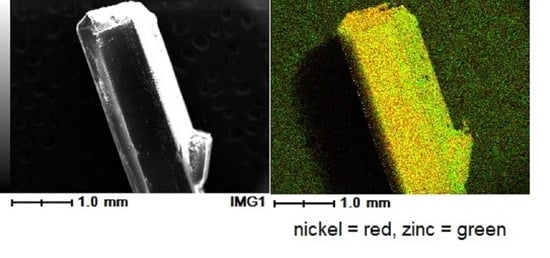
Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2
Abstract
Share and Cite
Serdan, U.; Robin, L.; Marchivie, M.; Gonidec, M.; Rosa, P.; Duverger-Nédellec, E.; Pouget, E.; Sainctavit, P.; Arrio, M.-A.; Juhin, A.; et al. Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry 2023, 5, 255-268. https://doi.org/10.3390/chemistry5010020
Serdan U, Robin L, Marchivie M, Gonidec M, Rosa P, Duverger-Nédellec E, Pouget E, Sainctavit P, Arrio M-A, Juhin A, et al. Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry. 2023; 5(1):255-268. https://doi.org/10.3390/chemistry5010020
Chicago/Turabian StyleSerdan, Ugo, Lucas Robin, Mathieu Marchivie, Mathieu Gonidec, Patrick Rosa, Elen Duverger-Nédellec, Emilie Pouget, Philippe Sainctavit, Marie-Anne Arrio, Amélie Juhin, and et al. 2023. "Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2" Chemistry 5, no. 1: 255-268. https://doi.org/10.3390/chemistry5010020
APA StyleSerdan, U., Robin, L., Marchivie, M., Gonidec, M., Rosa, P., Duverger-Nédellec, E., Pouget, E., Sainctavit, P., Arrio, M.-A., Juhin, A., Rogalev, A., Wilhelm, F., & Hillard, E. A. (2023). Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry, 5(1), 255-268. https://doi.org/10.3390/chemistry5010020