Gas Chromatography–Mass Spectrometry Chemical Profiling and Radical Scavenging Potential of Sesquiterpene-Rich Essential Oil of Polygonum equisetiforme Sm.: In Silico Study on Hematopoietic Cell Kinase (Hck) and Human Peroxiredoxin 5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection, Authentication, and Preparation
2.2. Chemical and Drugs
2.3. EO Extraction
2.4. EO Analysis by Gas Chromatography–Mass Spectroscopy (GC-MS)
2.5. DPPH and ABTS Scavenging Activity
2.6. Data Treatments and Statistical Analysis
2.7. Molecular Docking Studies
3. Results and Discussions
3.1. Chemical Profiling of P. equisetiforme EO
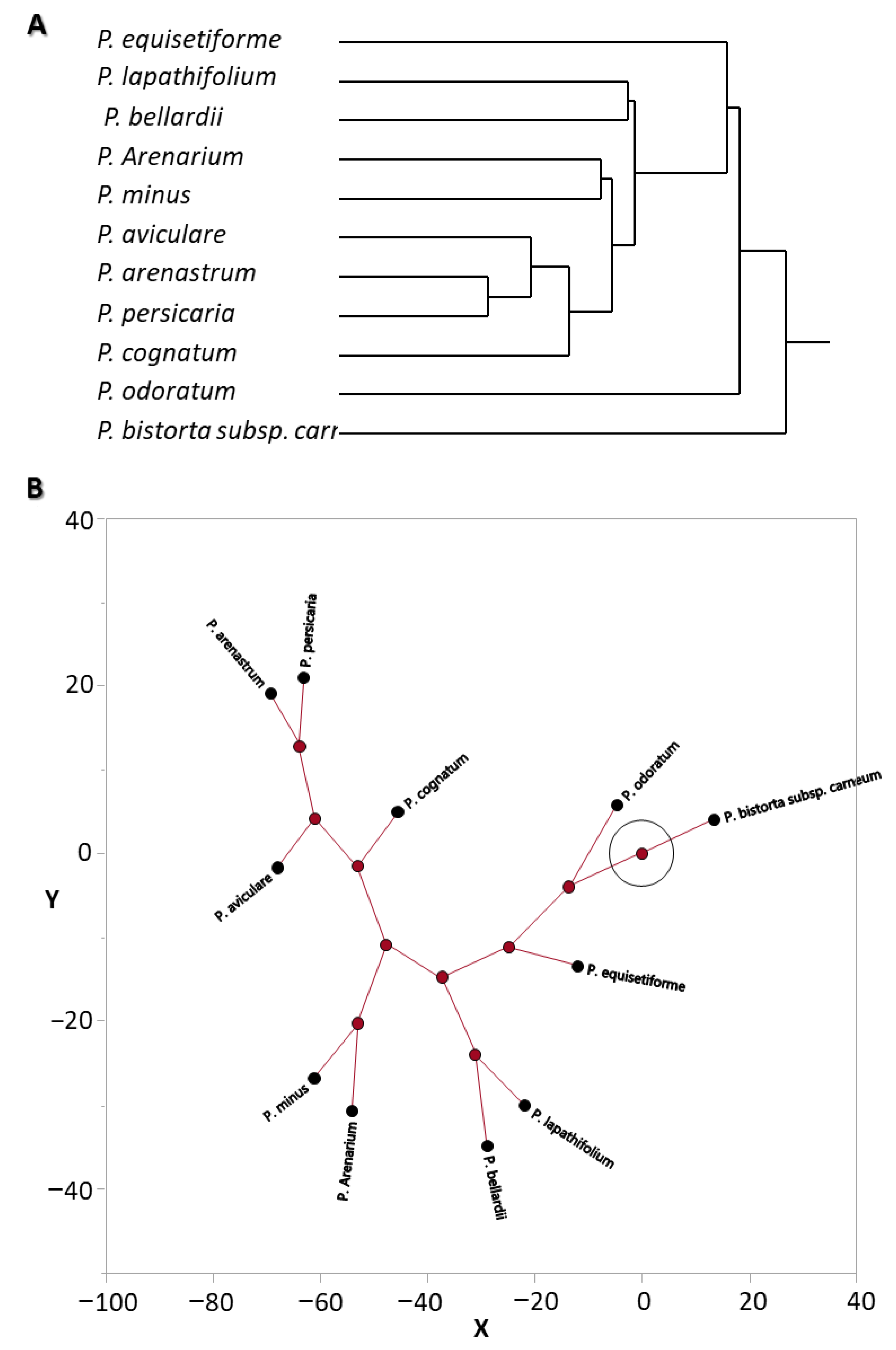
3.2. Chemosystematic Significance via Multivariate Analysis
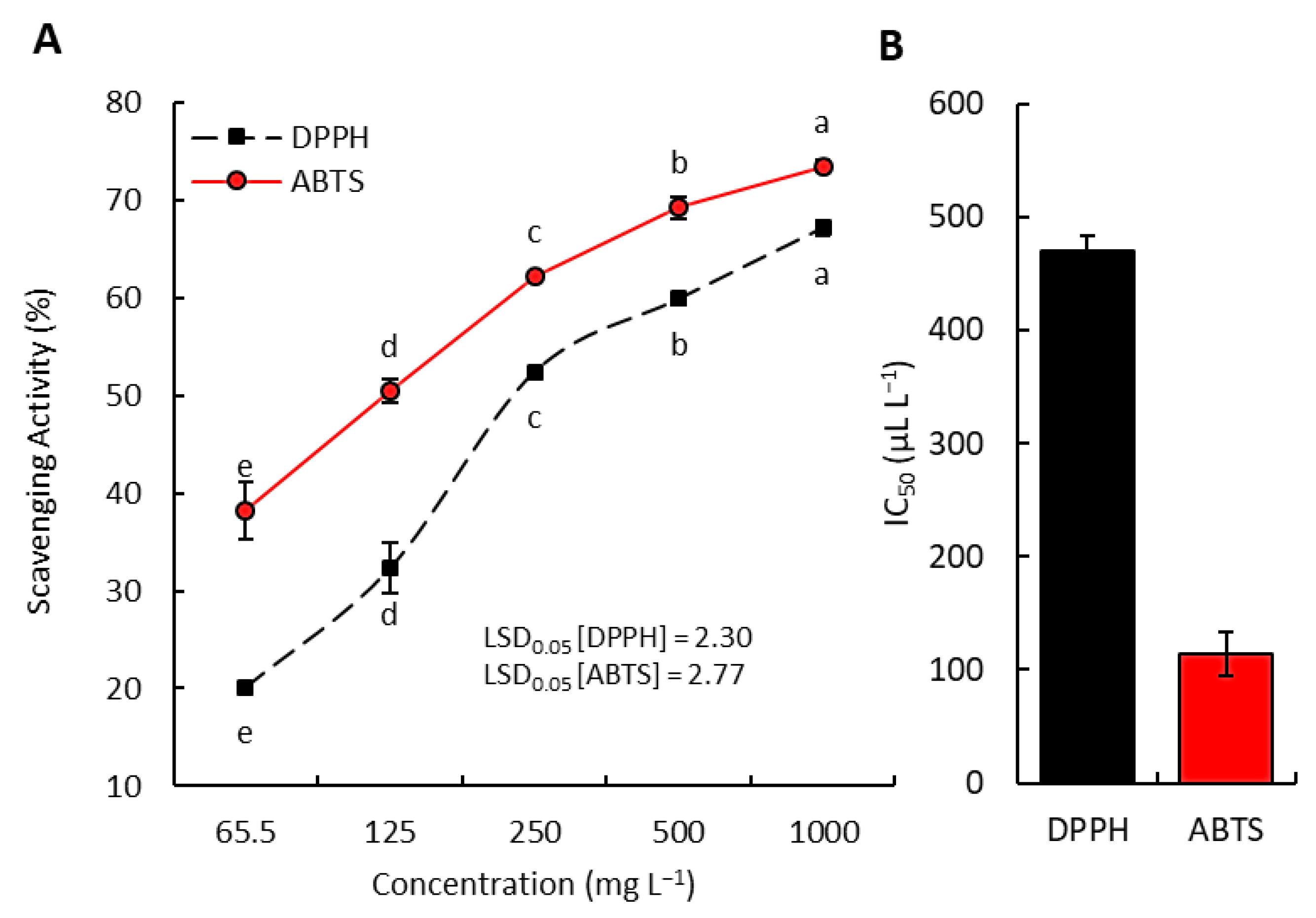
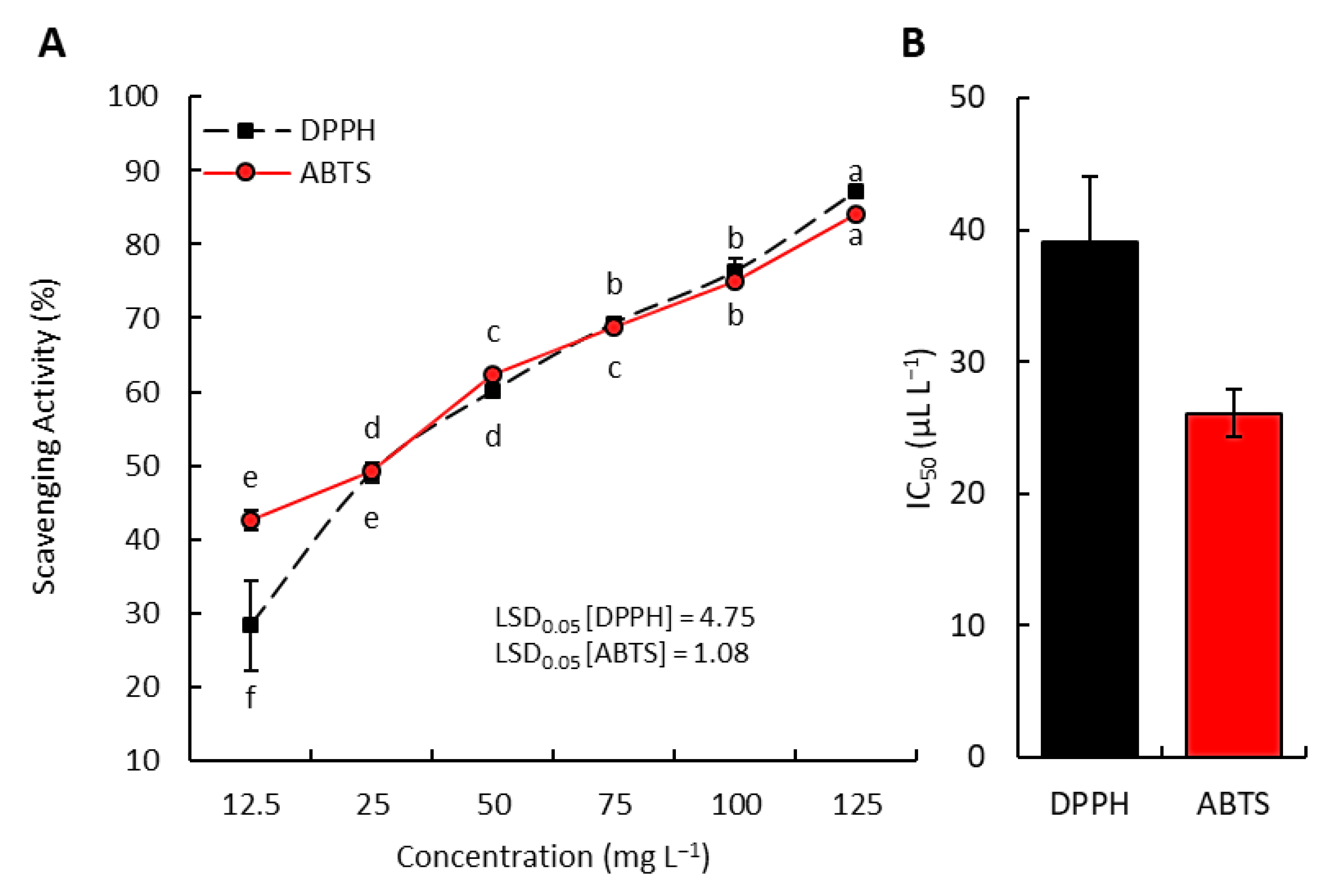
3.3. Radical Scavenging Activity
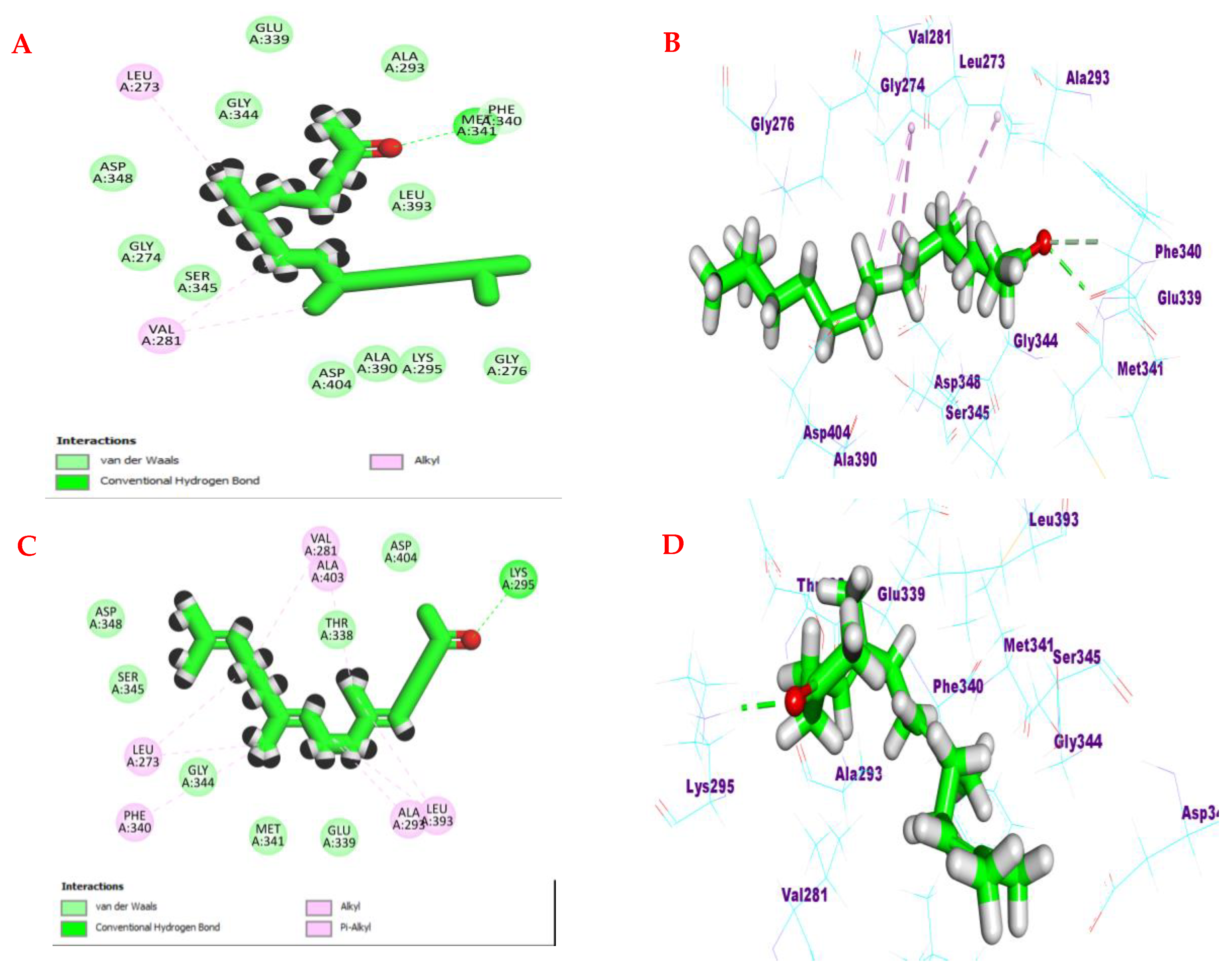
3.4. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, T.; Lupo, A.T., Jr.; Chinn, J.W., Jr.; Kang, R.K. Biological activity of essential oils and their constituents. Stud. Nat. Prod. Chem. 2000, 21, 571–631. [Google Scholar]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, A., Ed.; Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 203–238. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Boughalleb, F.; Bouhamda, T.; Abdellaoui, R.; Nasri, N. Unexploited Polygonum equisetiforme seeds: Potential source of useful natural bioactive products. Ind. Crop. Prod. 2018, 122, 349–357. [Google Scholar] [CrossRef]
- Budel, J.M.; Farago, P.V.; Duarte, M.d.R.; Takeda, I.J. Morpho-anatomical study of the cladodes of Homalocladium platycladum (FJ Muell.) LH Bailey (Polygonaceae). Rev. Bras. Farmacogn. 2007, 17, 39–43. [Google Scholar] [CrossRef]
- Koochak, H.; Seyyednejad, S.M.; Motamedi, H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran). Asian Pac. J. Trop. Med. 2010, 3, 180–184. [Google Scholar] [CrossRef]
- Chen, K.-T.; Zhou, W.-L.; Liu, J.-W.; Zu, M.; He, Z.-N.; Du, G.-H.; Chen, W.-W.; Liu, A.-L. Active neuraminidase constituents of Polygonum cuspidatum against influenza A (H1N1) influenza virus. China J. Chin. Mater. Med. 2012, 37, 3068–3073. [Google Scholar]
- Kuhnlein, H.V.; Turner, N.J. Traditional Plant Foods of Canadian Indigenous Peoples: Nutrition, Botany, and Use; Taylor & Francis: New York, NY, USA, 1991; Volume 8. [Google Scholar]
- Yang, X.; Zou, Y.; Ye, J.; Bao, Z.; Ding, Z. Chemical studies on the plants of Polygonum. Yun Chem. Technol. 2003, 30, 31–33. [Google Scholar]
- Mouldi, G. Natural vegetation cover dynamic under grazing-rotation managements in desert rangelands of Tunisia. Arid Ecosyst. 2014, 20, 66–75. [Google Scholar]
- Khafagi, I.K.; Dewedar, A. The efficiency of random versus ethno-directed research in the evaluation of Sinai medicinal plants for bioactive compounds. J. Ethnopharmacol. 2000, 71, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Hussein, S.; Usama, E.-M.; Tantawy, M.; Kawashty, S.; Saleh, N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arab. J. Chem. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Salib, J.Y.; Shafik, N.H.; Elkarim, A.; Farag, A. New flavonoids from the aerial parts of Polygonum equisetiforme SM (Polygonaceae). Int. J. Pharm. Pharm. Sci. 2017, 9, 166–170. [Google Scholar] [CrossRef]
- El-Toumy, S.A.H.; Salib, J.Y.; Shafik, N.H.; Abd Elkarim, A.S.; Salama, A.; Omara, E.A.A.; Micky, J. Evaluation of hepatoprotective activity of Polygonum equisetiforme methanolic extract. J. Appl. Pharm. Sci. 2019, 9, 054–059. [Google Scholar]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a new type of mammalian Peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef]
- Poh, A.R.; O’Donoghue, R.J.; Ernst, M. Hematopoietic cell kinase (HCK) as a therapeutic target in immune and cancer cells. Oncotarget 2015, 6, 15752–15771. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt; Al-Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- El Gendy, A.E.-N.G.; Essa, A.F.; El-Rashedy, A.A.; Elgamal, A.M.; Khalaf, D.D.; Hassan, E.M.; Abd-ElGawad, A.M.; Elgorban, A.M.; Zaghloul, N.S.; Alamery, S.F. Antiviral potentialities of chemical characterized essential oils of Acacia nilotica bark and fruits against hepatitis A and herpes simplex viruses: In vitro, in silico, and molecular dynamics studies. Plants 2022, 11, 2889. [Google Scholar] [CrossRef]
- Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd El-Rahman, S.S.; Elgamal, A.M.; Elshamy, A.I. Oral and topical anti-inflammatory and antipyretic potentialities of Araucaria bidiwillii shoot essential oil and its nanoemulsion in relation to chemical composition. Molecules 2021, 26, 5833. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), 2015. Chemical Computing Group. Canada, Montreal. Available online: http://www.chemcomp.com (accessed on 6 September 2023).
- Declercq, J.-P.; Evrard, C.; Clippe, A.; Vander Stricht, D.; Bernard, A.; Knoops, B. Crystal structure of human Peroxiredoxin 5, a novel type of mammalian Peroxiredoxin at 1.5 Å resolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sicheri, F.; Moarefi, I.; Kuriyan, J. Crystal structure of the Src family tyrosine kinase Hck. Nature 1997, 385, 602–609. [Google Scholar] [CrossRef]
- Demirpolat, A. Chemical Composition of Essential Oils of Seven Polygonum Species from Turkey: A Chemotaxonomic Approach. Molecules 2022, 27, 9053. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, K.B. Essential Oil of Polygonum minus Huds. J. Essent. Oil Res. 1990, 2, 167–172. [Google Scholar] [CrossRef]
- Iskender, N.Y.; Güleç, C.A.; Yücel, M.; Sinek, K.; Yayli, N. Analysis of the essential oil from the flower of Polygonum bistorta L. subsp. carneum (Koch). Asian J. Chem. 2011, 23, 1940–1942. [Google Scholar]
- Alarcón, L.; Peña, A.; Velasco, J.; Baptista, J.G.; Rojas, L.; Aparicio, R.; Usubillaga, A. Chemical composition and antibacterial activity of the essential oil of Ruilopezia bracteosa. Nat. Prod. Commun. 2015, 10, 655–656. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.J.; Wessjohann, L.; Setzer, W.N. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- Pereira, R.A.; Ramos, Y.J.; de Queiroz, G.A.; Guimarães, E.F.; Defaveri, A.C.A.; de Lima Moreira, D. Chemodiversity of Essential Oils in Piper L.(Piperaceae) Species from the Restinga of Marambaia Island, Rio de Janeiro-RJ, Brazil. Rev. Virtual Química 2021, 13, 1203–1215. [Google Scholar] [CrossRef]
- Martínez-Arévalo, J.V.; Cruz, S.M.; Apel, M.A.; Henriques, A.T.; Cáceres, A. Essential Oil of Piper oradendron from the Pacific Slope of Guatemala. Nat. Prod. Commun. 2019, 14, 79–81. [Google Scholar]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- Thulasiram, H.V.; Erickson, H.K.; Poulter, C.D. Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 2007, 316, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; AM El-Khrisy, E.E.D.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- El-Kashak, W.A.; Elshamy, A.I.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Saleh, I.A.; Umeyama, A. Rumpictuside A: Unusual 9, 10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydr. Res. 2017, 448, 74–78. [Google Scholar] [CrossRef]
- Hammad, H.M.; Albu, C.; Matar, S.A.; Litescu, S.-C.; Al Jaber, H.; Abualraghib, A.; Afifi, F.U. Biological activities of the hydro-alchoholic and aqueous extracts of Achillea biebersteinii Afan.(Asteraceae) grown in Jordan. Afr. J. Pharm. Pharmacol. 2013, 7, 1686–1694. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2016, 72, 474–480. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.N.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical composition of Kickxia aegyptiaca essential oil and its potential antioxidant and antimicrobial activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.; El Gendy, A.E.-N.; El-Amier, Y.; Gaara, A.; Omer, E.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef]
- Cipriano, R.R.; Maia, B.H.; Deschamps, C. Chemical variability of essential oils of Eugenia uniflora L. genotypes and their antioxidant activity. An. Acad. Bras. Ciências 2021, 93, e20181299. [Google Scholar] [CrossRef]
- Yagi, S.; Babiker, R.; Tzanova, T.; Schohn, H. Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac. J. Trop. Med. 2016, 9, 763–770. [Google Scholar] [CrossRef]
- Gourine, N.; Yousfi, M.; Bombarda, I.; Nadjemi, B.; Stocker, P.; Gaydou, E. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crop. Prod. 2010, 31, 203–208. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef] [PubMed]

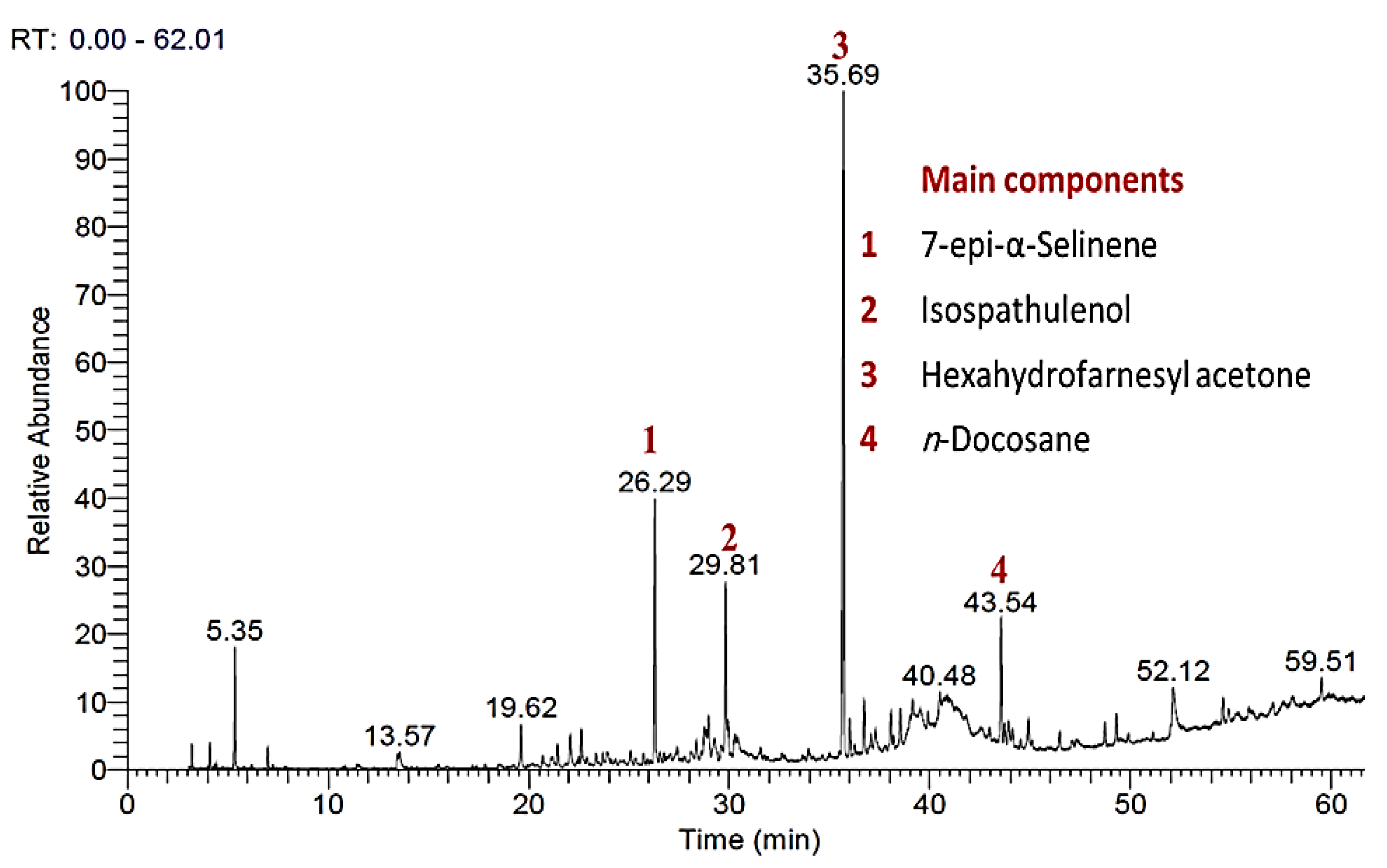


| Rt | RA (%) | Compound | Class | KIit. | KIcal. | IM |
|---|---|---|---|---|---|---|
| 3.22 | 0.32 ± 0.01 | α-Pinene | MH | 939 | 937 | KI and Ms |
| 4.12 | 0.49 ± 0.02 | β-Pinene | MH | 979 | 980 | KI and Ms |
| 5.35 | 2.55 ± 0.07 | d-Limonene | MH | 1029 | 1032 | KI and Ms |
| 7.00 | 0.49 ± 0.01 | α-terpinolene | MH | 1088 | 1089 | KI and Ms |
| 13.46 | 0.53 ± 0.01 | Cumin aldehyde | OM | 1241 | 1243 | KI and Ms |
| 13.57 | 0.56 ± 0.02 | β-Longipinene | SH | 1400 | 1399 | KI and Ms |
| 19.62 | 1.48 ± 0.05 | α-Cedrene | OS | 1411 | 1410 | KI and Ms |
| 21.45 | 0.74 ± 0.03 | trans-Caryophyllene | SH | 1419 | 1417 | KI and Ms |
| 22.08 | 1.08 ± 0.04 | Phytane | DH | 1425 | 1425 | KI and Ms |
| 22.62 | 1.18 ± 0.05 | (E)-α-Ionone | Car | 1430 | 1432 | KI and Ms |
| 22.92 | 0.32 ± 0.01 | Neryl acetone | OM | 1436 | 1438 | KI and Ms |
| 23.35 | 0.44 ± 0.02 | n-Pentadecane | H | 1500 | 1498 | KI and Ms |
| 23.69 | 0.38 ± 0.01 | (Z)-α-Bisabolene | SH | 1507 | 1506 | KI and Ms |
| 23.92 | 0.55 ± 0.02 | Modhephen-8-β-ol | SH | 1513 | 1511 | KI and Ms |
| 25.72 | 0.40 ± 0.01 | γ-Cadinene | SH | 1513 | 1513 | KI and Ms |
| 26.29 | 14.45 ± 0.12 | 7-epi-α-Selinene | SH | 1522 | 1524 | KI and Ms |
| 26.54 | 0.48 ± 0.01 | E-Nerolidol | OS | 1563 | 1561 | KI and Ms |
| 27.41 | 0.56 ± 0.01 | Hexahydrofarnesol | OS | 1563 | 1563 | KI and Ms |
| 28.09 | 0.51 ± 0.01 | Spathulenol | OS | 1578 | 1576 | KI and Ms |
| 28.36 | 0.72 ± 0.02 | Caryophyllene oxide | OS | 1583 | 1584 | KI and Ms |
| 28.75 | 1.16 ± 0.04 | Veridiflorol | OS | 1590 | 1588 | KI and Ms |
| 28.86 | 0.96 ± 0.03 | Globulol | OS | 1590 | 1592 | KI and Ms |
| 28.96 | 1.54 ± 0.06 | n-Hexadecane | H | 1600 | 1602 | KI and Ms |
| 29.27 | 1.60 ± 0.07 | Humulene epoxide II | OS | 1608 | 1609 | KI and Ms |
| 29.81 | 8.35 ± 0.11 | Isospathulenol | OS | 1623 | 1620 | KI and Ms |
| 29.93 | 1.51 ± 0.05 | Cubenol | OS | 1646 | 1644 | KI and Ms |
| 30.27 | 1.70 ± 0.06 | ar-Turmerone | OS | 1669 | 1671 | KI and Ms |
| 32.62 | 0.33 ± 0.01 | Hexadecanal | OH | 1819 | 1821 | KI and Ms |
| 35.69 | 29.45 ± 0.23 | Hexahydrofarnesyl acetone | OS | 1846 | 1846 | KI and Ms |
| 35.99 | 1.38 ± 0.12 | 2-Heptadecanone | OH | 1875 | 1876 | KI and Ms |
| 36.25 | 0.38 ± 0.01 | n-Nonadecane | OH | 1900 | 1900 | KI and Ms |
| 36.72 | 1.92 ± 0.04 | 5E,9E-Farnesyl acetone | OS | 1913 | 1915 | KI and Ms |
| 37.28 | 0.93 ± 0.01 | Methyl palmitate | OH | 1921 | 1920 | KI and Ms |
| 38.05 | 1.58 ± 0.05 | Carissone | OS | 1927 | 1929 | KI and Ms |
| 38.52 | 1.29 ± 0.03 | Phytol | OD | 1943 | 1942 | KI and Ms |
| 38.99 | 0.34 ± 0.01 | n-Eicosane | H | 2000 | 2002 | KI and Ms |
| 39.14 | 1.36 ± 0.04 | 9-Octadecenoic acid (Z)-, methyl ester | OH | 2085 | 2088 | KI and Ms |
| 40.48 | 1.57 ± 0.04 | n-Heneicosane | H | 2100 | 2100 | KI and Ms |
| 42.58 | 0.41 ± 0.01 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | OH | 2101 | 2103 | KI and Ms |
| 43.54 | 6.79 ± 0.09 | n-Docosane | H | 2200 | 2200 | KI and Ms |
| 43.9 | 1.67 ± 0.05 | n-Pentacosane | H | 2500 | 2498 | KI and Ms |
| 44.89 | 1.08 ± 0.04 | n-Hexacosane | H | 2600 | 2603 | KI and Ms |
| 54.59 | 0.95 ± 0.03 | n-Dotriacontane | H | 3200 | 3200 | KI and Ms |
| 3.85 | Monoterpene hydrocarbons (MH) | |||||
| 0.85 | Oxygen containing monoterpenes (OM) | |||||
| 17.08 | Sesquiterpene hydrocarbons (SH) | |||||
| 51.98 | Oxygen containing sesquiterpenes (OS) | |||||
| 1.08 | Diterpene hydrocarbons (DH) | |||||
| 1.29 | Oxygen containing diterpenes (OD) | |||||
| 14.38 | Hydrocarbons (H) | |||||
| 4.79 | Oxygen containing hydrocarbons (OH) | |||||
| 1.18 | Carotenoid-derived compounds (Car) | |||||
| Total | 96.48 | |||||
| Compound | ΔG a (kcal/mol) | |
|---|---|---|
| Tyrosine Kinase Hck | Human Peroxiredoxin 5 | |
| D-Limonene | −5.17 | −4.46 |
| 7-epi-α-Selinene | −4.93 | −4.86 |
| Isospathulenol | −5.41 | −4.70 |
| Cubenol | −5.41 | −4.84 |
| ar-Turmerone | −6.14 | −4.93 |
| Hexahydrofarnesyl acetone | −6.83 | −5.34 |
| 5E,9E-Farnesyl acetone | −7.08 | −5.47 |
| Carissone | −5.35 | −4.77 |
| n-Docosane | −6.79 | −5.25 |
| Vitamin C (reference) | −5.12 | −4.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Ahmed, R.F.; Essa, A.F.; Gendy, A.E.-N.G.E.; El-Newary, S.A.; Elshamy, A.I.; Sarker, T.C.; El-Amier, Y.A. Gas Chromatography–Mass Spectrometry Chemical Profiling and Radical Scavenging Potential of Sesquiterpene-Rich Essential Oil of Polygonum equisetiforme Sm.: In Silico Study on Hematopoietic Cell Kinase (Hck) and Human Peroxiredoxin 5. Chemistry 2023, 5, 2257-2272. https://doi.org/10.3390/chemistry5040151
Abd-ElGawad AM, Ahmed RF, Essa AF, Gendy AE-NGE, El-Newary SA, Elshamy AI, Sarker TC, El-Amier YA. Gas Chromatography–Mass Spectrometry Chemical Profiling and Radical Scavenging Potential of Sesquiterpene-Rich Essential Oil of Polygonum equisetiforme Sm.: In Silico Study on Hematopoietic Cell Kinase (Hck) and Human Peroxiredoxin 5. Chemistry. 2023; 5(4):2257-2272. https://doi.org/10.3390/chemistry5040151
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Rania F. Ahmed, Ahmed F. Essa, Abd El-Nasser G. El Gendy, Samah A. El-Newary, Abdelsamed I. Elshamy, Tushar C. Sarker, and Yasser A. El-Amier. 2023. "Gas Chromatography–Mass Spectrometry Chemical Profiling and Radical Scavenging Potential of Sesquiterpene-Rich Essential Oil of Polygonum equisetiforme Sm.: In Silico Study on Hematopoietic Cell Kinase (Hck) and Human Peroxiredoxin 5" Chemistry 5, no. 4: 2257-2272. https://doi.org/10.3390/chemistry5040151
APA StyleAbd-ElGawad, A. M., Ahmed, R. F., Essa, A. F., Gendy, A. E.-N. G. E., El-Newary, S. A., Elshamy, A. I., Sarker, T. C., & El-Amier, Y. A. (2023). Gas Chromatography–Mass Spectrometry Chemical Profiling and Radical Scavenging Potential of Sesquiterpene-Rich Essential Oil of Polygonum equisetiforme Sm.: In Silico Study on Hematopoietic Cell Kinase (Hck) and Human Peroxiredoxin 5. Chemistry, 5(4), 2257-2272. https://doi.org/10.3390/chemistry5040151









