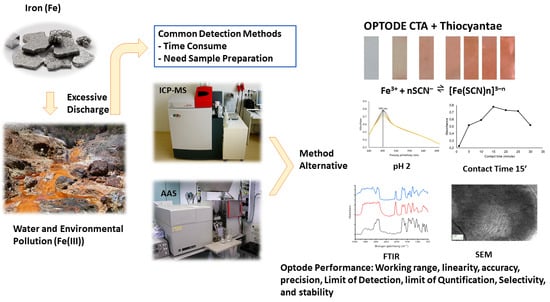
A Sensor (Optode) Based on Cellulose Triacetate Membrane for Fe(III) Detection in Water Samples
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Procedure
2.3.1. NH4SCN Optimisation and CTA Optode Membrane Fabrication
2.3.2. Characterisation of CTA Optode Membrane
2.3.3. Determination of Optimum pH and Contact Time
2.3.4. Determination of Working Range and Optode Membrane Linearity Test
2.3.5. Limit of Detection and Limit of Quantity Test
2.3.6. Precision and Accuracy Test
2.3.7. Selectivity Test
2.3.8. Colour Complex Stability Test
2.3.9. Detection of Fe(III) in Water Samples with CTA Optode Membrane
3. Results and Discussion
3.1. Optimisation of NH4SCN and CTA Optode Membrane Fabrication
3.2. Characterisation of the Optode Membrane
3.2.1. Physical Characteristics
3.2.2. SEM
3.2.3. FTIR
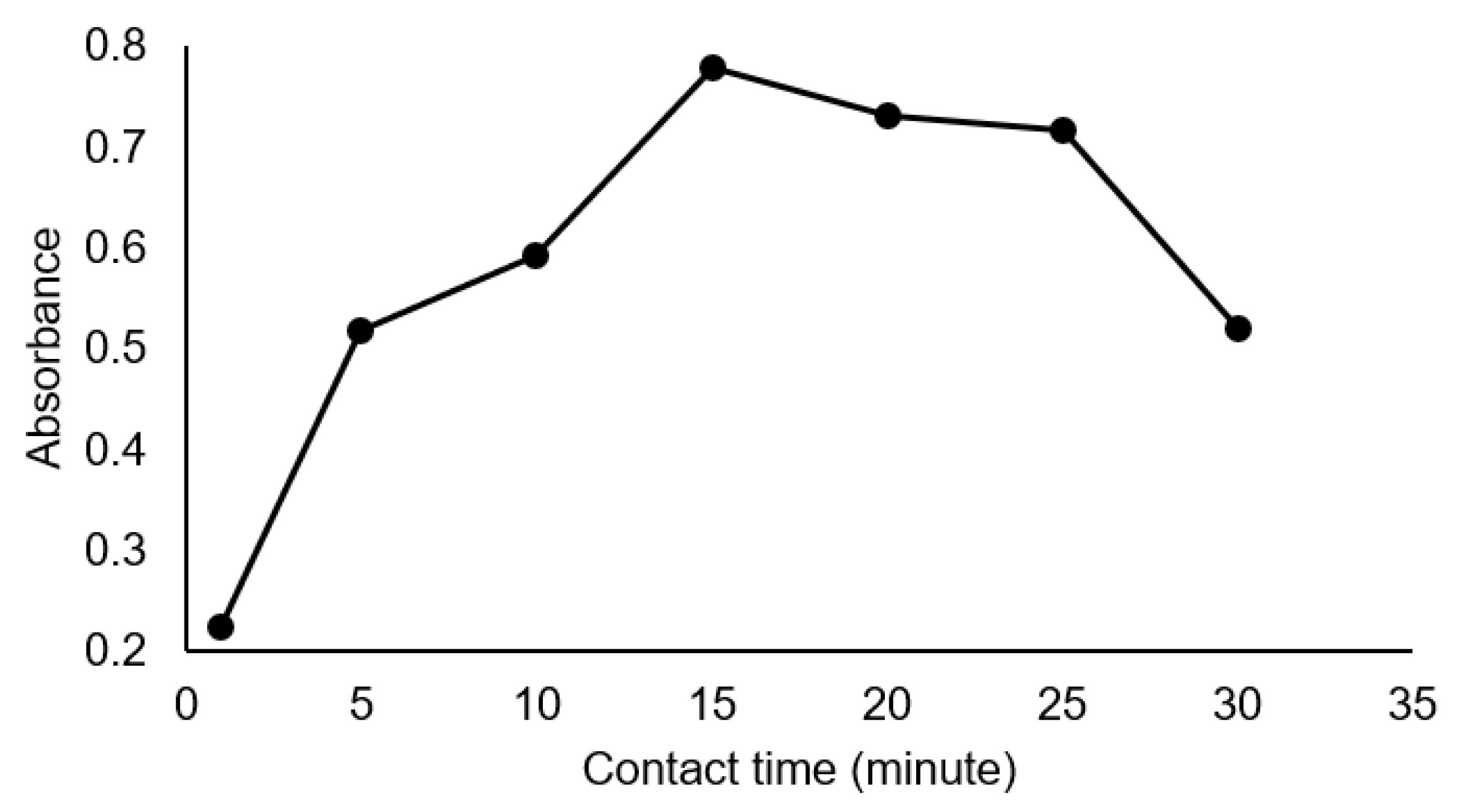
3.3. Optimization of pH and Contact Time
3.4. Working Range and Linearity
3.5. Limit of Detection and Limit of Quantity
3.6. Precision and Accuracy
3.7. Selectivity and Complex Colour Stability
3.8. Detection of Fe(III) in Water Samples with CTA Optode Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamsipur, M.; Sadeghi, M.; Garau, A.; Lippolis, V. An efficient and selective fluorescent chemical sensor based on 5-(8-hydroxy-2-quinolinylmethyl)-2,8-dithia-5-aza-2,6-pyridinophane as a new fluoroionophore for determination of iron(III) ions a novel probe for iron speciation. Anal. Chim. Acta 2013, 761, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Prianto, E.; Husnah, H. Penambangan timah inkonvensional: Dampaknya terhadap kerusakan biodiversitas perairan umum di pulau bangka. BAWAL 2009, 2, 193–198. [Google Scholar] [CrossRef]
- Regulation the Minister of Health of the Republic of Indonesia. Regulation of the Minister of Health of the Republic of Indonesia Number 32 of 2017 Concerning Environmental Health Quality Standards and Water Health Requirements for Sanitary Hygiene, Swimming Pools, Solus Per Aqua, and Public Baths; Minister of Health of the Republic of Indonesia: Jakarta, Indonesia, 2017.
- Ghaedi, M.; Shahamiri, A.; Hajati, S.; Mirtamizdoust, B. A novel PVC-membrane optical sensor for high sensitive and selective determination of Cu2+ ion based on synthesized (E)-N′-(pyridin-2-ylmethylene)isonicotin-ohydrazide. J. Mol. Liquids 2014, 199, 483–488. [Google Scholar] [CrossRef]
- Scindia, Y.M.; Pandey, A.K.; Reddy, A.V.R.; Manohar, S.B. Chemically selective membrane optode for Cr(VI) determination in aqueous samples. J. Anal. Chem. Acta 2004, 515, 311–321. [Google Scholar] [CrossRef]
- Verma, C.; Tapadia, K.; Soni, A.B. Determination of iron(III) in food, biological and environmental samples. Food Chem. 2017, 221, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, N.; Wagner-Wysiecka, E. Salicylaldimine-based receptor as a material for iron(III) selective optical sensing. J. Photochem. Photobiol. A Chem. 2017, 346, 318–326. [Google Scholar] [CrossRef]
- ICH Expert Working Group. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 2 June 2014. [Google Scholar]
- Harmita, H. Petunjuk pelaksanaan validasi metode dan cara perhitungannya. Maj. Ilmu Kefarmasian 2004, 1, 117–135. [Google Scholar] [CrossRef]
- Harvey, D. Analytical Chemistry 2.1; McGraw-Hill Companies: New York, NY, USA, 2016. [Google Scholar]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Couvrat, N.; Fatyeyeva, K. Polymer inclusion membranes based on CTA/PBAT blend containing aliquot 336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. React. Funct. Polym. 2019, 139, 120–132. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, A.; Heng, L.Y. An optical sensor for Al3+ ion based on immobilized eriochrome cyanine r in polymer inclusion membrane using absorption sensing principle. Malay. J. Anal. Sci. 2021, 25, 296–310. [Google Scholar]
- Regmi, C.; Ashtiani, S.; Sofer, Z.; Hrdlička, Z.; Průša, F.; Vopička, O.; Friess, K. CeO2-blended cellulose triacetate mixed-matrix membranes for selective CO2 separation. Membranes 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Rastegarzadeh, S.; Nahid, P.; Zahra, J. Design of a sensitive membrane optode for iron determination. Instrum. Sci. Technol. 2013, 41, 290–300. [Google Scholar] [CrossRef]
- Pedreno-Sanches, C.; Ortuno, J.A.; Albero, M.I.; Garcia, M.S.; Valero, M.V. Development of a new bulk optode membrane for the determination of mercury(II). Anal. Chim. Acta 2000, 414, 195–203. [Google Scholar] [CrossRef]
- Luke, C.L. New Spectrophomotometric Thiocyanate Determination of Iron In Metals, Alloys, Acids, and Salts. Anal. Chim. Acta 1966, 36, 122–126. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 4th ed.; Cengange Learning: Washington, DC, USA, 2009. [Google Scholar]
- Nyasulu, F.; Barlag, R. Colorimetric Determination of the Iron(III)-Thiocyanate Reaction Equilibrium Constant with Calibration and Equilibrium Solutions Prepared in a Cuvette by Sequential Additions of One Reagent to the Other. J. Chem. Edu. 2011, 88, 313–314. [Google Scholar] [CrossRef]
- Lestari, I.; Afrida, A.; Sanova, A. Sintesis dan karakterisasi senyawa kompleks logam kadmium(II) dengan ligan kufperon. J. Penelit. Univ. Jambi Seri Sains 2014, 16, 1–8. [Google Scholar]
- Kong, F.; Ni, Y. Determination of Cr(VI) concentration in diluted samples based on the paper test strip method. Water Sci. Technol. 2009, 60, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Regulation the Minister of Health of the Republic of Indonesia. Regulation of the Minister of Health of the Republic of Indonesia Number 492 of 2010 Concerning Requirements for Drinking Water Quality; Minister of Health of the Republic of Indonesia: Jakarta, Indonesia, 2010.
- Association of Official Analytical Chemist. Official Method of Analysis of AOAC International, 20th ed.; AOAC Pr: Washington, DC, USA, 2013. [Google Scholar]
- Bakker, E.; Simon, W. Selectivity of ion-sensitive bulk optodes. Anal Chem. 1992, 64, 1805–1812. [Google Scholar] [CrossRef]
- Muirhead, K.A.; Haight, G.P. Kinetics and mechanism of the oxidation of thiocyanate ion by chromium(VI). Inorg. Chem. 1973, 12, 1116–1120. [Google Scholar] [CrossRef]
- Nashukha, H.L.; Sulistyarti, H.; Sabarudin, A. Uji linieritas, selektivitas, dan validitas metode analisis merkuri(ii) secara spektrofotometri berdasarkan penurunan absorbansi kompleks besi(iii) tiosianat. Kim. Stud. J. 2014, 2, 492–498. [Google Scholar]
- Suah, F.B.M.; Ahmad, M.; Heng, L.Y. A novel polymer inclusion membranes based optode for sensitive determination of Al3+ ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 81–87. [Google Scholar] [CrossRef] [PubMed]


 pH 1,
pH 1,  pH 2,
pH 2,  pH 3,
pH 3,  pH 4.
pH 4.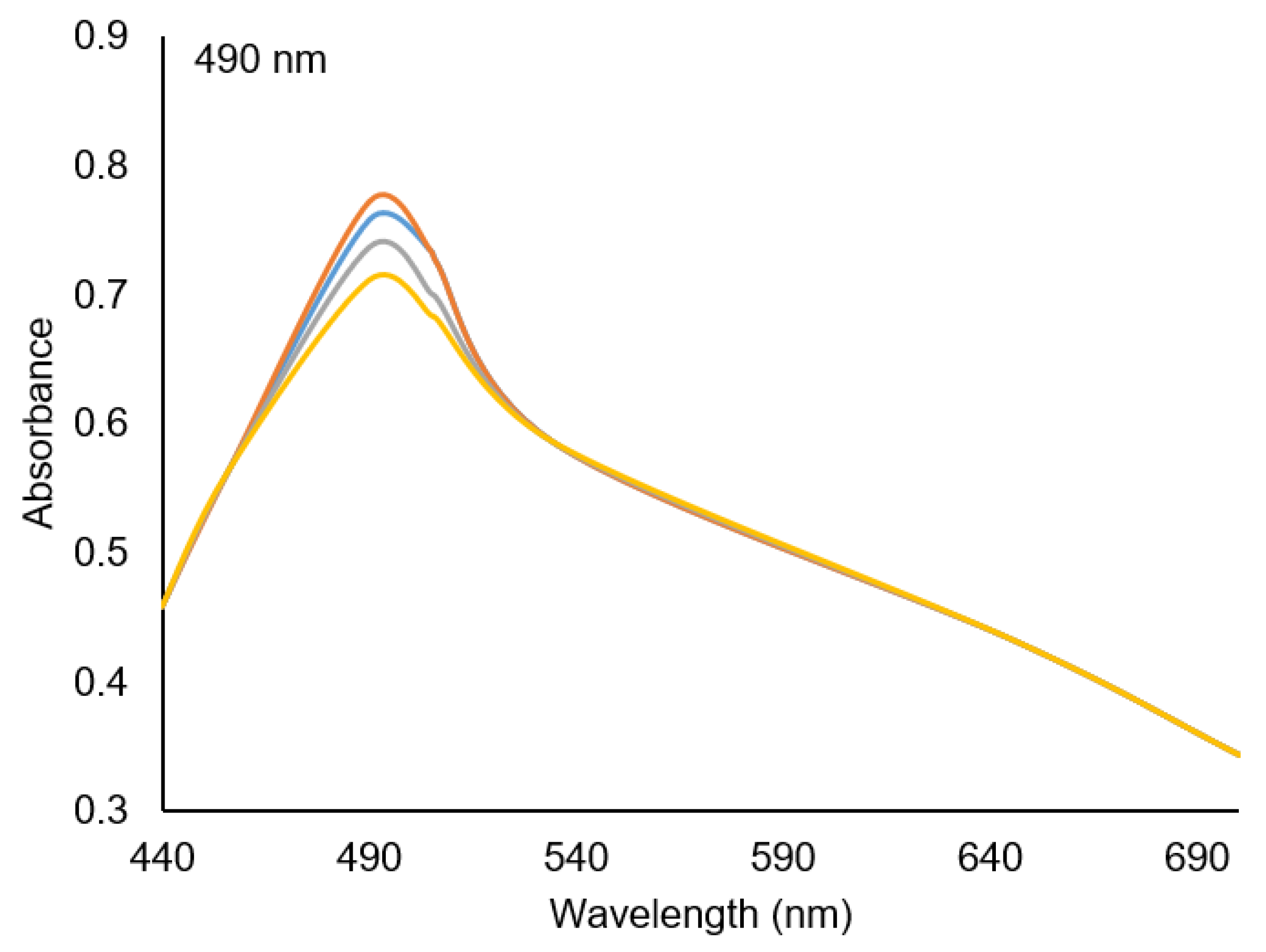
| Standard Concentration of Fe(III) (mg/L) | Concentration NH4SCN (M) | Corrected Absorbance |
|---|---|---|
| 12 | 0.1 | −0.0085 |
| 0.5 | 0.5950 | |
| 1 | 0.7324 |
| Membrane | Repetition | Thickness | Average | ||
|---|---|---|---|---|---|
| Inch | mm | Inch | mm | ||
| 1 | 1 | 0.0017 | 0.043 | 0.0017 | 0.042 |
| 2 | 0.0016 | 0.041 | |||
| 3 | 0.0017 | 0.043 | |||
| 4 | 0.0016 | 0.041 | |||
| 5 | 0.0017 | 0.043 | |||
| 2 | 1 | 0.0018 | 0.046 | 0.0018 | 0.046 |
| 2 | 0.0018 | 0.046 | |||
| 3 | 0.0019 | 0.048 | |||
| 4 | 0.0017 | 0.043 | |||
| 5 | 0.0018 | 0.046 | |||
| 3 | 1 | 0.0017 | 0.043 | 0.0017 | 0.044 |
| 2 | 0.0017 | 0.043 | |||
| 3 | 0.0016 | 0.041 | |||
| 4 | 0.0018 | 0.046 | |||
| 5 | 0.0018 | 0.046 | |||
| Average | 0.0017 | 0.044 | |||
| SD | 7.02 × 10−5 | 1.78 × 10−3 | |||
| %RSD | 4.07 | 4.07 | |||
| Wavenumber (cm−1) | Wavenumber (cm−1) [17] | |
|---|---|---|
| C−O stretching | 1055 | 1300–1000 |
| 1245 | ||
| C=O streching | 1735 | 1750–1730 |
| S−CN streching | 2061 | 2200–2000 |
| C−H streching | 2935 | 3000–2850 |
| Repetition | Slope | Intercept |
|---|---|---|
| 1 | 0.2966 | 0.1088 |
| 2 | 0.2983 | 0.1116 |
| 3 | 0.2950 | 0.1061 |
| Average | 0.2966 | 0.1088 |
| SDi | 0.0022 | |
| LoD | 0.0250 | |
| LoQ | 0.0757 |
| Repetition | Corrected Absorbance |
|---|---|
| 1 | 0.7064 |
| 2 | 0.7480 |
| 3 | 0.7691 |
| 4 | 0.7186 |
| 5 | 0.7111 |
| 6 | 0.7380 |
| Average | 0.7319 |
| SD | 0.0242 |
| %RSD | 3.31 |
| [Fe(III)]standard (mg/L) | Repetition | Corrected Absorbance | [Fe(III)]experiment (mg/L) | %R | Average %R |
|---|---|---|---|---|---|
| 1 | 1 | 0.4044 | 0.9966 | 99.66 | 100.13 112 |
| 2 | 0.4034 | 0.9933 | 99.33 | ||
| 3 | 0.4096 | 1.0142 | 101.42 | ||
| 2 | 1 | 0.7144 | 2.0418 | 102.09 | 102.33 0.56 |
| 2 | 0.7134 | 2.0384 | 101.92 | ||
| 3 | 0.7196 | 2.0593 | 102.97 | ||
| 4 | 1 | 1.1948 | 3.6615 | 91.54 | 99.01 6.61 |
| 2 | 1.3122 | 4.0573 | 101.43 | ||
| 3 | 1.3435 | 4.1628 | 104.07 |
| Ion Standard Solution | Corrected Absorbance | KA | KI | KA,I | |||
|---|---|---|---|---|---|---|---|
| U1 | U2 | U3 | Average | ||||
| Fe(III) | 0.6980 | 0.7605 | 0.7644 | 0.7410 | 0.3705 | ||
| Fe(III) + Cr(VI) | 0.5572 | 0.4704 | 0.3961 | 0.4746 | −0.1332 | −0.3595 | |
| Fe(III) + Pb(II) | 0.3468 | 0.3560 | 0.4680 | 0.3903 | −0.1754 | −0.4733 | |
| Day | Corrected Absorbance | Average | Decreased of Absorbance | % Decreased Colour Stability | ||
|---|---|---|---|---|---|---|
| U1 | U2 | U3 | ||||
| 0 | 0.7812 | 0.5368 | 0.7388 | 0.6856 | - | - |
| 1 | 0.7219 | 0.4999 | 0.6639 | 0.6286 | 0.0570 | 8.32 |
| 2 | 0.7695 | 0.5134 | 0.7635 | 0.6821 | 0.0035 | 0.51 |
| 3 | 0.7599 | 0.5239 | 0.7399 | 0.6746 | 0.0110 | 1.61 |
| 6 | 0.7699 | 0.6110 | 0.6751 | 0.6853 | 0.0003 | 0.04 |
| 7 | 0.6859 | 0.5781 | 0.6961 | 0.6534 | 0.0322 | 4.70 |
| 8 | 0.6645 | 0.6081 | 0.6553 | 0.6426 | 0.0430 | 6.27 |
| 9 | 0.6989 | 0.5949 | 0.7302 | 0.6747 | 0.0109 | 1.59 |
| 10 | 0.6951 | 0.5995 | 0.7514 | 0.6820 | 0.0036 | 0.53 |
| Average | 0.6677 | |||||
| SD | 0.0209 | |||||
| %RSD | 3.14 | |||||
| Sample Solution | Corrected Absorbance | [Fe(III)]optode (mg/L) | [Fe(III)]UV-Vis (mg/L) |
|---|---|---|---|
| U1 | 0.1523 | 0.2449 | 0.2499 |
| U2 | 0.1534 | 0.2511 | 0.2555 |
| U3 | 0.1561 | 0.2663 | 0.2550 |
| Average | 0.2541 | 0.2535 | |
| SD | 0.0110 | 0.0030 |
| Repetition | Corrected Absorbance |
|---|---|
| 1 | 0.1380 |
| 2 | 0.1315 |
| 3 | 0.1399 |
| 4 | 0.1378 |
| 5 | 0.1387 |
| 6 | 0.1447 |
| Average | 0.1384 |
| SD | 0.0042 |
| %RSD | 3.07 |
| Solution (mg/L) | Corrected Absorbance | [Fe(III)]obtained (mg/L) | %R | Average %R |
|---|---|---|---|---|
| Sample U1 | 0.1506 | 0.1409 | - | - |
| Sample U2 | 0.1513 | 0.1433 | ||
| Sample U3 | 0.1527 | 0.1480 | ||
| Sample + 1 | 0.4299 | 1.0826 | 93.73 | 106.25 13.39 |
| Sample + 1 | 0.4624 | 1.1922 | 104.67 | |
| Sample + 1 | 0.5090 | 1.3493 | 120.36 | |
| Sample + 2 | 0.7689 | 2.2256 | 101.71 | 105.10 4.22 |
| Sample + 2 | 0.7813 | 2.2674 | 103.75 | |
| Sample + 2 | 0.8182 | 2.3918 | 109.83 | |
| Sample + 4 | 1.0388 | 3.1355 | 75.53 | 75.58 0.21 |
| Sample + 4 | 1.0421 | 3.1467 | 75.81 | |
| Sample + 4 | 1.0373 | 3.1305 | 75.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, Z.; Sugiarti, S.; Rohaeti, E.; Batubara, I. A Sensor (Optode) Based on Cellulose Triacetate Membrane for Fe(III) Detection in Water Samples. Chemistry 2024, 6, 81-94. https://doi.org/10.3390/chemistry6010005
Arif Z, Sugiarti S, Rohaeti E, Batubara I. A Sensor (Optode) Based on Cellulose Triacetate Membrane for Fe(III) Detection in Water Samples. Chemistry. 2024; 6(1):81-94. https://doi.org/10.3390/chemistry6010005
Chicago/Turabian StyleArif, Zulhan, Sri Sugiarti, Eti Rohaeti, and Irmanida Batubara. 2024. "A Sensor (Optode) Based on Cellulose Triacetate Membrane for Fe(III) Detection in Water Samples" Chemistry 6, no. 1: 81-94. https://doi.org/10.3390/chemistry6010005
APA StyleArif, Z., Sugiarti, S., Rohaeti, E., & Batubara, I. (2024). A Sensor (Optode) Based on Cellulose Triacetate Membrane for Fe(III) Detection in Water Samples. Chemistry, 6(1), 81-94. https://doi.org/10.3390/chemistry6010005