---|---|---|
| Crystal system | trigonal | tetragonal |
| Space group | P-31c | P4/mbm |
| a, Å | 10.2040(3) | 16.040(2) |
| c, Å | 17.1278(5) | 3.9231(6) |
| V, Å3 | 1544.45(10) | 1009.3(3) |
| F(000) | 2800 | 1936 |
| Density | 7.351 | 7.815 |
| Radiation, wavelength, Å | MoKα, 0.71073 | |
| Ranges of h, k, l | –13 ≤ h ≤ 12, –13 ≤ k ≤ 12, –22 ≤ l ≤ 21 | −18 ≤ h ≤ 18, −18 ≤ k ≤ 18, −4 ≤ l ≤ 4 |
| Number of reflections | 8347 | 15,047 |
| Number of unique reflections | 1093 | 525 |
| 2θmin–2θmax | 3.313–27.987 | 1.79–24.63 |
| Rint/Rsigma | 0.029/0.043 | 0.029/0.094 |
| R1 [F > 4σ(F)]/wR1 | 0.029/0.053 | 0.045/0.12 |
| R2/wR2 | 0.039/0.091 | 0.064/0.13 |
| GOF | 1.162 | 1.151 |
| CCDC | 2,214,650 | 2,214,660 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarchuk, E.V.; Siidra, O.I.; Charkin, D.O.; Tagirova, Y.G. U(VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]. Chemistry 2022, 4, 1515-1523. https://doi.org/10.3390/chemistry4040100
Nazarchuk EV, Siidra OI, Charkin DO, Tagirova YG. U(VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]. Chemistry. 2022; 4(4):1515-1523. https://doi.org/10.3390/chemistry4040100
Chicago/Turabian StyleNazarchuk, Evgeny V., Oleg I. Siidra, Dmitri O. Charkin, and Yana G. Tagirova. 2022. "U(VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]" Chemistry 4, no. 4: 1515-1523. https://doi.org/10.3390/chemistry4040100
APA StyleNazarchuk, E. V., Siidra, O. I., Charkin, D. O., & Tagirova, Y. G. (2022). U(VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]. Chemistry, 4(4), 1515-1523. https://doi.org/10.3390/chemistry4040100