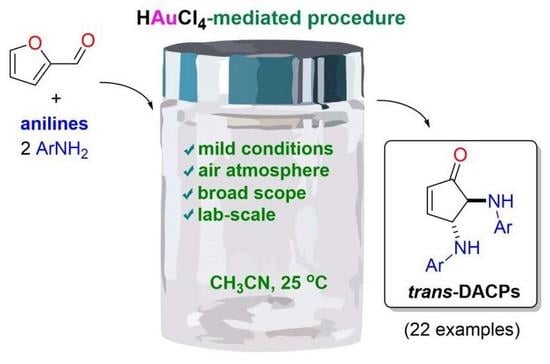
Gold(III) Chloride-Mediated Transformation of Furfural to the trans-N,N-4,5-Diaminocyclopent-2-enones in the Presence of Anilines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Instrumentations
2.3. Heterogeneous Catalytic Reaction between Furfural and Amines
2.4. Homogeneous Catalytic Reaction between Furfural and Secondary Amines
2.5. Homogeneous Catalytic Reaction between Furfural and Substituted Anilines
3. Results and Discussion
3.1. Evaluation of Catalytic Conditions for the Reaction of Furfural with Morpholine
 | |||
| Solvent [a] | 1 (%) | 3 (%) | 4 (%) |
| CH3CN | - | - | 100 |
| EtOAc | 8 | 75 | 17 |
| MeOH | - | 63 | 37 |
| EtOH | - | 67 | 33 |
| DCM | 13 | 67 | 20 |
| DCE | 9 | 75 | 16 |
| DMC | 10 | 70 | 20 |
| Toluene | 8 | 80 | 12 |
| CHCl3 | 12 | 79 | 9 |
3.2. Application of Catalytic Conditions to the Synthesis of trans-DACPs 4, 4a–4d, and 5a–5o
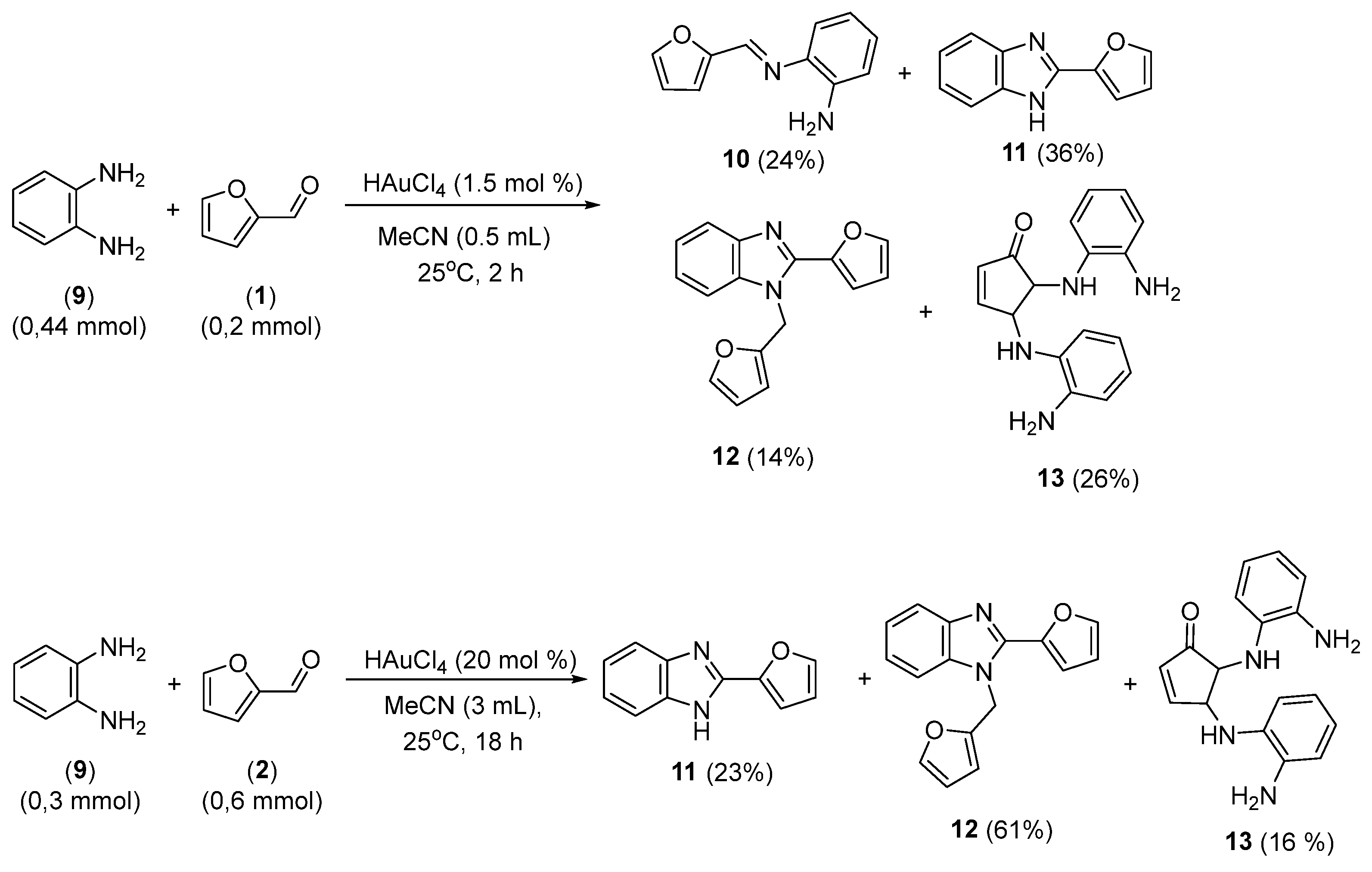
3.3. Reaction Profile Evaluation of the Reaction between Furfural and o-Phenylenediamine
3.4. Proposed Mechanistic Pathway for the Reaction of Furfural with Anilines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and Heterogeneous Gold Catalysis for Materials Science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ma, X.; Cheng, X.; Zhao, K.; Gutman, K.; Li, T.; Zhang, L. Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev. 2021, 121, 8979–9038. [Google Scholar] [CrossRef] [PubMed]
- Mato, M.; Franchino, A.; Garcıa-Morales, C.; Echavarren, A.M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N. Gold and Silver Assisted Synthesis of Five-Membered Oxygen and Nitrogen Containing Heterocycles. Synth. Commun. 2019, 49, 1459–1485. [Google Scholar] [CrossRef]
- Pflasterer, D.; Hashmi, A.S.K. Gold Catalysis in Total Synthesis –Recent Achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. Dual Gold Catalysis. Acc. Chem. Res. 2014, 47, 864–876. [Google Scholar] [CrossRef]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; Garcıa, H. Catalytic Activity of Unsupported Gold Nanoparticles. Catal. Sci. Technol. 2013, 3, 58–69. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2022, 120, 464–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratakis, M.; Lykakis, I.N. Nanogold(0)-Catalyzed Addition of Heteroelement σ-Linkages to Functional Groups. Synthesis 2019, 51, 2435–2454. [Google Scholar] [CrossRef]
- Hutchings, G.J. Heterogeneous Gold Catalysis. ACS Cent. Sci. 2018, 4, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Takale, B.S.; Bao, M.; Yamamoto, Y. Gold Nanoparticle (AuNPs) and Gold Nanopore (AuNPore) Catalysts in Organic Synthesis. Org. Biomol. Chem. 2014, 12, 2005–2027. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Liu, Y.-M.; Cao, Y. Supported Gold Catalysis: From Small Molecule Activation to Green Chemical Synthesis. Acc. Chem. Res. 2014, 47, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomea, T.; Kaneda, K. Gold Nanoparticle Catalysts for Selective Hydrogenations. Green Chem. 2013, 15, 2636–2654. [Google Scholar] [CrossRef]
- Mielby, J.; Kegnζs, S.; Fristrup, P. Gold Nanoparticle-Catalyzed Formation of Nitrogen Containing Compounds—From Mechanistic Understanding to Synthetic Exploitation. ChemCatChem 2012, 4, 1037–1047. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef] [Green Version]
- Fürstner, A. Gold and Platinum Catalysis—A Convenient Tool for Generating Molecular Complexity. Chem. Soc. Rev. 2009, 38, 3208–3221. [Google Scholar] [CrossRef]
- Zi, W.; Toste, F.D. Recent Advances in Enantioselective Gold Catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589. [Google Scholar] [CrossRef]
- Sengupta, S.; Shi, X. Recent Advances in Asymmetric Gold Catalysis. ChemCatChem 2010, 2, 609–619. [Google Scholar] [CrossRef]
- Meera, G.; Rohit, K.R.; Treesa, G.S.S.; Anilkumar, G. Advances and Prospects in Gold-Catalyzed C−H Activation. Asian J. Org. Chem. 2020, 9, 144–161. [Google Scholar] [CrossRef]
- de Haro, T.; Nevado, C. On Gold-Mediated C-H Activation Processes. Synthesis 2011, 16, 2530–2539. [Google Scholar]
- Shu, X.; Zhang, M.; He, Y.; Frei, H.; Toste, F.D. Dual Visible Light Photoredox and Gold-Catalyzed Arylative Ring Expansion. J. Am. Chem. Soc. 2014, 136, 5844–5847. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Hopkinson, M.N.; Glorius, F. Combining Gold and Photoredox Catalysis: Visible Light-Mediated Oxy- and Aminoarylation of Alkenes. J. Am. Chem. Soc. 2013, 135, 5505–5508. [Google Scholar] [CrossRef]
- Witzel, S.; Hashmi, A.S.K.; Xie, J. Light in Gold Catalysis. Chem. Rev. 2021, 121, 8868–8925. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; Garcıa, H. Titania Supported Gold Nanoparticles as Photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef]
- Rocchigiani, L.; Bochmann, M. Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121, 8364–8451. [Google Scholar] [CrossRef]
- Collado, A.; Nelson, D.J.; Nolan, S.P. Optimizing Catalyst and Reaction Conditions in Gold(I) Catalysis−Ligand Development. Chem. Rev. 2021, 121, 8559–8612. [Google Scholar] [CrossRef]
- Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)- and (Aryl)-(amino)carbene Coinage Metal Complexes and Their Applications. Chem. Rev. 2020, 120, 4141–4168. [Google Scholar] [CrossRef]
- Lu, Z.; Hammond, G.B.; Xu, B. Improving Homogeneous Cationic Gold Catalysis through a Mechanism-Based Approach. Acc. Chem. Res. 2019, 52, 1275–1288. [Google Scholar] [CrossRef]
- Kumar, R.; Nevado, C. Cyclometalated Gold(III) Complexes: Synthesis, Reactivity, and Physicochemical Properties. Angew. Chem. Int. Ed. 2017, 56, 1994–2015. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, S.M.; Hirao, H.; Hong, S.H. Gold(I)/Gold(III)-Catalyzed Selective Synthesis of N -Sulfonyl Enaminone Isomers from Sulfonamides and Ynones via Two Distinct Reaction Pathways. Org. Lett. 2017, 19, 4734–4737. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Giuseppe, S.D.; Marinelli, F.; Rossi, E. Conversion of Homochiral Amines and α-Amino Esters to their Chiral 1,2,3,5-Substituted Pyrrole Derivatives via Gold-Catalysed Amination/Annulation Reactions of 2-Propynyl-1,3-dicarbonyl Compounds. Tetrahedron Asymmetry 2001, 19, 2715–2720. [Google Scholar] [CrossRef]
- Shu, X.-Z.; Liu, X.-Y.; Xiao, H.-Q.; Ji, K.-G.; Guo, L.-N.; Liang, Y.-M. Tandem Gold(III)-Catalyzed Amination-Intramolecular Hydroamination Reactions of 1-En-4-yn-3-ols with Sulfonamides: Efficient Approach to Highly Substituted Pyrroles. Adv. Synth. Catal. 2008, 350, 243–248. [Google Scholar] [CrossRef]
- Atechian, S.; Nock, N.; Norcross, R.D.; Ratni, H.; Thomas, A.W.; Verron, J.; Masciadri, R. New Vistas in Quinoline Synthesis. Tetrahedron 2007, 63, 2811–2823. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Ding, D.; Sun, J.; Ji, Y.; Dong, J. Gold(III)-Catalyzed Three-Component Coupling Reaction (TCC) Selective toward Furans. Org. Lett. 2013, 15, 2884–2887. [Google Scholar] [CrossRef]
- Wachenfeldt, H.V.; Röse, P.; Paulsen, F.; Loganathan, N.; Strand, D. Catalytic Three-Component Domino Reaction for the Preparation of Trisubstituted Oxazoles. Chem. Eur. J. 2013, 19, 7982–7988. [Google Scholar] [CrossRef]
- Kidwai, M.; Jahan, A.; Mishra, N.K. Gold(III) Chloride (HAuCl4·3H2O) in PEG: A New and Efficient Catalytic System for the Synthesis of Functionalized Spirochromenes. Appl. Catal. A Gen. 2012, 425–426, 35–43. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Peters, F.N. Industrial Uses of Furans. Ind. Eng. Chem. 1939, 31, 178–180. [Google Scholar] [CrossRef]
- Lukes, R.M.; Wilson, C.L. Reactions of Furan Compounds. XI. Side Chain Reactions of Furfural and Furfuryl Alcohol over Nickel-Copper and Iron-Copper Catalysts. J. Am. Chem. Soc. 1951, 73, 4790–4794. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Wölfle, M.; Teles, J.; Frey, W. Bisphenols from Furfurals by Organocatalysis and Gold Catalysis. Synlett 2007, 11, 1747–1752. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Tšupova, S.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Synthesis of Phenols from Hydroxymethylfurfural (HMF). Green Chem. 2016, 18, 5800–5805. [Google Scholar] [CrossRef]
- Simeonov, S.P.; Nunes, J.P.M.; Guerra, K.; Kurteva, V.B.; Afonso, C.A.M. Synthesis of Chiral Cyclopentenones. Chem. Rev. 2016, 116, 5744–5893. [Google Scholar] [CrossRef] [PubMed]
- Takano, I.; Yasuda, I.; Nishijima, M.; Hitotsuyanagi, Y.; Takeya, K.; Itokawa, H. New Cephalotaxus Alkaloids from Cephalotaxus harringtonia var. drupacea. J. Nat. Prod. 1996, 59, 965–967. [Google Scholar] [CrossRef]
- Inagaki, F.; Kinebuchi, M.; Miyakoshi, N.; Mukai, C. Formal Synthesis of (+)-Nakadomarin A. Org. Lett. 2010, 12, 1800–1803. [Google Scholar] [CrossRef]
- Dong, G. Recent Advances in the Total Synthesis of Agelastatins. Pure Appl. Chem. 2010, 82, 2231–2246. [Google Scholar] [CrossRef] [Green Version]
- Kusama, T.; Tanaka, N.; Sakai, K.; Gonoi, T.; Fromont, J.; Kashiwada, Y.; Kobayashi, J. Agelamadins A and B, Dimeric Bromopyrrole Alkaloids from a Marine Sponge Agelas sp. Org. Lett. 2014, 16, 3916–3918. [Google Scholar] [CrossRef]
- Araki, A.; Tsuda, M.; Kubota, T.; Mikami, Y.; Fromont, J.; Kobayashi, J. Nagelamide J, a Novel Dimeric Bromopyrrole Alkaloid from a Sponge Agelas Species. Org. Lett. 2007, 9, 2369–2371. [Google Scholar] [CrossRef]
- Hanessian, S.; Vakiti, R.R.; Dorich, S.; Banerjee, S.; Lecomte, F.; DelValle, J.R.; Zhang, J.; Deschênes-Simard, B. Total Synthesis of Pactamycin. Angew. Chem. Int. Ed. 2011, 50, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Mulquiney, C. Rearrangements in the Furan Series. II. The Reaction Between Furfuraldehyde and Aromatic Amines. Aust. J. Chem. 1979, 32, 1079–1092. [Google Scholar] [CrossRef]
- Lewis, K.G.; Mulquiney, C.E. Aspects of the Formation and use of Stenhouse Salts and Related Compounds. Tetrahedron 1977, 33, 463–475. [Google Scholar] [CrossRef]
- Hofmann, T. Characterization of the Chemical Structure of Novel Colored Maillard Reaction Products from Furan-2-carboxaldehyde and Amino Acids. J. Agric. Food Chem. 1998, 46, 932–940. [Google Scholar] [CrossRef]
- Li, S.-W.; Batey, R.A. Mild Lanthanide(iii) Catalyzed Formation of 4,5-Diaminocyclopent-2-enones from 2-Furaldehyde and Secondary Amines: A Domino Condensation/Ring-Opening/Electrocyclization Process. Chem. Commun. 2007, 36, 3759–3761. [Google Scholar] [CrossRef]
- Nunes, J.P.M.; Afonso, C.A.M.; Caddick, S. Synthesis of 2,4-Bifunctionalised Cyclopentenones from 2-Furaldehyde. RSC Adv. 2013, 3, 14975. [Google Scholar] [CrossRef]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) Chloride in Ethyl Lactate as a Smart Ecofriendly System for Efficient and Rapid Stereoselective Synthesis of trans -4,5-Diaminocyclopent-2-enones. ACS Sustain. Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Ramesh, D.; Reddy, T.S.; Narasimhulu, M.; Rajaram, S.; Suryakiran, N.; Mahesh, K.C.; Venkateswarlu, Y. Efficient and Rapid Stereoselective Synthesis of trans -4,5-Diaminocyclopent-2-enones by Acidic Ionic Liquid under Solvent-free Conditions. Chem. Lett. 2009, 38, 586–587. [Google Scholar] [CrossRef]
- Hiscox, A.; Ribeiro, K.; Batey, R.A. Lanthanide(III)-Catalyzed Synthesis of trans -Diaminocyclopentenones from Substituted Furfurals and Secondary Amines via a Domino Ring-Opening/4π-Electrocyclization Pathway. Org. Lett. 2018, 20, 6668–6672. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Esteves, N.R.; Coelho, J.A.S.; Afonso, C.A.M. Copper(II) Triflate As a Reusable Catalyst for the Synthesis of trans -4,5-Diamino-cyclopent-2-enones in Water. J. Org. Chem. 2018, 83, 7509–7513. [Google Scholar] [CrossRef]
- Peewasan, K.; Merkel, M.P.; Fuhr, O.; Powell, A.K. A Designed and Potentially Decadentate Ligand for use in Lanthanide(III) Catalysed Biomass Transformations: Targeting Diastereoselective trans-4,5-Diaminocyclopentenone Derivatives. Dalton Trans. 2020, 49, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Estevão, M.S.; Afonso, C.A.M. Synthesis of trans-4,5-diaminocyclopent-2-enones from furfural catalyzed by Er(III) immobilized on silica. Tetrahedron Lett. 2017, 58, 302–304. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, R. Heterogeneous MOF Catalysts for the Synthesis of trans-4,5-Diaminocyclopent-2-enones from Furfural and Secondary Amines. Catal. Commun. 2019, 120, 11–16. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Cavaca, L.A.S.; Goncalves, J.M.; Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Afonso, C.A.M. Silica-Supported Copper for the Preparation of Trans- 4,5-Diamino-Cyclopent-2-Enones under Continuous Flow Conditions. ACS Sustain. Chem. Eng. 2021, 9, 16038–16043. [Google Scholar] [CrossRef]
- Griffiths, K.; Kumar, P.; Mattock, J.D.; Abdul-Sada, A.; Pitak, M.B.; Coles, S.J.; Navarro, O.; Vargas, A.; Kostakis, G.E. Efficient Ni II 2 Ln III 2 Electrocyclization Catalysts for the Synthesis of trans-4,5-Diaminocyclopent-2-enones from 2-Furaldehyde and Primary or Secondary Amines. Inorg. Chem. 2016, 55, 6988–6994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, K.; Gallop, C.W.D.; Abdul-Sada, A.; Vargas, A.; Navarro, O.; Kostakis, G.E. Heteronuclear 3 d/Dy III Coordination Clusters as Catalysts in a Domino Reaction. Chem. Eur. J. 2015, 21, 6358–6361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampani, S.I.; McGown, A.; Vargas, A.; Abdul-Sada, A.; Tizzard, G.J.; Coles, S.J.; Spencer, J.; Kostakis, G.E. Solvent-Free Synthesis and Key Intermediate Isolation in Ni2Dy2 Catalyst Development in the Domino Ring-Opening Electrocyclization Reaction of Furfural and Amines. J. Org. Chem. 2019, 84, 6858–6867. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water Excellent Solvent for the Synthesis of Bifunctionalized Cyclopentenones from Furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; Zhu, M.; Li, J.; Zheng, X.; Wang, L. Novel Role of p-Toluenesulfonamide in the Preparation of 4,5-Diaminocyclopent-2-enones. Synthesis 2013, 45, 2165–2170. [Google Scholar]
- Di Gioia, M.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [Green Version]
- Nánási, D.E.; Kunfi, A.; Abrahám, A.; Mayer, P.J.; Mihály, J.; Samu, G.F.; Kiss, E.; Mohai, M.; London, G. Construction and Properties of Donor−Acceptor Stenhouse Adducts on Gold Surfaces. Langmuir 2021, 37, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Tzani, M.A.; Fountoulaki, S.; Lykakis, I.N. Polyoxometalate-Driven Ease Conversion of Valuable Furfural to trans-N,N-4,5-Diaminocyclopenten-2-ones. J. Org. Chem. 2022, 87, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.G.; António, J.P.M.; Mendonça, R.; Gomes, R.F.A.; Afonso, C.A.M. Rediscovering Aminal Chemistry: Copper(II) Catalysed Formation under Mild Conditions. Green Chem. 2020, 22, 7484–7490. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gabriel, C.; Lykakis, I.N. Selective Synthesis of Benzimidazoles from o-Phenylenediamine and Aldehydes Promoted by Supported Gold Nanoparticles. Nanomaterials 2020, 10, 2405. [Google Scholar] [CrossRef]



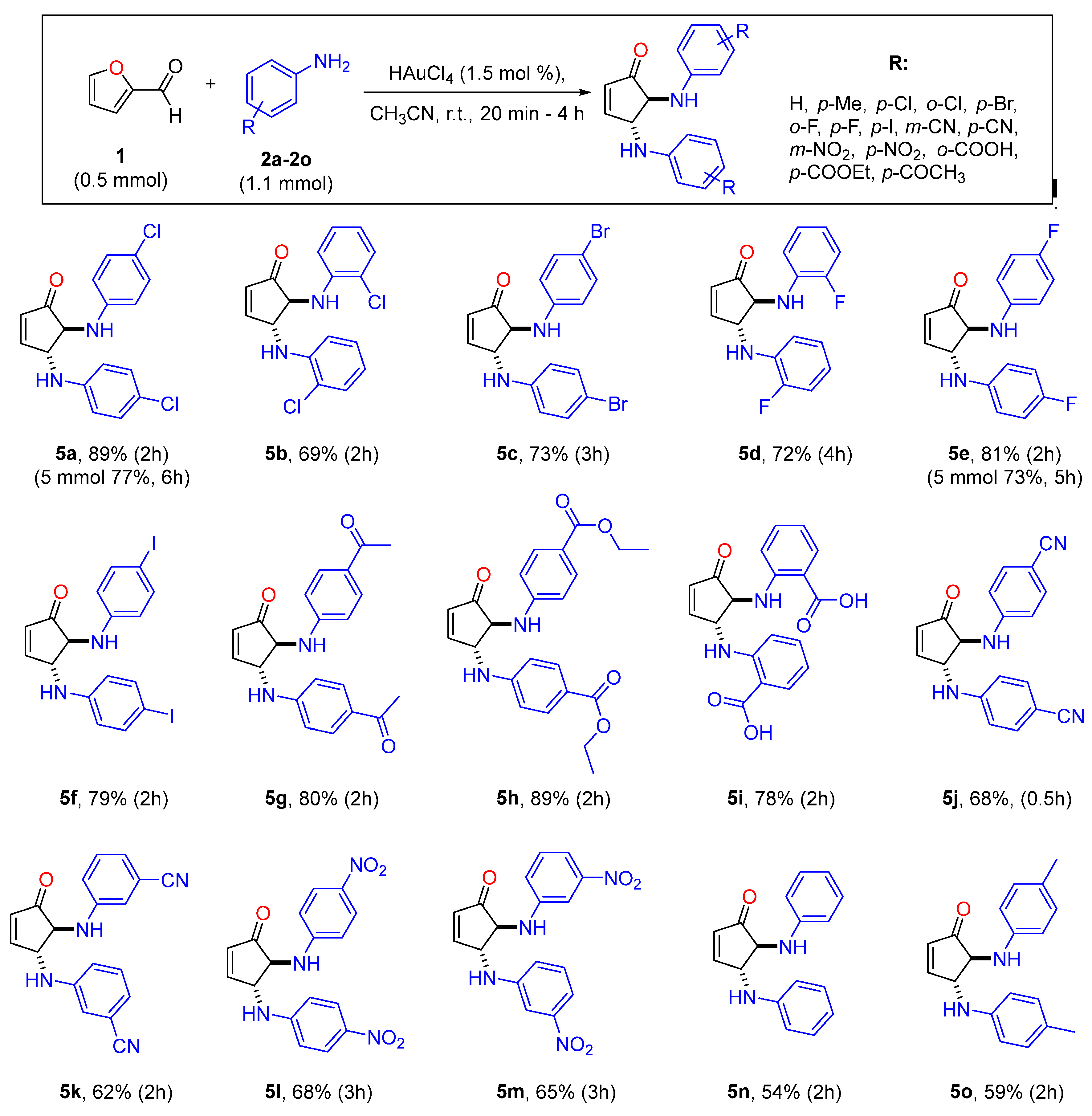


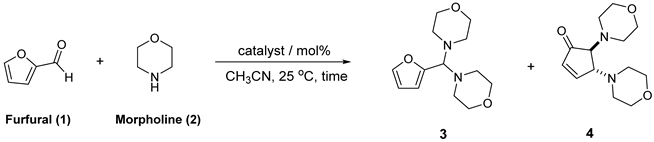 | |||||
| Entry | Catalyst/mol% [a] | Time | 1 (%) | 3 (%) | 4 (%) |
| 1 | No catalyst | 1 h | 18 | 58 | 24 |
| 1 | Au/TiO2 / 1 mol% | 1 h | - | 100 | - |
| 2 | Au/Al2O3 / 1 mol% | 1 h | 8 | 92 | - |
| 3 | Au/ZnO / 1 mol% | 1 h | 13 | 74 | 13 |
| 4 | TiO2 | 1 h | 32 | 68 | - |
| 5 | Al2O3 | 1 h | 25 | 75 | - |
| 6 | AuCl / 3 mol% | 1 h | 12 | 51 | 37 |
| 7 [b] | AuCl / 3 mol% | 1 h | 16 | 37 | 47 |
| 8 [c] | (pTolyl)3PAuCl / 3 mol% | 1 h | 14 | 33 | 33 |
| 9 | HAuCl4 / 3 mol% | 1 h | - | - | 100 |
| 10 | HAuCl4/ 3 mol% | 15 min | 14 | 18 | 68 |
| 11 | HAuCl4/ 1.5 mol% | 2 h | - | - | 100 |
| 12 | HAuCl4/ 1 mol% | 2 h | 9 | 14 | 77 |
| 13 [b] | HAuCl4/ 3 mol% | 15 min | - | - | 100 |
| 14 [d] | HAuCl4/ 3 mol% | 30 min | - | - | 100 |
| 15 [b,e] | HCl / 3 mol% | 1 h | - | 29 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzani, M.A.; Lykakis, I.N. Gold(III) Chloride-Mediated Transformation of Furfural to the trans-N,N-4,5-Diaminocyclopent-2-enones in the Presence of Anilines. Chemistry 2023, 5, 393-405. https://doi.org/10.3390/chemistry5010029
Tzani MA, Lykakis IN. Gold(III) Chloride-Mediated Transformation of Furfural to the trans-N,N-4,5-Diaminocyclopent-2-enones in the Presence of Anilines. Chemistry. 2023; 5(1):393-405. https://doi.org/10.3390/chemistry5010029
Chicago/Turabian StyleTzani, Marina A., and Ioannis N. Lykakis. 2023. "Gold(III) Chloride-Mediated Transformation of Furfural to the trans-N,N-4,5-Diaminocyclopent-2-enones in the Presence of Anilines" Chemistry 5, no. 1: 393-405. https://doi.org/10.3390/chemistry5010029
APA StyleTzani, M. A., & Lykakis, I. N. (2023). Gold(III) Chloride-Mediated Transformation of Furfural to the trans-N,N-4,5-Diaminocyclopent-2-enones in the Presence of Anilines. Chemistry, 5(1), 393-405. https://doi.org/10.3390/chemistry5010029