Abstract
Exploring affordable and efficient platform for innovative DNA computing is of great significance. Herein, by coupling 2-aminopurine (2AP) with DNA copper nanoparticles (CuNPs) as two universal opposite outputs, we, for the first time, fabricated a rapid and enzyme-free system for operating DNA contrary logic pairs (D-CLPs). Notably, derived from the rapid and concomitant response of both fluorescent probes, different D-CLPs can be achieved via a “double-results-half-efforts” manner in less than 20 min with low-cost. Moreover, based on the same system, the smart ratiometric analysis of target DNA was realized by employing the high reliability and accuracy of D-CLPs, providing a robust and typical paradigm for the exploration of smart nucleic acid sensors.
1. Introduction
In recent decades, we have witnessed the significant impacts generated by silicon-templated semiconductor computers on various aspects of our lives. Meanwhile, when it comes to the word “Molecular Computation”, this can be traced back to the groundbreaking work of Prof. de Silva in 1993 [1]. Through mimicking the Boolean operation (0/1) at the molecular level, different output signals can be obtained after inputting specific binary stimuli to the appropriate platforms. Since the first molecular AND gate was designed [1], this area has flourished by utilizing multifarious components (proteins, enzymes, antibodies, nanomaterials, etc.) as building blocks [2,3,4,5,6,7], and recent efforts have been largely directed to the fields “where silicon counterparts cannot go”.
Among the various molecular logic systems, DNA-based ones have aroused substantial interest from molecular engineers. Because of the affordability, controllable design, and accuracy of Watson–Crick pairs, this kind of genetic material has been recognized as the most outstanding and interesting candidate [8,9,10,11,12,13]. With the surprising progress of DNA nanotechnology over the past several decades [14,15,16,17,18,19], DNA-based computing systems have been extensively explored and widely applied to logic-controlled biosensing, cancer diagnosis, drug release, subcellular imaging, and other smart bio-applications [20,21,22,23,24,25,26]. In particular, Dong’s group proposed a novel concept of DNA “contrary logic pair” (CLP) not long ago, and designed an intelligent platform for operating CLPs and combinatorial circuits based on the peroxidase-like property of G-quadruplex DNAzyme [27,28], in which logic gates with opposite functions (e.g., YES + NOT) were executed via the same DNA reaction simultaneously. After that, electrochemical [29] and electro-chemiluminescent [30] CLP systems were successively reported by using toehold-mediated strand displacement (TMSD) reactions and exonuclease III (Exo III). Despite the above achievements, there are still great restrictions in current CLP platforms. For example, the expensive tool enzymes (such as Exo III) that require critical conditions and nanomaterials (upconversion nanoparticles (UNCPs) and C3N4 nanosheets) that need tedious synthesis steps [28,31] were frequently used, resulting in almost unchanged long operation times (2–6 h) and high costs. Taking the above drawbacks into account, there is an urgent need to develop a rapid and enzyme-free system for executing CLP functions.
Functional nucleic acids (FNAs) are DNA/RNA molecules with specific structures or functions, such as G-quadruplex (G4), i-motif, DNA-templated nanomaterials, etc. [32,33,34,35,36,37,38]. Similar to FNAs, some nucleoside bases with specific properties are also promising building blocks for DNA computing. Among these, 2-aminopurine (2AP), a fluorescent analogue of adenine [39,40], has been confirmed to act as excellent quencher-free fluorescent probe and the fluorescence of 2AP greatly relies on its surrounding microenvironment. In particular, Li’s group demonstrated that the fluorescence intensity at 370 nm of single-base looped-out 2AP in duplex can be greatly improved, when compared with that of 2AP in single-stranded DNA and fully complemented duplexes [41]. This phenomenon can be attributed to the destruction of electron transfer quenching of π-π aromatic stacking between two neighboring bases. Based on the instant and sensitive fluorescence properties of 2AP, many versatile biosensors have been constructed [42,43,44]. Apart from 2AP, DNA-templated copper nanoparticles (CuNPs) are also excellent fluorophores via reducing Cu2+ on DNA strands using ascorbic acid. Specifically, Wang’s group found that poly-thymine strands could work as satisfactory templates for the formation of CuNPs at minute levels, which can exhibit high fluorescence intensity at 625 nm [45,46]. Moreover, subsequent works also reported that poly-adenine strands could work as excellent blocking elements for poly-T CuNPs [45]. Due to the easy synthesis, instant response, and flexible design, DNA CuNPs have been widely employed in biosensing, biocomputing, and even cellular imaging. The characteristic fluorescence emissions and fast responses of these two probes inspired us to explore the possibility of integrating them together as a universal dual-output to design CLPs.
Herein, by coupling 2AP with DNA CuNPs as universal opposite dual output, we, for the first time, report a rapid and enzyme-free system for operating DNA CLPs (D-CLPs). It should be noted that, benefiting from the rapid and concomitant response of both fluorescent probes, different D-CLPs were achieved via a “double-results-half-efforts” way in just less than 20 min. Moreover, based on the above system and taking A25 strand as a model target, the smart ratiometric analysis of poly-A DNA was achieved by taking advantage of the high reliability and accuracy of D-CLPs.
2. Discussion and Results
2.1. Experimental Section
2.1.1. Materials and Reagents
All DNA samples were obtained from Shanghai Sangon Biotechnology Co. (Shanghai, China) and the sequences are shown in Table S1 (in the Supplementary Information). The oligonucleotides were dissolved in ultrapure water as stock solutions. All experiments were conducted using 3-(N-morpholino) propanesulfonic acid (MOPS) buffer (10 mM MOPS, 150 mM NaCl, pH 7.5). MOPS was purchased from Aldrich (St. Louis, MO, USA); CuSO4 and ascorbic acid (AA) were obtained from Sinopharm Chemical Regent Co. (Shanghai, China). All chemicals were of analytical grade and the water used in the experiments was purified by a Millipore system.
2.1.2. Synthesis of CuNPs and Characterization
In a typical process, the stock solutions of template DNA (2AP-T20) were diluted to the desired concentrations (250 nM) with 1× MOPS buffer. After that, 2 mM AA was added, followed by shaking for 1 min. Then, 100 μM of CuSO4 was added, followed by another 2 min of shaking. After incubation at room temperature for approximately 10 min, the fluorescence spectra of the CuNPs were collected. High-resolution transmission electron microscopy (HRTEM) tests of CuNPs were performed using an FEI-TECNAI-G2-F20 microscope that operates at 200 KV.
2.1.3. Apparatus
The fluorescence spectra were collected using a F-4700 Fluorescence Spectrofluorometer (Hitachi High-Tech Science Corporation, Tokyo, Japan). The emission spectra of 2AP were collected from 325 to 550 nm with an excitation wavelength of 300 nm. The slit widths for the excitation and emission were 5 nm and 10 nm, respectively. The fluorescence spectra of the CuNPs were obtained from 550 nm to 660 nm after excitation at 340 nm and the excitation and emission slit widths were both 10 nm.
2.1.4. Native Polyacrylamide Gel Electrophoresis (PAGE)
The DNA stock solutions were diluted to a suitable concentration by 1 × MOPS buffer and heated at 88 °C for 8 min, then slowly cooled down to room temperature. After that, the desired volume of the 2AP-T20 and A20-C2 solution was mixed and added into 1× MOPS buffer to give a final concentration of 2 μM. After a 30 min incubation, the DNA solutions were analyzed using a 15% native polyacrylamide gel. Electrophoresis was conducted in 1 × TBE (17.80 mM Tris, 17.80 mM boric acid, 2 mM EDTA, pH 8.0) at a constant voltage of 60 V for 2.5 h. After staining with gel dye, the gels were scanned by a UV transilluminator.
2.1.5. Operation of YES/NOT Gate
Firstly, all the DNA solutions were heated at 88 °C for 8 min and slowly cooled down to room temperature. In addition, three important elements of 250 nM 2AP-T20, 2 mM AA, and 100 μM Cu2+ were used as the platform of the contrary logical pairs in this work.
For the construction of the YES/NOT gate, 300 nM A20-C1 was used as the input; 300 nM A20-C1 was incubated with 250 nM 2AP-T20 for 15 min in 1× MOPS buffer. Then, the collected fluorescence signal of 2AP at 370 nm was the output of the YES gate. After that, 2 mM AA was added to the solution, followed by the addition of 100 μM Cu2+. After shaking, the mixture was reacted in a dark environment for another 10 min. The fluorescence intensity of the CuNPs at 625 nm (output of the NOT gate) was measured under excitation at 340 nm.
2.1.6. Operation of OR/NOR Gate
The annealing process of all DNAs and the platform were the same as the YES/NOT gate mentioned above. For the construction of the OR/NOR gate, 300 nM A20-C1 and 300 nM A22-C1 were used as two inputs. Different input combinations were added into the platform for 15 min. The subsequent steps were the same as those for the construction of the YES/NOT gate, after which, the fluorescence signals of 2AP and CuNPs were collected.
2.1.7. Ratiometric Fluorescent Detection of A25
Firstly, 250 nM T30-2AP and different concentrations of A25 were annealed. Then, both were hybridized in 1× MOPS buffer with a final volume of 460 μL for 15 min and the fluorescence spectra of 2AP were collected. Subsequently, the CuNPs could be synthesized and their fluorescence signals were also obtained after following the steps mentioned above.
2.2. Mechanism and Verification Experiments
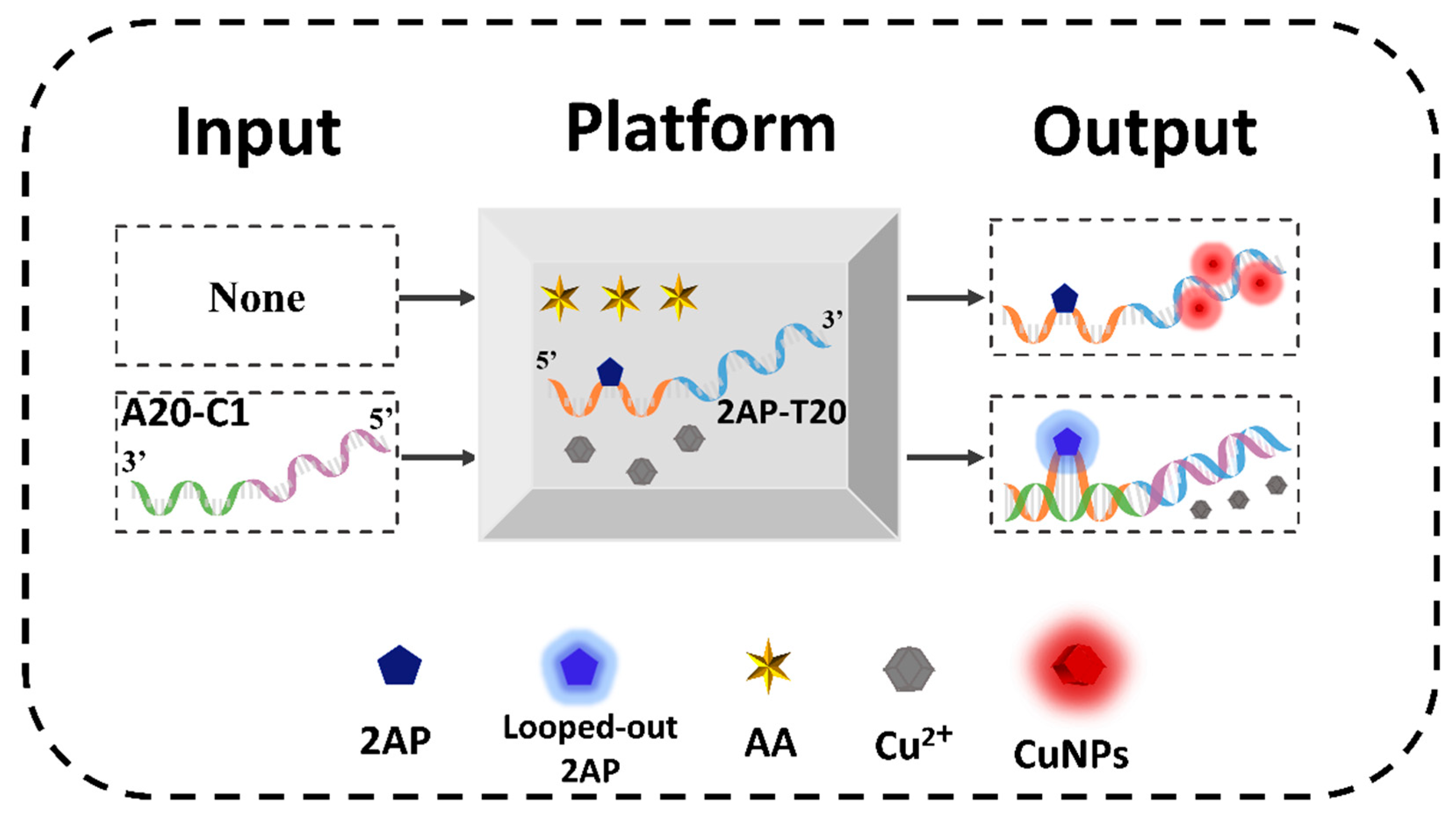
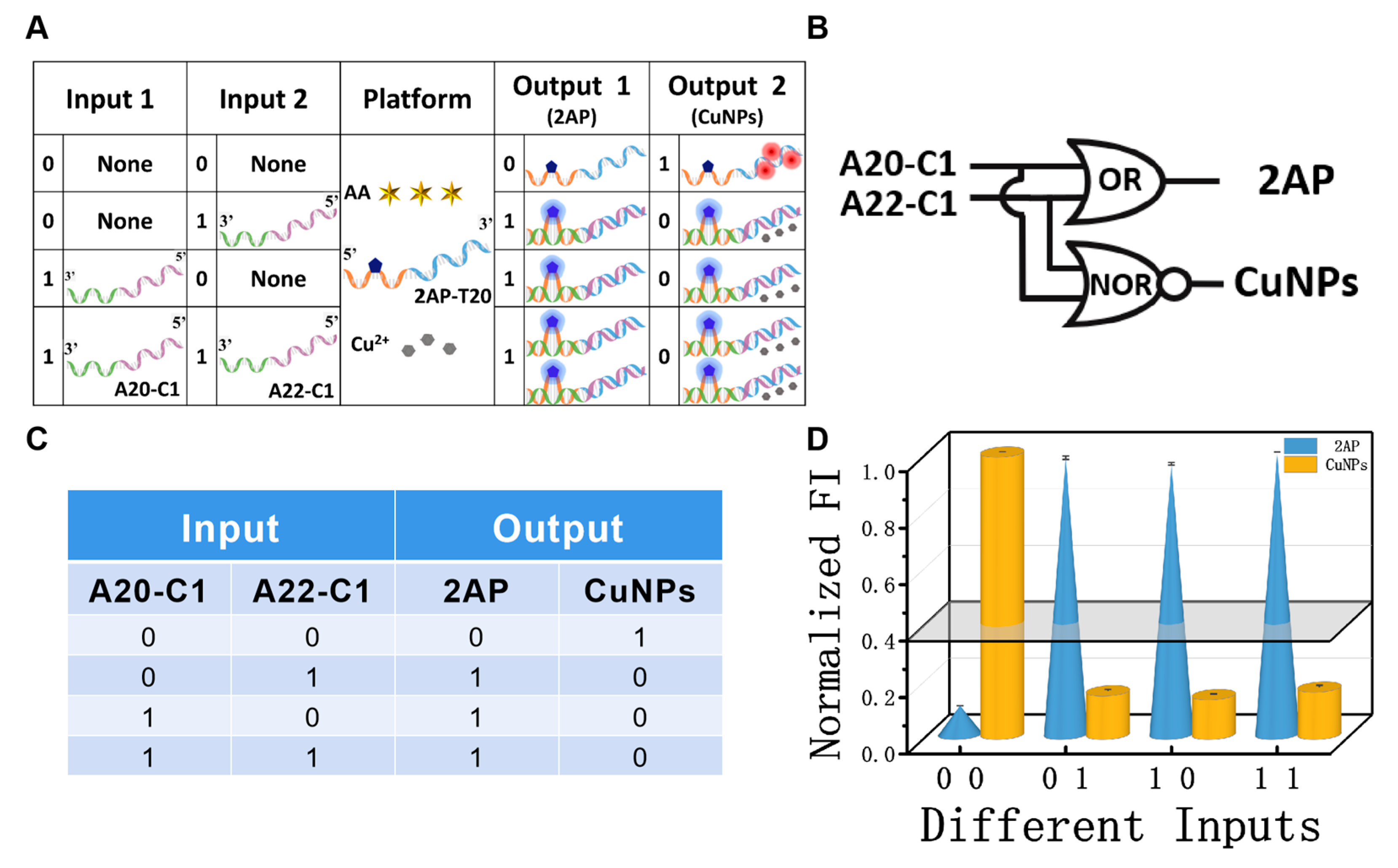
Before the operation of DNA CLPs (D-CLPs), the definitions of platform, inputs, and outputs are illustrated and the corresponding verification experiments were performed. The mixture of AA, Cu2+, and 2AP-T20 was used as the universal platform (Scheme 1), in which 2AP-T20 is a single strand that integrates 20 consecutive T bases at the 3′ end (blue color) with 21 random sequences (X part, orange color) at the 5′ end, and 2AP was inserted as the 11th base of the X part. The fluorescence emission of 2AP at 370 nm and that of the CuNPs at 625 nm were employed as the positive Output 1 and negative Output 2 of the D-CLPs, respectively (Scheme 1). A normalized fluorescence intensity of 0.40 was set as the universal threshold value to judge the high and low outputs. The high fluorescence signal was defined as “1”, and conversely, the low one was defined as “0”. To achieve different logic functions, distinct poly-A strands with subtle design were used as inputs.
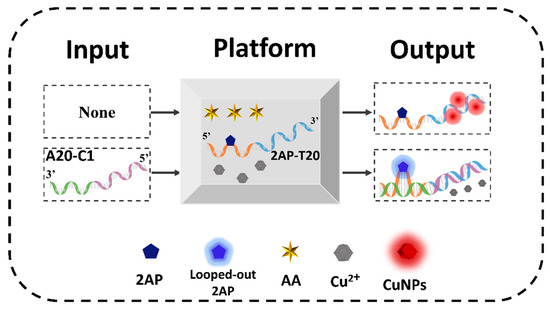
Scheme 1.
Mechanism of DNA contrary logic pairs based on the integration of 2AP and CuNPs.
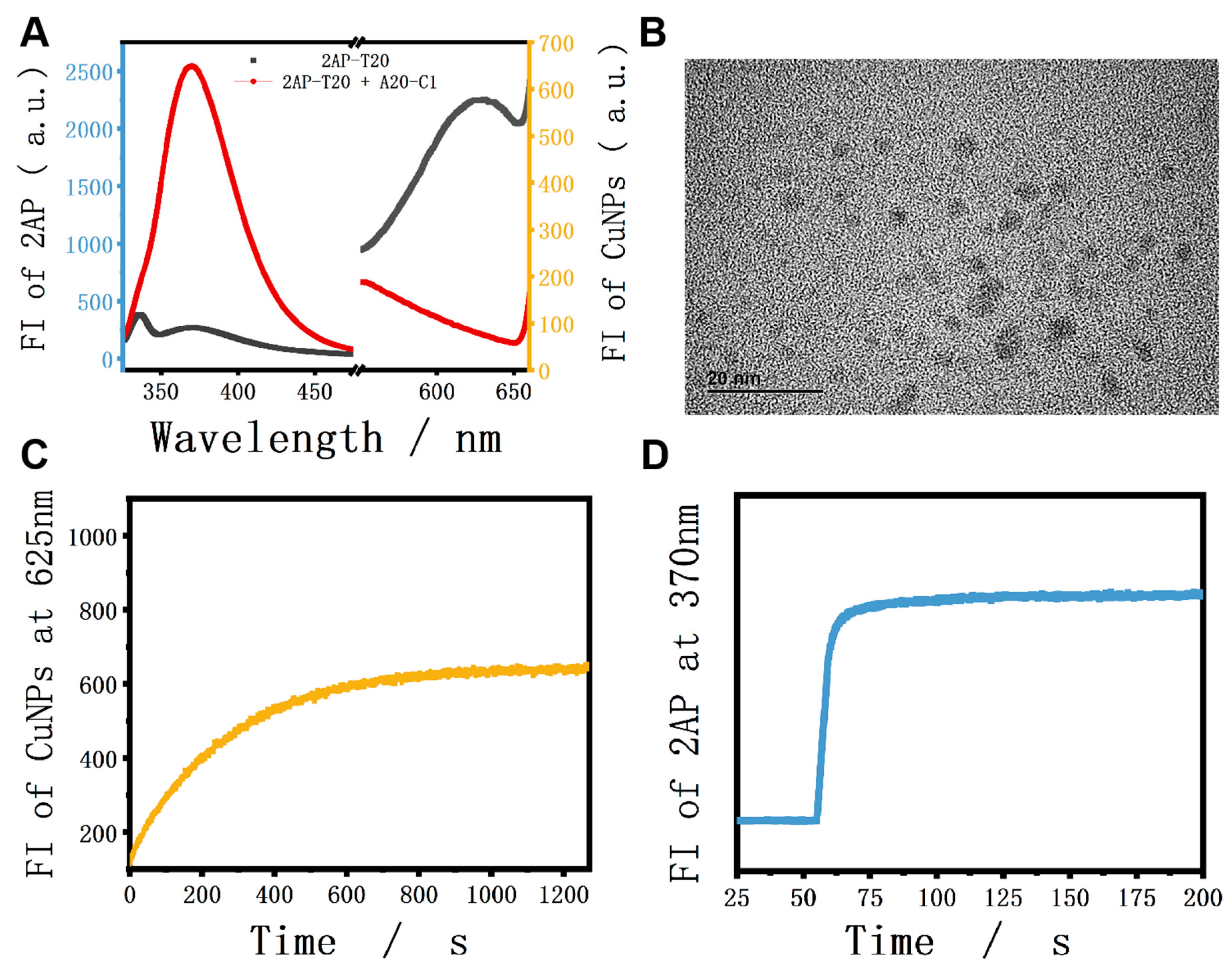
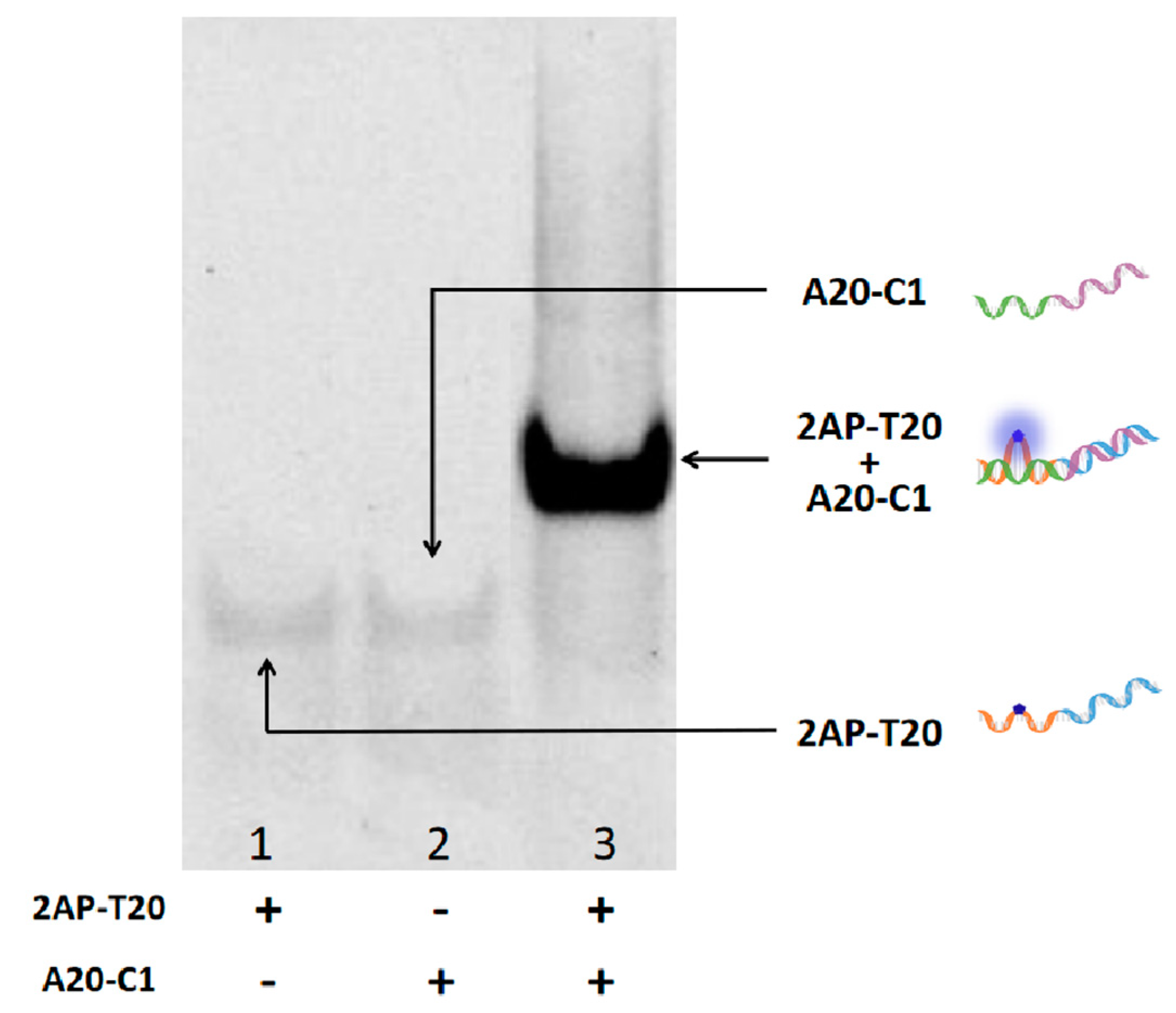
As for the verification experiments, strand A20-C1 was introduced into the system to interact with 2AP-T20. To make it clear, A20-C1 is another single strand that is fully complementary to the X and T20 sections of 2AP-T20 (green and purple parts, respectively). As shown in Figure 1A, only 2AP-T20 exhibited relatively low fluorescence at 370 nm, but could generate high fluorescence at 625 nm after the formation of CuNPs (HRTEM image in Figure 1B). However, after adding a certain concentration of A20-C1 to the platform, it will hybridize with 2AP-T20 and form duplex. Accordingly, the 2AP base will be looped out, yielding remarkably high fluorescence at 370 nm because of the disruption of π-π stacking electron transfer quenching. Meanwhile, the generation of CuNPs will be blocked as a result of the absence of a poly-T template, yielding an ultra-low fluorescence signal at 625 nm. Moreover, the fluorescent kinetics of CuNPs in the absence and presence of a poly-T template were also tested (Figure S1 and Figure 1C). The fluorescence intensity at 625 nm increased gradually after reducing Cu2+ using AA and reached a plateau in less than 20 min, indicating the surprising rapid response of both probes. It should be noted that the kinetics of 2AP demonstrated almost an instant response (Figure 1D). Moreover, the hybridization between 2AP-T20 and A20-C1 was vividly identified by the PAGE experiment (Figure 2). The disappearance of single-stranded bands and appearance of a new band in Lane 3 indicated the formation of a 2AP-T20/A20-C1 duplex. All the above phenomena prove the reliable looping out of the 2AP base, the successful preparation of CuNPs, and the possibility of integrating them together as dual output to construct D-CLPs.
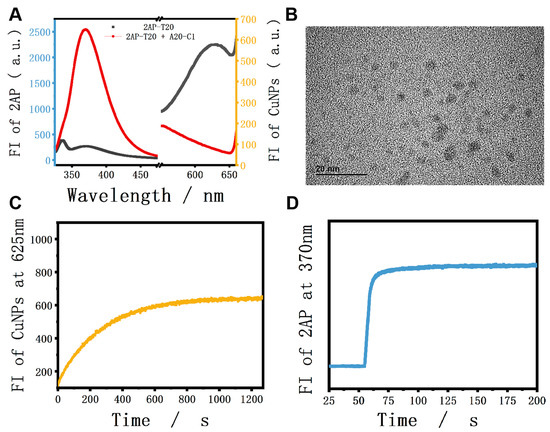
Figure 1.
(A) Fluorescence spectra of 2AP and CuNPs in the absence and presence of A20-C1; (B) HRTEM image of CuNPs; (C) fluorescence kinetics of CuNPs in the presence of 250 nM 2AP-T20 strand; (D) fluorescence kinetics of 2AP after the addition of 300 nM A20-C1 strand.
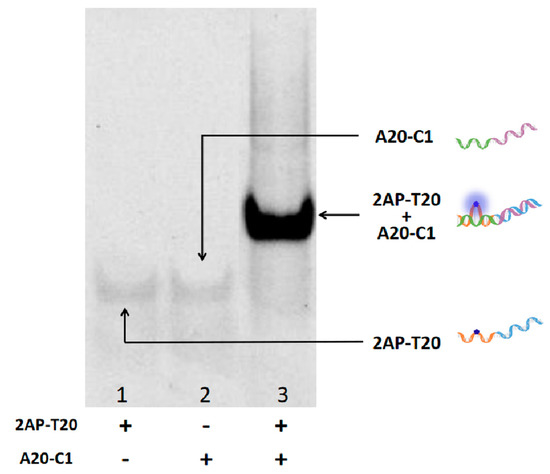
Figure 2.
A 15% native polyacrylamide gel showing the interaction between A20-C1 and 2AP-T20. Lane 1: 2AP-T20, Lane 2: A20-C1, Lane 3: 2AP-T20+A20-C1.
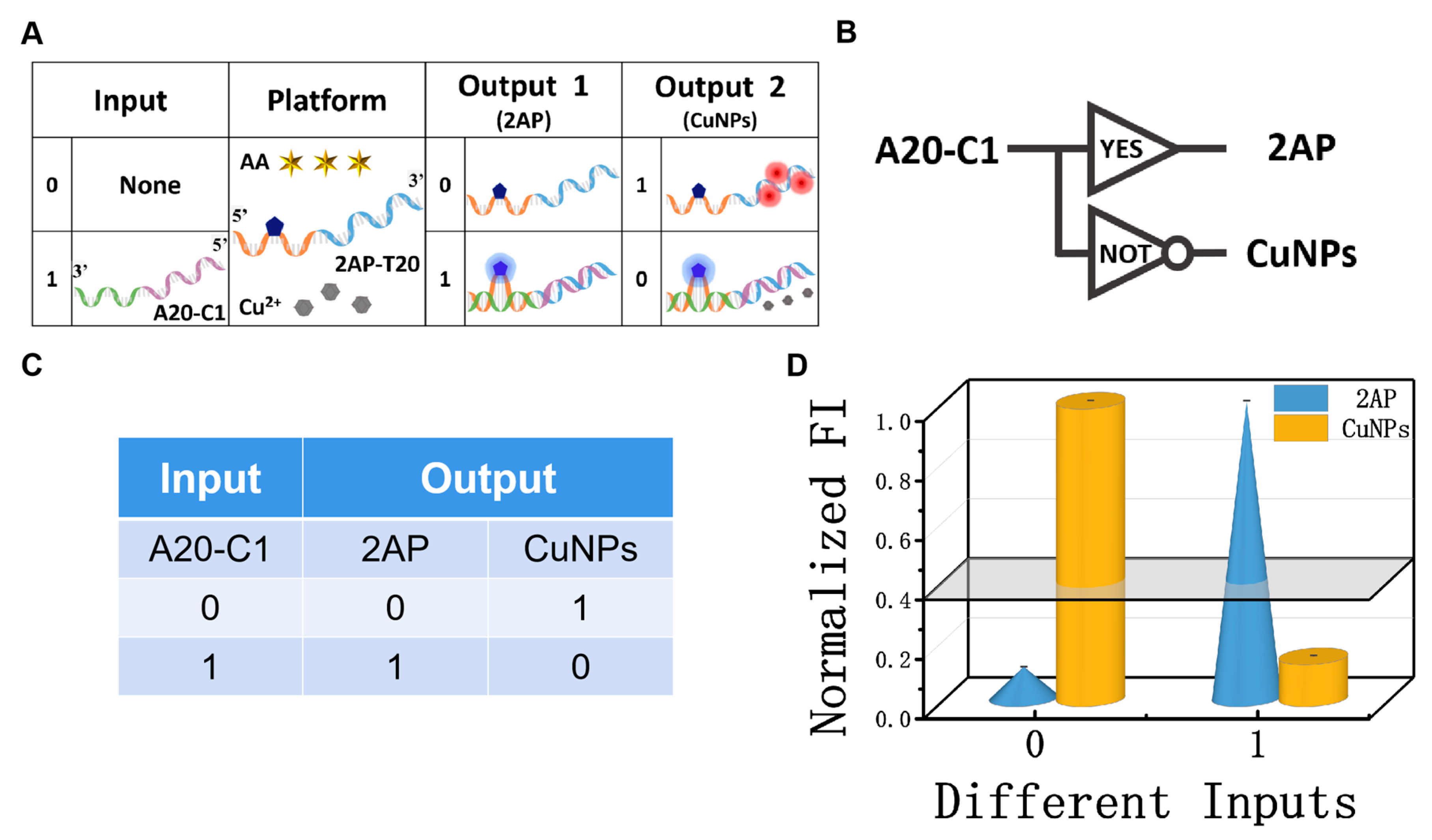
2.2.1. Construction of YES/NOT Logic Pair
Among the various molecular logic devices, the YES and NOT logic gates are the simplest and the most essential ones [47,48,49]. Again, the mixture of AA, Cu2+, and 2AP-T20 was used as the platform of the YES/NOT logic pair (Figure 3A,B), and strand A20-C1 was the input. The absence/presence of strand A20-C1 were assigned as the input state of “0” and “1”, respectively. The detailed operating principles of the YES/NOT D-CLP are illustrated in Figure 3A. In the absence of any strand, the negligibly low signal at 370 nm of free 2AP was obtained, and the high fluorescence intensity of the CuNPs (625 nm) could be clearly observed at the same time, generating the output state “0, 1”. However, after adding suitable concentrations of A20-C1 to the solution, 2AP will be looped out and the interaction between 2AP-T20 and A20-C1 will inhibit the formation of CuNPs, yielding an output state of “1, 0”. The fluorescence spectra of the YES/NOT logic gate and the essential optimization experiments can be found in Figures S2 and S3, respectively. The CLPs were implemented under the optimal conditions. The corresponding fluorescent column bars are depicted in Figure 3D. The above operating principle featured the characteristics and truth table of YES/NOT CLPs (Figure 3C,D), proving its reasonable operation.
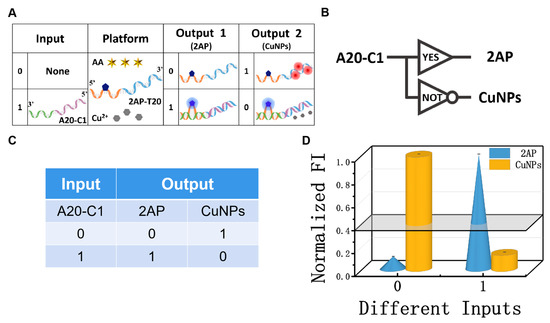
Figure 3.
(A) Detailed operation of the ‘‘YES/NOT’’ logic pair; (B) equivalent logic symbol of “YES/NOT” logic pair; (C) truth table of ‘‘YES/NOT’’ logic pair; (D) normalized fluorescent column bars of the YES (blue, 2AP) and NOT (yellow, CuNPs) gates under different input variations.
2.2.2. Operation of OR/NOR Logic Pair
Apart from the YES/NOT pair, the OR/NOR pair was also constructed to testify the feasibility of this universal platform. Herein, the platform was the same as described above, while strands A20-C1 and A22-C1 were used as two inputs of the OR/NOR logic pair. Notably, A22-C1 is another single strand that is complementary to the X and T20 parts of 2AP-T20, but with two more A bases at the 5′ end (Figure 4A). Therefore, the function of A22-C1 during the construction of the CLPs is analogous to that of A20-C1. The absence/presence of input strands were designated as the input state of “0” and “1”, respectively. The computing illustrations of the OR/NOR D-CLP are shown in Figure 4A,B and the fluorescence spectra of the OR/NOR logic gate are presented in Figure S4. In the absence of any strand, the ultra-low signal at 370 nm of free 2AP and the high one at 625 nm from the CuNPs could be observed, producing the output state “0, 1”. Meanwhile, the presence of any one of the two inputs will induce the formation of the duplex, accompanied with the looping out of 2AP and no formation of CuNPs, corresponds to an output state of “1, 0”. The fluorescent column bars under the different input states are given in Figure 4D, which was mostly consistent with the truth table (Figure 4C).
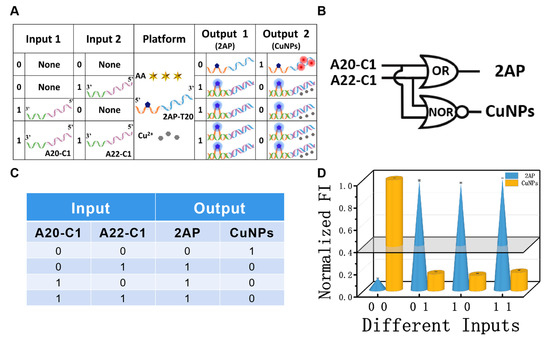
Figure 4.
(A) Detailed operation of the ‘‘OR/NOR’’ logic pair using strands A20-C1 and A22-C1 as inputs and the mixture of 2AP-T20, AA, and Cu2+ as platform; (B) equivalent logic symbol of “OR/NOR” logic pair; (C) truth table of ‘‘OR/NOR’’ logic pair; (D) normalized fluorescent column bars of the OR (blue, 2AP) and NOR (yellow, CuNPs) gates under different input combinations.
2.2.3. Ratiometric Fluorescent Detection of Poly-A Strand
A versatile logic platform could not only perform DNA computing, but also is capable of biosensing [50,51,52,53]. Inspired by the rapid and efficient response of both probes and based on the above platform, we further applied the contrary logic response to ratiometric fluorescent analysis of nucleic acids. It has been reported that poly-A tails can regulate the expression and stability of mRNA and is significant for the initiation of translation [54,55,56]. Therefore, the analysis of poly-A sequences could provide informative evidence for the early diagnosis of diseases.
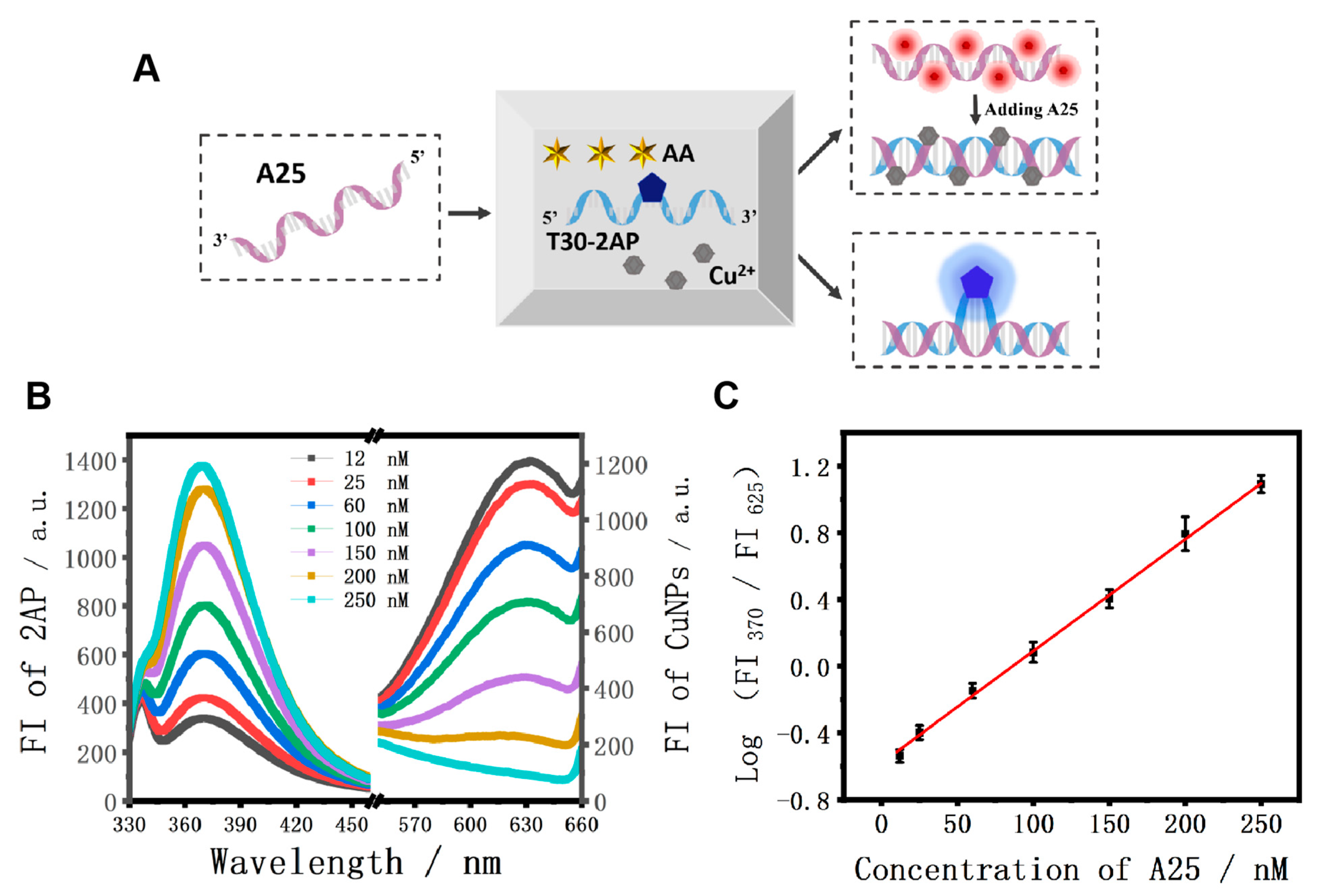
Different from the above logic system, the mixture of AA, Cu2+, and T30-2AP strands was employed as the initial platform for the nucleic acid analysis and strand A25 was used as the model target (Figure 5A). It should be noted that T30-2AP is a single-stranded DNA with 30 consecutive T bases, in which 2AP is at the 16th position. Figure 5B displays the fluorescence emission spectra of 2AP and the CuNPs in the presence of different concentrations of A25. With the addition of strand A25, the FI370 values were enhanced gradually, accompanied by the corresponding decrease in FI625 values as the concentration of A25 increased from 12 nM to 250 nM. Accordingly, an excellent calibration curve of the ratiometric values of FI370/FI625 as a function of various concentrations of A25 is presented in Figure 5C. The linear regression equation was F = −0.592 + 0.0068 C (F is the ratiometric value of FI370/FI625, and C is the concentration of strand A25), and the limit of detection (LOD) was estimated to be as low as 17.2 nM according to 3σ/s, which is comparable to most previously reported DNA sensors [57].
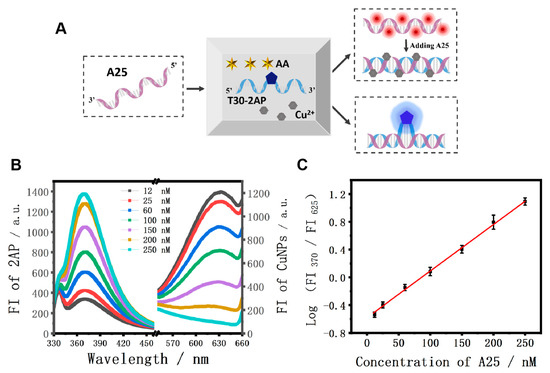
Figure 5.
(A) Scheme of the A25 fluorescent detection platform; (B) fluorescence spectra of 2AP and CuNPs with the different concentrations of A25; (C) corresponding calibration graph for the A25 detection. Linear relationship between the logarithmic values of the FI370/FI625 and A25 concentration in the range from 12 nM to 250 nM. The error bars were obtained via three independent experiments.
3. Conclusions
To summarize, we, for the first time, constructed a rapid and enzyme-free platform for constructing DNA CLPs by integrating poly-T CuNPs and 2AP as universal dual output generators. Surprisingly, the YES/NOT and OR/NOR CLPs can be operated in just less than 20 min without the participation of any enzymes. Additionally, the ratiometric analysis of poly-A DNA was also realized based on the same platform by taking advantage of the high reliability and accuracy of the D-CLPs. This work not only provided a low-cost, fast-response, and typical paradigm for DNA computing, but also demonstrated the fabrication of novel ratiometric fluorescent sensors for the analysis of nucleic acids and diagnosis of diseases in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5030108/s1, Table S1: Sequences of DNA strands used in this work; Figure S1: Fluorescence kinetics of CuNPs after adding 300 nM A20-C1 to the solution of 250 nM 2AP-T20 strand; Figure S2: Fluorescence spectra of YES/NOT logic gate under different inputs; Figure S3: Optimization of the concentration of A20-C1 used in D-CLPs via recording the fluorescence signal of 2AP and CuNPs; Figure S4: Fluorescence spectra of OR/NOR logic gate under different inputs; Figure S5: The different poly-As are set as control sequences to demonstrate the specific response of 2AP-T20 system. Figure S6. Different single bases are inserted into the poly-A tail to clarify the T30-2AP system’s specificity.
Author Contributions
Conceptualization, J.H. (Jiawen Han), D.F. and S.D.; Methodology, J.W., X.L., J.H. (Jingyu Hou) and D.F.; Validation, J.W., J.H. (Jiawen Han), X.L., J.H. (Jingyu Hou) and D.F.; Investigation, J.W., J.H. (Jiawen Han) and D.F.; Writing—original draft, J.W. and D.F.; Writing—review & editing, J.H. (Jiawen Han) and D.F.; Supervision, J.H. (Jiawen Han), D.F. and S.D.; Project administration, D.F.; Funding acquisition, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Nos. ZR2022QB010 and ZR2022QB197), National Natural Science Foundation of China (No. 22204153), Shandong Excellent Young Scientists Fund Program (Overseas, No. 2023HWYQ-054), Open Funds of the State Key Laboratory of Electroanalytical Chemistry (Nos. SKLEAC202306 and SKLEAC202307), and Fundamental Research Funds for the Central Universities (Cultivation Plan for Excellent Young Scientists Program of OUC, No. 202341013).
Data Availability Statement
All the data generated in this study is included in this article and the Supplementary Information.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (Nos. ZR2022QB010 and ZR2022QB197), National Natural Science Foundation of China (No. 22204153), Shandong Excellent Young Scientists Fund Program (Overseas, No. 2023HWYQ-054), Open Funds of the State Key Laboratory of Electroanalytical Chemistry (Nos. SKLEAC202306 and SKLEAC202307), and Fundamental Research Funds for the Central Universities (Cultivation Plan for Excellent Young Scientists Program of OUC, No. 202341013). D.F. also thanks the starting support from the Ocean University of China (OUC) and National Laboratory for Marine Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest. This paper dedicated to the Special Issue of Prof. Itamar Willner.
References
- De Silva, A.P.; Gunaratne, H.Q.N.; McCoy, C.P. A molecular photoionic and gate based on fluorescent signaling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- Feng, C.; Chen, T.S.; Mao, D.S.; Zhang, F.; Tian, B.; Zhu, X.L. Construction of a Ternary Complex Based DNA Logic Nanomachine for a Highly Accurate Imaging Analysis of Cancer Cells. ACS Sens. 2020, 5, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Merkx, M.; Janssen, B.; van Rosmalen, M.; van Beek, L. Antibody Activation using DNA-Based Logic Gates. Protein Sci. 2015, 24, 35. [Google Scholar]
- Shin, T.H.; Choi, J.S.; Yun, S.; Kim, I.S.; Song, H.T.; Kim, Y.; Park, K.I.; Cheon, J. T-1 and T-2 Dual-Mode MRI Contrast Agent for Enhancing Accuracy by Engineered Nanomaterials. ACS Nano 2014, 8, 3393–3401. [Google Scholar] [CrossRef]
- Strack, G.; Ornatska, M.; Pita, M.; Katz, E. Biocomputing security system: Concatenated enzyme-based logic gates operating as a biomolecular keypad lock. J. Am. Chem. Soc. 2008, 130, 4234. [Google Scholar] [CrossRef]
- Mailloux, S.; Gerasimova, Y.V.; Guz, N.; Kolpashchikov, D.M.; Katz, E. Bridging the Two Worlds: A Universal Interface between Enzymatic and DNA Computing Systems. Angew. Chem. Int. Edit. 2015, 54, 6562–6566. [Google Scholar] [CrossRef]
- Prokup, A.; Deiters, A. Interfacing Synthetic DNA Logic Operations with Protein Outputs. Angew. Chem. Int. Edit. 2014, 53, 13192–13195. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Q.; Wang, J.; Wang, E.K.; Dong, S.J. Propelling DNA Computing with Materials’ Power: Recent Advancements in Innovative DNA Logic Computing Systems and Smart Bio-Applications. Adv. Sci. 2020, 7, 25. [Google Scholar] [CrossRef]
- Jiao, K.; Au, B.; Guo, L.J.; Zhou, H.B.; Wang, F.; Zhang, X.L.; Shi, J.Y.; Li, Q.; Wang, L.H.; Li, J.; et al. Programming Switchable Transcription of Topologically Constrained DNA. J. Am. Chem. Soc. 2020, 142, 10739–10746. [Google Scholar] [CrossRef]
- Prokup, A.; Hemphill, J.; Deiters, A. DNA Computation: A Photochemically Controlled AND Gate. J. Am. Chem. Soc. 2012, 134, 3810–3815. [Google Scholar] [CrossRef]
- Wilkins, M.H.F.; Stokes, A.R.; Wilson, H.R. Molecular structure of deoxypentose nucleic acids. Nature 1953, 171, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, X.Y.; Zheng, X.D.; Ke, Y.G.; Chen, K.T.; Liu, D.S.; Lu, Z.H.; Yang, J.; Yan, H. Programmable allosteric DNA regulations for molecular networks and nanomachines. Sci. Adv. 2022, 8, eabl4589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Chandrasekaran, A.R.; Li, Q.; Li, X.; Sha, R.J.; Seeman, N.C.; Mao, C.D. Post-Assembly Stabilization of Rationally Designed DNA Crystals. Angew. Chem. Int. Edit. 2015, 54, 9936–9939. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Schaap, I.A.T.; Tardin, C.F.; Erben, C.M.; Berry, R.M.; Schmidt, C.F.; Turberfield, A.J. Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science 2005, 310, 1661–1665. [Google Scholar] [CrossRef]
- Hu, Y.; Niemeyer, C.M. From DNA Nanotechnology to Material Systems Engineering. Adv. Mater. 2019, 31, 1806294. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, J.M.; Liu, L.F.; Jonchhe, S.; Rizzuto, F.J.; Mandal, S.; He, H.W.; Wei, S.S.; Sleiman, H.F.; Mao, H.B.; et al. A poly(thymine)-melamine duplex for the assembly of DNA nanomaterials. Nat. Mater. 2020, 19, 1012. [Google Scholar] [CrossRef]
- Pashuck, E.T.; Seeman, N.; Macfarlane, R. Self-assembly of bioinspired and biologically functional materials. MRS Bull. 2020, 45, 832–840. [Google Scholar] [CrossRef]
- Shen, H.J.; Wang, Y.Q.; Wang, J.; Li, Z.H.; Yuan, Q. Emerging Biomimetic Applications of DNA Nanotechnology. ACS Appl. Mater. Interfaces 2019, 11, 13859–13873. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Fan, D.Q.; Winner, I. Modeling Gene Expression Instability by Programmed and Switchable Polymerization/Nicking DNA Nanomachineries. ACS Nano 2020, 14, 5046–5052. [Google Scholar] [CrossRef]
- Du, Y.; Peng, P.; Li, T. DNA Logic Operations in Living Cells Utilizing Lysosome-Recognizing Framework Nucleic Acid Nanodevices for Subcellular Imaging. ACS Nano 2019, 13, 5778–5784. [Google Scholar] [CrossRef]
- Fan, D.Q.; Shang, C.S.; Gu, W.L.; Wang, E.K.; Dong, S.J. Introducing Ratiometric Fluorescence to MnO2 Nanosheet-Based Biosensing: A Simple, Label-Free Ratiometric Fluorescent Sensor Programmed by Cascade Logic Circuit for Ultrasensitive GSH Detection. ACS Appl. Mater. Interfaces 2017, 9, 25870–25877. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Q.; Zhu, X.Q.; Zhai, Q.F.; Wang, E.K.; Dong, S.J. Polydopamine Nanotubes as an Effective Fluorescent Quencher for Highly Sensitive and Selective Detection of Biomolecules Assisted with Exonuclease III Amplification. Anal. Chem. 2016, 88, 9158–9165. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.X.; Zheng, J.; Ning, L.M.; Huang, Y.; Sheng, A.Z.; Chen, T.S.; Xiang, Y.; Zhu, X.L.; Li, G.X. Dual-Responsive DNA Nanodevice for the Available Imaging of an Apoptotic Signaling Pathway in Situ. ACS Nano 2019, 13, 12840–12850. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, C.C.; Liu, Y.; Cui, C.; Ge, J.; Tan, W.H. Multibranched Linear DNA-Controlled Assembly of Silver Nanoclusters and Their Applications in Aptamer-Based Cell Recognition. ACS Appl. Mater. Interfaces 2022, 14, 14953–14960. [Google Scholar] [CrossRef]
- Yan, N.; Lin, L.; Xu, C.N.; Tian, H.Y.; Chen, X.S. A GSH-Gated DNA Nanodevice for Tumor-Specific Signal Amplification of microRNA and MR Imaging-Guided Theranostics. Small 2019, 15, 1903016. [Google Scholar] [CrossRef]
- Yue, R.Y.; Chen, M.; Ma, N. Dual MicroRNA-Triggered Drug Release System for Combined Chemotherapy and Gene Therapy with Logic Operation. ACS Appl. Mater. Interfaces 2020, 12, 32493–32502. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Q.; Wang, E.K.; Dong, S.J. An intelligent universal system yields double results with half the effort for engineering a DNA “Contrary Logic Pairs” library and various DNA combinatorial logic circuits. Mater. Horiz. 2017, 4, 924–931. [Google Scholar] [CrossRef]
- Fan, D.Q.; Zhu, J.B.; Zhai, Q.F.; Wang, E.K.; Dong, S.J. Cascade DNA logic device programmed ratiometric DNA analysis and logic devices based on a fluorescent dual-signal probe of a G-quadruplex DNAzyme. Chem. Commun. 2016, 52, 3766–3769. [Google Scholar] [CrossRef]
- Zhu, L.P.; Yu, L.Y.; Yang, X.R. Electrochemical-Based DNA Logic Devices Regulated by the Diffusion and Intercalation of Electroactive Dyes. ACS Appl. Mater. Interfaces 2021, 13, 42250–42257. [Google Scholar] [CrossRef]
- Zhu, L.P.; Yu, L.Y.; Meng, T.; Peng, Y.; Yang, X.R. Contrary Logic Pair Library, Parity Generator/Checker and Various Concatenated Logic Circuits Engineered by a Label-Free and Immobilization-Free Electrochemiluminescence Resonance Energy Transfer System. Small 2021, 17, 2102881. [Google Scholar] [CrossRef]
- Bhasikuttan, A.C.; Mohanty, J. Targeting G-quadruplex structures with extrinsic fluorogenic dyes: Promising fluorescence sensors. Chem. Commun. 2015, 51, 7581–7597. [Google Scholar] [CrossRef] [PubMed]
- Alba, J.J.; Sadurni, A.; Gargallo, R. Nucleic Acid i-Motif Structures in Analytical Chemistry. Crit. Rev. Anal. Chem. 2016, 46, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.C.; Shi, Y.; Wang, Y.L.; Sun, Y.J.; Hu, J.T.; Ni, P.J.; Li, Z. Label-free turn-on fluorescent detection of melamine based on the anti-quenching ability of Hg2+ to gold nanoclusters. Biosens. Bioelectron. 2014, 53, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Q.; Wang, E.K.; Dong, S.J. Upconversion-chameleon-driven DNA computing: The DNA-unlocked inner-filter-effect (DU-IFE) for operating a multicolor upconversion luminescent DNA logic library and Its biosensing application. Mater. Horiz. 2019, 6, 375–384. [Google Scholar] [CrossRef]
- Fan, D.Q.; Zhai, Q.F.; Zhou, W.J.; Zhu, X.Q.; Wang, E.K.; Dong, S.J. A label-free colorimetric aptasensor for simple, sensitive and selective detection of Pt (II) based on platinum (II)-oligonucleotide coordination induced gold nanoparticles aggregation. Biosens. Bioelectron. 2016, 85, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Q.; Zhu, J.B.; Liu, Y.Q.; Wang, E.K.; Dong, S.J. Label-free and enzyme-free platform for the construction of advanced DNA logic devices based on the assembly of graphene oxide and DNA-templated AgNCs. Nanoscale 2016, 8, 3834–3840. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, K.; Liu, Y.L.; Wang, H.Y.; Wu, J.; Zhu, F.F.; Zou, P. Binding-induced and label-free colorimetric method for protein detection based on autonomous assembly of hemin/G-quadruplex DNAzyme amplification strategy. Biosens. Bioelectron. 2015, 64, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Zhou, Z.; Gou, X.L.; Shi, W.C.; Gong, Y.; Yi, M.; Cheng, W.; Song, F.Z. Light up multiple protein dimers on cell surface based on proximity-induced fluorescence activation of DNA-templated sliver nanoclusters. Biosens. Bioelectron. 2021, 179, 8. [Google Scholar] [CrossRef]
- Seio, K.; Kanamori, T.; Tokugawa, M.; Ohzeki, H.; Masaki, Y.; Tsunoda, H.; Ohkubo, A.; Sekine, M. Fluorescent properties of oligonucleotides doubly modified with an indole-fused cytosine analog and 2-aminopurine. Bioorgan. Med. Chem. 2013, 21, 3197–3201. [Google Scholar] [CrossRef]
- Jean, J.M.; Hall, K.B. 2-Aminopurine electronic structure and fluorescence properties in DNA. Biochemistry 2002, 41, 13152–13161. [Google Scholar] [CrossRef]
- Peng, P.; Du, Y.; Sun, Y.D.; Liu, S.N.; Mi, L.; Li, T. Probing the propeller-like loops of DNA G-quadruplexes with looped-out 2-aminopurine for label-free switchable molecular sensing. Analyst 2018, 143, 3814–3820. [Google Scholar] [CrossRef]
- Wang, X.L.; Zeng, R.; Chu, S.N.; Tang, W.; Lin, N.; Fu, J.; Yang, J.R.; Gao, B. A quencher-free DNAzyme beacon for fluorescently sensing uranyl ions via embedding 2-aminopurine. Biosens. Bioelectron. 2019, 135, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Geng, N.N.; Zheng, X.; Luo, X.R.; Wu, M.S.; Zhang, H. DNA logic circuits based amplification system for quencher-free and highly sensitive detection of DNA and adenosine triphosphate. J. Pharmaceut. Biomed. 2018, 161, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Zhu, Z.C.; Zou, R.; Wang, L.Y.; Gong, H.; Cai, C.Q. An enzyme-free three-dimensional DNA walker powered by catalytic hairpin assembly for H5N1 DNA ratiometric detection. Microchem. J. 2021, 170, 106728. [Google Scholar] [CrossRef]
- Fan, D.Q.; Wang, E.K.; Dong, S.J. Simple, fast, label-free, and nanoquencher-free system for operating multivalued DNA logic gates using poly-thymine templated CuNPs as signal reporters. Nano Res. 2017, 10, 2560–2569. [Google Scholar] [CrossRef]
- Qing, Z.H.; He, X.X.; He, D.G.; Wang, K.M.; Xu, F.Z.; Qing, T.P.; Yang, X. Poly(thymine)-Templated Selective Formation of Fluorescent Copper Nanoparticles. Angew. Chem. Int. Edit. 2013, 2, 9719–9722. [Google Scholar] [CrossRef]
- Fan, D.Q.; Wang, E.K.; Dong, S.J. A DNA-based parity generator/checker for error detection through data transmission with visual readout and an output-correction function. Chem. Sci. 2017, 8, 1888–1895. [Google Scholar] [CrossRef]
- Fan, D.Q.; Wang, J.; Han, J.W.; Wang, E.K.; Dong, S.J. Engineering DNA logic systems with non-canonical DNA-nanostructures: Basic principles, recent developments and bio-applications. Sci. China-Chem. 2022, 65, 284–297. [Google Scholar] [CrossRef]
- Fan, D.Q.; Wang, K.; Zhu, J.B.; Xia, Y.; Han, Y.C.; Liu, Y.Q.; Wang, E.K. DNA-based visual majority logic gate with one-vote veto function. Chem. Sci. 2015, 6, 1973–1978. [Google Scholar] [CrossRef]
- Pan, J.F.; He, Y.; Liu, Z.; Chen, J.H. Tetrahedron-Based Constitutional Dynamic Network for COVID-19 or Other Coronaviruses Diagnostics and Its Logic Gate Applications. Anal. Chem. 2022, 94, 714–722. [Google Scholar] [CrossRef]
- Han, J.W.; Wang, J.; Wang, J.; Fan, D.Q.; Dong, S.J. Recent advancements in coralyne (COR)-based biosensors: Basic principles, various strategies and future perspectives. Biosens. Bioelectron. 2022, 210, 114343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Yuan, Y.W.; Wen, X.L.; Li, Y.; Cao, C.; Xiong, Q.H. A coordination and ligand replacement based three-input colorimetric logic gate sensing platform for melamine, mercury ions, and cysteine. RSC Adv. 2015, 5, 59106–59113. [Google Scholar] [CrossRef]
- Han, J.W.; Ding, Y.R.; Lv, X.J.; Zhang, Y.W.; Fan, D.Q. Integration of G-Quadruplex and Pyrene as a Simple and Efficient Ratiometric Fluorescent Platform That Programmed by Contrary Logic Pair for Highly Sensitive and Selective Coralyne (COR) Detection. Biosensors 2023, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, T.; Mizoguchi, Y.; Sun, Y.; Aoki, F.; Imakawa, K.; Sakai, S. The casein mRNA decay changes in parallel with the poly(A) tail length in the mouse mammary gland. Mol. Cell. Endocrinol 2002, 190, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.G.; Jiao, F.; Liao, Q.; Luo, H.T.; Li, H.; Sun, L.; Bu, D.C.; Yu, K.T.; Zhao, Y.; Chen, R.S. Genome-wide identification of cancer-related polyadenylated and non-polyadenylated RNAs in human breast and lung cell lines. Sci. China-Life Sci. 2013, 56, 503–512. [Google Scholar] [CrossRef]
- Zheng, D.; Tian, B. Sizing up the poly(A) tail: Insights from deep sequencing. Trends Biochem.Sci. 2014, 39, 255–257. [Google Scholar] [CrossRef]
- Liao, X.F.; Luo, N.; Li, M.Y.; Fu, H.; Zou, L. Label-free and highly sensitive fluorescent detection of bleomycin based on CRISPR-Cas12a and G-quadruplex-thioflavin T. Sensor. Actuat. B-Chem. 2023, 381, 133459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).