Defective PrOx for Efficient Electrochemical NO2−-to-NH3 in a Wide Potential Range
Abstract
1. Introduction
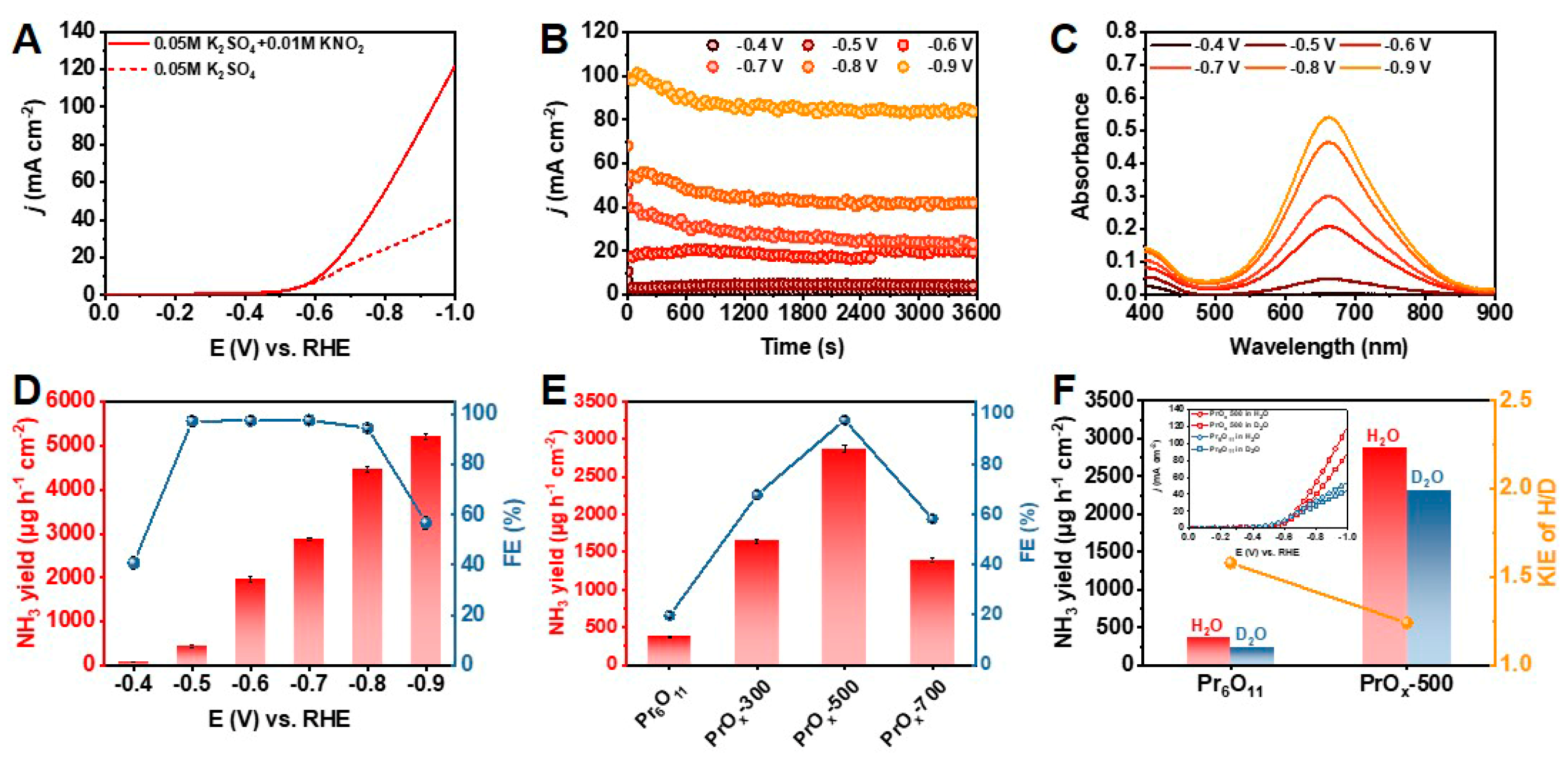
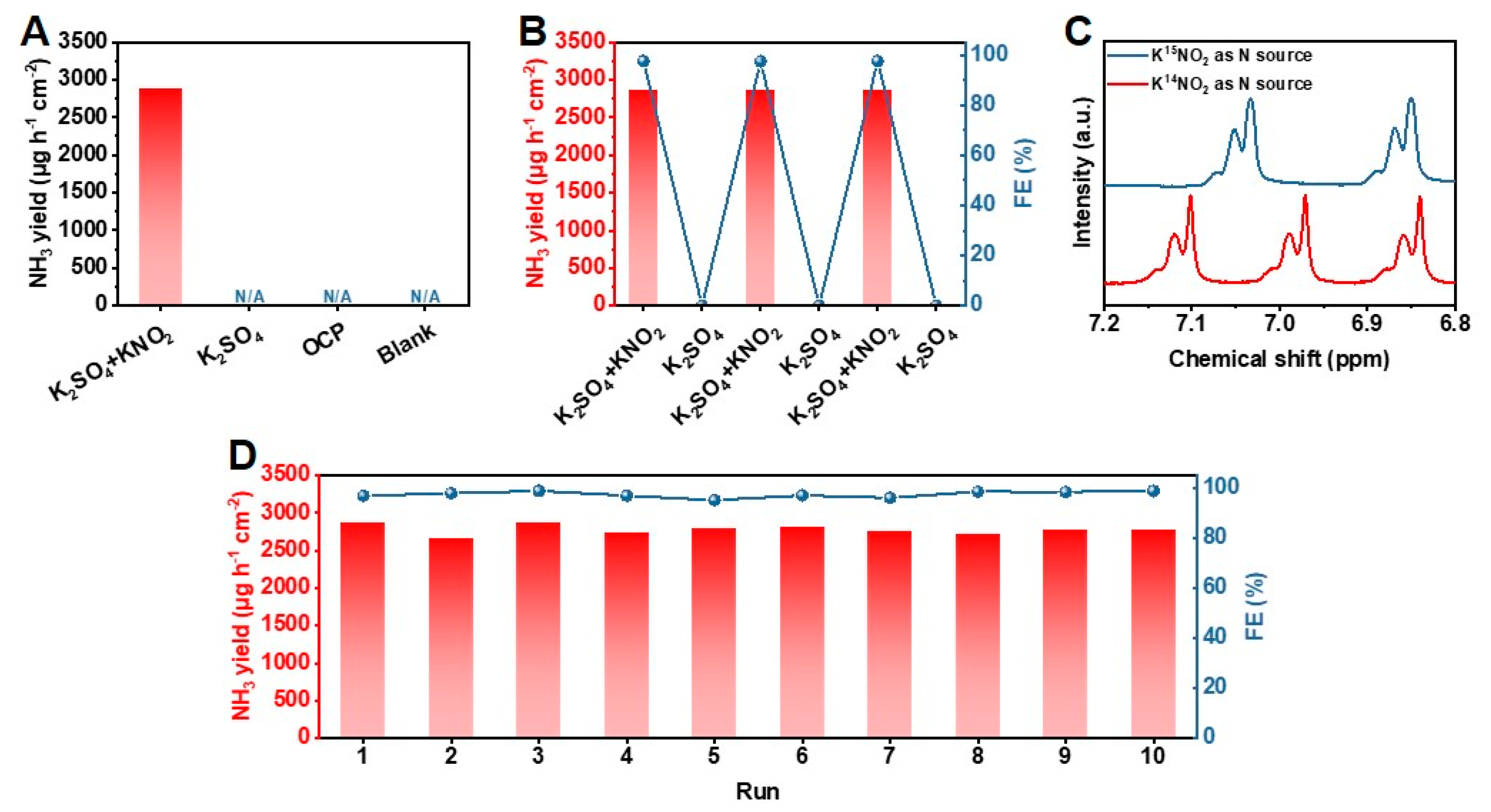
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Wang, B.; Ni, M.; Jiao, K. Green ammonia as a fuel. Sci. Bull. 2022, 67, 1530–1534. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Guan, Y.; Sun, Y.; Han, B. Green carbon science: Fundamental aspects. Natl. Sci. Rev. 2023, nwad046. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. Int. Ed. 2022, 61, e202112835. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef]
- Song, X.; Jia, S.; Xu, L.; Feng, J.; He, L.; Sun, X.; Han, B. Towards sustainable CO2 electrochemical transformation via coupling design strategy. Mater. Today Sustain. 2022, 19, 100179. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Wang, Y.; Guo, Y.; Xie, X.; Yu, Y.; Zhang, B. Recent advances in electrocatalytic nitrite reduction. Chem. Commun. 2022, 58, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, W.; Sun, X.; Han, B. Rational design of nanocatalysts for ambient ammonia electrosynthesis. Pure Appl. Chem. 2021, 93, 777–797. [Google Scholar] [CrossRef]
- Jia, S.; Wu, L.; Xu, L.; Sun, X.; Han, B. Multicomponent catalyst design for CO2/N2/NOx electroreduction. Ind. Chem. Mater. 2023, 1, 93–105. [Google Scholar] [CrossRef]
- Iriawan, H.; Andersen, S.Z.; Zhang, X.; Comer, B.M.; Barrio, J.; Chen, P.; Medford, A.J.; Stephens, I.E.L.; Chorkendorff, I.; Shao-Horn, Y. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Prim. 2021, 1, 56. [Google Scholar] [CrossRef]
- Stirling, A.; Papai, I.; Mink, J.; Salahub, D.R. Density functional study of nitrogen oxides. J. Chem. Phys. 1994, 100, 2910–2923. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Liu, S.; Feng, J.; Xu, L.; Tan, X.; Ma, X.; Sun, X. Promoting ambient ammonia electrosynthesis on modulated Cuδ+ catalysts by B-doping. J. Mater. Chem. A 2023, 11, 5520–5526. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Cui, X.; Zheng, Y.; Wang, X.; Xu, H.; Zhang, S.; Shao, T.; Davey, K.; Qiao, S.-Z. Efficient Nitrogen Fixation to Ammonia through Integration of Plasma Oxidation with Electrocatalytic Reduction. Angew. Chem. Int. Ed. 2021, 60, 14131–14137. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, C.; Wang, L.; Tan, X.; Wang, Z.; Wei, Q.; Zhang, Y.; Qiu, J. Microscopic-Level Insights into the Mechanism of Enhanced NH3 Synthesis in Plasma-Enabled Cascade N2 Oxidation–Electroreduction System. J. Am. Chem. Soc. 2022, 144, 10193–10200. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Xu, Y.; Du, Y.; Yan, C. Ultrathin 2D Rare-Earth Nanomaterials: Compositions, Syntheses, and Applications. Adv. Mater. 2020, 32, 1806461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saji, S.E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C.-H. Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications. Adv. Mater. 2021, 33, 2005988. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, Z.; Ouyang, L.; Fan, X.; Zhang, L.; Sun, S.; Liu, Q.; Alshehri, A.A.; Luo, Y.; Kong, Q.; et al. CeO2 nanoparticles with oxygen vacancies decorated N-doped carbon nanorods: A highly efficient catalyst for nitrate electroreduction to ammonia. Nano Res. 2022, 15, 8914–8921. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Guan, L.; Li, K.; Lin, Y. Oxygen Vacancy Regulation Strategy Promotes Electrocatalytic Nitrogen Fixation by Doping Bi into Ce-MOF-Derived CeO2 Nanorods. J. Phys. Chem. C 2020, 124, 18003–18009. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Z.; Han, M.; Zhang, S.; Zhao, C.; Chen, C.; Wang, G.; Zhang, H. Ambient Electrosynthesis of Ammonia on a Core–Shell-Structured Au@CeO2 Catalyst: Contribution of Oxygen Vacancies in CeO2. Chem. Eur. J. 2019, 25, 5904–5911. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, H.; Chen, C.; Zou, Y.; Wang, S. Defect Engineering Strategies for Nitrogen Reduction Reactions under Ambient Conditions. Small Methods 2019, 3, 1800331. [Google Scholar] [CrossRef]
- Yang, C.; Lu, Y.; Zhang, L.; Kong, Z.; Yang, T.; Tao, L.; Zou, Y.; Wang, S. Defect Engineering on CeO2-Based Catalysts for Heterogeneous Catalytic Applications. Small Struct. 2021, 2, 2100058. [Google Scholar] [CrossRef]
- Xu, L.; Feng, J.; Wu, L.; Song, X.; Tan, X.; Zhang, L.; Ma, X.; Jia, S.; Du, J.; Chen, A.; et al. Identifying the optimal oxidation state of Cu for electrocatalytic reduction of CO2 to C2+ products. Green Chem. 2023, 25, 1326–1331. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Liu, S.; Tan, X.; Wu, L.; Jia, S.; Xu, L.; Ma, X.; Song, X.; Ma, J.; et al. Atomically Dispersed Ni–Cu Catalysts for pH-Universal CO2 Electroreduction. Adv. Mater. 2023, 2209590. [Google Scholar] [CrossRef]
- Huang, P.X.; Wu, F.; Zhu, B.L.; Li, G.R.; Wang, Y.L.; Gao, X.P.; Zhu, H.Y.; Yan, T.Y.; Huang, W.P.; Zhang, S.M.; et al. Praseodymium Hydroxide and Oxide Nanorods and Au/Pr6O11 Nanorod Catalysts for CO Oxidation. J. Phys. Chem. B 2006, 110, 1614–1620. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Y.; Zhan, X.; Zhang, L.; Zhao, Y.; Zhu, X.; Wu, H.; Chen, H.; Shen, C.; Wang, L. Pr6O11: Temperature-Dependent Oxygen Vacancy Regulation and Catalytic Performance for Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2022, 14, 40975–40984. [Google Scholar] [CrossRef]
- Tankov, I.; Arishtirova, K.; Bueno, J.M.C.; Damyanova, S. Surface and structural features of Pt/PrO2–Al2O3 catalysts for dry methane reforming. App. Catal. A Gen. 2014, 474, 135–148. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Sanivarapu, S.R.; Lawrence, J.B.; Sreedhar, G. Role of Surface Oxygen Vacancies and Lanthanide Contraction Phenomenon of Ln(OH)3 (Ln = La, Pr, and Nd) in Sulfide-Mediated Photoelectrochemical Water Splitting. ACS Omega 2018, 3, 6267–6278. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen Vacancies Confined in Ultrathin Indium Oxide Porous Sheets for Promoted Visible-Light Water Splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, D.; Harle, G.; Qin, Q.; Guo, L.; Zheng, T.; Yin, X.; Du, J.; Zhao, Y. Ammonia removal in selective catalytic oxidation: Influence of catalyst structure on the nitrogen selectivity. J. Hazard. Mater. 2021, 416, 125782. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J.; et al. Highly Efficient CO2 Electroreduction to Methanol through Atomically Dispersed Sn Coupled with Defective CuO Catalysts. Angew. Chem. Int. Ed. 2021, 60, 21979–21987. [Google Scholar] [CrossRef]
- Yang, Y.; Agarwal, R.G.; Hutchison, P.; Rizo, R.; Soudackov, A.V.; Lu, X.; Herrero, E.; Feliu, J.M.; Hammes-Schiffer, S.; Mayer, J.M.; et al. Inverse kinetic isotope effects in the oxygen reduction reaction at platinum single crystals. Nat. Chem. 2022, 15, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xie, W.; Li, J.; Sun, Y.; Xu, P.; Tang, Y.; Li, Z.; Shao, M. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Sang, X.; Yang, B.; Li, Z.; Zheng, S.; Zhang, Q.; Yao, S.; Yang, X.; Lei, L.; et al. Atomically Dispersed Zinc(I) Active Sites to Accelerate Nitrogen Reduction Kinetics for Ammonia Electrosynthesis. Adv. Mater. 2022, 34, 2103548. [Google Scholar] [CrossRef]
- Jia, S.; Ma, X.; Sun, X.; Han, B. Electrochemical Transformation of CO2 to Value-Added Chemicals and Fuels. CCS Chem. 2022, 4, 3213–3229. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lin, Z.; Liang, Y.; Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 2021, 4, 725–730. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Tan, X.; Wu, L.; Feng, J.; Zhang, L.; Xu, L.; Wang, R.; Sun, X.; Han, B. Defective PrOx for Efficient Electrochemical NO2−-to-NH3 in a Wide Potential Range. Chemistry 2023, 5, 753-761. https://doi.org/10.3390/chemistry5020053
Jia S, Tan X, Wu L, Feng J, Zhang L, Xu L, Wang R, Sun X, Han B. Defective PrOx for Efficient Electrochemical NO2−-to-NH3 in a Wide Potential Range. Chemistry. 2023; 5(2):753-761. https://doi.org/10.3390/chemistry5020053
Chicago/Turabian StyleJia, Shunhan, Xingxing Tan, Limin Wu, Jiaqi Feng, Libing Zhang, Liang Xu, Ruhan Wang, Xiaofu Sun, and Buxing Han. 2023. "Defective PrOx for Efficient Electrochemical NO2−-to-NH3 in a Wide Potential Range" Chemistry 5, no. 2: 753-761. https://doi.org/10.3390/chemistry5020053
APA StyleJia, S., Tan, X., Wu, L., Feng, J., Zhang, L., Xu, L., Wang, R., Sun, X., & Han, B. (2023). Defective PrOx for Efficient Electrochemical NO2−-to-NH3 in a Wide Potential Range. Chemistry, 5(2), 753-761. https://doi.org/10.3390/chemistry5020053







