Mechanochemistry through Extrusion: Opportunities for Nanomaterials Design and Catalysis in the Continuous Mode
Abstract
:1. Introduction
2. Materials and Methods
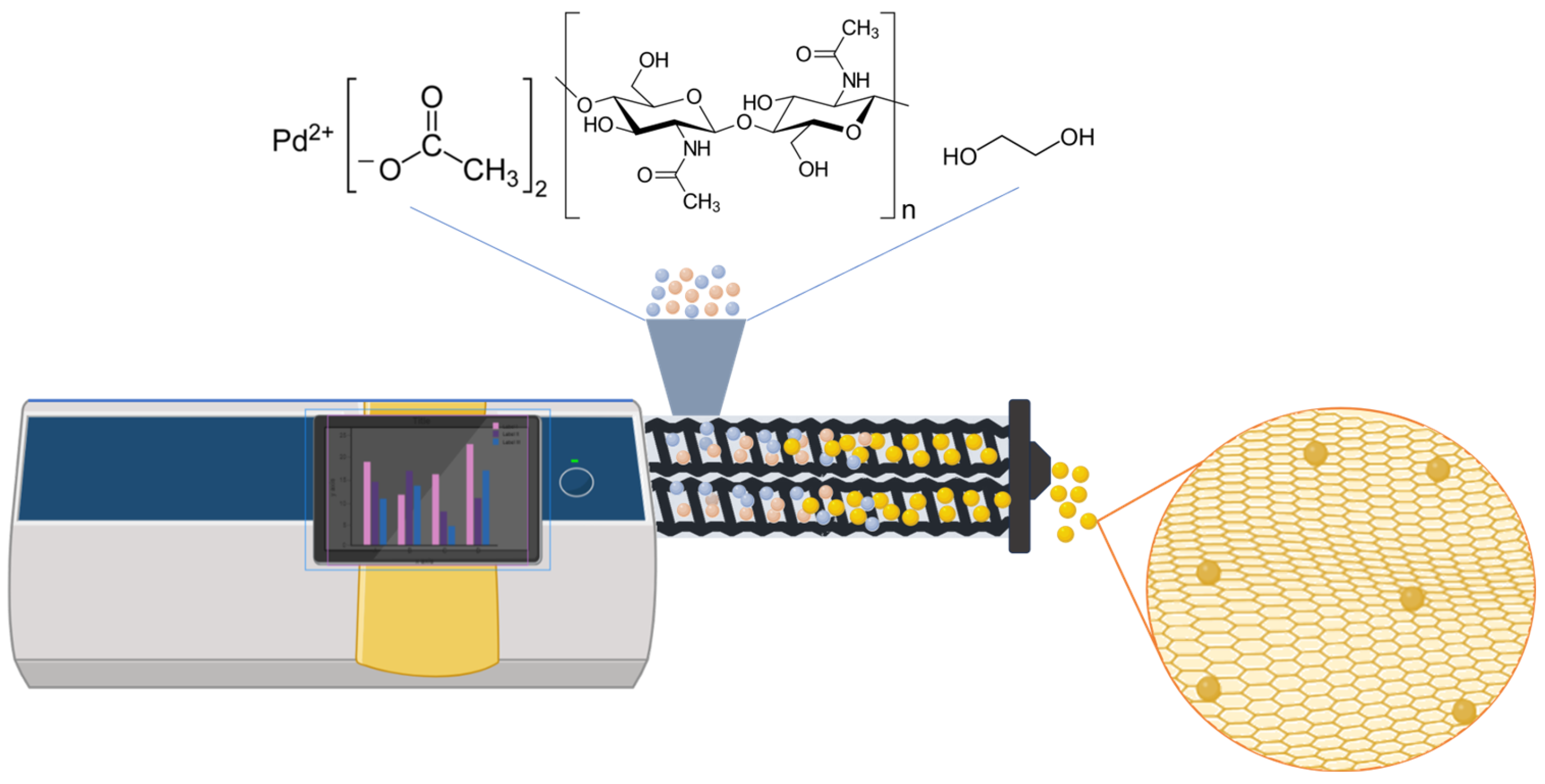
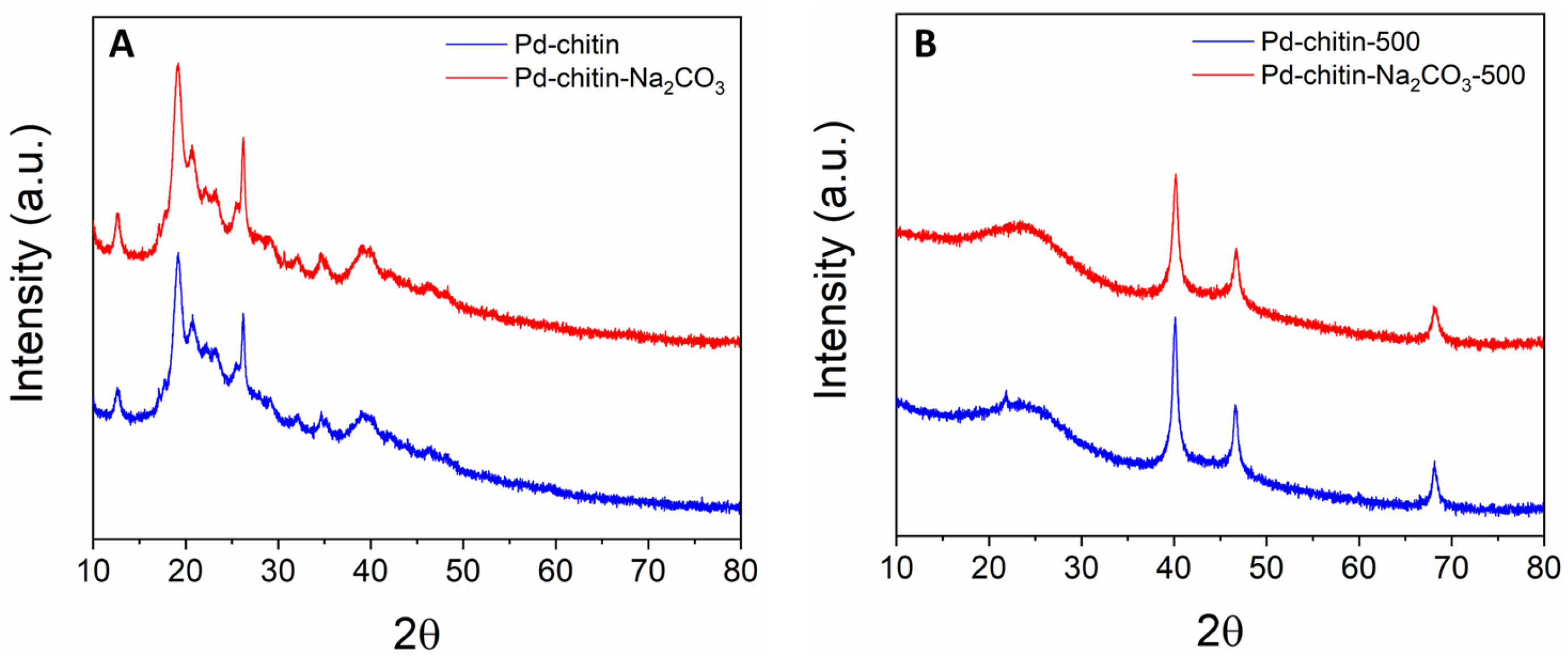
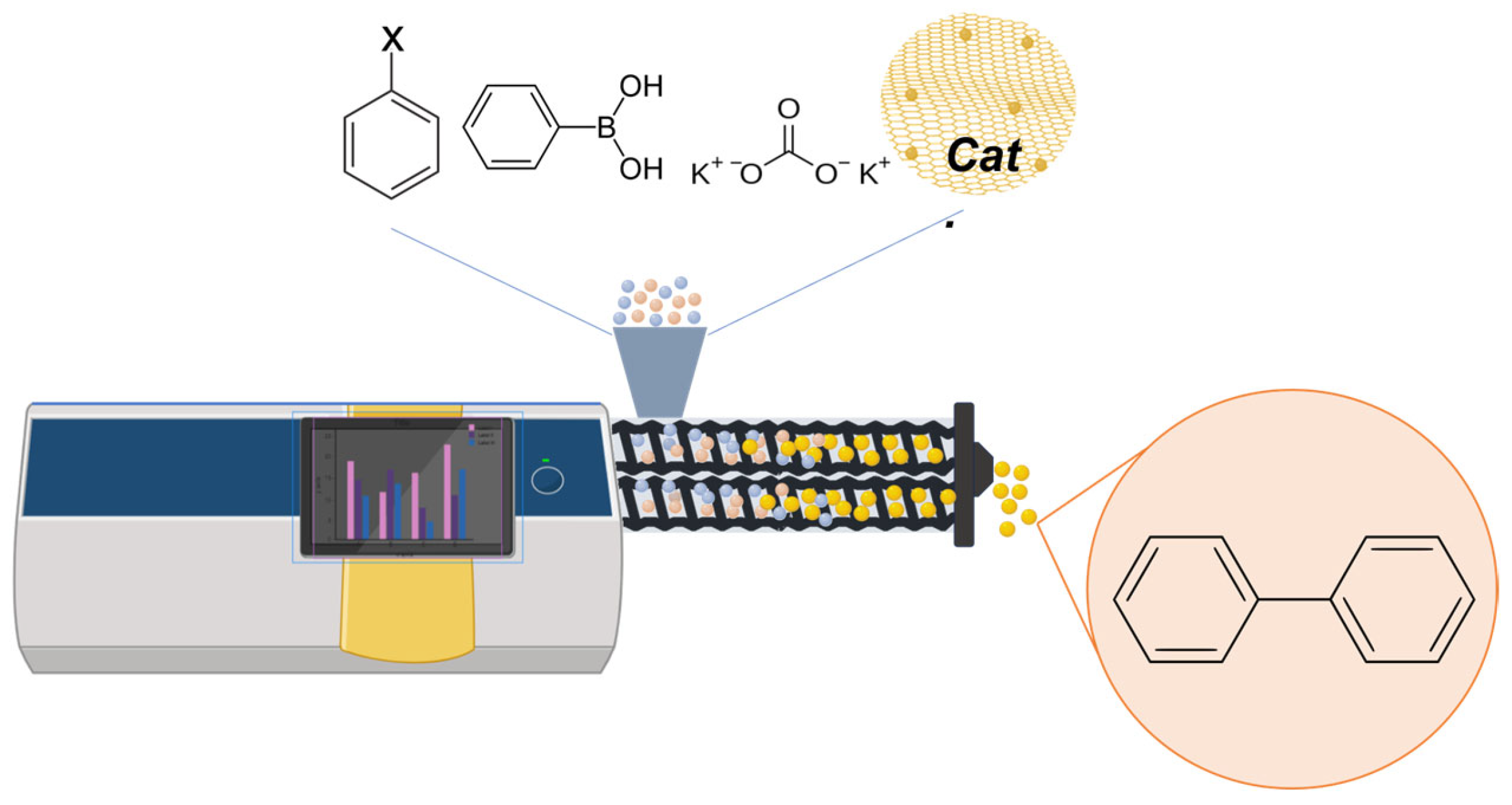
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-Batista, M.J.; Rodriguez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward Sustainable Design of Advanced Nanomaterials for Electrochemical Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Highly Efficient Direct Oxygen Electro-Reduction by Partially Unfolded Laccases Immobilized on Waste-Derived Magnetically Separable Nanoparticles. Nanoscale 2018, 10, 3961–3968. [Google Scholar] [CrossRef] [PubMed]
- García-Espejo, G.; Rodríguez-Padrón, D.; Luque, R.; Camacho, L.; De Miguel, G. Mechanochemical Synthesis of Three Double Perovskites: Cs2AgBiBr6, (CH3NH3)2TlBiBr6 and Cs2AgSbBr6. Nanoscale 2019, 11, 16650–16657. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padrón, D.; Jodlowski, A.D.; De Miguel, G.; Puente-Santiago, A.R.; Balu, A.M.; Luque, R. Synthesis of Carbon-Based Fluorescent Polymers Driven by Catalytically Active Magnetic Bioconjugates. Green Chem. 2018, 20, 225–229. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Benign-by-Design Orange Peellated Nanocatalysts for Continuous Flow Conversion of Levulinic Acid to N-Heterocycles. ACS Sustain. Chem. Eng. 2018, 6, 16637–16644. [Google Scholar] [CrossRef]
- Rodriguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Romero, A.A.; Luque, R. Solventless Mechanochemical Preparation of Novel Magnetic Bioconjugates. Chem. Commun. 2017, 53, 7635–7637. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Rodríguez-Padrón, D.; Muñoz-Batista, M.J.; Kubacka, A.; Luque, R.; Fernández-García, M. Facile Synthesis of B/g-C3N4composite Materials for the Continuous-Flow Selective Photo-Production of Acetone. Green Chem. 2020, 22, 4975–4984. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Caballero, A.; Benítez, A.; Balu, A.M.; Romero, A.A.; Luque, R. Mechanochemical Design of Hemoglobin-Functionalised Magnetic Nanomaterials for Energy Storage Devices. J. Mater. Chem. A 2017, 5, 16404–16411. [Google Scholar] [CrossRef]
- García-Espejo, G.; Rodríguez-Padrón, D.; Pérez-Morales, M.; Luque, R.; De Miguel, G.; Camacho, L. Mechanochemical Synthesis of One-Dimensional (1D) Hybrid Perovskites Incorporating Polycyclic Aromatic Spacers: Highly Fluorescent Cation-Based Materials. J. Mater. Chem. C 2018, 6, 7677–7682. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef]
- Jiao, Y.; Nan, Y.; Wu, Z.; Wang, X.; Zhang, J.; Zhang, B.; Huang, S.; Shi, J. Mechanochemical Synthesis of Enzyme@covalent Organic Network Nanobiohybrids. Appl. Mater. Today 2022, 26, 101381. [Google Scholar] [CrossRef]
- Espro, C.; Rodríguez-Padrón, D. Re-Thinking Organic Synthesis: Mechanochemistry as a Greener Approach. Curr. Opin. Green Sustain. Chem. 2021, 30, 100478. [Google Scholar] [CrossRef]
- Martín-Perales, A.I.; Balu, A.M.; Malpartida, I.; Luque, R. Prospects for the Combination of Mechanochemistry and Flow Applied to Catalytic Transformations. Curr. Opin. Green Sustain. Chem. 2022, 38, 100714. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Mechanochemistry and Organic Synthesis: From Mystical to Practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- James, S.L.; Frišcic, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Do, J.; Titi, H.M.; Auvray, T.; Lennox, C.B.; Cuccia, L.A. Rapid, room-temperature, solvent-free mechanochemical oxidation of elemental gold into organosoluble gold salts †. Green Chem. 2023, 25, 5774. [Google Scholar] [CrossRef]
- Brekalo, I.; Martinez, V.; Karadeniz, B.; Orešković, P.; Drapanauskaite, D.; Vriesema, H.; Stenekes, R.; Etter, M.; Dejanović, I.; Baltrusaitis, J.; et al. Scale-Up of Agrochemical Urea-Gypsum Cocrystal Synthesis Using Thermally Controlled Mechanochemistry. ACS Sustain. Chem. Eng. 2022, 10, 6743–6754. [Google Scholar] [CrossRef]
- Bolt, R.R.A.; Raby-Buck, S.E.; Ingram, K.; Leitch, J.A.; Browne, D.L. Temperature-Controlled Mechanochemistry for the Nickel-Catalyzed Suzuki-Miyaura-Type Coupling of Aryl Sulfamates via Ball Milling and Twin-Screw Extrusion. Angew. Chem. Int. Ed. 2022, 61, e202210508. [Google Scholar] [CrossRef]
- Andersen, J.; Brunemann, J.; Mack, J. Exploring Stable, Sub-Ambient Temperatures in Mechanochemistry: Via a Diverse Set of Enantioselective Reactions. React. Chem. Eng. 2019, 4, 1229–1236. [Google Scholar] [CrossRef]
- Bolt, R.R.A.; Leitch, J.A.; Jones, A.C.; Nicholson, W.I.; Browne, D.L. Continuous Flow Mechanochemistry: Reactive Extrusion as an Enabling Technology in Organic Synthesis. Chem. Soc. Rev. 2022, 51, 4243–4260. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Grammatikos, S.; Papadakis, V.; Kechagias, J.D.; Das, S.C. On the Thermal and Mechanical Performance of Polycarbonate/Titanium Nitride Nanocomposites in Material Extrusion Additive Manufacturing. Compos. Part C Open Access 2022, 8, 100291. [Google Scholar] [CrossRef]
- Galant, O.; Cerfeda, G.; McCalmont, A.S.; James, S.L.; Porcheddu, A.; Delogu, F.; Crawford, D.E.; Colacino, E.; Spatari, S. Mechanochemistry Can Reduce Life Cycle Environmental Impacts of Manufacturing Active Pharmaceutical Ingredients. ACS Sustain. Chem. Eng. 2022, 10, 1430–1439. [Google Scholar] [CrossRef]
- Xu, J.; Hou, S.; Niu, Q.; Zhang, P.; Luo, Z.H. Cyclic Extrusion Synthesis of Mesoporous Carbon-Supported Metal Catalysts for Selective Hydrogenation. AIChE J. 2023, 69, e18073. [Google Scholar] [CrossRef]
- Sonei, S.; Taghavi, F.; Khojastehnezhad, A.; Gholizadeh, M. Copper-Functionalized Silica-Coated Magnetic Nanoparticles for an Efficient Suzuki Cross-Coupling Reaction. ChemistrySelect 2021, 6, 359–368. [Google Scholar] [CrossRef]
- Musza, K.; Szabados, M.; Ádám, A.A.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemically Modified Hydrazine Reduction Method for the Synthesis of Nickel Nanoparticles and Their Catalytic Activities in the Suzuki-Miyaura Cross-Coupling Reaction. React. Kinet. Mech. Catal. 2019, 126, 857–868. [Google Scholar] [CrossRef]
- Beromi, M.M.; Nova, A.; Balcells, D.; Brasacchio, A.M.; Brudvig, G.W.; Guard, L.M.; Hazari, N.; Vinyard, D.J. Mechanistic Study of an Improved Ni Precatalyst for Suzuki-Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species. J. Am. Chem. Soc. 2017, 139, 922–936. [Google Scholar] [CrossRef] [Green Version]
- Hergert, T.; Varga, B.; Thurner, A.; Faigl, F.; Mátravölgyi, B. Copper-Facilitated Suzuki-Miyaura Coupling for the Preparation of 1,3-Dioxolane-Protected 5-Arylthiophene-2-Carboxaldehydes. Tetrahedron 2018, 74, 2002–2008. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Chen, F.; Fu, Q. Tannic Acid: A Green and Efficient Stabilizer of Au, Ag, Cu and Pd Nanoparticles for the 4-Nitrophenol Reduction, Suzuki-Miyaura Coupling Reactions and Click Reactions in Aqueous Solution. J. Colloid Interface Sci. 2021, 604, 281–291. [Google Scholar] [CrossRef]
- Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Solid-State Suzuki-Miyaura Cross-Coupling Reactions: Olefin-Accelerated C-C Coupling Using Mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. [Google Scholar] [CrossRef] [Green Version]
- Seo, T.; Kubota, K.; Ito, H. Mechanochemistry-Directed Ligand Design: Development of a High-Performance Phosphine Ligand for Palladium-Catalyzed Mechanochemical Organoboron Cross-Coupling. J. Am. Chem. Soc. 2023, 145, 6823–6837. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Ma, Y.; Szostak, M. Mechanochemical Synthesis of Ketones via Chemoselective Suzuki-Miyaura Cross-Coupling of Acyl Chlorides. Org. Lett. 2022, 24, 2338–2343. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Shao, L.; Wang, R.; Ma, Y.; Szostak, M. Mechanochemical Solvent-Free Suzuki-Miyaura Cross-Coupling of Amides via Highly Chemoselective N−C Cleavage. Angew. Chem. Int. Ed. 2022, 61, e202114146. [Google Scholar] [CrossRef]
- Zuliani, A.; Balu, A.M.; Luque, R. Efficient and Environmentally Friendly Microwave-Assisted Synthesis of Catalytically Active Magnetic Metallic Ni Nanoparticles. ACS Sustain. Chem. Eng. 2017, 5, 11584–11587. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of Marine (Fish and Crustaceans) Biowaste for High Added-Value Molecules and Bio(Nano)-Materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef]
- Polidoro, D.; Perosa, A.; Rodríguez-Castellón, E.; Canton, P.; Castoldi, L.; Rodríguez-Padrón, D.; Selva, M. Metal-Free N-Doped Carbons for Solvent-Less CO2Fixation Reactions: A Shrimp Shell Valorization Opportunity. ACS Sustain. Chem. Eng. 2022, 10, 13835–13848. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-Wealth: Biowaste Valorization into Valuable Bio(Nano)Materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Zhao, D.; Carrillo-Carrion, C.; Morales-Torres, C.; Elsharif, A.M.; Balu, A.M.; Luque, R.; Len, C. Exploring the Potential of Biomass-Templated Nb/ZnO Nanocatalysts for the Sustainable Synthesis of N-Heterocycles. Catal. Today 2021, 368, 243–249. [Google Scholar] [CrossRef]
- Martín-Perales, A.I.; Rodríguez-Padrón, D.; García Coleto, A.; Len, C.; De Miguel, G.; Muñoz-Batista, M.J.; Luque, R. Photocatalytic Production of Vanillin over CeOxand ZrO2Modified Biomass-Templated Titania. Ind. Eng. Chem. Res. 2020, 59, 17085–17093. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin Characterization by SEM, FTIR, XRD, And13C Cross Polarization/Mass Angle Spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Cui, D.; Yang, J.; Lu, B.; Deng, L.; Shen, H. Extraction and Characterization of Chitin from Oratosquilla Oratoria Shell Waste and Its Application in Brassica campestris L.ssp. Int. J. Biol. Macromol. 2022, 198, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tamoradi, T.; Ghorbani-Choghamarani, A.; Ghadermazi, M. Synthesis of a New Pd(0)-Complex Supported on Magnetic Nanoparticles and Study of Its Catalytic Activity for Suzuki and Stille Reactions and Synthesis of 2,3-Dihydroquinazolin-4(1H)-One Derivatives. Polyhedron 2018, 145, 120–130. [Google Scholar] [CrossRef]
- Baruah, D.; Das, R.N.; Hazarika, S.; Konwar, D. Biogenic Synthesis of Cellulose Supported Pd(0) Nanoparticles Using Hearth Wood Extract of Artocarpus Lakoocha Roxb—A Green, Efficient and Versatile Catalyst for Suzuki and Heck Coupling in Water under Microwave Heating. Catal. Commun. 2015, 72, 73–80. [Google Scholar] [CrossRef]
- Polidoro, D.; Rodriguez-Padron, D.; Perosa, A.; Luque, R.; Selva, M. Chitin-Derived Nanocatalysts for Reductive Amination Reactions. Materials 2023, 16, 575. [Google Scholar] [CrossRef]
- Masood, M.H.; Haleem, N.; Shakeel, I.; Jamal, Y. Carbon Dioxide Conversion into the Reaction Intermediate Sodium Formate for the Synthesis of Formic Acid. Res. Chem. Intermed. 2020, 46, 5165–5180. [Google Scholar] [CrossRef]
- Kirik, S.D.; Mulagaleev, R.F.; Blokhin, A.L. [Pd(CH3COO)2]n from X-Ray Powder Diffraction Data. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, 449–450. [Google Scholar] [CrossRef] [Green Version]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B.W. Strain and Grain Size of TiO2 Nanoparticles from TEM, Raman Spectroscopy and XRD: The Revisiting of the Williamson-Hall Plot Method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation. ” Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Karandikar, P.; Patil, K.R.; Mitra, A.; Kakade, B.; Chandwadkar, A.J. Synthesis and Characterization of Mesoporous Carbon through Inexpensive Mesoporous Silica as Template. Microporous Mesoporous Mater. 2007, 98, 189–199. [Google Scholar] [CrossRef]
- Polidoro, D.; Ballesteros-Plata, D.; Perosa, A.; Rodríguez-Castellón, E.; Rodríguez-Padrón, D.; Selva, M. Controlled Alcohol Oxidation Reactions by Supported Non-Noble Metal Nanoparticles on Chitin-Derived N-Doped Carbons. Catal. Sci. Technol. 2023, 13, 2223–2238. [Google Scholar] [CrossRef]
- Bao, G.; Bai, J.; Li, C. Synergistic Effect of the Pd-Ni Bimetal/Carbon Nanofiber Composite Catalyst in Suzuki Coupling Reaction. Org. Chem. Front. 2019, 6, 352–361. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. A Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef]
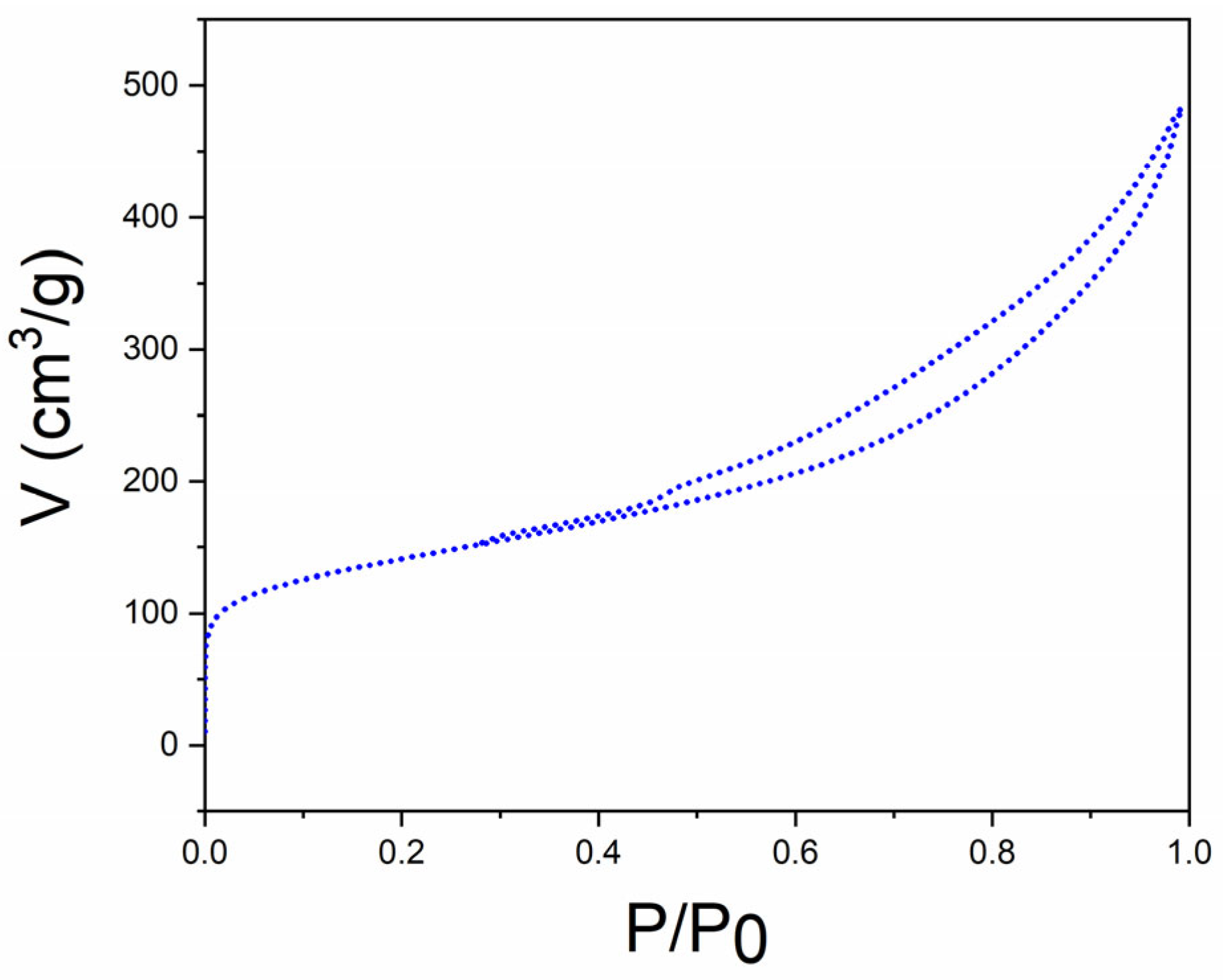
| Material | SBET [m2g−1] a | DBJH (nm) b | VBJH [cm3/g] c | Pd conc./mg g−1 d | Pd Particle Size (nm) e |
|---|---|---|---|---|---|
| Pd-chitin-500 | 498 | 4.9 | 0.61 | 4.3 | 9.7 |
| Pd-chitin-Na2CO3-500 | 452 | 5.3 | 0.53 | 3.9 | 6.7 |
| Entry | Catalyst | Base | Conversion (%) a | Selectivity (%) b |
|---|---|---|---|---|
| 1 | Pd-chitin-500 | / | <10 | >99 |
| 2 | Pd-chitin-Na2CO3 | / | <10 | >99 |
| 3 | Pd-chitin-Na2CO3-500 | / | <10 | >99 |
| 4 | Pd-chitin | K2CO3 | 73 | >99 |
| 5 | Pd-chitin-500 | K2CO3 | 81 | >99 |
| 6 | Pd-chitin-Na2CO3-500 | K2CO3 | 78 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentin, O.; Polidoro, D.; Perosa, A.; Rodríguez-Castellon, E.; Rodríguez-Padrón, D.; Selva, M. Mechanochemistry through Extrusion: Opportunities for Nanomaterials Design and Catalysis in the Continuous Mode. Chemistry 2023, 5, 1760-1769. https://doi.org/10.3390/chemistry5030120
Trentin O, Polidoro D, Perosa A, Rodríguez-Castellon E, Rodríguez-Padrón D, Selva M. Mechanochemistry through Extrusion: Opportunities for Nanomaterials Design and Catalysis in the Continuous Mode. Chemistry. 2023; 5(3):1760-1769. https://doi.org/10.3390/chemistry5030120
Chicago/Turabian StyleTrentin, Oscar, Daniele Polidoro, Alvise Perosa, Enrique Rodríguez-Castellon, Daily Rodríguez-Padrón, and Maurizio Selva. 2023. "Mechanochemistry through Extrusion: Opportunities for Nanomaterials Design and Catalysis in the Continuous Mode" Chemistry 5, no. 3: 1760-1769. https://doi.org/10.3390/chemistry5030120
APA StyleTrentin, O., Polidoro, D., Perosa, A., Rodríguez-Castellon, E., Rodríguez-Padrón, D., & Selva, M. (2023). Mechanochemistry through Extrusion: Opportunities for Nanomaterials Design and Catalysis in the Continuous Mode. Chemistry, 5(3), 1760-1769. https://doi.org/10.3390/chemistry5030120










