Synthesis of the Bipyridine-Type Ligand 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine and Structural Elucidation of Its Cu(I) and Ag(I) Complexes
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods of Study
2.2. Synthesis of the Diimine Ligand
2.3. Synthesis of the Complexes
2.4. Crystallography
3. Results and Discussion
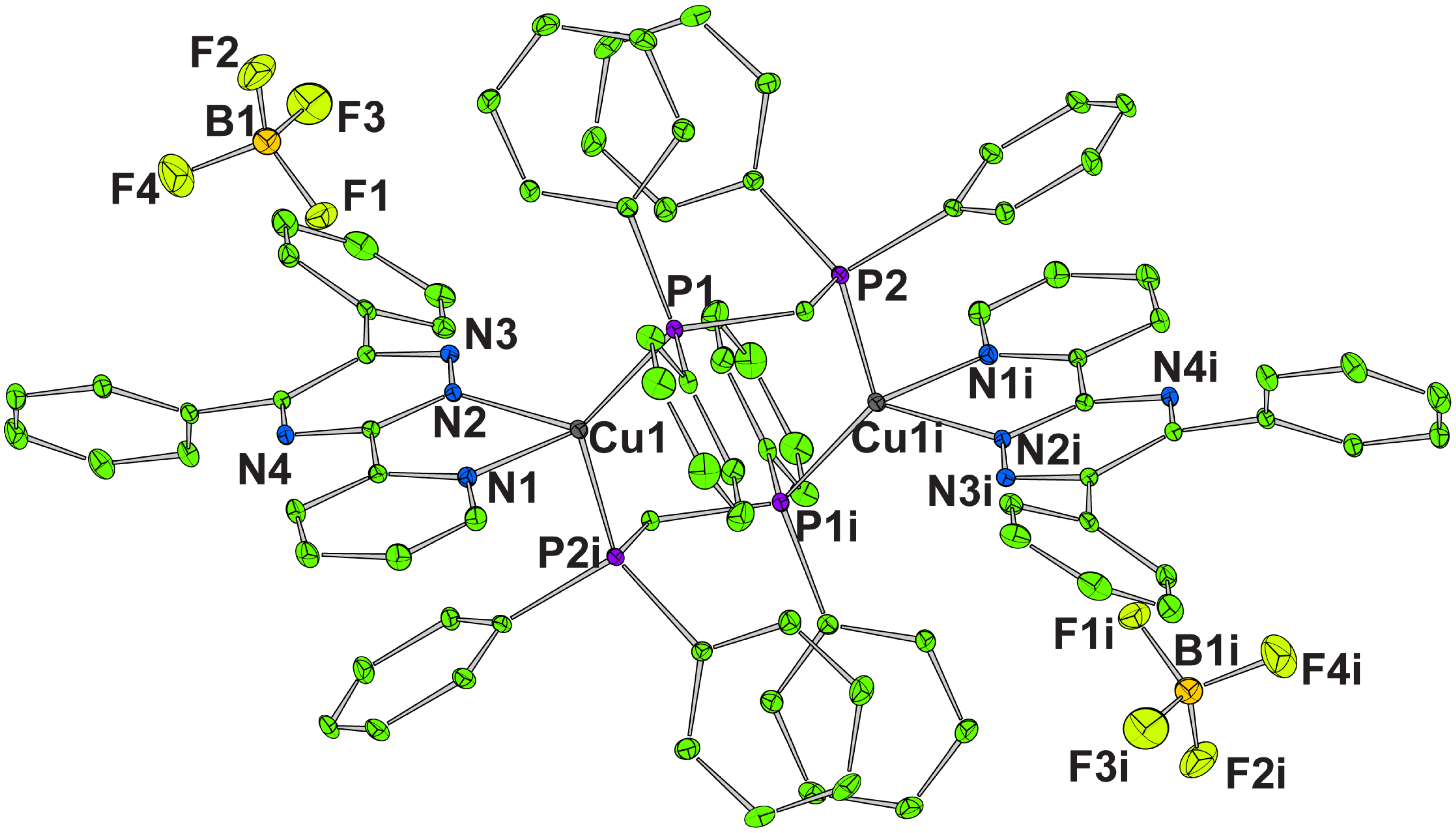
3.1. Crystal Structure of [{Cu(L)}2(μ-dppm)2](BF4)2
3.2. Crystal Structure of [{Ag(L)}2(μ-dppm)2] (NO3)2 1.5(H2O)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van Koten, G.; Vrieze, K. 1,4-Diaza-1,3-butadiene (α-diimine) ligands: Their coordination modes and the reactivity of their metal complexes. Adv. Organomet. Chem. 1982, 21, 151–239. [Google Scholar]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Kyzioł, A.; Cierniak, A.; Gubernator, J.; Markowski, A.; Jezowska-Bojczuk, M.; Komarnicka, U.K. Copper(I) complexes with phosphine derived from sparfloxacin. Part III: Multifaceted cell death and preliminary study of liposomal formulation of selected copper(I) complexes. JCS Dalton Trans. 2018, 47, 1981–1992. [Google Scholar] [CrossRef]
- Gandin, V.; Porchia, M.; Tisato, F.; Zanella, A.; Severin, E.; Dolmella, A.; Marzano, C. Novel mixed-ligand copper(I) complexes: Role of diimine ligands on cytotoxicity and genotoxicity. J. Med. Chem. 2013, 56, 7416–7430. [Google Scholar] [CrossRef] [PubMed]
- Barnard, P.J.; Berners-Price, S.J. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Fischer, S.; Jurrat, M.; Luo, S.P.; Rockstroh, N.; Junge, H.; Ludwig, R.; Beller, M. Copper-Based Photosensitisers in Water Reduction: A More Efficient In Situ Formed System and Improved Mechanistic Understanding. Chem. Eur. J. 2016, 22, 1233–1238. [Google Scholar] [CrossRef]
- Reiser, O. Shining Light on Copper: Unique Opportunities for Visible-Light-Catalyzed Atom Transfer Radical Addition Reactions and Related Processes. Acc. Chem. Res. 2016, 49, 1990–1996. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards efficient sustainable full-copper dye-sensitized solar cells. JCS Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Appleby, M.V.; Walker, P.G.; Pritchard, D.; van Meurs, S.; Booth, C.M.; Robertson, C.; Ward, M.D.; Kelly, D.J.; Weinstein, J.A. Cu(I) diimine complexes as immobilised antibacterial photosensitisers operating in water under visible light. Mater. Adv. 2020, 1, 3417–3427. [Google Scholar] [CrossRef]
- McMillin, D.R.; Kirchhoff, J.R.; Goodwin, K.V. Exciplex quenching of photo-excitd copper complexes. Coord. Chem. Rev. 1985, 64, 83–92. [Google Scholar] [CrossRef]
- Miller, M.T.; Gantzel, P.K.; Karpishin, T.B. Structures of the Copper(I) and Copper(II) Complexes of 2,9-Diphenyl-1,10-phenanthroline: Implications for Excited-State Structural Distortion. Inorg. Chem. 1999, 38, 2285–2290. [Google Scholar] [CrossRef]
- Smith, C.S.; Branham, C.W.; Marquardt, B.J.; Mann, K.R. Oxygen gas sensing by luminescence quenching in crystals of Cu(xantphos)(phen)+ complexes. J. Am. Chem. Soc. 2010, 132, 14079–14085. [Google Scholar] [CrossRef]
- Keller, S.; Brunner, F.; Junquera-Hernández, J.M.; Pertegás, A.; La-Placa, M.-G.; Prescimone, A.; Bolink, H.J.; Orti, E.; Constable, E.C.; Housecroft, C.E. CF3 Substitution of [Cu(P^P)(bpy)][PF6] Complexes: Effects on Photophysical Properties and Light-Emitting Electrochemical Cell Performance. ChemPlusChem 2018, 83, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schulz, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Heterolepticdiimine–diphosphine Cu(I) complexes as an alternative towards noble-metal based photosensitizers: Design strategies, photophysical properties and perspective applications. Coord. Chem. Rev. 2018, 356, 127–146. [Google Scholar]
- Wallesch, M.; Volz, D.; Zink, D.M.; Schepers, U.; Nieger, M.; Baumann, T.; Bräse, S. Bright Coppertunities: Multinuclear CuI Complexes with N–P Ligands and Their Applications. Chem. Eur. J. 2014, 20, 6578–6590. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Copper(I) and silver(I) complexes of 9,9-dimethyl-4,5-bis(di-tert-butylphosphino)xanthene: Photophysical properties and structural rigidity under pressure. Photochem. Photobiol. Sci. 2018, 17, 375–385. [Google Scholar] [CrossRef]
- Hsu, C.W.; Lin, C.C.; Chung, M.W.; Chi, Y.; Lee, G.H.; Chou, P.T.; Chang, C.H.; Chen, P.Y. Systematic Investigation of the Metal-Structure–Photophysics Relationship of Emissive d10-Complexes of Group 11 Elements: The Prospect of Application in Organic Light Emitting Devices. J. Am. Chem. Soc. 2011, 133, 12085–12099. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Santos, C.I.M.; Welter, R.; Aullón, G.; Lodeiro, C.; Avilés, T. Comparison of the Structure and Stability of New α-Diimine Complexes of Copper(I) and Silver(I): Density Functional Theory versus Experimental. Inorg. Chem. 2010, 49, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Babaei, A.; Pertegás, A.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. Phosphane tuning in heteroleptic [Cu(N^N)(P^P)]+ complexes for light-emitting electrochemical cells. JCS Dalton Trans. 2019, 48, 446–460. [Google Scholar] [CrossRef]
- Figeys, H.P.; Mathy, A. Diels-alder reactions with inverse electron demand. II. The reaction of benzamidine with π-deficient heteroaromatic compounds. Tetrahedron Lett. 1981, 22, 1393–1396. [Google Scholar] [CrossRef]
- Zhao, Z.; Leister, W.H.; Strauss, K.A.; Wisnoski, D.D.; Lindsley, G.W. Broadening the scope of 1,2,4-triazine synthesis by the application of microwave technology. Tetrahedron Lett. 2003, 44, 1123–1127. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Systems, Inc. Apex2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Siemens Industrial Automation, Inc. SADABS: Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. Software for Guided Crystal Structure Analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Watkin, D.J.; Prout, C.K.; Pearce, L.J. Chemical Crystallography Laboratory; University of Oxford: Oxford, UK, 1996. [Google Scholar]
- DIAMOND—Crystal and Molecular Structure Visualization, Version 3.1c; Crystal Impact: Bonn, Germany, 2006.
- Uma, R.; Palaniandavar, M.; Butcher, R.J. Synthesis, structure, spectra and redox interconversions in copper(II) complexes of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. J. Chem. Soc. Dalton Trans. 1996, 10, 2061–2066. [Google Scholar] [CrossRef]
- Eltayeb, N.E.; Teoh, S.G.; Ng, S.-L.; Funb, H.-K.; Ibrahim, K. 5,6-Diphenyl-3-(2-pyridyl)-1,2,4-triazine. Acta Cryst. 2007, E63, o1041–o1042. [Google Scholar] [CrossRef]
- James, J.P. Stewart; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2016; Available online: http://OpenMOPAC.net (accessed on 20 November 2022).
- Keyes, T.E.; WeIdon, F.; Muller, E.; Pechy, P.; Gratzel, M.; Vos, J.G. Application of Deuteriation to Determine the Location of the Emitting State in Mixed-ligand RuII Polypyridyl Complexes. J. Chem. Soc. Dalton Trans. 1995, 16, 2705–2706. [Google Scholar] [CrossRef]
- Hobbollahi, E.; Himmelsbach, M.; List, M.; Monkowius, U. Synthesis and characterization of dinuclear silver(I) complexes with exchangeable nitrile ligands. Inorg. Chem. Commun. 2016, 71, 105. [Google Scholar] [CrossRef]
- Hoffmann, M.; Dagorne, S.; Pale, P.; Blanc, A.; de Frémont, P. Dinuclear Silver(I) and Gold(I) Complexes with Chiral Oxazoline-NHC Ligands. J. Organomet. Chem. 2022, 979, 122507. [Google Scholar] [CrossRef]



| Crystal Data | ||
| Chemical formula Moiety formula | C90H72B2Cu2F8N8P4 C90H72Cu2N8P4, 2(BF4) | C90H75Ag2N10O7.50P4 C90H72Ag2N8P4, 2(NO3), 1.5(H2O) |
| Mr | 1690.21 | 1756.28 |
| Crystal system, Space group | Monoclinic P21/n | Triclinic Pī |
| Temperature (K) | 295 | 295 |
| a (Å) b (Å) c (Å) α (°) | 11.4136 (12) 25.281 (3) 14.3061 (17) 90 | 15.771 (2) 17.242 (2) 19.635 (3) 64.301 (4) |
| β (°) γ (°) | 104.919 (4) 90 | 68.549 (4) 62.873 (4) |
| V (Å3) | 3988.8 (8) | 4186.8 (10) |
| Z | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.69 | 0.61 |
| Crystal size (mm) | 0.14 × 0.13 × 0.11 | 0.20 × 0.19 × 0.12 |
| Tmin, Tmax | 0.91, 0.93 | 0.89, 0.93 |
| No. of reflections | ||
| Measured Independent Observed [I > 2.0σ(I)] | 35,566 7633 5673 | 70,037 16,094 11,509 |
| Rint | 0.055 | 0.029 |
| (sin θ/λ)max (Å−1) | 0.612 | 0.615 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.045 0.096 1.00 | 0.046 0.108 1.00 |
| No. of reflections | 5673 | 11,509 |
| No. of parameters | 514 | 1018 |
| No. of restraints | - | 8 |
| Δρmax, Δρmin (e Å−3) | 0.57–0.39 | 1.27–0.74 |
| Cu1—P2i | 2.2610 (8) | P2i—Cu1—P1 | 130.85 (3) |
| Cu1—P1 | 2.2259 (8) | P2i—Cu1—N1 | 106.49 (7) |
| Cu1—N1 | 2.122 (3) | P1—Cu1—N1 | 107.45 (7) |
| Cu1—N2 | 2.113 (2) | P2i—Cu1—N2 | 93.42 (7) |
| Ag1—P1 | 2.4526 (10) | P1—Ag1—P3 | 159.91 (4) |
| Ag1—P3 | 2.4168 (10) | P1—Ag1—N1 | 98.05 (8) |
| Ag1—N1 | 2.463 (4) | P3—Ag1—N1 | 100.75 (8) |
| Ag1—N2 | 2.565 (3) | P1—Ag1—N2 | 94.86 (8) |
| Ag2—P2 | 2.4197 (10) | P3—Ag1—N2 | 99.43 (8) |
| Ag2—P4 | 2.4544 (10) | N1—Ag1—N2 | 64.99 (11) |
| Ag2—N5 | 2.491 (4) | P2—Ag2—P4 | 159.83 (4) |
| Ag2—N6 | 2.598 (3) | P2—Ag2—N5 | 101.69 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatzidimitriou, A.; Stamatiou, A.; Tzimopoulos, D.; Akrivos, P.D. Synthesis of the Bipyridine-Type Ligand 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine and Structural Elucidation of Its Cu(I) and Ag(I) Complexes. Chemistry 2023, 5, 1508-1517. https://doi.org/10.3390/chemistry5030103
Hatzidimitriou A, Stamatiou A, Tzimopoulos D, Akrivos PD. Synthesis of the Bipyridine-Type Ligand 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine and Structural Elucidation of Its Cu(I) and Ag(I) Complexes. Chemistry. 2023; 5(3):1508-1517. https://doi.org/10.3390/chemistry5030103
Chicago/Turabian StyleHatzidimitriou, Antonios, Antonios Stamatiou, Dimitrios Tzimopoulos, and Pericles D. Akrivos. 2023. "Synthesis of the Bipyridine-Type Ligand 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine and Structural Elucidation of Its Cu(I) and Ag(I) Complexes" Chemistry 5, no. 3: 1508-1517. https://doi.org/10.3390/chemistry5030103
APA StyleHatzidimitriou, A., Stamatiou, A., Tzimopoulos, D., & Akrivos, P. D. (2023). Synthesis of the Bipyridine-Type Ligand 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine and Structural Elucidation of Its Cu(I) and Ag(I) Complexes. Chemistry, 5(3), 1508-1517. https://doi.org/10.3390/chemistry5030103







