Abstract
Here, five bonds to carbon through tri-coordination are theoretically established in the global minimum energy isomers of anion (1a) and neutral (1n) for the first time. Various isomers of are theoretically identified using density functional theory at the PBE0-D3/def2-TZVP level. Chemical bonding features are thoroughly analyzed for these two isomers (1a and 1n) with different bonding and topological quantum chemical tools, such as adaptive natural density partitioning (AdNDP), Wiberg Bond Indices (WBIs), nucleus-independent chemical shifts (NICS), and atoms in molecules (AIM) analyses. The structure of isomer 1a is planar with C2v symmetry, whereas its neutral counterpart 1n is non-planar with C2 symmetry, in which its terminal aluminum atoms are out of the plane. The central allenic carbon atom of isomers 1a and 1n exhibits tri-coordination and thus makes it a case of five bonds to carbon, which is confirmed through their total bond order as observed in WBI. Both the isomers show σ- and π-aromaticity and are predicted with the NICS and AdNDP analyses. Further, the results of ab initio molecular dynamics simulations reveal their kinetic stability at room temperature; thus, they are experimentally viable systems.
1. Introduction
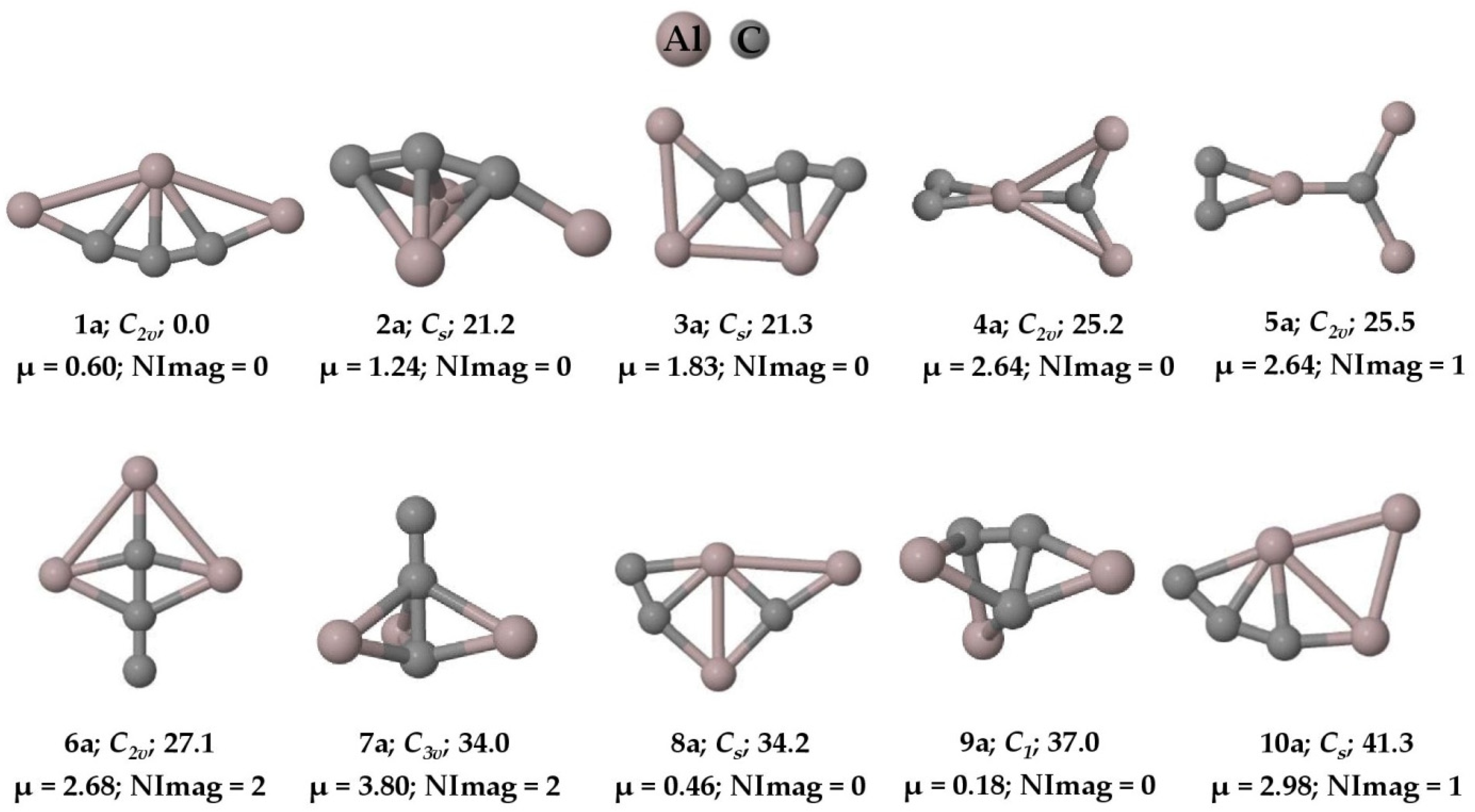
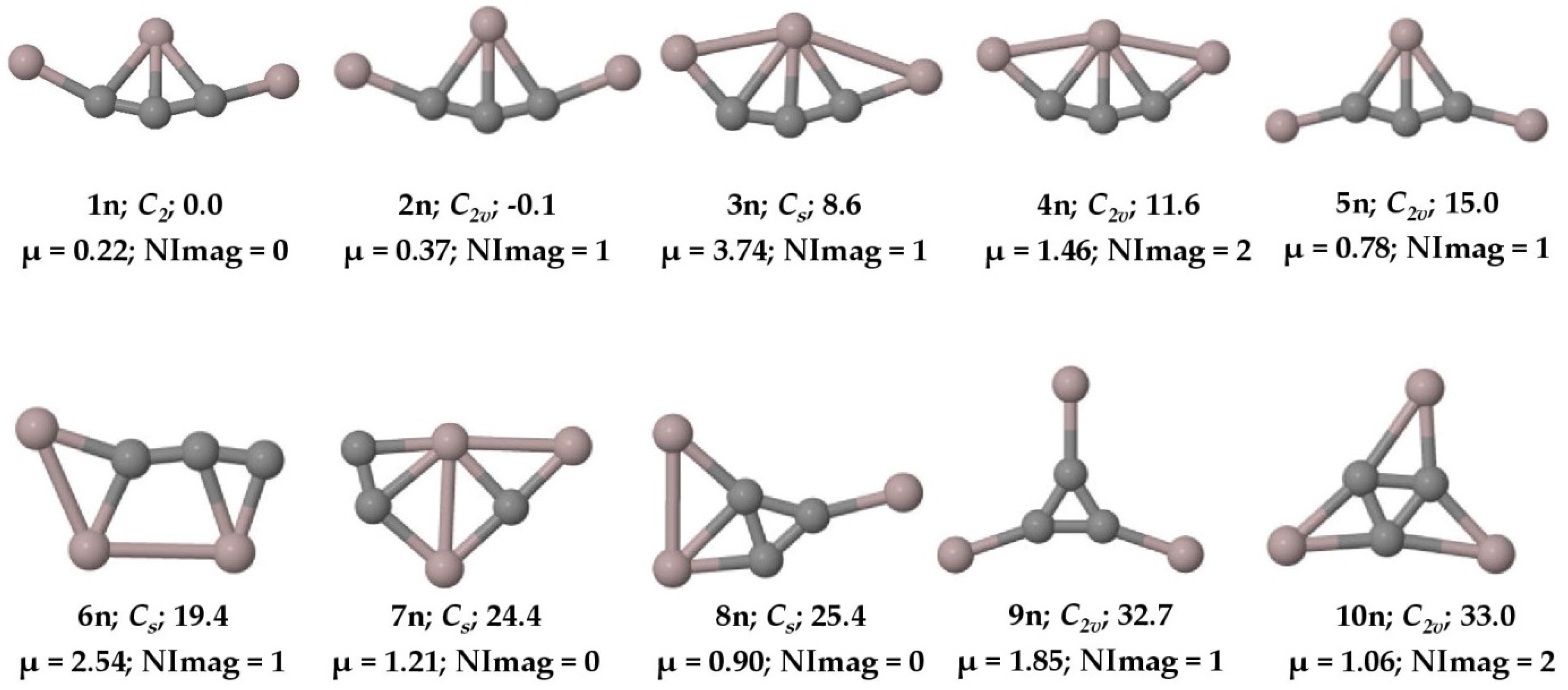
The concept of five bonds to carbon became indispensable since the discovery of methanium ion () in the laboratory in 1950 [1]. Recording the infrared spectra of this simple protonated methane molecule was quite challenging, as it took almost five decades from its discovery [2]. The theoretical investigation of lithium carbides such as CLi5 and CLi6 [3] and the experimental realization of CLi6 through mass spectroscopic measurements further motivated the interest in hyper-coordinate carbon molecules [4]. While computational studies on Si2(CH3 [5] and C(CH3 [6] provided further guidance on hyper-coordinate behavior of group 14 elements, it is the experimental observations such as [CCH3, HC[Au(PPh3), [(C6H5)3PAu5C]+, [(Ph3PAu)6C]2+, C6[CH3, etc., that gave chemists the real grandeur of hyper-coordinate carbon molecules [7,8,9,10,11]. Akiba and co-workers have shown penta- and hexa-coordinate anthracene moieties through x-ray crystallography and ab initio calculations [12,13]. The iron–molybdenum nitrogenase cofactor existing in diazotrophs is a clear example of hexa-coordinate carbon in biological systems [14]. From the well-known concept of molecules with a planar tetra-coordinate carbon (ptC) atom [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30], the idea was extended to planar penta-coordinate carbon (ppC) [31,32,33,34,35,36,37,38,39,40] and planar hexa-coordinate carbon (phC) [41,42,43,44]. Hill and coworkers experimentally reported the existence of a penta-coordinate carbon atom in 1981 [45]. The penta-coordinate carbon atom was also theoretically reported by Gleiter and coworkers [46] in the Cp2Zr[CH2(BH{C6F5}2)2] complex. In 1996, the experimental proof of the first complex with a hyper-coordinate ylidic carbon atom was also reported by Jones and coworkers [47]. While a gradual amount of progress has been made in these classes of molecules to date, to a larger extent in the literature, the concept of making hyper-coordinate carbon molecules was predominantly focused on making single bonds to the central carbon atom irrespective of whether they are planar or non-planar [48]. However, in this article, the intent is to make five bonds to a carbon atom through tri-coordination instead of penta-coordination, as is normally carried out. To this end, we have theoretically investigated the aluminum–carbon cluster, , in both its anion and neutral forms and established the fact that the global minimum isomers (1a and 1n) contain five bonds to carbon through tri-coordination (see Figure 1 and Figure 2).
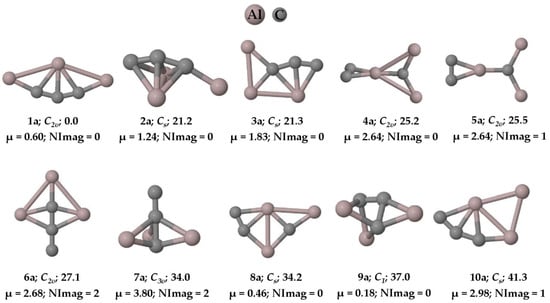
Figure 1.
Ten low-lying isomers of with the ZPVE-corrected relative energies (in kcal mol−1), dipole moments (in Debye), and the number of imaginary frequencies (NImag) obtained at the PBE0-D3/def2-TZVP level.
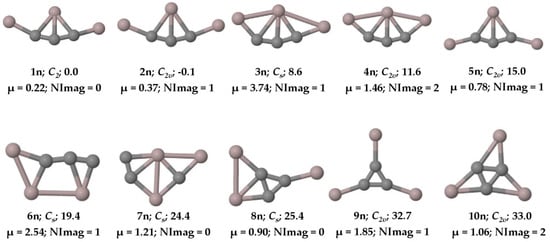
Figure 2.
Ten low-lying isomers of with ZPVE-corrected relative energies (in kcal mol−1), dipole moments (in Debye), and the number of imaginary frequencies (NImag) obtained at the PBE0-D3/def2-TZVP level.
Various aluminum–carbon clusters have continuously been investigated [16,17,49,50,51,52] as they have potential applications in energy storage [53] and the production of nano-powders [54,55] and solar cells [56]. Zheng and coworkers theoretically and experimentally explored the system and found that the global minimum isomer in the neutral state contains the planar hexa-coordinate aluminum [57]. In 2022, Kalita et al. reported the planar penta-coordinate Al and Ga centers in and systems, which were global minimum structures, and found that the stabilizing factor was σ-aromaticity [58]. Recently, Malhan et al. reported the Al2C4H2 system with ptC, planar tetra-coordinate aluminum (ptAl), and planar penta-coordinate aluminum (ppAl) atoms with aromatic characteristics [59]. The aforementioned discoveries have also inspired researchers to look for further systems containing hyper-coordinate main group elements, such as group 13 elements. Wang and coworkers reported and clusters exhibiting planar hepta- and octa-coordinate central boron atoms with combined experimental and computational studies [60]. Li et al. reported the global minimum structure of the system with planar penta-coordinate boron (ppB) [61]. The global minimum isomer containing the ppB atom in the system with aromatic characteristics was reported by Wu and coworkers. In 2021, Khatun et al. reported BAl4Mg−/0/+ [62], in which the global minimum structures were found to have a planar tetra-coordinate boron (ptB) atom in its anionic and cationic forms as well as a ppB atom in the neutral state. In 2022, Das and coworkers explored the potential energy surface of CB6Al0/+ [63] and found that both neutral and cation contain planar hexa-coordinate boron (phB) atoms in their global minima. Thompson et al. experimentally reported the ptAl species [64]. The ptAl species of calix [4] pyrrole aluminate was also experimentally reported by Greb and coworkers in 2019 [65]. In 2023, Merino and coworkers also reported a quasi-ptC atom in the system [66]. These distinctive bonding arrangements demonstrate not only the fundamental importance of improving our knowledge of chemical bonding but also a completely new class of molecules in the world of chemistry. Herein, the present work reports the system with tri-coordination; that is, five bonds to the central allenic carbon atom via computational quantum chemical modeling. The aluminum and carbon-based molecules have potential applications ranging from cluster assembled materials [67,68], energy storage [69], and two-dimensional donor materials in solar cells [70]. Dong et al. [71] have already reported the isomer 2n of the system with hydrogen storage properties both experimentally and theoretically at MP2/6-311+G* level of theory, which gives us confidence that the system investigated here, has a high chance of synthetic viability in the future.
2. Computational Methodology
The initial geometries of the system were first generated through chemical intuition, and then, using the in-house Python code, all other possible geometries were explored on these two potential energy surfaces (PESs) using density functional theory (DFT). All geometries were fully optimized using the hybrid functional PBE0 [72] coupled with Grimme’s dispersion correction (D3) [73,74] and the def2-TZVP [75,76] basis set. Frequency calculations were carried out at the same level of theory to ensure whether the optimized geometries are true minima or maxima or higher-order saddle points. To get more characteristic features on the chemical bonding of isomers 1a and 1n, the natural bond order (NBO) analysis [77], adaptive natural density partitioning (AdNDP) analysis [78,79], and Wiberg bond indices (WBIs) [80] were performed at the PBE0-D3/def2-TZVP level. The nucleus-independent chemical shift (NICS) [81] calculations for 1a and 1n structures were carried out at the same level to analyze the aromatic behavior of these systems. Atoms in molecules (AIM) analysis [82] of the Laplacian of electron density and electron localization function (ELF) [83] were carried out for isomers 1a and 1n using the wave function file generated by the Gaussian program [84] at the PBE0-D3/def2-TZVP level. The dynamic stabilities of 1a and 1n were evaluated using the atom-centered density matrix propagation (ADMP) [85] at the same level of theory. The AdNDP and ELF were analyzed through the Multiwfn program [86]. All the calculations were performed using the Gaussian 16 package [84].
3. Results and Discussion
The PESs of the are explored, and we found that the global minimum energy geometry (1a and 1n) of both the anion and neutral system contains a carbon atom (C4) with two π bonds and three σ bonds, exhibiting a total of five bonds through tri-coordination. The ten low-lying isomers of the anion and the neutral system are given in Figure 1 and Figure 2, respectively. All other isomers on the PES of the system are given in the Supplementary Materials in Figures S1 and S2, respectively. The system has a singlet spin state and the system corresponds to a doublet. Isomer 1a is a planar structure with C2v symmetry, whereas isomer 1n shows C2 symmetry, in which the terminal aluminum atoms are out of the plane (see Figure 3). The system also has structures that exhibit planar penta-coordinate aluminum (ppAl) and planar tetra-coordinate carbon (ptC) atoms as local minimum energy isomers (8a, 7n; and 3a, 13a, and 8n, respectively) on their PESs. Nevertheless, our focus here is on the global minimum energy isomers, 1a and 1n, which exhibit five bonds to carbon through tri-coordination.
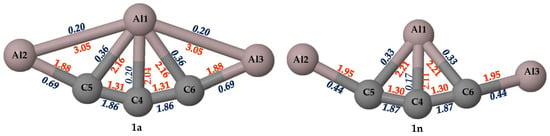
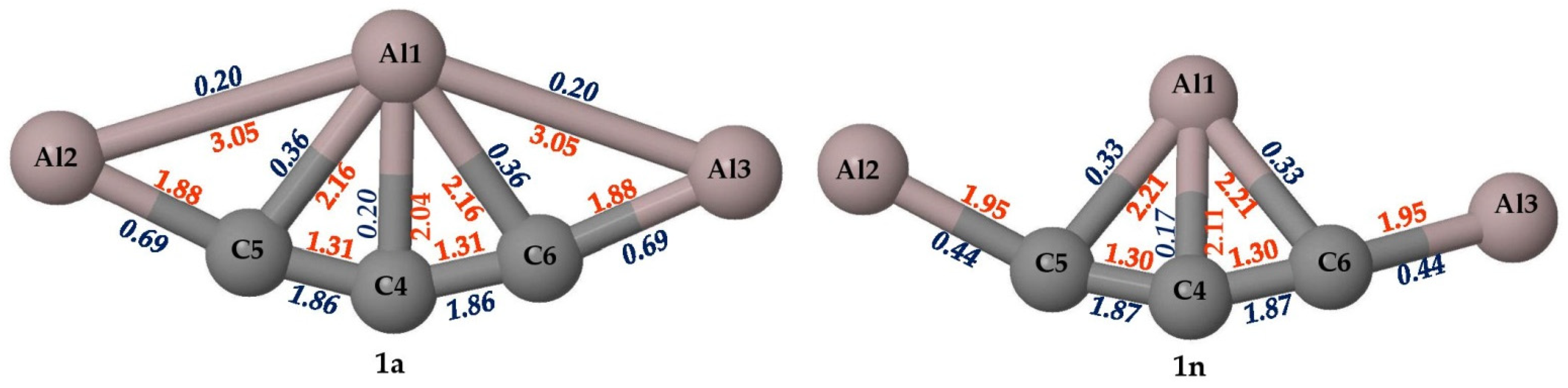
Figure 3.
The bond lengths (in Å, red) and the Wiberg bond orders (in blue) are obtained at the PBE0-D3/def2-TZVP level for isomers 1a and 1n.
3.1. Wiberg Bond Indices
The WBI values obtained from NBO analysis for the allenic carbon (C4) atom in isomers 1a and 1n are critically analyzed. The WBI values and the bond distances are given in Figure 3. The standard covalent bond lengths of C–Al and C=C are 2.01 and 1.34 Å, respectively, which are in close agreement with the obtained values. The C4–Al1 bond length of isomer 1n having 2.11 Å is slightly higher than that of isomer 1a with 2.04 Å. The WBI values for the C=C bond in isomers 1a and 1n are 1.86 and 1.87, respectively, which confirms the presence of π bonds in both isomers. This further proves that in both cases, the C4 atom makes two π bonds with neighboring atoms. The C4–Al1 bond with WBI values of 0.20 and 0.17 in isomers 1a and 1n suggests the dative bonding nature of the fifth bond. The total bond order of C4 in isomers 1a and 1n is 3.99 and 3.97, respectively. This indicates that the allenic carbon atom, C4, is surrounded by eight electrons but still makes two π bonds with neighboring carbon atoms and also forms an additional dative bond with the central aluminum atom.
3.2. Adaptive Natural Density Partitioning (AdNDP) Analysis
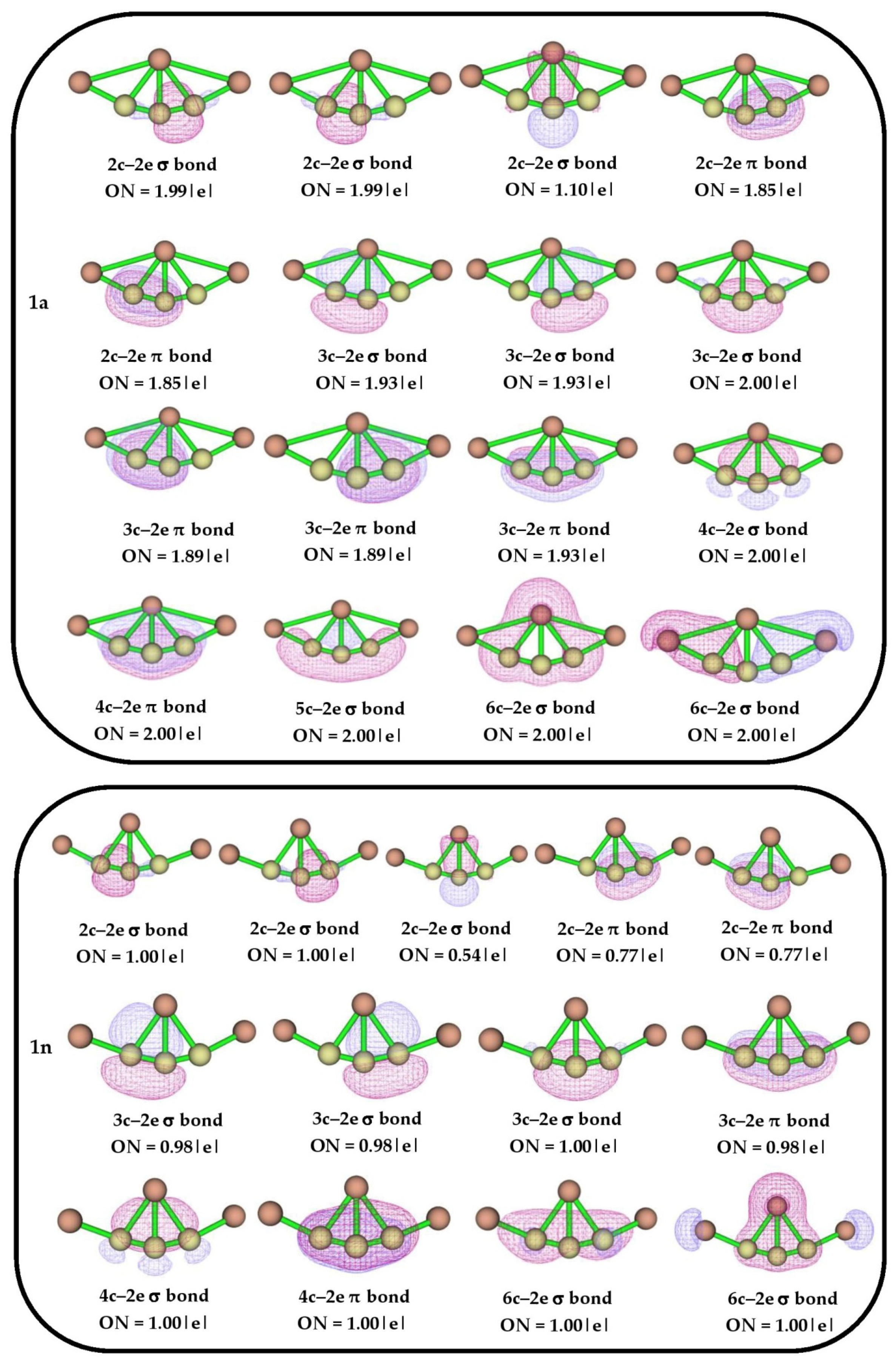
To further analyze the bonding scenario, the AdNDP analysis was carried out for the delineation of n-center 2-electron (nc-2e) bonds in the investigated systems. The generated AdNDP orbitals with occupation numbers (ON) for isomers 1a and 1n are shown in Figure 4. As the neutral system is in a doublet state, only alpha orbitals are considered for this analysis. The tri-coordinated C4 atom has two 2c–2e σ and two 2c–2e π bonds with its neighboring carbon atoms, with ON 1.99 |e| and 1.85 |e|, respectively, which confirms the presence of alternating π bonds in the isomer 1a. It also exhibits delocalization of electron densities through 3c–2e σ, 4c–2e σ, 3c–2e π, and 4c–2e π bonds with ON ranging from 1.89 |e| to 2.00 |e, which support the tri-coordination in the structure. The two 2c–2e π bonds in isomer 1n confirm the presence of alternating π bonds which also exhibit delocalization of electron densities through multi-center 2e σ and π bonds, which supports the structural stability. To support the observed AdNDP bonding pattern, the nucleus-independent chemical shift (NICS) values are also calculated for isomers 1a and 1n, which are shown in Figure S3. The negative values of NICS (0) and NICS (1) also confirm the presence σ- and π-aromatic nature in both the isomers, respectively.
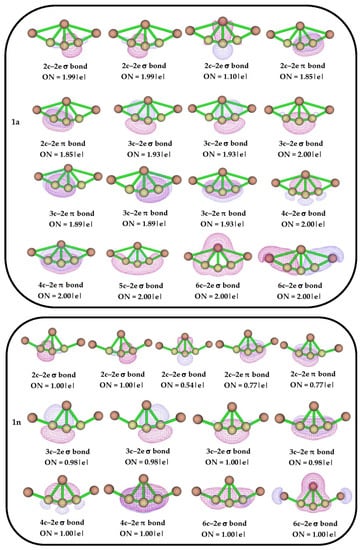
Figure 4.
AdNDP bonding patterns with occupation numbers (ONs) for isomers 1a and 1n.
3.3. Atoms in Molecule (AIM) Analysis
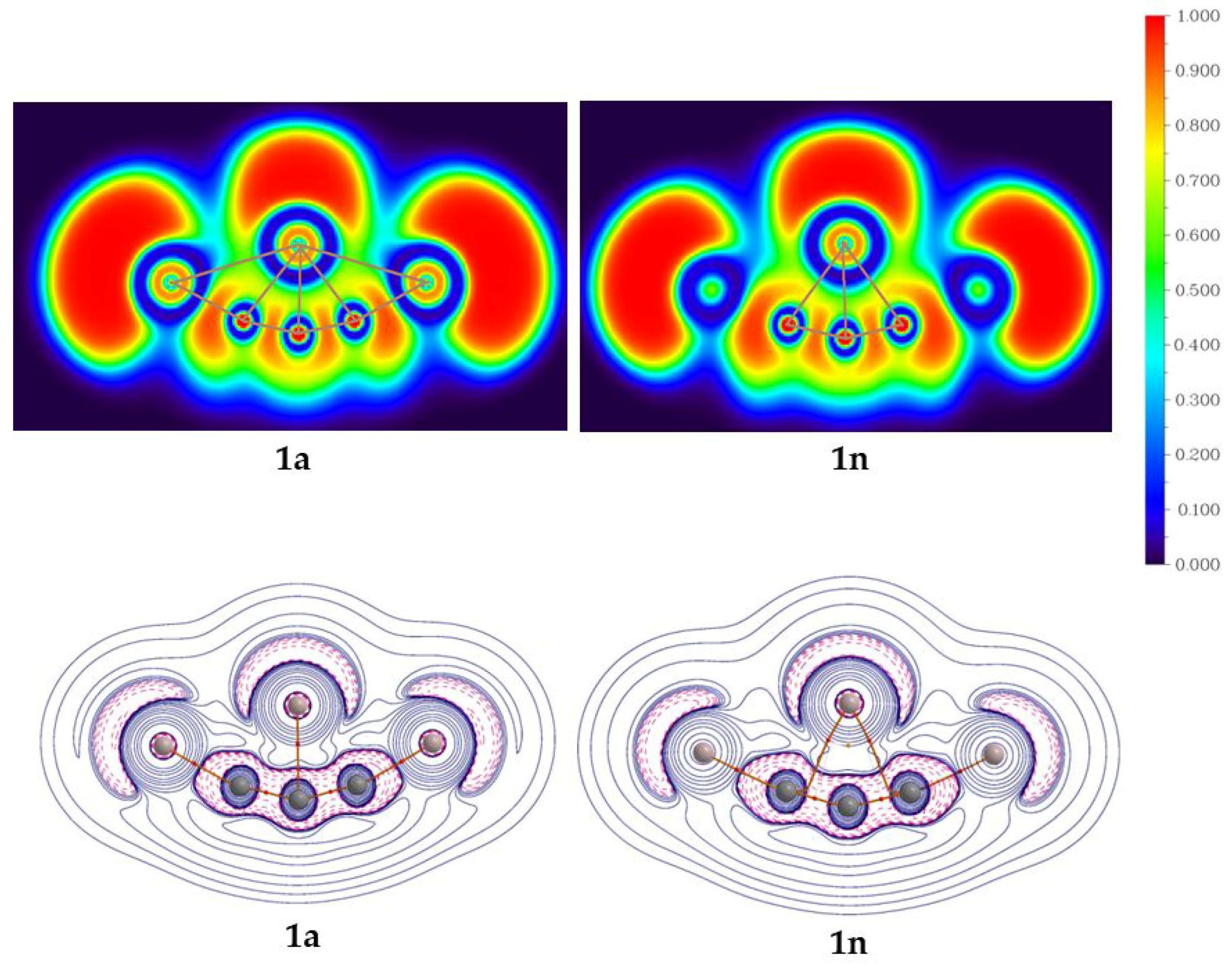
The AIM analysis is carried out to gain insight into the bonding characteristic features. The color-filled plots of the electron localization function (ELF) and contour diagram for the Laplacian of electron density (∆2ρ(r)) for isomers 1a and 1n are shown in Figure 5. The ELF plot of isomers 1a indicates the interaction of the C4 atom with its neighboring atoms and supports the delocalization of electron densities within the molecule. Apart from the terminal Al2 and Al3 atoms, which are out of the plane, isomer 1n also supports the delocalization of electron densities around the C4 atom. In 1a, bond critical points (BCPs) between the C4 and its neighboring atoms support the existence of bond paths. Isomer 1n has BCPs between C4 and its adjacent carbon atoms and also has a ring critical point (RCP) which dictates the dominant aromatic characteristics in the structure.
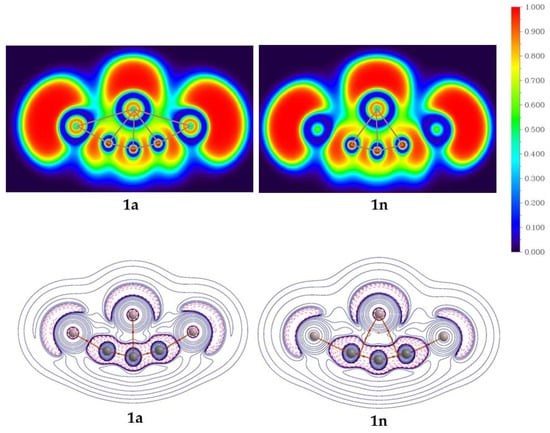
Figure 5.
The color-filled map of ELF (top row) and the Laplacian of electron density (∆2ρ(r)) with bond paths (bottom row) for isomers 1a and 1n at the PBE0-D3/def2-TZVP level.
3.4. Kinetic Stability
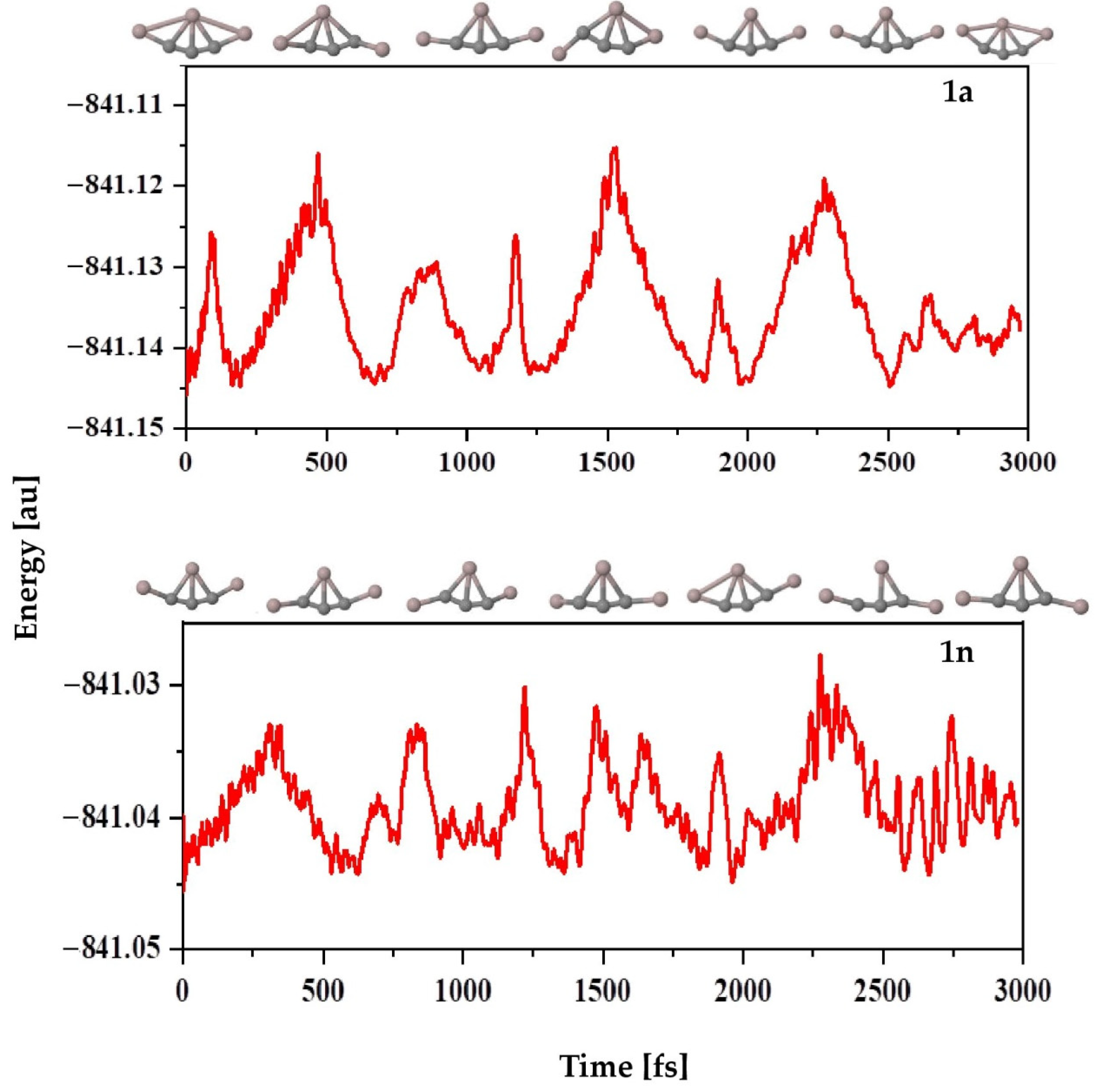
The ab initio molecular dynamics (AIMD) simulations are carried out for 3000 fs at 298 K and 1 atm pressure using the ADMP approach to explore the kinetic stability of the investigated structures. The time evolution of energy plots for isomers 1a and 1n are given in Figure 6. Slight structural deformation occurs during the simulation, which causes an increase in nuclear kinetic energy. However, the present data reveal that the overall geometry is not completely destroyed, which indicates that these molecules are kinetically stable apart from their thermodynamic stability. As expected, for both isomers 1a and 1n, the five bonds to the C4 atom remain the same throughout the simulation period. The structural stability of these isomers is well maintained during the simulation, and no isomerization or other structural modifications occur in these molecules, suggesting that they are indeed kinetically stable.
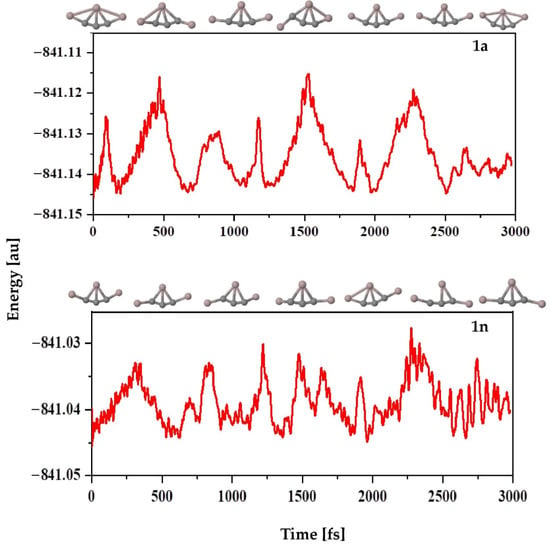
Figure 6.
Time evolution of the total energy of isomers 1a (top) and 1n (bottom) calculated at the PBE0-D3/def2-TZVP level.
4. Conclusions
Using density functional theory, various isomers of the system were explored first by chemical intuition, and other possible isomers are then identified with the help of the in-house Python code. The global minimum isomers 1a and 1n exhibit five bonds to carbon through tri-coordination. The present work reports for the first time five bonds to carbon through tri-coordination in the system as observed in isomers 1a and 1n, respectively. Isomer 1a is a planar structure with C2v symmetry, whereas isomer 1n shows C2 symmetry with the terminal aluminum atoms out of the plane. WBI analysis indicates that the central allenic carbon atom in both the isomers (1a and 1n) forms five bonds through tri-coordination and also obeys the octet rule simultaneously. The BCPs from AIM analysis confirm the presence of bond paths between allenic carbon and its adjacent atoms. The aromatic nature that stabilizes both the isomers 1a and 1n is well supported by AdNDP, ELF, and NICS analyses. Both the isomers are kinetically stable as inferred from the ab initio molecular dynamics simulations at 1 atm pressure and 298 K temperature up to 3000 fs of time. The obtained results on the system via computational calculations may encourage experimentalists to design this new class of molecules in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020076/s1, The optimized geometries of all and isomers are given in Figures S1 and S2, respectively, NICSs in ppm for the isomers 1a and 1n are given in Figure S3, AdNDP bonding patterns with occupation numbers (ONs) for isomers 1a and 1n are given in Figures S4 and S5, respectively, total energies (in a.u), Zero-point vibrational energies (ZPVEs; in a.u), ZPVE corrected total energies (E+ZPVE; in a.u), relative energies (ΔE+ZPVE; in kcal mol−1), and the number of imaginary frequencies (NImag) of all and isomers at PBE0-D3/def2-TZVP level are given in Tables S1 and S2, respectively, and Cartesian coordinates of all and isomers at the PBE0-D3/def2-TZVP level are given in Tables S3 and S4, respectively.
Author Contributions
Conceptualization, V.S.T. and K.T.; Investigation, A.H.M., V.S.T. and K.T.; Methodology, A.H.M., V.S.T. and K.T.; Supervision, V.S.T. and K.T.; Visualization, A.H.M. and K.T.; Writing—original draft, A.H.M. and V.S.T.; Writing—review and editing, V.S.T. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the article or Supplementary Materials.
Acknowledgments
The computational facility provided at the VIT, Vellore, to carry out this work is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tal’rose, V.L.; Lyubimova, A.K. Secondary Processes in the Ion Source of the Mass Spectrometer. Dokl. Akad. Nauk SSSR 1952, 86, 909–912. [Google Scholar]
- White, E.T.; Tang, J.; Oka, T. CH5+: The Infrared Spectrum Observed. Science 1999, 284, 135–137. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Wuerthwein, E.U.; Kaufmann, E.; Clark, T.; Pople, J.A. Effectively Hypervalent Molecules. 2. Lithium Carbide (CLi5), Lithium Carbide (CLi6), and the Related Effectively Hypervalent First Row Molecules, CLi5-NHn and CLi6-NHn. J. Am. Chem. Soc. 1983, 105, 5930–5932. [Google Scholar] [CrossRef]
- Kudo, H. Observation of Hypervalent CLi6 by Knudsen-Effusion Mass Spectrometry. Nature 1992, 355, 432–434. [Google Scholar] [CrossRef]
- Dávalos, J.Z.; Herrero, R.; Abboud, J.-L.M.; Mó, O.; Yáñez, M. How can a Carbon Atom Be Covalently Bound to Five Ligands? The Case of Si2(CH3)7+. Angew. Chem. Int. Ed. 2006, 46, 381–385. [Google Scholar] [CrossRef]
- McKee, W.C.; Agarwal, J.; Schaefer, H.F.; Schleyer, P.V.R. Covalent Hypercoordination: Can Carbon Bind Five Methyl Ligands? Angew. Chem. Int. Ed. 2014, 53, 7875–7878. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W. Direct observation of a remarkably stable dication of unusual structure: (CCH3)62+. Tetrahedron Lett. 1973, 14, 1665–1670. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Gabbaie, F.; Schier, A.; Riede, J. Hypercoordinate Carbon in Protonated Tetraauriomethane Molecules. Organometallics 1995, 14, 4969–4971. [Google Scholar] [CrossRef]
- Scherbaum, F.; Grohmann, A.; Müller, G.; Schmidbaur, H. Synthesis, Structure, and Bonding of the Cation [(C6H5)3PAu5C]+. Angew. Chem. Int. Ed. 1989, 28, 463–465. [Google Scholar] [CrossRef]
- Scherbaum, F.; Grohmann, A.; Huber, B.; Krüger, C.; Schmidbaur, H. “Aurophilicity” as a Consequence of Relativistic Effects: The Hexakis (Triphenylphosphaneaurio) Methane Dication [(Ph3PAu)6C]2+. Angew. Chem. Int. Ed. 1988, 27, 1544–1546. [Google Scholar] [CrossRef]
- Malischewski, M.; Seppelt, K. Crystal Structure Determination of the Pentagonal-Pyramidal Hexamethylbenzene Dication C6 (CH3)62+. Angew. Chem. Int. Ed. 2017, 56, 368–370. [Google Scholar] [CrossRef]
- Akiba, K.; Yamashita, M.; Yamamoto, Y.; Nagase, S. Synthesis and Isolation of Stable Hypervalent Carbon Compound (10-C-5) Bearing a 1,8-Dimethoxyanthracene Ligand. J. Am. Chem. Soc. 1999, 121, 10644–10645. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamamoto, Y.; Kinoshita, D.; Akiba, K.; Zhang, Y.; Reed, C.A.; Hashizume, D.; Iwasaki, F. Synthesis and Structure of a Hexacoordinate Carbon Compound. J. Am. Chem. Soc. 2008, 130, 6894–6895. [Google Scholar] [CrossRef]
- Lancaster, K.M.; Roemelt, M.; Ettenhuber, P.; Hu, Y.; Ribbe, M.W.; Neese, F.; Bergmann, U.; DeBeer, S. X-Ray Emission Spectroscopy Evidences a Central Carbon in the Nitrogenase Iron-Molybdenum Cofactor. Science 2011, 334, 974–977. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alder, R.W.; Wilcox, C.F. Planar Tetracoordinate Carbon. J. Am. Chem. Soc. 1970, 92, 4992–4993. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.-S.; Boldyrev, A.I.; Simons, J. Tetracoordinated Planar Carbon in the Al4C− Anion. A Combined Photoelectron Spectroscopy and Ab Initio Study. J. Am. Chem. Soc. 1999, 121, 6033–6038. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.-F.; Wang, L.-S.; Geske, G.D.; Boldyrev, A.I. Pentaatomic Tetracoordinate Planar Carbon, [CAl4]2−: A New Structural Unit and Its Salt Complexes. Angew. Chem. 2000, 39, 3630–3632. [Google Scholar] [CrossRef]
- Keese, R. Carbon Flatland: Planar Tetracoordinate Carbon and Fenestranes. Chem. Rev. 2006, 106, 4787–4808. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Yu, S.; Ding, Y.; Bowen, K.H. Identifying the Hydrogenated Planar Tetracoordinate Carbon: A Combined Experimental and Theoretical Study of CAl4H and CAl4H–. J. Phys. Chem. Lett. 2017, 8, 2263–2267. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Du, Z. 18-Valence-Electron Rule Lighted Planar Tetracoordinate Carbon and Nitrogen: The Global Energy Minima of CAl4Zn and NAl4Zn+. Phys. Chem. Chem. Phys. 2023, 25, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Khatun, M.; Anoop, A.; Chattaraj, P.K. CSinGe4−n2+ (n = 1–3): Prospective Systems Containing Planar Tetracoordinate Carbon (PtC). Phys. Chem. Chem. Phys. 2022, 24, 16701–16711. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chattaraj, P.K. CSiGaAl2−/0 and CGeGaAl2−/0 Having Planar Tetracoordinate Carbon Atoms in Their Global Minimum Energy Structures. J. Comput. Chem. 2022, 43, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Parra, L.; Inostroza, D.; Yañez, O.; Cruz, J.C.; Garza, J.; García, V.; Tiznado, W. Persistent Planar Tetracoordinate Carbon in Global Minima Structures of Silicon-Carbon Clusters. Atoms 2022, 10, 27. [Google Scholar] [CrossRef]
- Liu, F.-L.; Guo, J.-C. Ternary CE2Ba2 (E = As, Sb) Clusters: New Pentaatomic Planar Tetracoordinate Carbon Species with 18 Valence Electrons. J. Mol. Model. 2022, 28, 230. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Chandrasekaran, V.; Cooksy, A.L.; Thimmakondu, V.S. Kinetic Stability of Si2C5H2 Isomer with a Planar Tetracoordinate Carbon Atom. Chemistry 2020, 3, 13–27. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Karton, A.; Thimmakondu, V.S. From High-Energy C7H2 Isomers with a Planar Tetracoordinate Carbon Atom to an Experimentally Known Carbene. J. Phys. Chem. A 2018, 122, 9054–9064. [Google Scholar] [CrossRef] [PubMed]
- Thimmakondu, V.S.; Thirumoorthy, K. Si3C2H2 Isomers with a Planar Tetracoordinate Carbon or Silicon Atom(s). Comput. Theor. Chem. 2019, 1157, 40–46. [Google Scholar] [CrossRef]
- Jayakumari, C.; Nag, P.; Isukapalli, S.; Vennapusa, S. Exploring the Excited-State Nonadiabatic Effects in the Semisaturated Planar Tetracoordinated Carbon Molecule C7H4. Atoms 2022, 10, 10. [Google Scholar] [CrossRef]
- Job, N.; Khatun, M.; Thirumoorthy, K.; CH, S.S.R.; Chandrasekaran, V.; Anoop, A.; Thimmakondu, V.S. CAl4Mg0/−: Global Minima with a Planar Tetracoordinate Carbon Atom. Atoms 2021, 9, 24. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Thimmakondu, V.S. Flat Crown Ethers with Planar Tetracoordinate Carbon Atoms. Int. J. Quantum Chem. 2021, 121, e26479. [Google Scholar] [CrossRef]
- Pei, Y.; An, W.; Ito, K.; Schleyer, P.V.R.; Xiao, C.Z. Planar Pentacoordinate Carbon in CAI5+: A Global Minimum. J. Am. Chem. Soc. 2008, 130, 10394–10400. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Halla, J.O.C.; Wu, Y.-B.; Wang, Z.-X.; Islas, R.; Heine, T.; Merino, G. CAl4Be and CAl3Be2−: Global Minima with a Planar Pentacoordinate Carbon Atom. Chem. Commun. 2010, 46, 8776. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Cabellos, J.L.; Orozco-Ic, M.; Chattaraj, P.K.; Zhao, L.; Merino, G. Planar Pentacoordinate Carbon in CGa5+ Derivatives. Phys. Chem. Chem. Phys. 2018, 20, 12350–12355. [Google Scholar] [CrossRef]
- Das, P.; Chattaraj, P.K. Structure and Bonding in Planar Hypercoordinate Carbon Compounds. Chemistry 2022, 4, 1723–1756. [Google Scholar] [CrossRef]
- Sarkar, P.; Thirumoorthy, K.; Anoop, A.; Thimmakondu, V.S. Planar Pentacoordinate Carbon in [XC7H2]2+ (X = Be and Mg) and Its Derivatives. Phys. Chem. Chem. Phys. 2022, 24, 27606–27611. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Jin, B.; Huo, B.; Yuan, C.; Zhai, H.-J.; Wu, Y.-B. Planar Pentacoordinate Carbon in a Sulphur-Surrounded Boron Wheel: The Global Minimum of CB5S5+. Chem. Commun. 2022, 58, 2552–2555. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, X.-F.; Jin, B.; Huo, B.; Bian, J.-H.; Guan, X.-L.; Yuan, C.; Wu, Y.-B. Influence of Stepwise Oxidation on the Structure, Stability, and Properties of Planar Pentacoordinate Carbon Species CAl5+. Phys. Chem. Chem. Phys. 2020, 22, 17062–17067. [Google Scholar] [CrossRef]
- Das, P.; Pan, S.; Chattaraj, P.K. Planar Hypercoordinate Carbon. In Atomic Clusters with Unusual Structure, Bonding and Reactivity; Elsevier: Amsterdam, The Netherlands, 2023; pp. 357–372. [Google Scholar]
- Vassilev-Galindo, V.; Pan, S.; Donald, K.J.; Merino, G. Planar Pentacoordinate Carbons. Nat. Rev. Chem. 2018, 2, 0114. [Google Scholar] [CrossRef]
- Yang, L.-M.; Ganz, E.; Chen, Z.; Wang, Z.-X.; Schleyer, P.V.R. Four Decades of the Chemistry of Planar Hypercoordinate Compounds. Angew. Chem. Int. Ed. 2015, 54, 9468–9501. [Google Scholar] [CrossRef]
- Exner, K.; Schleyer, P.V.R. Planar Hexacoordinate Carbon: A Viable Possibility. Science 2000, 290, 1937–1940. [Google Scholar] [CrossRef]
- Ito, K.; Chen, Z.F.; Corminboeuf, C.; Wannere, C.S.; Zhang, X.H.; Li, Q.S.; Schleyer, P.v.R. Myriad Planar Hexacoordinate Carbon Molecules Inviting Synthesis. J. Am. Chem. Soc. 2007, 129, 1510–1511. [Google Scholar] [CrossRef]
- Inostroza, D.; Leyva-Parra, L.; Yañez, O.; Solar-Encinas, J.; Vásquez-Espinal, A.; Valenzuela, M.L.; Tiznado, W. Searching for Systems with Planar Hexacoordinate Carbons. Atoms 2023, 11, 56. [Google Scholar] [CrossRef]
- Leyva-Parra, L.; Diego, L.; Yañez, O.; Inostroza, D.; Barroso, J.; Vásquez-Espinal, A.; Merino, G.; Tiznado, W. Planar Hexacoordinate Carbons: Half Covalent, Half Ionic. Angew. Chem. Int. Ed. 2021, 60, 8700–8704. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Ansell, G.B.; Leonowicz, M.E.; Hill, E.W. Synthesis and Molecular Structure of. Mu.4-Carbido-.Mu.2-Carbonyl-Dodecacarbonyltetrairon, a Neutral Iron Butterfly Cluster Bearing an Exposed Carbon Atom. J. Am. Chem. Soc. 1981, 103, 4968–4970. [Google Scholar] [CrossRef]
- Radius, U.; Silverio, S.J.; Hoffmann, R.; Gleiter, R. A Five-Coordinate Carbon Center and Zr to H, B, and C Bonding in Cp2Zr[CH2(BH{C6F5}2)2]. Organometallics 1996, 15, 3737–3745. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Guerrero, R.; Jones, P.G. Synthesis of the First Complex with a Hypercoordinate Ylidic Carbon Atom. Crystal and Molecular Structure of [{Au(PPh3)}4CS(=O)Me2](ClO4)2. J. Am. Chem. Soc. 1996, 118, 699–700. [Google Scholar] [CrossRef]
- Shajan, S.; Guo, J.-C.; Sinjari, A.; Thirumoorthy, K.; Thimmakondu, V.S. Pentacoordinate Carbon Atoms in a Ferrocene Dication Derivative—[Fe(Si2-H5-C5H2)2]2+. Chemistry 2022, 4, 1092–1100. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Wang, P.; Xu, X.-L.; Xu, H.-G.; Zheng, W.-J. Photoelectron Spectroscopy and Theoretical Study of AlnC5 −/0 (n = 1–5) Clusters: Structural Evolution, Relative Stability of Star-like Clusters, and Planar Tetracoordinate Carbon Structures. Phys. Chem. Chem. Phys. 2021, 23, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Ravell, E.; Jalife, S.; Barroso, J.; Orozco-Ic, M.; Hernández-Juárez, G.; Ortiz-Chi, F.; Pan, S.; Cabellos, J.L.; Merino, G. Structure and Bonding in CE5− (E=Al-Tl) Clusters: Planar Tetracoordinate Carbon versus Pentacoordinate Carbon. Chem. Asian J. 2018, 13, 1467–1473. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, J.; Lu, H.; Wang, Z.; Perez-Peralta, N.; Islas, R.; Contreras, M.; Merino, G.; Wu, J.I.; von Ragué Schleyer, P. Starlike Aluminum–Carbon Aromatic Species. Chem. A Eur. J. 2011, 17, 714–719. [Google Scholar] [CrossRef]
- Bai, L.-X.; Guo, J.-C. CAl4X4 (X = Te, Po): Double Aromatic Molecular Stars Containing Planar Tetracoordinate Carbon Atoms. Molecules 2023, 28, 3280. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Chen, H. Dissociation of H2 on Carbon Doped Aluminum Cluster Al6C. J. Chem. Phys. 2014, 141, 064302. [Google Scholar] [CrossRef] [PubMed]
- Ermoline, A.; Schoenitz, M.; Dreizin, E.; Yao, N. Production of Carbon-Coated Aluminium Nanopowders in Pulsed Microarc Discharge. Nanotechnology 2002, 13, 638–643. [Google Scholar] [CrossRef]
- Kotov, Y.A.; Beketov, I.V.; Medvedev, A.I.; Murzakaev, A.M.; Timoshenkova, O.P.; Demina, T.M. Forming a Carbide Coating on the Surface of Aluminum Nanoparticles and Producing Nanopowders from Al-Al4C3 Using the Method of Electric Explosion of Wire. Nanotechnol. Russ. 2010, 5, 831–836. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; Schleyer, P.v.R.; Chen, Z. Al2C Monolayer: The Planar Tetracoordinate Carbon Global Minimum. Nanoscale 2014, 6, 10784. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Xu, H.-G.; Xu, X.-L.; Zheng, W.-J. Anion Photoelectron Spectroscopy and Theoretical Studies of Al4C6–/0: Global Minimum Triangle-Shaped Structures and Hexacoordinated Aluminum. J. Phys. Chem. A 2021, 125, 302–307. [Google Scholar] [CrossRef]
- Kalita, A.J.; Sarmah, K.; Yashmin, F.; Borah, R.R.; Baruah, I.; Deka, R.P.; Guha, A.K. σ-Aromaticity in Planar Pentacoordinate Aluminium and Gallium Clusters. Sci. Rep. 2022, 12, 10041. [Google Scholar] [CrossRef]
- Malhan, A.H.; Sobinson, S.; Job, N.; Shajan, S.; Mohanty, S.P.; Thimmakondu, V.S.; Thirumoorthy, K. Al2C4H2 Isomers with the Planar Tetracoordinate Carbon (PtC)/Aluminum (PtAl). Atoms 2022, 10, 112. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Alexandrova, A.N.; Birch, K.A.; Boldyrev, A.I.; Wang, L.-S. Hepta- and Octacoordinate Boron in Molecular Wheels of Eight- and Nine-Atom Boron Clusters: Observation and Confirmation. Angew. Chem. 2003, 115, 6186–6190. [Google Scholar] [CrossRef]
- Li, S.D.; Miao, C.Q.; Ren, G.M. D5h Cu5H5X: Pentagonal Hydrocopper Cu5H5 Containing Pentacoordinate Planar Nonmetal Centers (X = B, C, N, O). Eur. J. Inorg. Chem. 2004, 2232–2234. [Google Scholar] [CrossRef]
- Khatun, M.; Roy, S.; Giri, S.; CH, S.S.R.; Anoop, A.; Thimmakondu, V.S. BAl4Mg−/0/+: Global Minima with a Planar Tetracoordinate or Hypercoordinate Boron Atom. Atoms 2021, 9, 89. [Google Scholar] [CrossRef]
- Das, P.; Patra, S.G.; Chattaraj, P.K. CB6Al0/+: Planar Hexacoordinate Boron (PhB) in the Global Minimum Structure. Phys. Chem. Chem. Phys. 2022, 24, 22634–22644. [Google Scholar] [CrossRef]
- Thompson, E.J.; Myers, T.W.; Berben, L.A. Synthesis of Square-Planar Aluminum(III) Complexes. Angew. Chem. Int. Ed. 2014, 53, 14132–14134. [Google Scholar] [CrossRef]
- Ebner, F.; Wadepohl, H.; Greb, L. Calix [4]Pyrrole Aluminate: A Planar Tetracoordinate Aluminum(III) Anion and Its Unusual Lewis Acidity. J. Am. Chem. Soc. 2019, 141, 18009–18012. [Google Scholar] [CrossRef]
- Bai, L.-X.; Barroso, J.; Orozco-Ic, M.; Ortiz-Chi, F.; Guo, J.-C.; Merino, G. : A Molecular Rotor with a Quasi-Planar Tetracoordinate Carbon. Chem. Commun. 2023, 59, 4966–4969. [Google Scholar] [CrossRef] [PubMed]
- Leskiw, B.D.; Castleman, A.W. The Interplay between the Electronic Structure and Reactivity of Aluminum Clusters: Model Systems as Building Blocks for Cluster Assembled Materials. Chem. Phys. Lett. 2000, 316, 31–36. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Zhai, H.; Zhou, R.; Ni, G.; Xu, Z. Mass Spectrometric and First Principles Study of AlnC− Clusters. Solid State Commun. 2002, 122, 543–547. [Google Scholar] [CrossRef]
- Maatallah, M.; Guo, M.; Cherqaoui, D.; Jarid, A.; Liebman, J.F. Aluminium Clusters for Molecular Hydrogen Storage and the Corresponding Alanes as Fuel Alternatives: A Structural and Energetic Analysis. Int. J. Hydrogen Energy 2013, 38, 5758–5767. [Google Scholar] [CrossRef]
- Dai, J.; Wu, X.; Yang, J.; Zeng, X.C. AlxC Monolayer Sheets: Two-Dimensional Networks with Planar Tetracoordinate Carbon and Potential Applications as Donor Materials in Solar Cell. J. Phys. Chem. Lett. 2014, 5, 2058–2065. [Google Scholar] [CrossRef]
- Dong, F.; Heinbuch, S.; Xie, Y.; Rocca, J.J.; Bernstein, E.R. Experimental and Theoretical Study of Neutral AlmCn and AlmCnHx Clusters. Phys. Chem. Chem. Phys. 2010, 12, 2569. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Perdew, J.P. Generalized Gradient Approximation to the Angle- and System-Averaged Exchange Hole. J. Chem. Phys. 1998, 109, 3313–3320. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Developing Paradigms of Chemical Bonding: Adaptive Natural Density Partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing Intuitively Assessable Chemical Bonding Patterns in Organic Aromatic Molecules via Adaptive Natural Density Partitioning. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Richard, F.W. Bader Atoms in Molecules. A Quantum Theory; Oxford University Press: Oxford, UK, 1990; ISBN 9780198558651. [Google Scholar]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schlegel, H.B.; Millam, J.M.; Iyengar, S.S.; Voth, G.A.; Daniels, A.D.; Scuseria, G.E.; Frisch, M.J. Ab Initio Molecular Dynamics: Propagating the Density Matrix with Gaussian Orbitals. J. Chem. Phys. 2001, 114, 9758–9763. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).