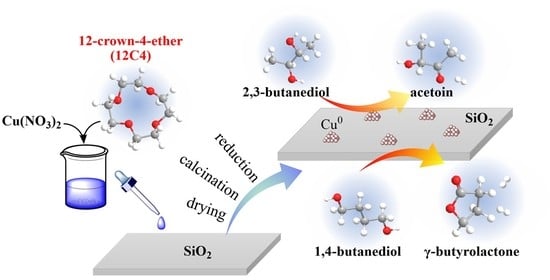
Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
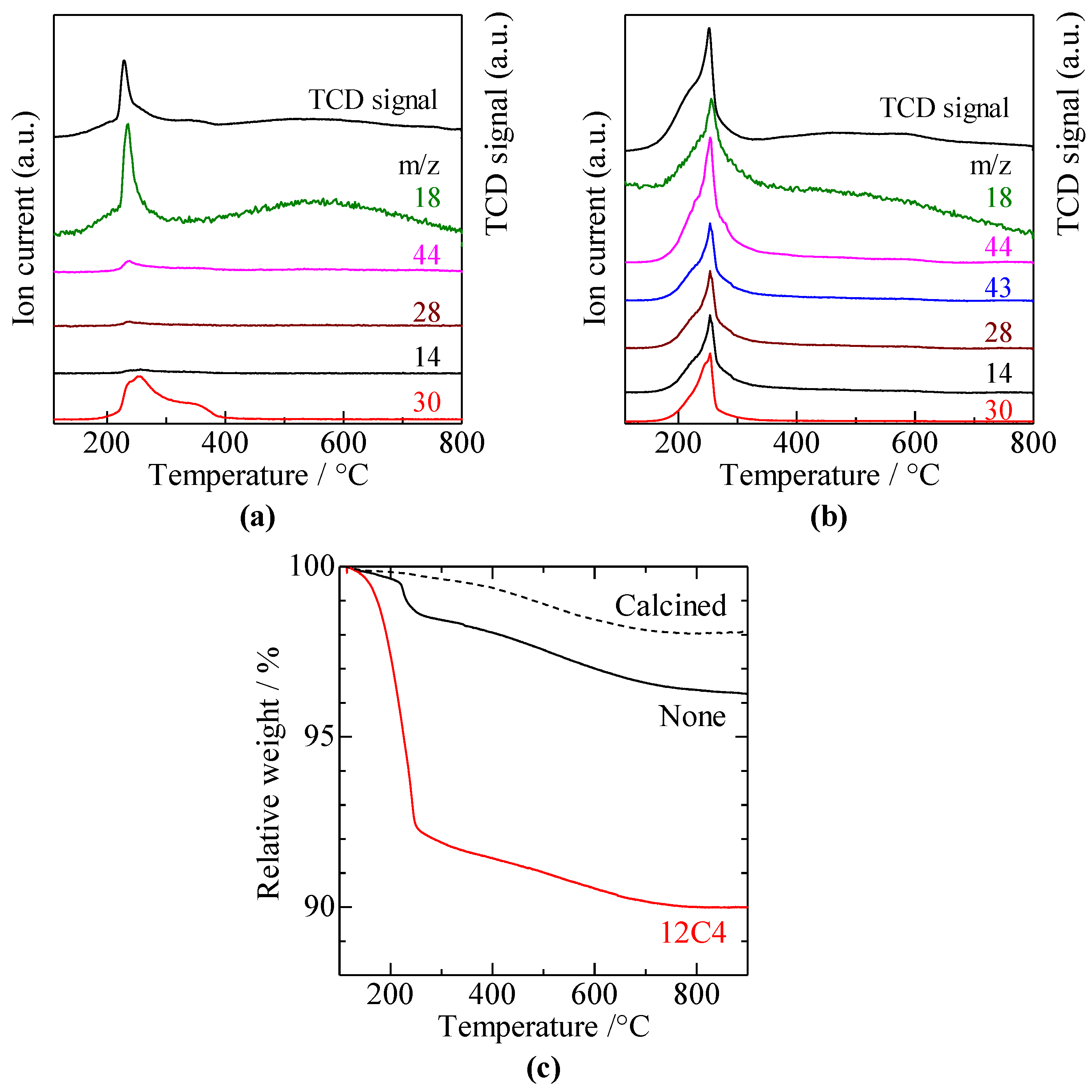
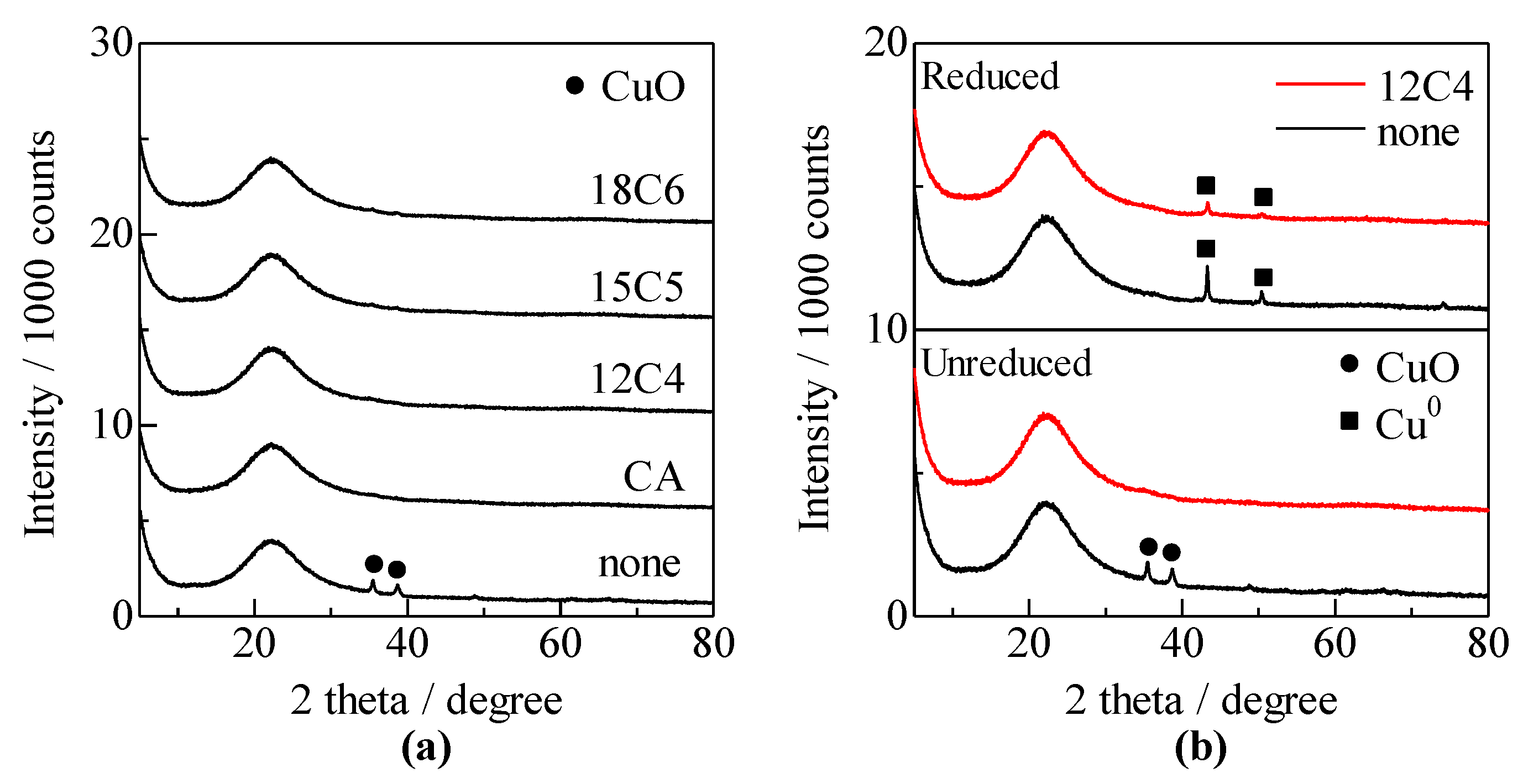
2.2. Catalyst Characterization
2.3. Vapor-Phase Dehydrogenation of 2,3-BDO and 1,4-BDO
3. Results
3.1. Catalyst Characterization
3.2. Comparison of Organic Additives
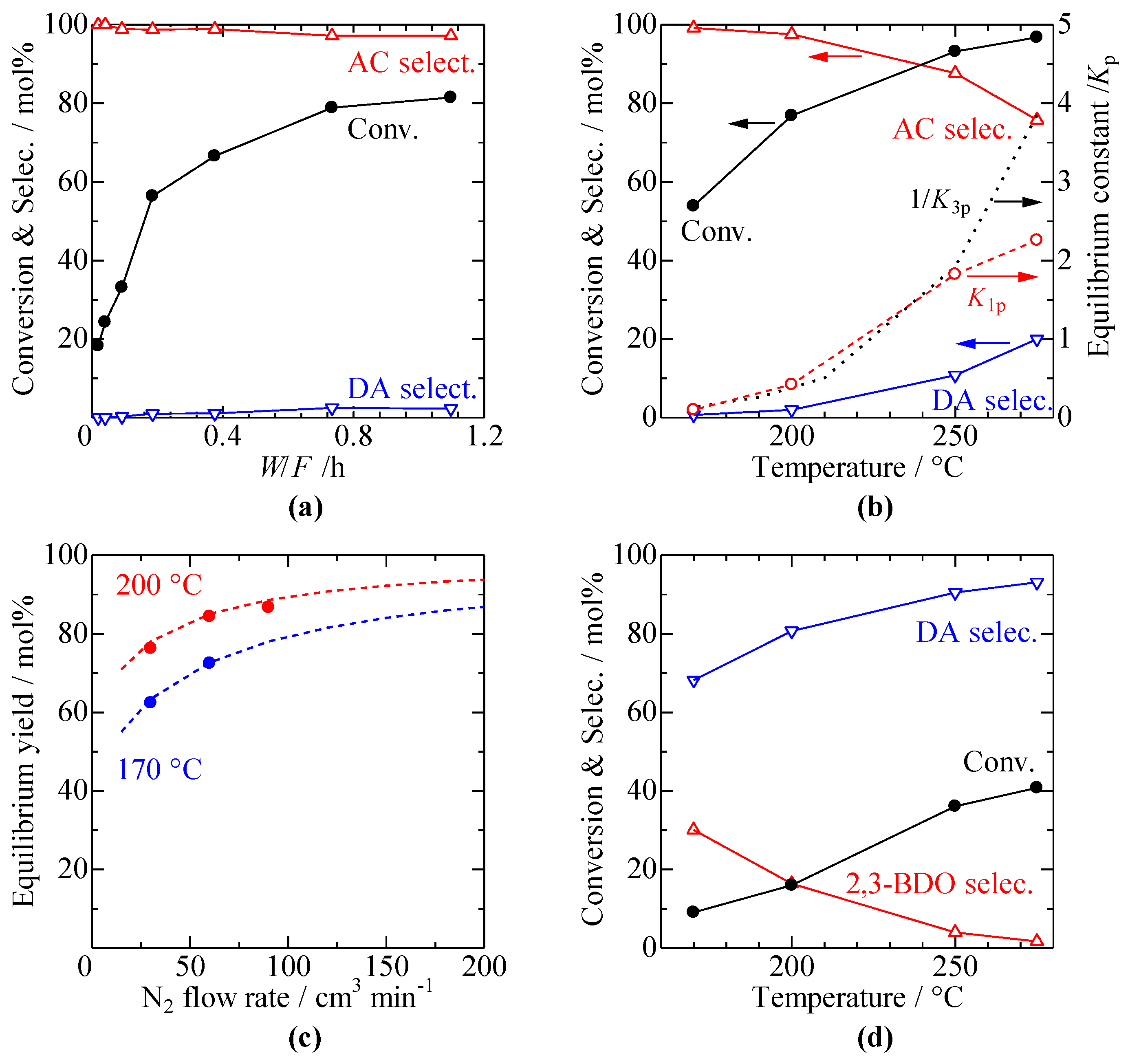
3.3. Effect of Reaction Conditions such as W/F, Temperature, and N2 Flow Rate
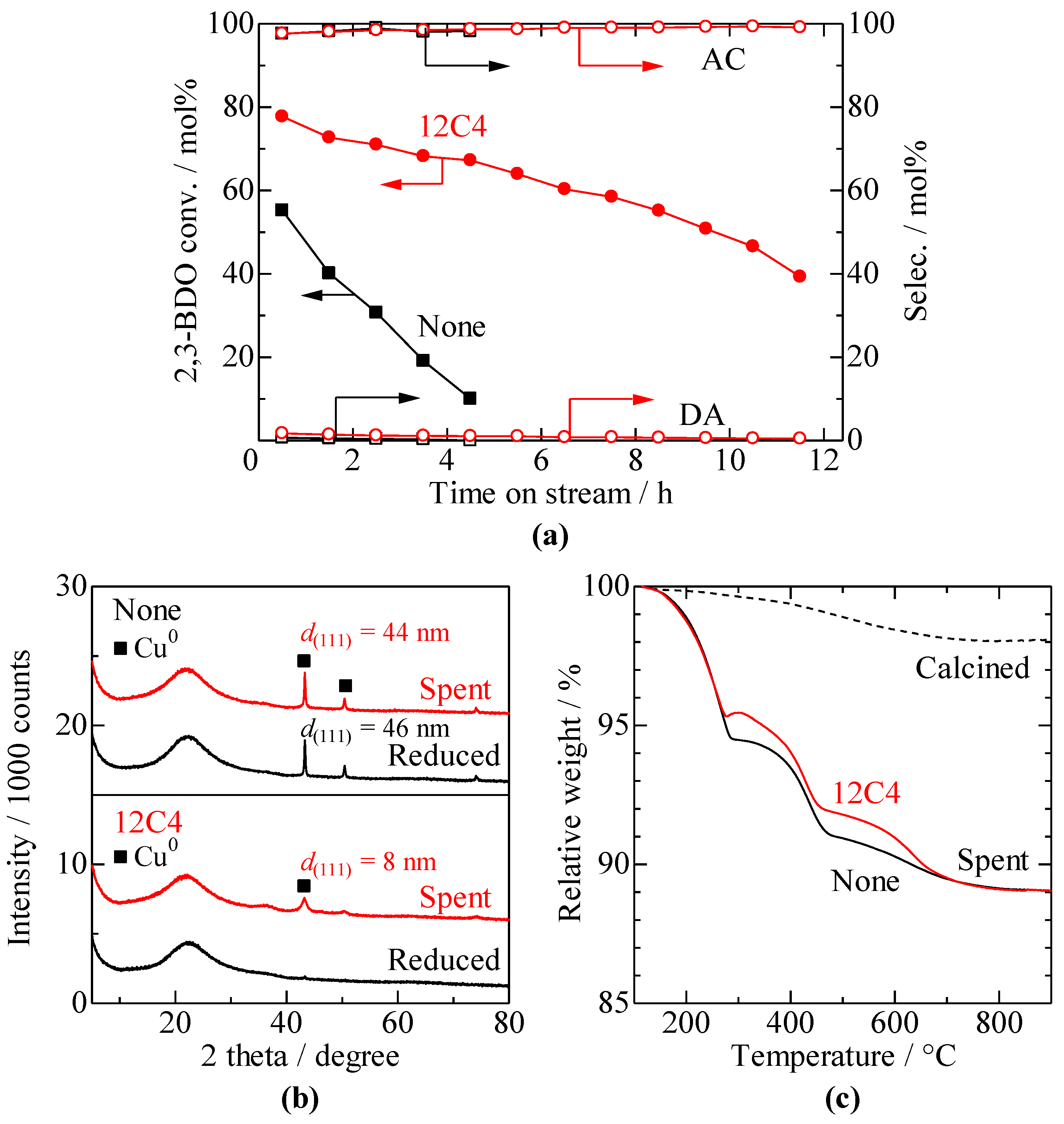
3.4. Stability of Cu/SiO2 Catalysts
3.5. Effect of Cu Content and SACu on the Formation Rate of Acetoin
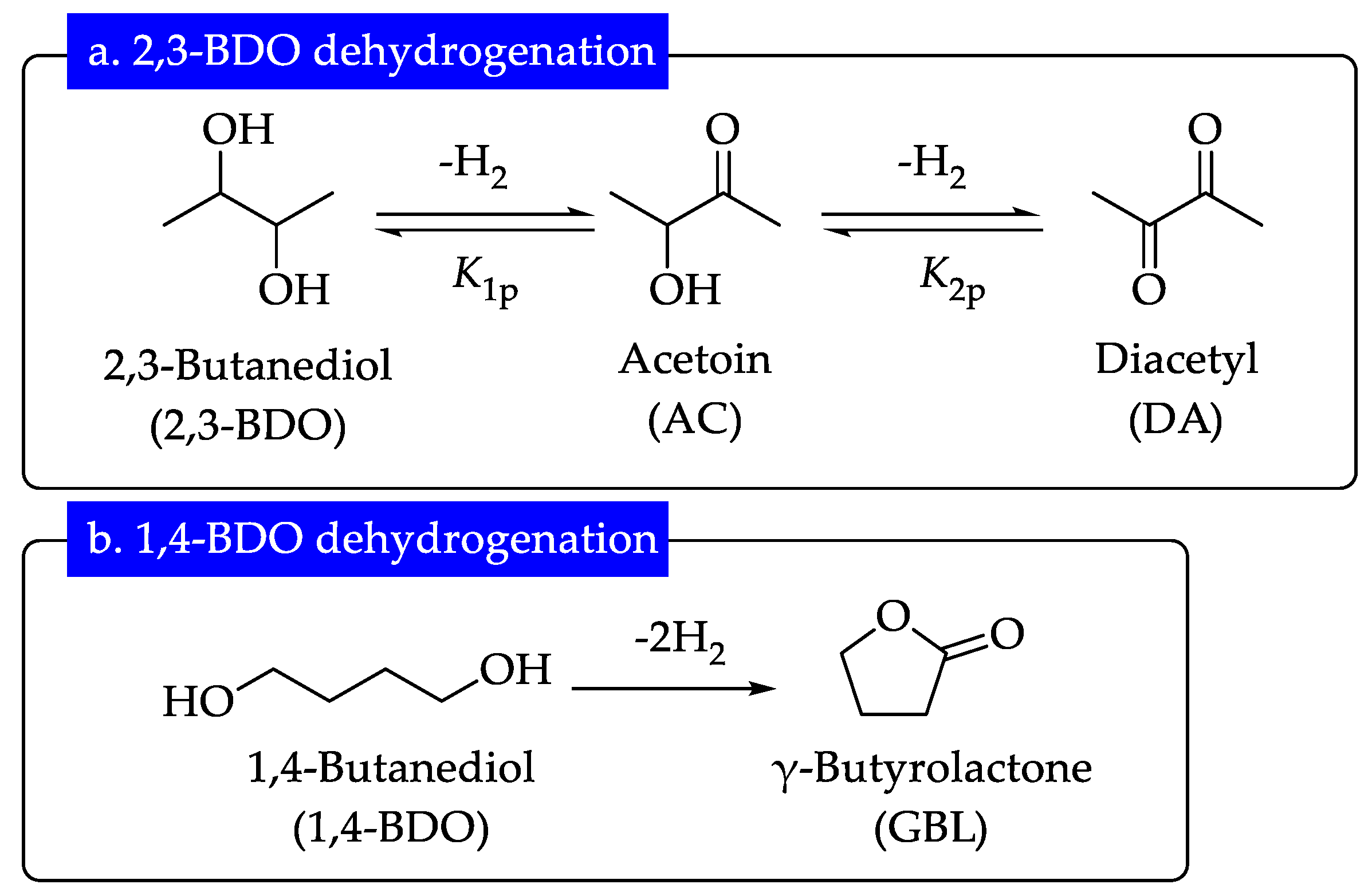
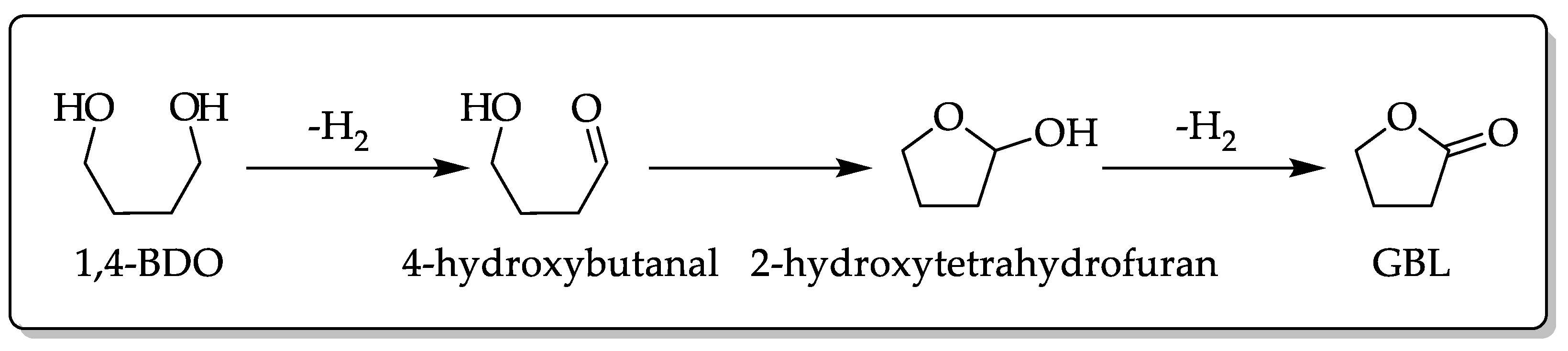
3.6. Dehydrogenation of 1,4-BDO over Cu/SiO2 Catalyst
3.7. Vapor-Phase Oxidant-Free Dehydrogenation over Cu Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Valizadeh, S.; Hakimian, H.; Farooq, A.; Jeon, B.H.; Chen, W.H.; Hoon Lee, S.; Jung, S.C.; Won Seo, M.; Park, Y.K. Valorization of biomass through gasification for green hydrogen generation: A comprehensive review. Bioresour. Technol. 2022, 365, 128143. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Dumeignil, F.; Capron, M.; Katryniok, B.; Wojcieszak, R.; Löfberg, A.; Girardon, J.S.; Desset, S.; Araque-Marin, M.; Jalowiecki-Duhamel, L.; Paul, S. Biomass-derived platform molecules upgrading through catalytic processes: Yielding chemicals and fuels. J. Jpn. Pet. Inst. 2015, 58, 257–273. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Fukuoka, A. Catalytic transformation of cellulose into platform chemicals. Appl. Catal. B Environ. 2014, 145, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Xu, S.; Zhou, C.; Xiao, Y.; Hu, C.; Tsang, D.C.W. Biomass-derived polyols valorization towards glycolic acid production with high atom-economy. Appl. Catal. B Environ. 2022, 317, 121785. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Chitta, D.; Karanjikar, M.; Vadlani, P.V. Appropriate lignocellulosic biomass processing strategies for efficient 2,3-butanediol production from biomass-derived sugars using Bacillus licheniformis DSM 8785. Food Bioprod. Process. 2017, 104, 147–158. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Vadlani, P.V. 2,3-Butanediol production using Klebsiella oxytoca ATCC 8724: Evaluation of biomass derived sugars and fed-batch fermentation process. Process Biochem. 2017, 58, 25–34. [Google Scholar] [CrossRef]
- Sathesh-Prabu, C.; Kim, D.; Lee, S.K. Metabolic engineering of Escherichia coli for 2,3-butanediol production from cellulosic biomass by using glucose-inducible gene expression system. Bioresour. Technol. 2020, 309, 123361. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Zheng, L. Achievements and perspectives in 1,4-butanediol production from engineered microorganisms. J. Agric. Food Chem. 2021, 69, 10480–10485. [Google Scholar] [CrossRef]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 444–445. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Zhu, G.; Song, W.; Yi, C. Cleaner production and downstream processing of bio-based 2,3-butanediol: A review. J. Clean. Prod. 2022, 343, 131033. [Google Scholar] [CrossRef]
- Burgard, A.; Burk, M.J.; Osterhout, R.; Van Dien, S.; Yim, H. Development of a commercial scale process for production of 1,4-butanediol from sugar. Curr. Opin. Biotechnol. 2016, 42, 118–125. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kashinathan, P.; Lee, J.M.; Lee, J.H.; Lee, U.; Hwang, J.S.; Hwang, Y.K.; Chang, J.S. Production of γ-butyrolactone from biomass-derived 1,4-butanediol over novel copper-silica nanocomposite. Green Chem. 2011, 13, 1672–1675. [Google Scholar] [CrossRef]
- Bhanushali, J.T.; Prasad, D.; Patil, K.N.; Ramesh Babu, G.V.; Kainthla, I.; Rama Rao, K.S.; Jadhav Arvind, H.; Nagaraja, B.M. The selectively regulated vapour phase dehydrogenation of 1,4-butanediol to γ-butyrolactone employing a copper-based ceria catalyst. New J. Chem. 2019, 43, 11968–11983. [Google Scholar] [CrossRef]
- Mitsudome, T.; Mikami, Y.; Funai, H.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Oxidant-free alcohol dehydrogenation using a reusable hydrotalcite-supported silver nanoparticle catalyst. Angew. Chem. 2008, 120, 144–147. [Google Scholar] [CrossRef]
- Gunasekaran, N. Aerobic oxidation catalysis with air or molecular oxygen and ionic liquids. Adv. Synth. Catal. 2015, 357, 1990–2010. [Google Scholar] [CrossRef]
- Pandey, P.; Dutta, I.; Bera, J.K. Acceptorless alcohol dehydrogenation: A mechanistic perspective. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2016, 86, 561–579. [Google Scholar] [CrossRef]
- Challa, P.; Enumula, S.S.; Paleti, G.; Dosali, M.; Burri, D.R.; Kamaraju, S.R.R. Support Effect in Cu-Based Catalysts for Vapor Phase Dehydrogenation of 1-Decanol to Decanal Using CO2 as a Soft Oxidant. Int. J. Energy Res. 2022, 46, 9166–9177. [Google Scholar] [CrossRef]
- Poreddy, R.; Engelbrekt, C.; Riisager, A. Copper oxide as efficient catalyst for oxidative dehydrogenation of alcohols with air. Catal. Sci. Technol. 2015, 5, 2467–2477. [Google Scholar] [CrossRef]
- Putro, W.S.; Kojima, T.; Hara, T.; Ichikuni, N.; Shimazu, S. Acceptorless dehydrogenation of alcohols using cu-fe catalysts prepared from Cu-Fe layered double hydroxides as precursors. Catal. Sci. Technol. 2018, 8, 3010–3014. [Google Scholar] [CrossRef]
- Kurniawan, E.; Hara, T.; Permana, Y.; Ichikuni, N.; Shimazu, S. In situ generation of catalytically active Cu0 species derived from Cu-Al layered double hydroxides for acceptorless alcohol dehydrogenation. Chem. Lett. 2022, 51, 334–337. [Google Scholar] [CrossRef]
- Bhuyan, B.; Paul, A.; Devi, M.; Dhar, S.S. A silver NP-dispersed water extract of fly ash as a green and efficient medium for oxidant-free dehydrogenation of benzyl alcohols. RSC Adv. 2018, 8, 1313–1319. [Google Scholar] [CrossRef]
- Zeng, G.; Sakaki, S.; Fujita, K.I.; Sano, H.; Yamaguchi, R. Efficient catalyst for acceptorless alcohol dehydrogenation: Interplay of theoretical and experimental studies. ACS Catal. 2014, 4, 1010–1020. [Google Scholar] [CrossRef]
- Wang, Q.; Chai, H.; Yu, Z. Acceptorless Dehydrogenation of N-Heterocycles and Secondary Alcohols by Ru(II)-NNC Complexes Bearing a Pyrazoyl-Indolyl-Pyridine Ligand. Organometallics 2018, 37, 584–591. [Google Scholar] [CrossRef]
- Paul, B.; Sharma, S.; Purkayastha, D.D.; Dhar, S.S.; Bal, R. Facile synthesis of size-controlled Ag supported on WO3 nanorods and their application as novel and active catalyst in oxidant-free dehydrogenation of benzyl alcohols. Catal. Commun. 2019, 132, 105804. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Q.; Chen, J.; Deng, W.; Wang, Y. Gold nanoparticles on hydrotalcites as efficient catalysts for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2010, 46, 1547–1549. [Google Scholar] [CrossRef]
- Kon, K.; Hakim Siddiki, S.M.A.; Shimizu, K.I. Size and support-dependent pt nanocluster catalysis for oxidant-free dehydrogenation of alcohols. J. Catal. 2013, 304, 63–71. [Google Scholar] [CrossRef]
- Nicolau, G.; Tarantino, G.; Hammond, C. Acceptorless alcohol dehydrogenation catalysed by Pd/C. ChemSusChem 2019, 12, 4953–4961. [Google Scholar] [CrossRef] [PubMed]
- Crivello, M.E.; Pérez, C.F.; Mendieta, S.N.; Casuscelli, S.G.; Eimer, G.A.; Elías, V.R.; Herrero, E.R. N-Octyl alcohol dehydrogenation over copper catalysts. Catal. Today 2008, 133–135, 787–792. [Google Scholar] [CrossRef]
- Mitsudome, T.; Mikami, Y.; Ebata, K.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Copper nanoparticles on hydrotalcite as a heterogeneous catalyst for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2008, 39, 4804–4806. [Google Scholar] [CrossRef]
- Wang, F.; Shi, R.; Liu, Z.Q.; Shang, P.J.; Pang, X.; Shen, S.; Feng, Z.; Li, C.; Shen, W. Highly efficient dehydrogenation of primary aliphatic alcohols catalyzed by Cu nanoparticles dispersed on rod-shaped La2O2CO3. ACS Catal. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Yuan, Q.; Pang, J.; Yu, W.; Zheng, M. Vapor-phase furfural decarbonylation over a high-performance catalyst of 1%Pt/SBA-15. Catalysts 2020, 10, 1304. [Google Scholar] [CrossRef]
- Yuan, E.; Ni, P.; Zhuang, W.; Jian, R.; Jian, P. Synergic catalysis by a CuO-like phase and Cu0 for anaerobic dehydrogenation of 2,3-butanediol. J. Catal. 2020, 382, 256–268. [Google Scholar] [CrossRef]
- Yuan, E.; Ni, P.; Xie, J.; Jian, P.; Hou, X. Highly efficient dehydrogenation of 2,3-butanediol induced by metal-support interface over Cu-SiO2 catalysts. ACS Sustain. Chem. Eng. 2020, 8, 15716–15731. [Google Scholar] [CrossRef]
- Al-Auda, Z.; Li, X.; Hohn, K.L. Dehydrogenation of 2,3-butanediol to acetoin using copper catalysts. Ind. Eng. Chem. Res. 2022, 61, 3530–3538. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, F.; Ma, H.; Chen, L.; Su, J.; Yuan, X.; Zhang, J. Dehydrogenation of 2,3-butanediol to 3-hydroxybutanone over CuZnAl catalysts: Effect of lithium cation as promoter. Top. Catal. 2020, 63, 866–874. [Google Scholar] [CrossRef]
- Ichikawa, N.; Sato, S.; Takahashi, R.; Sodesawa, T.; Inui, K. Dehydrogenative cyclization of 1,4-butanediol over copper-based catalyst. J. Mol. Catal. A Chem. 2004, 212, 197–203. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Modification of the supported Cu/SiO2 catalyst by alkaline earth metals in the selective conversion of 1,4-butanediol to γ-butyrolactone. Appl. Catal. A Gen. 2012, 443–444, 191–201. [Google Scholar] [CrossRef]
- Nagaiah, P.; Rao, M.V.; Thirupathaiah, K.; Venkateshwarlu, V.; Raju, B.D.; Rao, K.S.R. Selective vapour phase dehydrogenation of biomass-derived 1,4-butanediol to gamma butyrolactone over Cu/ZrO2 catalysts: Influence of La2O3 promotor. Res. Chem. Intermed. 2018, 44, 5817–5831. [Google Scholar] [CrossRef]
- Raju, M.A.; Gidyonu, P.; Nagaiah, P.; Rao, M.V.; Raju, B.D.; Rao, K.S.R. Mesoporous silica–supported copper catalysts for dehydrogenation of biomass-derived 1,4-butanediol to gamma butyrolactone in a continuous process at atmospheric pressure. Biomass Convers. Biorefinery 2019, 9, 719–726. [Google Scholar] [CrossRef]
- Bhanushali, J.T.; Prasad, D.; Patil, K.N.; Saidulu Reddy, K.; Kainthla, I.; Kamaraju, S.R.R.; Jadhav Arvind, H.; Nagaraja, B.M. Tailoring the catalytic activity of basic mesoporous Cu/CeO2 catalyst by Al2O3 for selective lactonization and dehydrogenation of 1,4-butanediol to γ-butyrolactone. Catal. Commun. 2020, 143, 106049. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Xiang, H.W.; Wu, G.S.; Bai, L.; Li, Y.W. A Novel route for synthesis of γ-butyrolactone through the coupling of hydrogenation and dehydrogenation. Chem. Commun. 2002, 3, 254–255. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Yang, J.; Zhu, Y.L.; Zhao, G.W. Synthesis of γ-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation over copper-chromite catalyst. React. Kinet. Catal. Lett. 2004, 82, 263–269. [Google Scholar] [CrossRef]
- Hari Prasad Reddy, K.; Rahul, R.; Sree Vardhan Reddy, S.; David Raju, B.; Rama Rao, K.S. Coupling of 1,4-butanediol dehydrogenation reaction with the hydrogenation of nitrobenzene over Cu/MgO catalysts. Catal. Commun. 2009, 10, 879–883. [Google Scholar] [CrossRef]
- Bhanushali, J.T.; Prasad, D.; Patil, K.N.; Reddy, K.S.; Rama Rao, K.S.; Jadhav, A.H.; Nagaraja, B.M. Simultaneous dehydrogenation of 1,4- butanediol to γ-butyrolactone and hydrogenation of benzaldehyde to benzyl alcohol mediated over competent CeO2–Al2O3 supported Cu as catalyst. Int. J. Hydrogen Energy 2020, 45, 12874–12888. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Fukuda, H.; Sekine, T.; Tsukuda, E. Synthesis of α-hydroxyketones from 1,2-diols over Cu-based catalyst. Catal. Commun. 2005, 6, 607–610. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Fukuda, H.; Inui, K. Dehydrogenation of 1,3-butanediol over Cu-based catalyst. J. Mol. Catal. A Chem. 2007, 272, 164–216. [Google Scholar] [CrossRef]
- Dong, F.; Ding, G.; Zheng, H.; Xiang, X.; Chen, L.; Zhu, Y.; Li, Y. Highly dispersed cu nanoparticles as an efficient catalyst for the synthesis of the biofuel 2-methylfuran. Catal. Sci. Technol. 2016, 6, 767–779. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Liu, Q.; Liu, Z.; Peng, Z. Preparation of highly active Cu/SiO2 catalysts for furfural to 2-methylfuran by ammonia evaporation method. Catalysts 2022, 12, 276. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Conrad Zhang, Z. Highly efficient Cu/SiO2 catalyst derived from ethanolamine modification for furfural hydrogenation. Appl. Catal. A Gen. 2020, 598, 117598. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/MgO catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Yoshida, R.; Sun, D.; Yamada, Y.; Sato, S. Stable Cu-Ni/SiO2 catalysts prepared by using citric acid-assisted impregnation for vapor-phase hydrogenation of levulinic acid. Mol. Catal. 2018, 454, 70–76. [Google Scholar] [CrossRef]
- Nakazono, K.; Hosaka, S.; Yamada, Y.; Sato, S. Highly active Ni/SiO2 catalyst prepared through citric acid-assisted impregnation for the hydrogenation of acetoin to 2,3-butanediol. Bull. Chem. Soc. Jpn. 2022, 95, 443–450. [Google Scholar] [CrossRef]
- Hosaka, S.; Kurniawan, E.; Yamada, Y.; Sato, S. Vapor-phase dehydrogenation of 1-decanol to decanal over cu/sio2 catalyst prepared by organic additives-assisted impregnation. Appl. Catal. A Gen. 2023, 653, 119079. [Google Scholar] [CrossRef]
- Shi, R.; Wang, F.; Mu, X.; Li, Y.; Huang, X.; Shen, W. MgO-supported Cu nanoparticles for efficient transfer dehydrogenation of primary aliphatic alcohols. Catal. Commun. 2009, 11, 306–309. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yuma, K.I.; Obata, Y. Distinction between surface and bulk oxidation of cu through N2O decomposition. J. Catal. 2000, 196, 195–199. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Song, T.; Chen, W.; Qi, Y.; Lu, J.; Wu, P.; Li, X. Efficient synthesis of methanol and ethylene glycol via the hydrogenation of CO2-derived ethylene carbonate on Cu/SiO2 catalysts with balanced Cu+-Cu0 sites. Catal. Sci. Technol. 2020, 10, 5149–5162. [Google Scholar] [CrossRef]
- Kurniawan, E.; Hara, T.; Permana, Y.; Kojima, T.; Ichikuni, N.; Shimazu, S. Creation of highly reducible CuO species by high-temperature calcination of a Cu-Al layered double hydroxide: Selective hydrogenation of furfural into furfuryl alcohol with formic acid. Bull. Chem. Soc. Jpn. 2022, 95, 121–128. [Google Scholar] [CrossRef]
- Yang, H.; Coolman, R.; Karanjkar, P.; Wang, H.; Dornath, P.; Chen, H.; Fan, W.; Conner, W.C.; Mountziaris, T.J.; Huber, G. The effects of contact time and coking on the catalytic fast pyrolysis of cellulose. Green Chem. 2017, 19, 286–297. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Delgado, R.; Chaves, S. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers: (IUPAC technical report). Pure Appl. Chem. 2003, 75, 71–102. [Google Scholar] [CrossRef]
- Christy, F.A.; Shrivastav, P.S. Conductometric studies on cation-crown ether complexes: A review. Crit. Rev. Anal. Chem. 2011, 41, 236–269. [Google Scholar] [CrossRef]
- Sun, D.; Misu, T.; Yamada, Y.; Sato, S. Advantages of using Cu/SiO2 catalyst for vapor-phase dehydrogenation of 1-decanol into decanal. Appl. Catal. A Gen. 2019, 582, 117109. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. The role of copper particle size in low pressure methanol synthesis via CO2 hydrogenation over Cu/ZnO catalysts. Catal. Sci. Technol. 2015, 5, 869–881. [Google Scholar] [CrossRef]
- Torresi, P.A.; Díez, V.K.; Luggren, P.J.; Di Cosimo, J.I. Conversion of diols by dehydrogenation and dehydration reactions on silica-supported copper catalysts. Appl. Catal. A Gen. 2013, 458, 119–129. [Google Scholar] [CrossRef]
- Sun, D.; Takahashi, Y.; Yamada, Y.; Sato, S. Efficient formation of angelica lactones in a vapor-phase conversion of levulinic acid. Appl. Catal. A Gen. 2016, 526, 62–69. [Google Scholar] [CrossRef]
- Dvořák, B.; Pašek, J. Determination of the specific copper surface area by chromatographic technique. J. Catal. 1970, 18, 108–114. [Google Scholar] [CrossRef]
- Evans, J.W.; Wainwright, M.S.; Bridgewater, A.J.; Young, D.J. On the determination of copper surface area by reaction with nitrous oxide. Appl. Catal. 1983, 7, 75–83. [Google Scholar] [CrossRef]
- Chinchen, G.C.; Hay, C.M.; Vandervel, H.D.; Waugh, K.C. The measurement of copper surface areas by reactive frontal chromatography. J. Catal. 1987, 103, 79–86. [Google Scholar] [CrossRef]
- Jensen, J.R.; Johannessen, T.; Livbjerg, H. An improved N2O-method for measuring Cu-dispersion. Appl. Catal. A Gen. 2004, 266, 117–122. [Google Scholar] [CrossRef]



| Entry | Organic Additive | D | SACu/ m2 g−1 | Conversion a/ mol% | Selectivity a/mol% | |
|---|---|---|---|---|---|---|
| AC | DA | |||||
| 1 | none | 0.248 | 2.76 | 19.0 | 99.3 | 0.0 |
| 2 | 12C4 | 0.632 | 7.06 | 56.4 | 98.8 | 1.0 |
| 3 | 15C5 | 0.613 | 6.84 | 43.0 | 99.2 | 0.5 |
| 4 | 18C6 | 0.521 | 5.81 | 38.9 | 99.3 | 0.4 |
| 5 | CA | 0.549 | 7.12 | 50.2 | 99.0 | 1.0 |
| Catalyst | Temp. /°C | W/F /h | TOS /h | Conv. /mol% | AC Selec. /mol% | AC Prod. /g g−1 h−1 | Ref. |
|---|---|---|---|---|---|---|---|
| 20.3Cu/SBA-15 | 280 | - | - | 9.9 * | >90 | - | [37] |
| 20Cu/SiO2-AE b | 280 | - | 1 | 76.0 | 94.5 | - | [38] |
| Li-CuZnAl | 260 | 10.0 | 100 | 72.4 | 95.6 | 0.07 | [40] |
| 15Cu-Al2O3 | 170 | 4.00 | - | 63.0 | 96.0 | 0.15 | [39] |
| 12C4-10Cu/SiO2 | 200 | 0.14 | 12 | 60.9 | 98.7 | 4.20 | This work |
| 12C4-2Cu/SiO2 | 200 | 0.71 | 5 | 76.9 | 97.6 | 1.04 | |
| 170 | 1.43 | 5 | 53.9 | 99.2 | 0.36 |
| Catalyst | SACu /m2 g–1 | Temp. /°C | W/F /h | TOS a /h | Conv. /mol% | Select. /mol% | GBL Prod. /g gcat−1 h−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cu/ZnO/ZrO2/Al2O3 b | 40.3 | 240 | 0.08 | 5 | 84 | 98 | 9.63 | [41] |
| 12Cu/SiO2 | 3.90 | 250 | 0.50 | 10 | 100 | 98 | 1.92 | [17] |
| 10Cu/La2O3/ZrO2 | 8.40 | 250 | 1.00 | 13 | 97 | 96 | 0.91 | [43] |
| 10Cu/SBA-15 | 8.68 | 250 | 1.00 | 17 | 100 | 98 | 0.95 | [44] |
| 10Cu/CeO2 | 5.60 | 240 | 0.50 | 1 | 93 | 98 | 1.78 | [18] |
| 10Cu/CeO2-Al2O3 | - | 240 | 0.50 | 1 | 100 | 99 | 1.94 | [45] |
| 12C4-10Cu/SiO2 | 26.1 | 260 | 0.27 | 5 | 98.8 | 98.5 | 3.44 | This work |
| 0.054 | 5 | 81.4 | 95.7 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, E.; Hosaka, S.; Kobata, M.; Yamada, Y.; Sato, S. Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation. Chemistry 2023, 5, 406-421. https://doi.org/10.3390/chemistry5010030
Kurniawan E, Hosaka S, Kobata M, Yamada Y, Sato S. Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation. Chemistry. 2023; 5(1):406-421. https://doi.org/10.3390/chemistry5010030
Chicago/Turabian StyleKurniawan, Enggah, Shuya Hosaka, Masayuki Kobata, Yasuhiro Yamada, and Satoshi Sato. 2023. "Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation" Chemistry 5, no. 1: 406-421. https://doi.org/10.3390/chemistry5010030
APA StyleKurniawan, E., Hosaka, S., Kobata, M., Yamada, Y., & Sato, S. (2023). Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation. Chemistry, 5(1), 406-421. https://doi.org/10.3390/chemistry5010030