Abstract
We hereby report a simple and efficient method for the preparation of 4-methylcoumarins series, including Coumarin 120 (7-amino-4-methylcoumarin) from phenols (or naphthols) and ethyl acetoacetate in the presence of 3 mol% InCl3. Coumarins were obtained in good yields (52–92%) through Pechmann condensation, under a rapid and environmentally friendly protocol using a high-speed ball mill mixer at room temperature, with short reaction times, under solvent-free conditions.
1. Introduction
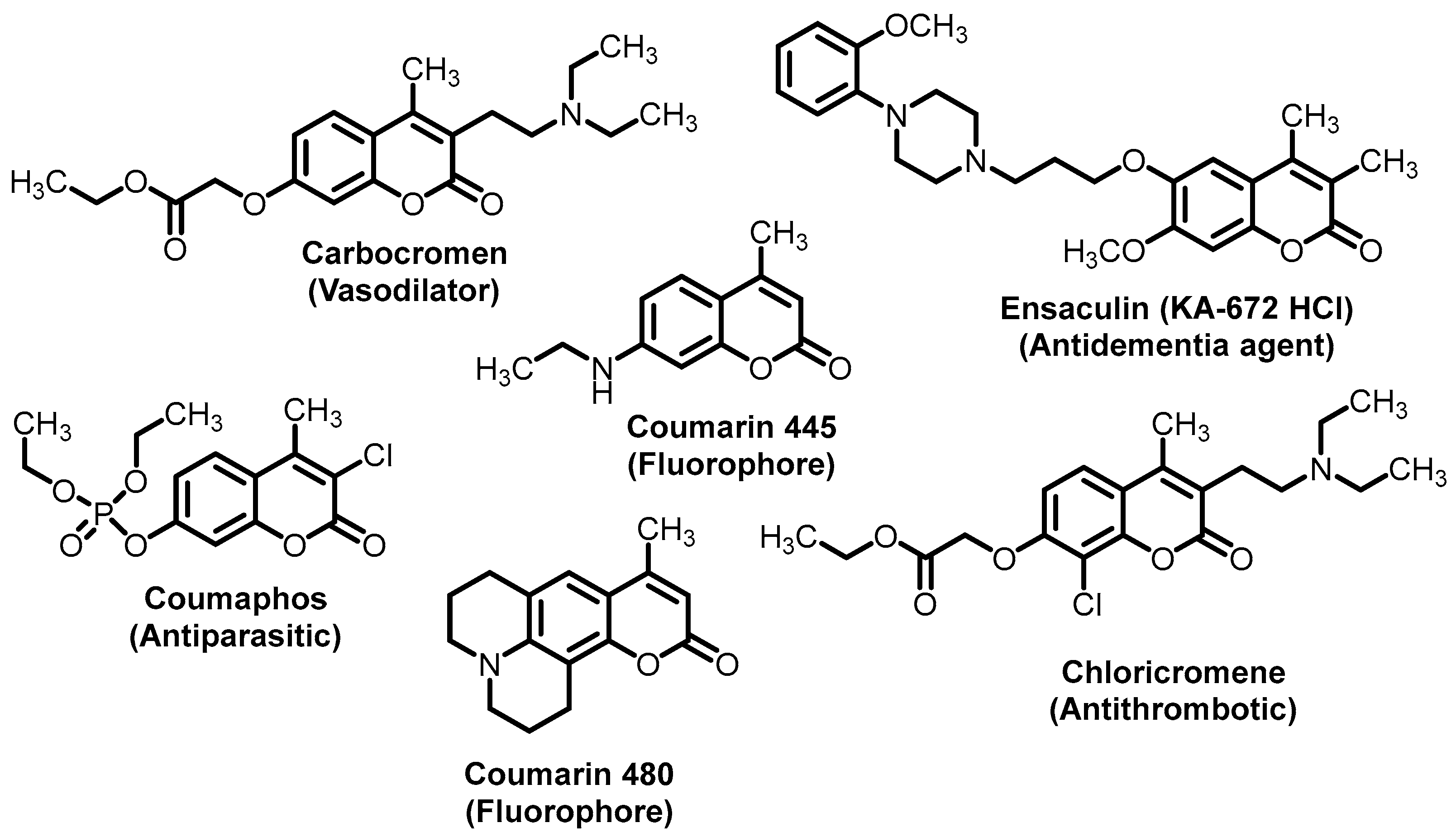
The coumarin ring is the main structural part for numerous secondary metabolites and privileged scaffolds in medicinal chemistry [1,2,3,4]. The chemical characteristics of the 2H-chromen-2-one core (aromatic ring, an oxygen atom, group C=O, π-conjugated lactone-(hetero)aryl motif) make simple functionalized coumarins attractive, suitable, and versatile models and./or precursors for diverse biological, pharmacological, photochemical/photophysical and agrochemical investigations and applications (Figure 1) [5,6,7,8,9,10,11,12]. Consequently, there is a growing demand and a comprehensible interest in their synthesis [13,14,15,16]. Historically, the coumarin ring could be constructed with many different named reactions such as the Perkin reaction, Claisen rearrangement, Knoevenagel condensation, Reformatsky reaction, Pechmann condensation, Wittig reaction, Kostanecki–Robinson reaction, and intramolecular alkyne hydroarylation reactions [17,18,19,20,21,22,23,24,25].
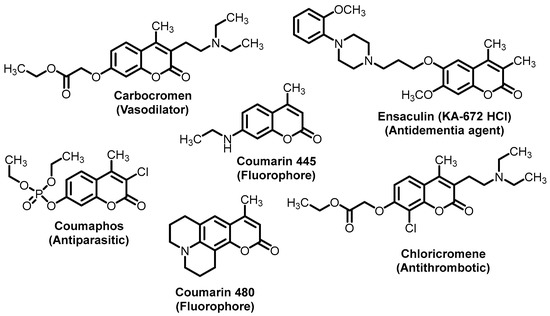
Figure 1.
Structures of selected 4-methylcoumarin derivatives as pharmaceuticals and fluorophores.
Among these methods, the Pechmann reaction is one of the simplest and most straightforward methods for the preparation of substituted coumarins using a wide spectrum of reaction parameters. The versatility of the Pechmann reaction allows the use of commercially available starting materials, substituted phenols, and β-ketoesters/acids in the presence of diverse acid catalysts such as conc. H2SO4, P2O5, trifluoroacetic acid, Nafion resin, TiCl4, ZrCl4, SnCl2·2H2O, HClO4/SiO2, LiBr, I2, phosphotungstic acid, zirconium phosphotungstate, Zn/I2, polyvinyl sulfonic acid, pentafluorophenylammonium triflate, (magnetic) nanocatalysts and ionic liquids, deep eutectic solvents, and many others [26,27,28,29,30,31,32,33,34,35].
These methods usually require harsh conditions (e.g., stoichiometric amounts of strong acids or bases, no reusability of catalyst, production of large acidic wastes, elevated temperature, and prolonged reaction time) with poor regioselectivity, limiting the substrate scope, or protocols that lead into a difficult purification process. Therefore, each developed protocol for coumarins preparation comes with these disadvantages and advantages. However, according to the significance of green and sustainable protocols, there is a perceptible need to develop new, eco-friendly, and simple procedures through a keen investigation of the existing strategies.
In this context, Pechmann condensation still attracts a lot of attention from synthetic chemists. Indeed, recently, various interesting protocols for coumarin ring construction based on green chemistry principles have been reported [36,37,38,39,40,41,42,43,44]. These procedures do not only focus on the effectiveness of a catalyst or its reusability but also its safety for both mankind and the environment using several alternative physical methods. Methodologies for the Pechmann reaction include microwave, sonication, photocatalysis, and mechanochemical approaches. The last one, i.e., mechanochemical activation, is the main current approach in free-solvent synthetic chemistry and relies on the application of mechanical energy produced from grinding or milling processes to achieve chemical transformations [45,46,47,48,49,50,51].
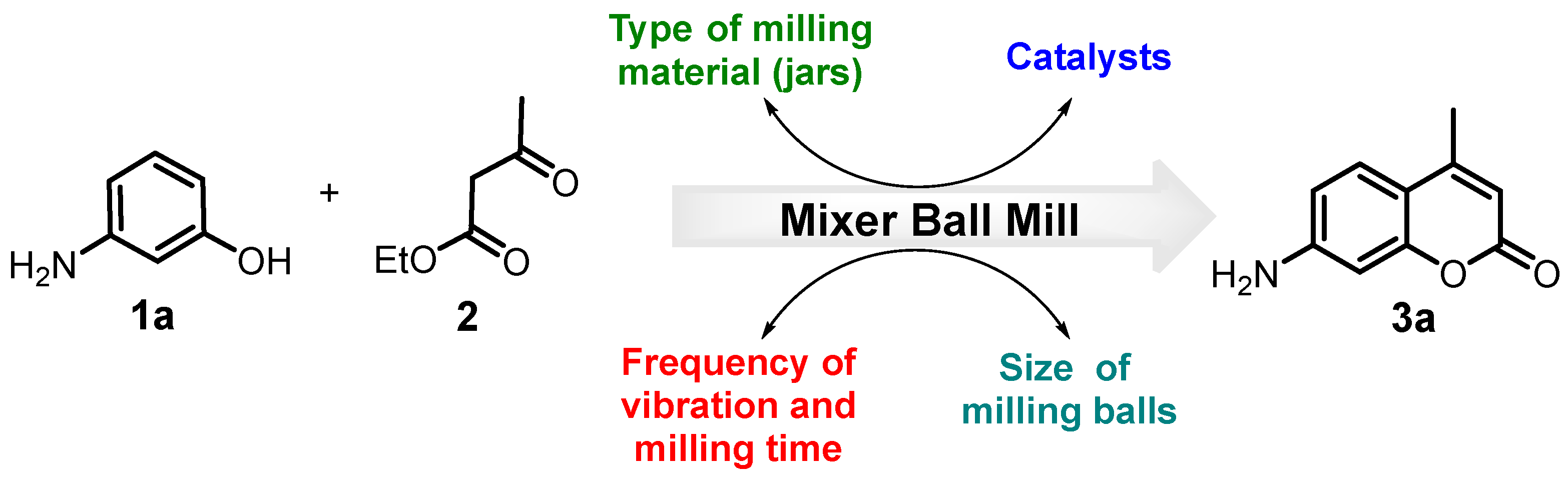
Despite the significance of the Pechmann condensation for the preparation of bioactive substituted 4-methylcoumarins (4-methyl-2H-chromen-2-ones) and the valuableness of a mechanochemical approach for chemical transformations as a greener way, there are very little synthetic methods that apply to the mechanochemical methodology to promote this reaction, in which only grinding (“in a mortar by a pestle” by a hand) and ball-milling (BM “in mill apparatus” by grinding balls) techniques were used (Figure 2). Using “porcelain mortar-pestle” as the manual grinding tool, Indian researchers described for the first time the synthesis of coumarins via Pechmann condensation under solvent-free conditions by applying a grinding technique. They reported higher yields of the obtained 4-methylcoumarin derivatives; however, two equivalents of catalysts (p-TsOH and P2O5) to the starting phenols were used [41,42,43]. It is known that manual grinding, carried out by a person, is a labor-intensive and non-automatic process, which often raises some concerns, including the kinetic parameters of the reaction and the reproducibility [51]. Although the hand-grinding technique is an easy and inexpensive approach, mechanical milling using a mixer mill or planetary mill by grinding balls (ball-milling) is becoming a more promising green tool for the synthesis of organic molecules, including heterocyclic compounds and condensation reactions [46]. Recently, a new protocol for the synthesis of simple coumarins under ball milling at an ambient temperature in the presence of methanesulfonic acid (MsOH) was developed by Sharapov et al. [44]. To obtain 4-methylcoumarin molecules with good yields, a Retsch PM 100 swing mill including 10 mL stainless steel ball mill jars and ten stainless steel balls 5 mm in diameter were used, but Pechmann condensations were carried out at 2 h, which is quite slow for this type of technique. Therefore, this topic is still an interesting research open window to investigate in the organic synthesis field.
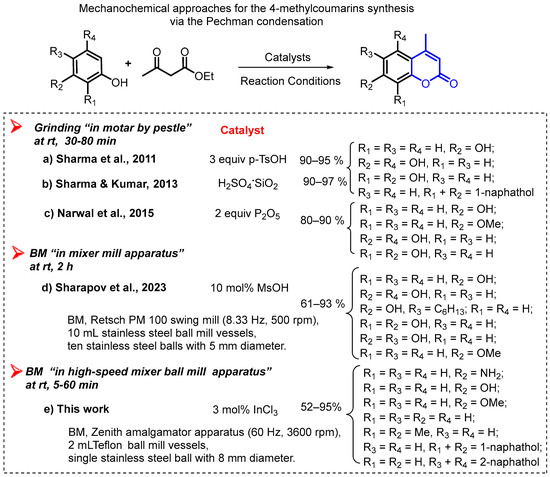
Figure 2.
Mechanical approaches for the 4-methylcoumarins synthesis via the Pechmann condensation [41,42,43,44].
Figure 2.
Mechanical approaches for the 4-methylcoumarins synthesis via the Pechmann condensation [41,42,43,44].
Considering the above-stated facts and our constant efforts to study the synthesis of new bioactive small heterocyclic molecules under sustainable reaction conditions [52,53,54,55], we developed a simple green procedure for the synthesis of 4-methylcoumarin derivatives, including 7-amino-4-methylcoumarin This molecule has been considered an important molecule and building block in biophysical research as a common fluorescent probe [56,57].
Thus, in this work, we described a practical method for the synthesis of the title compounds via an InCl3-catalyzed Pechmann condensation. As an important novelty of our research, we carried out coumarins synthesis under solvent-free conditions, using a high-speed mixer ball mill process with a milling frequency of 60 Hz, Teflon ball mill jars, and single stainless-steel balls 8 mm in diameter.
2. Materials and Methods
2.1. Materials and Instruments
The reagents and solvents used in the synthesis of intermediate and final compounds were of purity grade for synthesis. All reagents were purchased from Merck, J.T. Baker, Sigma, and Aldrich Chemical Co. and used without further purification. Reactions monitoring the purity of the final product were followed by thin layer chromatography (TLC) on Silufol UV254 plates of a 0.25 mm thickness. TLC plates were revealed in a UV light chamber of 254 nm or in an ethanolic solution of phosphomolybdic-sulfuric acids. Uncorrected melting points were measured in a Fisher-Johns melting point apparatus.
Reactions were carried out in a mixer mill apparatus (amalgamator Zenith, Z-1A) at a frequency of 60 Hz at room temperature using grinding jars of different materials (Nylon, Teflon, or stainless steel of 1.5–2 mL volume) and a single stainless-steel ball with different sizes: d = 5 mm (0.6401 g) and 8 mm (2.1031 g) (See, ESI: Table S1). All vessels were applied for 1–3 mmol runs.
The acquisition of nuclear magnetic resonance spectra 1H and 13C was performed in a Bruker Avance–400 spectrometer (400 MHz for 1H and 101 MHz for 13C). Chemical shifts were reported in ppm with solvent resonance as the internal standard (DMSO-d6: δ 2.50 ppm). Coupling constants (J) are given in Hz; a multiplicity of signals was expressed by the following abbreviations: (s) singlet, (d) doublet, (dd) doublet of doublets, and (m) multiplet.
Infrared spectra were recorded on an FTIR Bruker Tensor 27 spectrophotometer coupled to a Bruker platinum with an attenuated reflectance (ATR) cell at 31 scans and a 2 cm−1 resolution. Elemental analyses were performed on a Thermo Scientific CHNS-O analyzer (Model. Flash 2000) and were within ±0.4 of theoretical values.
2.2. General Procedure for the Synthesis of 4-Methylcoumarin Derivatives 3a–g
In a cylindrical Teflon vessel (8.1573 g, 2 mL), the respective phenol 1a–g (2.85 mmol) ethyl acetoacetate 2 (2.84 mmol) and InCl3 (3 mol%) were added. Subsequently, a stainless-steel ball of 8 mm in diameter with a weight of 2.1031 g was incorporated into the reaction mixture. Once the reactor was carefully sealed, it was subjected to a vibration of 60 Hz at a determined time in a mixer mill apparatus (amalgamator Zenith, Z-1A). This reaction was monitored by TLC, and once completed, the crude was poured onto crushed ice, obtaining the solids corresponding to products 3a–g, which were subsequently recrystallized in ethanol. All yields refer to the isolated products (Table 1). A total of 2 mL Teflon ball mill vessels were applied for 1–3 mmol runs.
2.2.1. The Characterization of 7-Amino-4-methyl-2H-chromen-2-one (7-Amino-4-methylcoumarin, 3a)
7-Amino-4-methyl-2H-chromen-2-one (7-Amino-4-methylcoumarin, 3a) was prepared according to the general procedure from m-aminophenol (0.31 g, 2.84 mmol), ethyl acetoacetate (0.37 g, 2.84 mmol), and InCl3 (19 mg, 3 mol%). 0.46 g (2.63 mmol) of a yellow solid was obtained in 10 min, with a yield of 92%. Rf = 0.50 (1:1, petroleum ether: ethyl acetate); Mp = 220–224 °C (ethanol). IR (KBr, νmax/cm−1): 3337–3245, 3072, 2984, 1680, 1542. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.29 (3H, s, 4-CH3), 5.90 (1H, s, 3-H), 6.09 (2H, s, NH2), 6.40 (1H, d, J = 1.9 Hz, 8-H), 6.56 (1H, dd, J = 8.5, 2.0 Hz, 6-H), 7.40 (1H, d, J = 8.6 Hz, 5-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 18.4, 98.94, 107.9, 109.3, 111.6, 126.6, 153.5, 154.2, 155.9, 161.2. MS (EI, 70 eV) m/z (%): 175 (M+, 100), 147 (80), 119 (26), 91 (6). Anal. calcd. for C10H9NO2 (175.19): C, 68.56; H, 5.18; N, 8.00%. Found: C, 68.35; H, 5.26; N, 8.15%.
2.2.2. The Characterization of 7-Hydroxy-4-methyl-2H-chromen-2-one
7-Hydroxy-4-methyl-2H-chromen-2-one (7-Hydroxy-4-methylcoumarin, 3b) was prepared according to the general procedure from m-hydroxyphenol (resorcinol) (0.31 g, 2.82 mmol), ethyl acetoacetate (0.37 g, 2.84 mmol), and InCl3 (19 mg, 3 mol%). 0.48 g (2.72 mmol) of an ivory white solid was obtained in 5 min, with a yield of 95%. Rf = 0.33 (2:1, petroleum ether: ethyl acetate); Mp = 184–186 °C (ethanol). IR (KBr, νmax/cm−1): 3493, 3117, 2994, 1669, 1517. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.35 (3H, d, J = 1.1 Hz, 4-CH3), 6.11 (1H, d, J = 1.1 Hz, 3-H), 6.69 (1H, d, J = 2.3 Hz, 8-H), 6.79 (1H, dd, J = 8.7, 2.3 Hz, 6-H), 7.58 (1H, d, J = 8.7 Hz, 5-H), 10.56 (1H, s, OH). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 18.1, 102.2, 110.3, 112.0, 112.9, 126.6, 153.6, 154.9, 160.3, 161.2. MS (EI, 70 eV) m/z (%): 176. (M+, 70), 148 (100), 147 (78), 91 (26). Anal. calcd. for C10H8O3 (176.17): C, 68.18; H, 4.58%. Found: C, 68.24; H, 4.66%.
2.2.3. The Characterization of 7-Methoxy-4-methyl-2H-chromen-2-one
7-Methoxy-4-methyl-2H-chromen-2-one (7-Methoxy-4-methylcoumarin or 4-methylumbelliferone, 3c) was prepared according to the general procedure from m-methoxyphenyl (0.10 g, 0.81 mmol), ethyl acetoacetate (0.10 g, 0.77 mmol), and InCl3 (6 mg, 3 mol%). 0.12 g (0.63 mmol) of a white solid was obtained in 20 min, with a yield of 80%. Rf = 0.50 (3:1, petroleum ether: ethyl acetate); Mp = 170–172 °C (ethanol). IR (KBr, νmax/cm−1): 2835, 3060, 2919, 1701, 1570. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.42 (3H, s, 4-CH3), 3.87 (3H, s, CH3O), 6.29 (3H, s, 3-H), 7.02 (1H, d, J = 2.6 Hz, 8-H), 7.11 (1H, dd, J = 9.0, 2.6 Hz, 6-H), 7.26 (1H, d, J = 9.0 Hz, 5-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 18.8, 55.9, 107.7, 115.6, 118.1, 118.8, 120.5, 147.98, 152.1, 156.1, 161.1. MS (EI, 70 eV) m/z (%): 190 (M+, 100), 162 (63), 147 (73), 91 (12). Anal. calcd. for C11H10O3 (190.20): C, 69.46; H, 5.30%. Found: C, 69.35; H, 5.61%.
2.2.4. The Characterization of 4-Methyl-2H-chromen-2-one
4-Methyl-2H-chromen-2-one (4-Methylcoumarin, 3d) was prepared according to the general procedure from phenol (0.29 g, 3.08 mmol), ethyl acetoacetate (0.5 g, 3.84 mmol), and InCl3 (20 mg, 3 mol%). 0.26 g (2.76 mmol) of a white solid was obtained in 60 min, with a yield of 52%. Rf = 0.33 (5:1, petroleum ether: ethyl acetate); Mp = 77–79 °C (ethanol). IR (KBr, νmax/cm−1): 3034, 2952, 1668, 1503. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.75 (3H s, 4-CH3), 5.71 (1H, s, 3-H), 6.75 (1H, dd, J = 4.8, 1.8 Hz, 8-H), 6.77 (2H, m, 6,7-H), 6.79 (1H, dd, J = 4.8, 1.8 Hz). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 21.7, 115.4, 116.5, 117.0, 125.6, 127.5, 143.1, 143.3 (2C), 154.2, 160.9. MS (EI, 70 eV) m/z (%): 160 (M+, 100), 132 (81), 31 (91). Anal. calcd. for C10H8O2 (160.17): C, 74.99; H, 5.03%. Found: C, 75.17; H, 5.14%.
2.2.5. The Characterization of 4,7,8-Trimethyl-2H-chromen-2-one
4,7,8-Trimethyl-2H-chromen-2-one (4,7,8-Trimethylcoumarin, 3e) was prepared according to the general procedure from 2,3-dimethylphenol (0.32 g, 2.66 mmol), ethyl acetoacetate (0.34 g, 2.62 mmol), and InCl3 (17 mg, 3 mol%). 0.28 g (1.49 mmol) of a purple solid was obtained in 45 min, with a yield of 56%. Rf = 0.40 (5:1, petroleum ether: ethyl acetate); Mp = 140–144 °C (ethanol). IR (KBr, νmax/cm−1): 2923, 3052, 2979, 1691, 1600. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.24 (3H, s, 4-CH3,), 2.33 (3H, s, 8-CH3), 2.38 (3H, s, 7-CH3), 6.28 (1H, s, 3-H), 7.16 (1H, d, J = 8.1 Hz, 6-H), 7.46 (1H, d, J = 8.0 Hz, 5-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 11.6, 18.6, 20.3, 113.3, 117.8, 122.5, 123.9, 125.9, 141.7, 151.4, 154.0, 160.5. MS (EI, 70 eV) m/z (%): 188 (M+, 100), 160 (63), 145 (50). Anal. calcd. for C12H12O2 (188.23): C, 76.57; H, 6.43%. Found: C, 76.34; H, 6.58%.
2.2.6. The Characterization of 4-Methyl-2H-benzo[h]chromen-2-one
4-Methyl-2H-benzo[h]chromen-2-one (3f) was prepared according to the general procedure from α-naphthol (0.34 g, 2.36 mmol), ethyl acetoacetate (0.31 g, 2.38 mmol), and InCl3 (16 mg, 3 mol%). 0.44 g (2.09 mmol) of an ocher solid was obtained in 12 min, with a yield of 88%. Rf = 0.36 (5:1, petroleum ether: ethyl acetate); Mp = 154–156 °C (ethanol). IR (KBr, νmax/cm−1): 3064, 3064, 2985, 1707, 1561. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.47 (1H, d, J = 1.2 Hz, 4-CH3), 6.32 (1H, d, J = 1.2 Hz, 3-H), 7.53 (1H, d, J = 8.7 Hz, 6-H), 7.59 (2H, m, 8,9-H), 7.64 (1H d, J = 8.7 Hz, 5-H), 7.83 (1H, m, 7-H), 8.50 (1H, m, 10-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 19.3, 114.3, 115.2, 120.4, 122.7, 123.2, 124.2, 127.2, 127.7, 128.6, 134.8, 150.6, 153.5, 160.9. MS (EI, 70 eV) m/z (%): 210 (M+, 90), 182 (100), 181 (48). Anal. calcd. for C14H10O2 (210.23): C, 79.98; H, 4.79%. Found: C, 79.83; H, 4.98%.
2.2.7. The Characterization of 1-Methyl-3H-benzo[f]chromen-3-one
1-Methyl-3H-benzo[f]chromen-3-one (3g) was prepared according to the general procedure from β-naphthol (0.34 g, 2.36 mmol), ethyl acetoacetate (0.31 g, 2.38 mmol), and InCl3 (16 mg, 3 mol%). 0.34 g (1.62 mmol) of an ocher solid was obtained in 15 min, with a yield of 68%. Rf = 0.43 (5:1, petroleum ether: ethyl acetate); Mp = 176–178 °C (ethanol). IR (KBr, νmax/cm−1): 3054, 3054, 2922, 1705, 1599. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.91 (3H, s, 4-CH3), 6.50 (1H, s, 3-H), 7.53 (1H, d, J = 9.0 Hz, 8-H), 7.60 (1H, m, 6-H), 7.70 (1H, m, 7-H), 8.06 (1H, d, J = 9.0 Hz, 9-H), 8.18 (1H, d, J = 9.0 Hz, 10-H), 8.67 (1H, d, J = 8.8 Hz, 5-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 20.4, 111.2, 115.8, 117.4, 118.6, 125.8, 126.1, 128.4, 129.7, 131.1, 134.1, 154.1, 155.3, 164.2. MS (EI, 70 eV) m/z (%): 210 (M+, 100), 182 (75). Anal. calcd. for C14H10O2 (210.23): C, 79.98; H, 4.79%. Found: C, 80.15; H, 4.66%.
3. Results and Discussion
The wide application of the Pechmann reaction arises from exceptionally simple procedures and reaction conditions using easily available simple reactants, substituted phenols and β-ketoesters (mainly ethyl acetoacetate) in the presence of acid catalysts [25]. Since, in our research, we needed 7-amino-4-methylcoumarin (coumarin 120), a rather expensive reagent, we resorted to the Pechmann condensation. Its preparation according to modified protocols based on this reaction is available in the chemical literature [58,59,60,61,62]. However, although we were able to reproduce a protocol synthesizing the desired coumarin via a three-step method proposed by Pozdnev [62,63] (See ESI, Scheme S1), we were not satisfied with the obtained results. Therefore, we decided to study the reaction of 3-aminophenol 1 and ethyl acetoacetate 2 to obtain straightforward 7-amino-4-methylcoumarin 3 (Scheme 1). Then, looking for the best green reaction conditions under solvent-free methodologies, we used a high-speed mixer ball mill (HSMBM) process at room temperature.
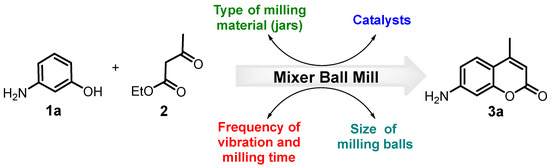
Scheme 1.
The model reaction of 1a and 2 to obtain 7-amino-4-methylcoumarin 3a and evaluated reaction parameters of the HSMBM process.
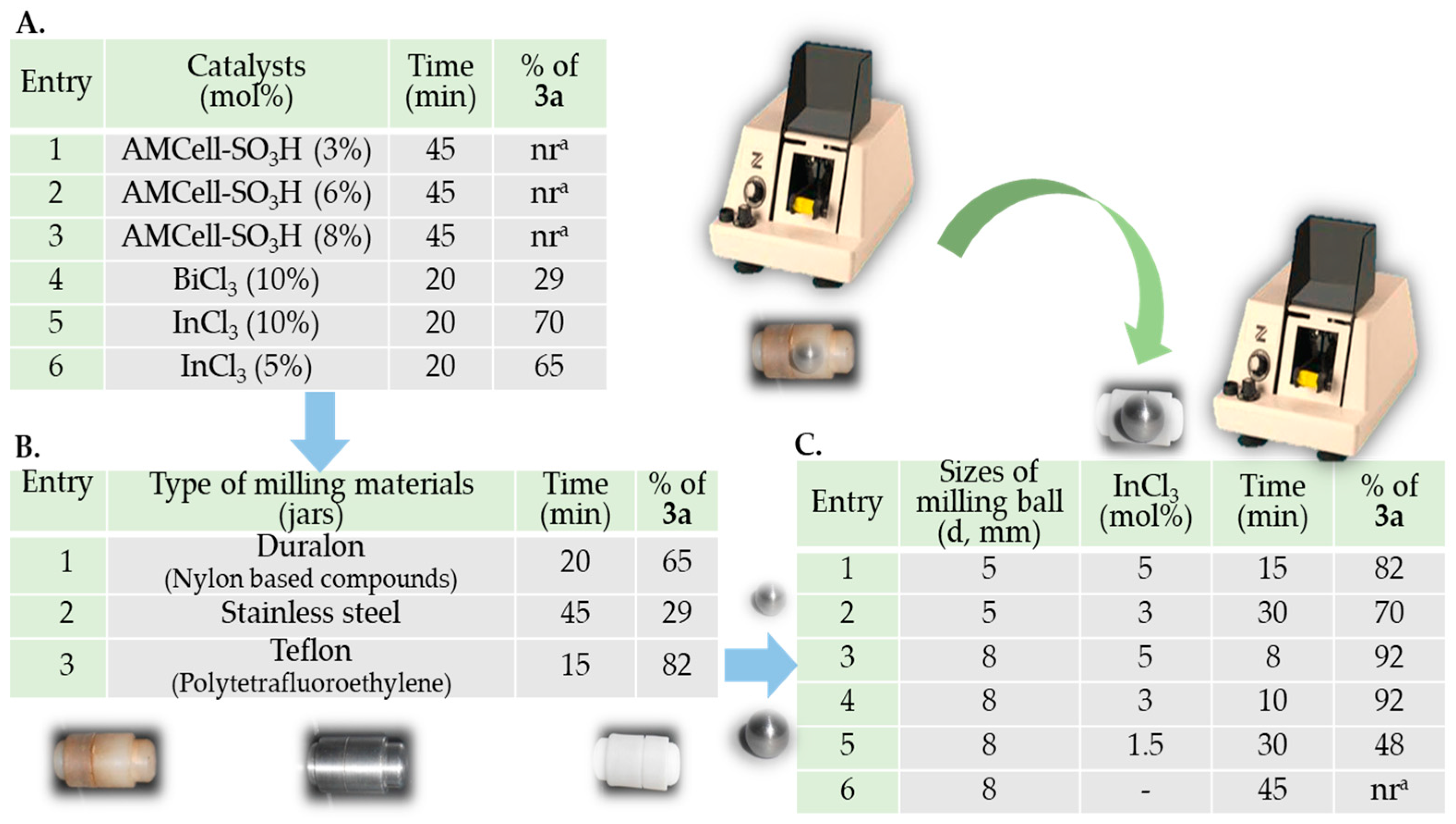
First, following the tutorial review of Stolle et al. on ball mills as chemical reactors [45], we studied several important technical variables of the ball mill process (frequency of vibration process, type of milling material, size of milling balls and milling time), in addition to the most important chemical parameter (nature of catalyst) for this model reaction (Figure 3). Our selection of the ball milling apparatus used fell to the Zenith amalgamator because it was simple to operate, cheap and affordable, and had three rotational speed ranges (4800, 4200, and 3600 rpm). We decided to carry out all planned reactions at a milling frequency of 60 Hz (i.e., working at 3600 rpm) to avoid secondary or consecutive reactions which could occur at higher frequencies (higher energies) [45]. In the initial stage, we used Duralon jars and a single stainless-steel ball with a diameter of 5 mm (0.6401 g). Based on our own experience with the solvent-less synthesis of heterocycles, which was catalyzed by amorphous milled cellulose sulfonic acid (AMCell-SO3H) [64], this promoter was the first choice. However, even the milling times (45 min) in the presence of this acid catalyst did not promote the formation of the desired product 3a (Figure 3A, entries 1–3). Similarly, it should be noted that when this catalyst was used in the traditional methodology of the Pechmann reaction, it did not lead to the synthesis of 3a. Therefore, we presumed that this unsuccessful result could be due to a lack of H+ ions in the media. This kind of solid support promoter can be used in Pechmann condensation under free solvent conditions; however, high temperatures (80–160 °C) or another additional energy source, such as an ultrasound, is necessary [26]. On the other hand, Lewis acids have been widely used as efficient catalysts in the conventional synthesis of 4-methylcoumarins, BiCl3, and InCl3 [65,66]. Therefore, we decided to use these compounds where bismuth(III) chloride (10 mol%) afforded the coumarin 3a in a poor yield (29%) (Entry 4), while indium(III) chloride with the same load gave 3a at a considerable yield (70%) highlighting that the milling time was only 20 min (Entry 5). Likewise, it was decided to reduce the InCl3 load (from 10 to 5 mol%) in the reaction, noting only a small decrease in its yield, which was not considered significant. Taking into account the fact that the grinding time was not increased and that only half of the catalyst originally used was effective (Entry 6), we used these reaction parameters to follow the optimization. Then, despite the fact that the desired 7-amino-4-methylcoumarin 3a was successfully obtained, it was observed how the precursors reacted with the vessel’s material, drastically deteriorating it. Therefore, it was necessary to change the reactors for others to be totally inert to the reagents.
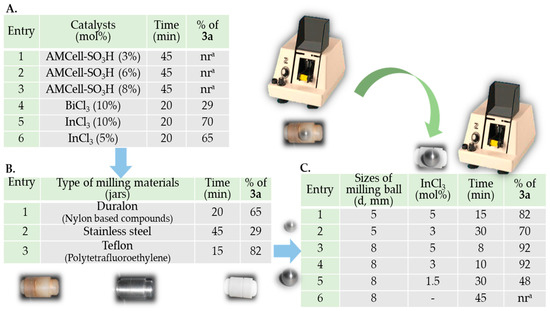
Figure 3.
Reaction optimization for the synthesis of 7-amino-4-methyl-coumarin 3a under HSMBM process at rt. (A). The activity of selected catalysts using mixer mill apparatus (amalgamator Zenith, Z-1A) at a frequency of 60 Hz, Duralon jars, and a single stainless-steel ball with a diameter of 5 mm. (B). Effects of the applied milling material (jars). (C). Effects of the diameter of the milling ball (d, mm) using Teflon jars (a Reaction did not occur).
With these findings, we studied Pechmann condensation and the influence of the milling materials’ nature, changing Duralon vessels with stainless steel and Teflon milling jars (Figure 3B). When 5 mol% InCl3-catalyzed condensations under the same technical parameters of the ball mill process were realized, we observed a strong difference in the reaction effectiveness. Then, operating with stainless steel jars, a large decrease in the reaction yield and an increase in the milling time were recorded, compared to the Duralon vessel (Figure 3B, entries 1 and 2, respectively). By contrast, in using a Teflon reactor, better results were obtained since the reaction was completed in just 15 min, and its yield increased to 82% (Entry 3). Teflon jars are resistant to reagents and therefore do not react with the precursors used, as well as lightweight, which allows the equipment to remain vibrating steadily. Conversely, using a stainless-steel capsule, a greater effort in the amalgamator to work normally was observed experimentally. This was due to the greater weight of the steel capsule. When using light containers, the activation energy was low, and the reactions were driven toward the formation of thermodynamically stable products, unlike what happens when the vessels are dense, requiring more energy to reach comparable levels of product yield [45].
In the next stage, with the parameters found so far, and a frequency at 60 Hz, the Teflon reactor, and InCl3 as the catalyst, we proceeded to study the behavior of the reaction, varying the size of the grinding body because it is known that larger balls lead to an increased energy input per collision [45,67] For this, stainless steel balls of 5 and 8 mm in diameter were used (Figure 3C). As can be seen in this Figure, by reducing the catalyst to 3 mol% and using the smallest ball (d, 5 mm), the reaction took twice as long, and its yield decreased (Entry 2 vs. Entry 1). Unlike the observation when an 8 mm diameter ball was used, product 3a was obtained with a 92% yield in just 8 min (Entry 3 vs. Entry 1).
Moreover, despite the catalyst amount being reduced, coumarin was isolated in the same yield after 10 min of grinding (Entry 4 vs. Entry 3). These results suggest that it is very likely that the increase in the size of the ball could counteract the decrease in the catalyst since its contact surface is greater and facilitates the crushing of the precursors. Considering these observations, we proceeded to decrease the InCl3 load to 1.5 mol%. However, the grinding time tripled, and its yield dropped to 48% (Entry 5). Likewise, when the reaction was carried out in the absence of a catalyst, it was found that the precursors remained unreacted after 45 min (Entry 6).
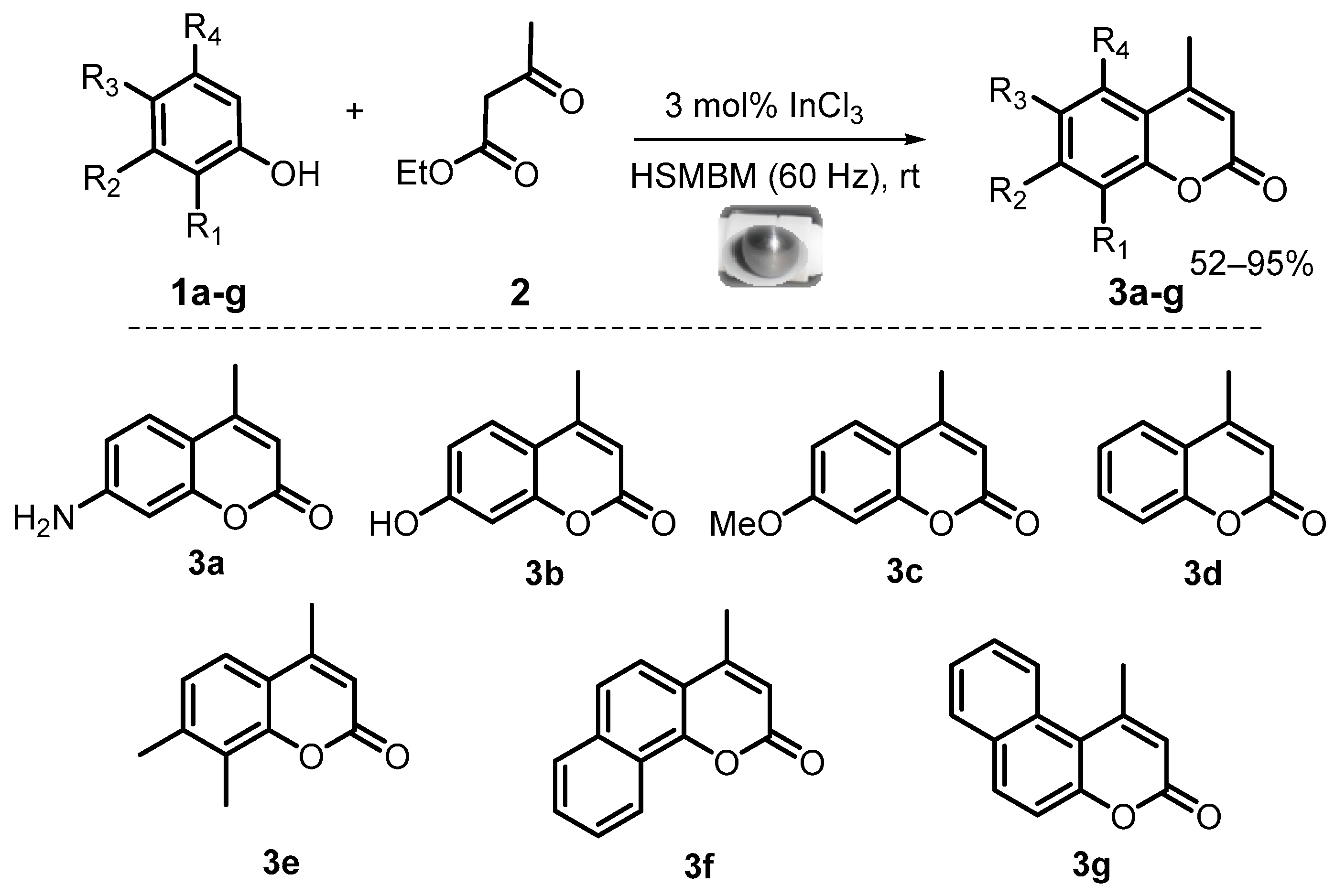
Therefore, the study of all these variables on a model reaction led us to develop a high-speed mixer ball mill protocol for Pechmann condensation. To evaluate its scope, other substituted phenols 1b–e (naphthols 1f–g) and ethyl acetoacetate 2, were used as the main precursors to obtain corresponding 4-methylcoumarins 3b–g in good yields under solvent-free conditions and at room temperature (Figure 4). It is important to note that no reported ball milling or grinding procedure [41,42,43,44] offered the direct preparation of 7-amino-4-methylcoumarin.
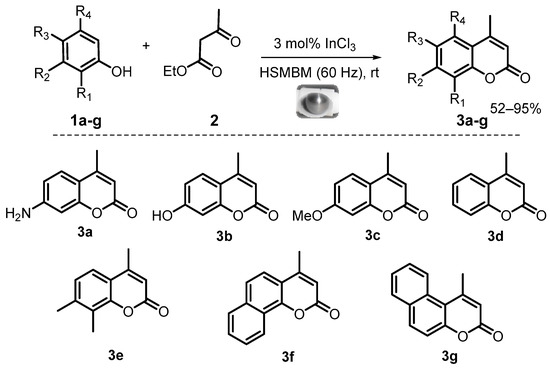
Figure 4.
Reaction scope of Pechmann condensation under HSMBM process at rt.
The identity of the products 3a–g (Table 1) was confirmed by their IR, 1H NMR, 13C NMR, and mass spectral data and by comparison with authentic samples.

Table 1.
Characteristics of 4-methylcoumarin derivatives obtained via InCl3-catalyzed Pechmann condensation under HSMBM process at rt.
4. Conclusions
During a detailed study of the reaction conditions, where technical parameters such as the vibration frequency of the equipment, material, and size of the grinding implements, as well as chemical aspects, catalysts, and precursors, were evaluated, it was possible to establish for the first time an appropriate methodology for the von Pechmann reaction via mechanochemical activation and under green chemistry postulates.
The synthesis of a series of 4-methylcoumarins 3a–g was carried out in short reaction times with good and excellent yields (52–95%) through a high-speed mixer ball mill process with a milling frequency of 60 Hz. The developed protocol avoided the use of auxiliary substances such as hazardous solvents or acid catalysts and long heating times. In addition, it allowed the isolation of products with a very high degree of purity, thus evading additional purification processes that lead to the unnecessary expense of more chemical substances. Particularly, 7-amino-4-methylcoumarin 3a, a compound of high commercial value, was synthesized in a short time (10 min) and with an excellent yield (92%) at rt. Due to the HSMBM process, it was possible to keep away from the generation of secondary products observed in traditional methodologies and the use of protective groups that would not only prolong the experimental process but also entail the use of greater quantities of reagents, solvents, and catalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020073/s1, Synthetic procedures FT-IR and NMR.
Author Contributions
Conceptualization, V.V.K.; methodology, S.J.B.-A.; formal analysis, S.J.B.-A., D.R.M.A. and V.V.K.; writing—original draft preparation, V.V.K.; writing—review and editing, D.R.M.A. and V.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Synthetic procedures and NMR are reported in the Supplementary Materials.
Acknowledgments
We are grateful to Escuela de Química of the Universidad Industrial de Santander for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 533–538. [Google Scholar]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Ortiz Villamizar, M.C.; Puerto Galvis, C.E.; Vargas Méndez, L.Y.; Kouznetsov, V.V. Coumarin-Based Molecules as Suitable Models for Developing New Neuroprotective Agents Through Structural Modification. In Discovery and Development of Neuroprotective Agents from Natural Products, Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–235. [Google Scholar]
- Hu, X.L.; Gao, C.; Xu, Z.; Liu, M.L.; Feng, L.S.; Zhang, G.D. Recent development of coumarin derivatives as potential antiplasmodial and antimalarial agents. Curr. Top. Med. Chem. 2018, 18, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, J.; Bartz, U.; Gütschow, M. Coumarin as a structural component of substrates and probes for serine and cysteine proteases. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140445. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.; Malak, L.G.; Khallaf, I.S.; Bringmann, G.; Farag, S.F. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef]
- Song, F.; Huo, X.; Guo, Z. Anti-breast cancer potential of natural and synthetic coumarin derivatives. Curr. Top. Med. Chem. 2021, 21, 1692–1709. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; ElGaby, M.S.A.; Abbas, S.Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F. Classical Approaches and their Creative Advances in the Synthesis of Coumarins: A Brief Review. J. Med. Chem. Sci. 2021, 4, 612–625. [Google Scholar]
- Adimule, V.M.; Nandi, S.S.; Kerur, S.S.; Khadapure, S.A.; Chinnam, S. Recent advances in the one-pot synthesis of coumarin derivatives from different starting materials using nanoparticles: A review. Top. Catal. 2022, 65, 1669–1674. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Johnson, J.R. The Perkin Reaction and Related Reactions. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 210–265. [Google Scholar]
- Shriner, R.L. The Reformatsky Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–37. [Google Scholar]
- Cairns, N.; Harwood, L.M.; Astles, D.P. Tandem Thermal Claisen−Cope Rearrangements of Coumarate Derivatives. Total Syntheses of the Naturally Occurring Coumarins: Suberosin, Demethylsuberosin, Ostruthin, Balsamiferone and Gravelliferone. J. Chem. Soc. Perkin Trans. 1 1994, 3101–3107. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Puerto-Galvis, C.E.; Ortiz Villamizar, M.C.; Vargas-Méndez, L.Y. Insights into the metal-catalyzed alkyne hydroarylation reactions and related processes for the synthesis of coumarins. Curr. Org. Chem. 2017, 21, 949–963. [Google Scholar] [CrossRef]
- Bhatia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Heterocycl. Compd. 2018, 54, 280–291. [Google Scholar] [CrossRef]
- Farid, S.M.; Seifinoferest, B.; Gholamhosseyni, M.; Larijani, B.; Mahdavi, M. Modern metal-catalyzed and organocatalytic methods for synthesis of coumarin derivatives: A review. Org. Biomol. Chem. 2022, 20, 4846–4883. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Zambare, A.S.; Kalam Khan, A.F.; Zambare, S.P.; Shinde, S.D.; Sangshetti, J.N. Recent advances in the synthesis of coumarin derivatives via Pechmann condensation. Curr. Org. Chem. 2016, 20, 798–828. [Google Scholar] [CrossRef]
- Gulati, S.; Singh, R.; Sangwan, S. A review on convenient synthesis of substituted coumarins using reuseable solid acid catalysts. RSC Adv. 2021, 11, 29130–29155. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.P.; Shivasankar, K.; Sivappa, R.; Kale, R. Zinc mediated transesterification of β-ketoesters and coumarin synthesis. Tetrahedron Lett. 2002, 43, 8583–8586. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Mishra, R.K.; Kumar, A. A convenient synthesis of some coumarin derivatives using SnCl2·2H2O as catalyst. Catal. Lett. 2008, 121, 118–120. [Google Scholar] [CrossRef]
- Smitha, G.; Sanjeeva Reddy, C. ZrCl4-catalyzed Pechmann reaction: Synthesis of coumarins under solvent-free conditions. Synth. Comm. 2004, 34, 3997–4003. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, A.; Sandhu, J.S. LiBr-Mediated, solvent free von Pechmann reaction: Facile and efficient method for the synthesis of 2H-chromen-2-ones. Arkivoc 2007, 15, 18–23. [Google Scholar] [CrossRef]
- Borah, K.J.; Borah, R. Poly (4-vinylpyridine)-supported sulfuric acid: An efficient solid acid catalyst for the synthesis of coumarin derivatives under solvent-free conditions. Monatsh. Chem. 2011, 142, 1253–1257. [Google Scholar] [CrossRef]
- Montazeri, N.; Khaksar, S.; Nazari, A.; Alavi, S.S.; Vahdat, S.M.; Tajbakhsh, M. Pentafluorophenylammonium triflate (PFPAT): An efficient, metal-free and reusable catalyst for the von Pechmann reaction. J. Fluor. Chem. 2011, 132, 450–452. [Google Scholar] [CrossRef]
- Heravi, M.M.; Khaghaninejad, S.; Mostofi, M. Pechmann reaction in the synthesis of coumarin derivatives. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 112, pp. 1–50. [Google Scholar]
- Samiei, Z.; Soleimani-Amiri, S.; Azizi, Z. Fe3O4@C@OSO3H as an efficient, recyclable magnetic nanocatalyst in Pechmann condensation: Green synthesis, characterization, and theoretical study. Mol. Divers. 2021, 25, 67–86. [Google Scholar] [CrossRef]
- Rather, I.A.; Ali, R. An efficient and versatile deep eutectic solvent-mediated green method for the synthesis of functionalized coumarins. ACS Omega 2022, 7, 10649–10659. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Eifler-Lima, V.L. Coumarin synthesis via Pechmann condensation: Toward a greener era (microreview). Chem. Heterocycl. Compd. 2021, 57, 734–736. [Google Scholar] [CrossRef]
- Borah, B.; Dwivedi, K.D.; Kumar, B.; Chowhan, L.R. Recent advances in the microwave-and ultrasound-assisted green synthesis of coumarin-heterocycles. Arab. J. Chem. 2021, 15, 103654. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimani-Amiri, S.; Azizi, Z. Heterogeneously catalyzed Pechmann condensation employing the HFe(SO4)2·4 H2O-Chitosan nano-composite: Ultrasound-accelerated green synthesis of coumarins. Polycycl. Aromat. Compd. 2022, 42, 6072–6089. [Google Scholar] [CrossRef]
- Feizpour Bonab, M.; Soleimani-Amiri, S.; Mirza, B. Fe3O4@C@PrS-SO3H: A Novel Efficient Magnetically Recoverable Heterogeneous Catalyst in the Ultrasound-Assisted Synthesis of Coumarin Derivatives. Polycycl. Aromat. Compd. 2022, 42, 1628–1643. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Makrandi, J.K. Modified Pechmann condensation using grinding technique under solvent-free condition at room temperature. Green Chem. Lett. Rev. 2011, 4, 127–129. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S. A facile synthesis of 2H-chromen-2-ones via Pechmann condensation under solvent free conditions using grinding technique. Green Process. Synth. 2013, 2, 151–155. [Google Scholar] [CrossRef]
- Narwal, J.K.; Malik, R.K.; Kumari, N. An efficient solvent free synthesis of coumarins via solid phase Pechmann reaction. Chem. Sci. Trans. 2015, 4, 1092–1094. [Google Scholar]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Sharutin, V.V.; Santra, S.; Zyryanov, G.V.; Chupakhin, O.N.; Ranu, B.C. Mechanochemical synthesis of coumarins via Pechmann condensation under solvent-free conditions: An easy access to coumarins and annulated pyrano [2,3-f] and [3,2-f] indoles. Green Chem. 2022, 24, 2429–2437. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Villacampa, M.; Menéndez, J.C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042–2064. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, C.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef]
- Banerjee, M.; Panjikar, P.C.; Das, D.; Iyer, S.; Bhosle, A.A.; Chatterjee, A. Grindstone chemistry: A “green” approach for the synthesis and derivatization of heterocycles. Tetrahedron 2022, 112, 132753. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Merchán-Arenas, D.R.; Martínez-Bonilla, C.A.; Macías, M.A.; Roussel, P.; Gauthier, G.H. Grinding and Milling: Two efficient methodologies in the solvent-free phosphomolybdic acid-catalyzed and mechanochemical synthesis of cis-4-Amido-N-yl-2-methyl-tetrahydroquinolines. J. Braz. Chem. Soc. 2016, 27, 2246–2255. [Google Scholar] [CrossRef]
- Ortiz-Villamizar, M.C.; Puerto-Galvis, C.E.; Kouznetsov, V.V. The A3 redox-neutral C1-alkynylation of tetrahydroisoquinolines: A comparative study between visible light photocatalysis and transition-metal catalysis. Synthesis 2021, 53, 547–556. [Google Scholar]
- Peñaranda Gómez, A.; Puerto Galvis, C.E.; Macías, M.A.; Ochoa-Puentes, C.; Kouznetsov, V.V. I2/DMSO-Promoted the synthesis of chromeno[4,3-b]quinolines through an imine formation/aza-Diels-Alder/aromatization tandem reaction under metal-catalyst and photosensitizer-free conditions. Synthesis 2022, 54, 1857–1869. [Google Scholar]
- Villamizar-Mogotocoro, A.F.; Bonilla-Castañeda, S.M.; Kouznetsov, V.V. Green conditions for the efficient two-step synthesis of new 6-arylphenanthridines from 2-bromoacetoanilides based on microwave-assisted Suzuki-Miyaura cross-coupling and modified Pictet-Spengler dehydrogenative cyclization in a zinc chloride/[Bmim]BF4 mixture. Green Chem. 2022, 24, 7996–8004. [Google Scholar]
- Sun, X.; Guo, Y.; Wen, R.; Li, H. A highly sensitive and selective ratiometric sensing platform based on 7-amino-4-methylcoumarin for naked-eye visual fluorescence sensing of Cu2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120627. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Murase, T.; Hachisuka, D.; Sakamoto, Y.; Sugiura, M.; Nakano, S.; Fujii, K.; Masaki, A.; Inagaki, H. 7-Amino-4-methylcoumarin as a fluorescent substitute for Schiff’s reagent: A new method that can be combined with hemalum and eosin staining on the same tissue section. Biotech. Histochem. 2023, 98, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Von Pechmann, H.; Schwarz, O. Studien über Cumarine.–III. Ueber das ρ-Amido-β-methylcumarin. Ber. 1899, 32, 3696–3699. [Google Scholar] [CrossRef]
- Zimmerman, M.; Yurewicz, E.; Patel, G. A new fluorogenic substrate for chymotrypsin. Anal. Biochem. 1976, 70, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Bissell, E.R.; Mitchell, A.R.; Smith, R.E. Synthesis and chemistry of 7-amino-4-(trifluoromethyl) coumarin and its amino acid and peptide derivatives. J. Org. Chem. 1980, 45, 2283–2287. [Google Scholar] [CrossRef]
- Bao, X.; Wang, G.; Tian, C.; Dong, X.; Xu, G.; Li, F.; Chen, D. Er(OTf)3-catalyzed synthesis of fluorescent 7-aminocoumarins. Tetrahedron 2022, 123, 132994. [Google Scholar] [CrossRef]
- Pozdnev, V.F. Improved method for synthesis of 7-amino-4-methylcoumarin. Chem. Heterocycl. Compd. 1990, 26, 264–265. [Google Scholar] [CrossRef]
- Reddy, T.S.; Reddy, A.R. Synthesis and fluorescence study of 6,7-diaminocoumarin and its imidazolo derivatives. Dyes Pigm. 2013, 96, 525–534. [Google Scholar] [CrossRef]
- Merchan Arenas, D.R.; Kouznetsov, V.V. Diastereoselective synthesis of dihydroisoindolo [2,1-a] quinolin-11-ones by solvent-free AMCell-SO3H-catalyzed imino Diels–Alder/intramolecular amide cyclization cascade reactions. J. Org. Chem. 2014, 79, 5327–5333. [Google Scholar] [CrossRef]
- Senda, N.; Miwa, Y.; Tanaka, J.; Momotake, A.; Arai, T. Tsukuba-green: A fluorescent dye that emits green fluorescence useful for live-cell imaging. Chem. Lett. 2010, 39, 308–310. [Google Scholar] [CrossRef]
- Bose, D.S.; Rudradas, A.P.; Babu, M.H. The indium (III) chloride-catalyzed von Pechmann reaction: A simple and effective procedure for the synthesis of 4-substituted coumarins. Tetrahedron Lett. 2002, 43, 9195–9197. [Google Scholar] [CrossRef]
- Fischer, F.; Fendel, N.; Greiser, S.; Rademann, K.; Emmerling, F. Impact Is Important-Systematic Investigation of the Influence of Milling Balls in Mechanochemical Reactions. Org. Process Res. Dev. 2017, 21, 655–659. [Google Scholar] [CrossRef]
- Robertson, A.; Sandrock, W.F.; Henry, C.B. Hydroxy-carbonyl compounds, part V: The preparation of coumarins and 1:4-pyrones from phenol, p-cresol, quinol, and α-naphthol. J. Chem. Soc. 1931, 2426–2432. [Google Scholar] [CrossRef]
- Goswami, P. Dually activated organo-and nano-cocatalyzed synthesis of coumarin derivatives. Synth. Commun. 2009, 39, 2271–2278. [Google Scholar] [CrossRef]
- Yamada, K.; Takada, S.; Nakamura, S.; Hirata, Y. The structures of anisatin and neoanisatin: Toxic sesquiterpenes from Illicium anisatum L. Tetrahedron 1968, 24, 199–229. [Google Scholar] [CrossRef]
- Soleimani, E.; Khodaei, M.M.; Batooie, N.; Samadi, S. Tetrakis (acetonitrile) copper (I) hexafluorophosphate catalyzed coumarin synthesis via Pechmann condensation under solvent-free condition. J. Heterocycl. Chem. 2012, 49, 409–412. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Mowlazadeh Haghighi, S.; Panahi, F. Nano-TiO2 on dodecyl-sulfated silica: As an efficient heterogeneous Lewis acid–surfactant-combined catalyst (HLASC) for reaction in aqueous media. ACS Sustain. Chem. Eng. 2013, 1, 1015–1023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).