Abstract
The global emergency of antimicrobial resistance has drawn several efforts to evaluate new drug candidates, such as natural defensive biomolecules. Ocellatins are a group of antimicrobial peptides found in anurans of the Leptodactylidae family. This work investigated the presence of antimicrobial peptides in the skin secretion of Leptodactylus vastus from the Brazilian northeast. The secretion was fractionated by RP-HPLC, and the fractions were screened for antibacterial activity. A peptide isolated from the most active fraction was characterized for primary structure and evaluated for antibacterial activity, cytotoxicity to murine melanoma cells (B16-F10), and hemolytic activity. The RP-HPLC profile displayed 26 fractions, with fraction 25 being the most active. One of the two peptides present in this fraction had the primary structure determined, belonging to the group of ocellatins. Since it was not identical to other ocellatins previously reported, it was named ocellatin-VT. This peptide especially inhibited Gram-negative bacteria growth, with the highest activity against Acinetobacter baumannii and Escherichia coli (growth inhibition was higher than 95% at 8 and 16 µM, respectively). Ocellatin-VT was weakly cytotoxic to B16-F10 cells and showed low hemolytic activity. In conclusion, a new ocellatin was isolated from L. vastus skin secretion that was active against non-resistant and multidrug-resistant bacteria.
1. Introduction
The order Anura comprises toads, frogs, and tree frogs, making it one of the most threatened groups in the world and the most representative among the amphibians [1,2]. They are distributed across all continents, except in the polar region, and have one of the greatest representations and diversity in Brazil, with 1188 species recorded [3]. Among the anuran families, the Leptodactylidae stand out with records of 177 species and thirteen genera. The species Leptodactylus vastus, popularly known as the pepper frog or “jia,” is endemic to Brazil and occurs in all states of the northeast region [4].
Anurans can be found in terrestrial and aquatic environments due to their development and morphological and physiological adaptations. The integument of anurans performs several functions that are necessary for the survival of the species, being constituted by the epidermis and dermis. It participates in gas exchange, osmoregulation, thermoregulation, defense against macropredators and micropredators, and skin lubrication [5]. The integument contains mucous and granular glands, which contribute to protection against predators [6].
The secretion released through the integument of anurans, mainly by the granular glands, is rich in bioactive peptides and has been the subject of different studies, especially on antimicrobial peptides (AMPs), which act on different targets such as fungi, bacteria, and viruses [7,8,9]. Ocellatins are AMPs found in the secretion of leptodactylid anurans [10]. More than forty ocellatins and their variations have been described in the literature. One example is ocellatin-P1, found for the first time in Leptodactylus pentadactylus [11]. Ocellatin-P1 showed antibacterial activity against Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis [12], as well as antitumor activity against the murine melanoma lineage [13].
Antimicrobial resistance is one of the most urgent global concerns, and the spread of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant bacteria is occurring at an increasingly alarming speed [14]. The knowledge of antimicrobial peptides from anuran secretions can significantly contribute to the development of new therapeutic agents. In this sense, the present work investigated the peptide profile of the skin secretion of L. vastus from the Brazilian northeast as well as the presence of AMPs. In addition, the biomedical potential of a new ocellatin isolated from this secretion was studied by assessing its antibacterial activity and its cytotoxicity activity on the tumor cell line. Finally, the effect of ocellatin on membranes of the non-target human cells (erythrocytes) was investigated through hemolysis assay.
2. Materials and Methods
2.1. Collection of Animals
The Matas do Sistema Gurjaú Wildlife Refuge (RVS Gurjaú) is a full-protection conservation area located (8°2′30″ S, 34°56′30″ W) between the municipalities of Jaboatão dos Guararapes, Cabo de Santo Agostinho, and Moreno, all belonging to the metropolitan region of Recife (Pernambuco, Brazil). It comprises an area of 1340.72 ha, made up of seventeen fragments formed by an evergreen forest [15]. The RVS Gurjaú is bathed by the Gurjaú River, a tributary of the Pirapama River, which supplies the city of Cabo de Santo Agostinho and part of Recife.
Twenty specimens of L. vastus (Figure 1) were collected through active nocturnal and auditory searches in different aquatic and humid habitats. During capture, net traps and cloth bags were used. After capture, the animals were transported in moistened plastic bags to the Protein Biochemistry Laboratory of the Universidade Federal de Pernambuco (UFPE). Subsequently, they were kept in plastic boxes, simulating their natural environment, to reduce stress. The Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) authorized (no. 71273) the collection of specimens, as well as the Agência Estadual de Meio Ambiente of Pernambuco (CPRH). The research was entered (no. A177772) in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SisGen).

Figure 1.
A specimen of Leptodactylus vastus was collected for the present study.
2.2. Extraction of L. vastus Skin Secretion (LVS)
The secretion extraction method was approved by the Ethics Committee on the Use of Animals in UFPE (protocol no. 132/2019). The animals were previously washed in running water and subsequently with Milli-Q water. Collection was conducted using electrical stimulation of the integument at 50 mV for 8 s [16]. Immediately, the secretion was stored in 50 mL Falcon tubes (TPP Techno Plastic Products AG, Trasadingen, Switzerland) and kept refrigerated (4 °C). The extracted secretion (LVS) underwent a freeze-drying process on a LIOTOP L101 lyophilizer (Liobras, São Carlos, Brazil) and was stored at −20 °C. After the procedures, four specimens were euthanized with a lethal dose of thiopental (30 mg/kg) to be used in another study regarding the skin histological organization. Next, they were fixed with 10% formalin for 24 h and preserved in 70% alcohol before being deposited in the amphibian collection of the Zoology Department of UFPE. The other sixteen animals were returned to their natural environment.
2.3. Chromatographic Fractionation of LVS
LVS (2.0 mg) was submitted to reverse-phase high-performance liquid chromatography (RP-HPLC) on a C18 Shim-pack CLC-ODS column (6 × 150 mm; Shimadzu Corp., Kyoto, Japan) at a flow rate of 1.0 mL/min. The column was previously equilibrated with 0.1% (v/v) trifluoroacetic acid (TFA) in water (solvent A). Elution was conducted as follows: 5 min—100% solvent A; 0–50% TFA 0.1% (v/v) in acetonitrile (solvent B) in 50 min; 50–100% solvent B in 1 min; and 100% solvent B for 5 min. The elution was monitored at 216 nm. The eluted fractions were collected manually, dried in a vacuum concentrator (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany), and stored at −20 °C.
2.4. Mass Spectrometry Analysis
The fractions with the highest absorbance intensity at 216 nm were subjected to matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) using the Autoflex II equipment (Bruker Daltonics, Bremen, Germany). Samples were resuspended in 0.12% (v/v) TFA in 50% (v/v) acetonitrile, and 1 μL of each sample was applied to a stainless-steel plate, followed by the application of 1 μL of α-cyano-4-hydroxycinnamic acid matrix [20 µg/mL in 0.12% (v/v) TFA in 50% (v/v) acetonitrile]. The samples were analyzed in positive reflected mode, with an m/z range from 1000 to 5000. Calibration was performed with the Peptide Calibration Standard II standard, containing the following peptides: bradykinin(1–7)_[M + H]+_mono (757.399 Da), angiotensin_I_[M + H]+_mono (1046.5418 Da), angiotensin_II_[M + H]+_mono (1296.6848 Da), substance_P_[M + H]+_mono (1347.7354 Da), bombesin_[M + H]+_mono (1619.8223 Da), ACTH_clip(1–17)_[M + H]+_mono (2093.0862 Da), ACTH_clip(18–39)_[M + H]+_mono (2465.1983 Da), and somatostatin(28)_[M + H]+_mono (3147.4710 Da).
2.5. Antimicrobial Screening of Chromatographic Fractions
The fractions obtained after chromatographic fractionation of LVS were evaluated for their ability to inhibit the growth of two pathogenic bacteria: the Gram-positive bacterium Staphylococcus aureus (ATCC 25923) and the Gram-negative Escherichia coli (ATCC 25922). The bacteria were cultivated in 7 mL of Mueller Hinton (MH) medium (Sigma-Aldrich, St. Louis, MO, USA) under shaking at 37 °C until reaching an optical density (OD) at 625 nm equal to 0.08–0.1. The culture was diluted 1:10 in MH and used to prepare the inoculum by diluting this suspension 1:20 (5 × 105 cells/mL).
Aliquots of each fraction were dissolved in Milli Q water, and 50 μL of each fraction were transferred to flat-bottom microplate wells. Then, 50 μL of the bacterial suspension was added. After 24 h of incubation at 37 °C, the OD was measured at 625 nm using a Multiskan FC microplate reader (Thermo Scientific, San Jose, CA, USA). The controls for no growth and full growth (100%) were 0.8% (v/v) formaldehyde and Milli Q water, respectively, both incubated with bacterial suspensions.
2.6. Peptide Isolation, Determination of Primary Structure, and Computational Analysis
The chromatographic fraction with the best antibacterial activity was resubmitted to RP-HPLC (as described in Section 2.4), and the peaks were analyzed on the MALDI-TOF mass spectrometer Autoflex II as described in Section 2.4, but in a m/z range from 500 to 5000 Da. The isolated peptide found in peak 1 was called ocellatin-VT, and its primary structure was determined by Edman degradation on a PPSQ-31A/33A automatic sequencer (Shimadzu, Kyoto, Japan), according to the manufacturer’s instructions. The BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 15 December 2023)) was used for similarity searches. It used the ExpasypI/Mw tool (http://web.expasy.org/compute_pi/ (accessed on 15 December 2023)) to calculate the theoretical monoisotopic mass [17] and Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 15 December 2023)) for multiple sequence alignment [18].
2.7. Antimicrobial Activity of the Isolated Peptide
Ocellatin-VT was evaluated for its ability to inhibit the growth of standard isolates of the Gram-negative bacteria S. aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), and Enterococcus faecalis (ATCC 29212), as well as of the Gram-negative bacteria Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 13883), and Pseudomonas aeruginosa (ATCC 27853), as described in Section 2.6. The peptide was also investigated against antibiotic-resistant clinical isolates of K. pneumoniae (producer of a KPC carbapenemase), P. aeruginosa (MDR), and the Gram-negative Acinetobacter baumannii (MDR). The peptide was evaluated at concentrations ranging from 1 to 128 µM.
2.8. Cytotoxicity Evaluation
Murine cutaneous melanoma cells B16-F10 (ATCC CRL-6475) were maintained at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) culture medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen, Waltham, MA, USA). For use in the assays, the cells (5 × 103/well) were cultured overnight in 96-well microplates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in DMEM complete medium. Next, 50 µL of ocellatin-VT (1–128 µM) or Milli-Q water (negative control) were added. After 24 h, the cell viability was determined by a 3-4,5-dimethylthiazole 2,5-biphenyl tetrazolium bromide (MTT, Invitrogen, USA) assay [19]. Briefly, 15 µL of MTT solution (5 mg/mL in PBS) was added to each well. After 3 h of incubation at 37 °C in 5% CO2, the culture medium was aspirated, and 100 µL of dimethyl sulfoxide was added. Absorbance at a wavelength of 595 nm was monitored using a microplate reader (Bio-Rad, Hércules, CA, USA).
2.9. Hemolytic Assay
Human erythrocytes were obtained from healthy donors as approved by the Human Ethics Committee of the Universidade de Brasília (protocol no. 45/2010). The erythrocytes were washed hree times with Tris buffer (0.01 M Tris-HCl, pH 7.4, containing 0.15 M NaCl). Next, a 1% (v/v) suspension of erythrocytes in Tris was prepared, and aliquots of 100 µL were incubated for 1 h at 25 °C with 100 µL of ocellatin-VT at different concentrations (1, 2, 4, 8, 16, 32, 64, and 128 µM). Next, centrifugation at 400× g for 5 min was performed, and the supernatant had its absorbance measured at 405 nm using the Multiskan FC microplate reader (Waltham, MA, USA) to measure hemoglobin release. Tris-buffered saline and 1% (v/v) Triton X-100 were used as negative and positive controls, respectively. Assays were performed in triplicate. Hemolytic activity was calculated using the following formula: % hemolysis = 100 × (Aocellatin − Anegative control)/(Apositive control − Anegative control), where “A” is the absorbance.
2.10. Statistical Analysis
Data were represented as mean ± standard deviation. Significant differences were checked through one-way analysis of variance (ANOVA) followed by Tukey’s test using GraphPad Prism version 5.04 (GraphPad Software, La Jolla, CA, USA). A p < 0.05 was considered the level of significance.
3. Results
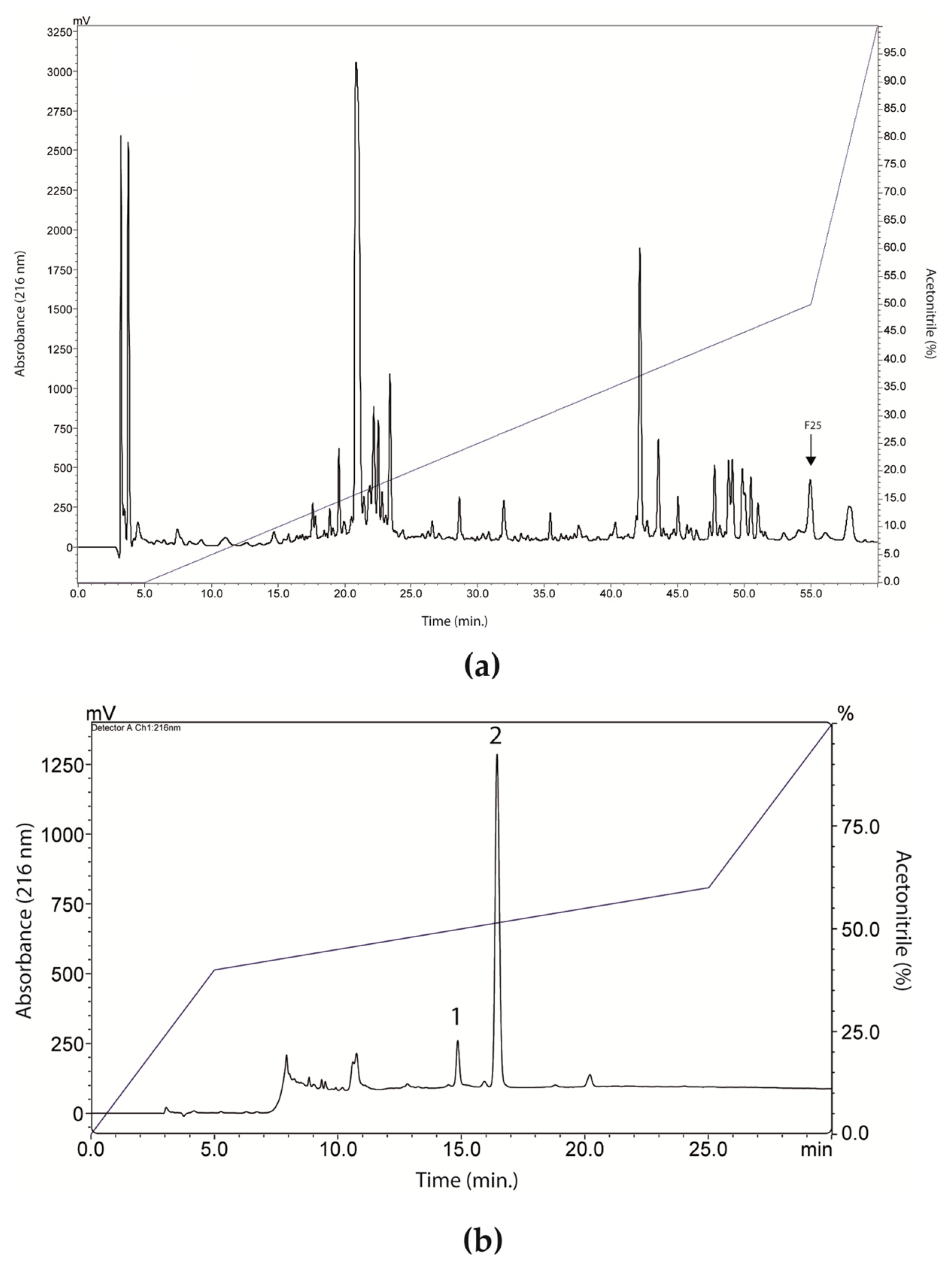
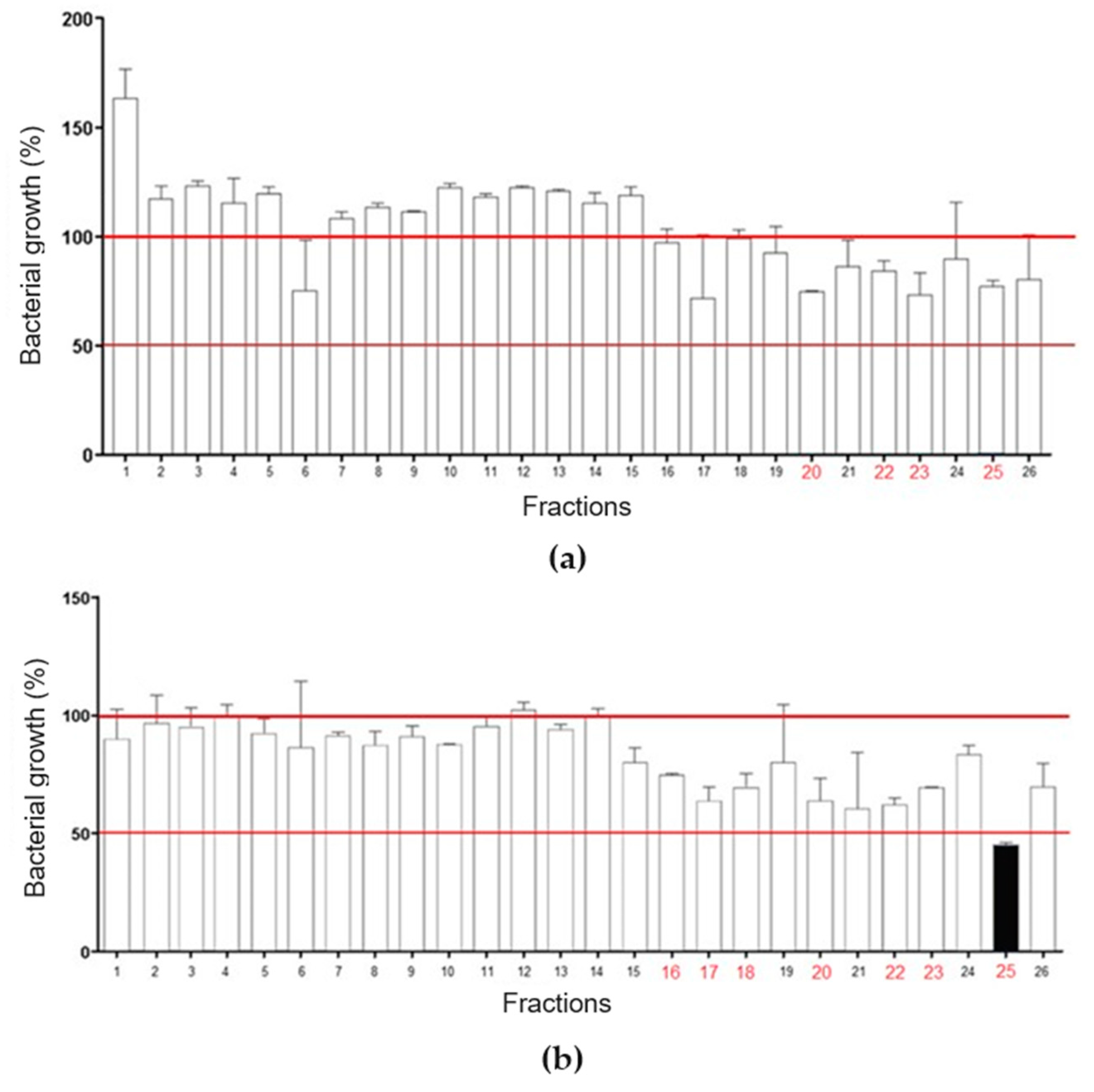
RP-HPLC of LVS on a Shim-pack CLC-ODS column resulted in the separation of twenty-six fractions (Figure 2a), which were evaluated for antibacterial activity against standard isolates of E. coli and S. aureus (Figure 3). Overall, there was a weak effect on E. coli growth, with only fractions 20, 22, 23, and 25 promoting inhibition, in all cases lower than or equal to 25% (Figure 3a). In Figure 3b, fraction 25 promoted a 50% inhibition of S. aureus growth, while fractions 16, 17, 18, 20, 22, and 23 caused a growth inhibition of up to 25%. These data show that SLV components performed better against S. aureus.
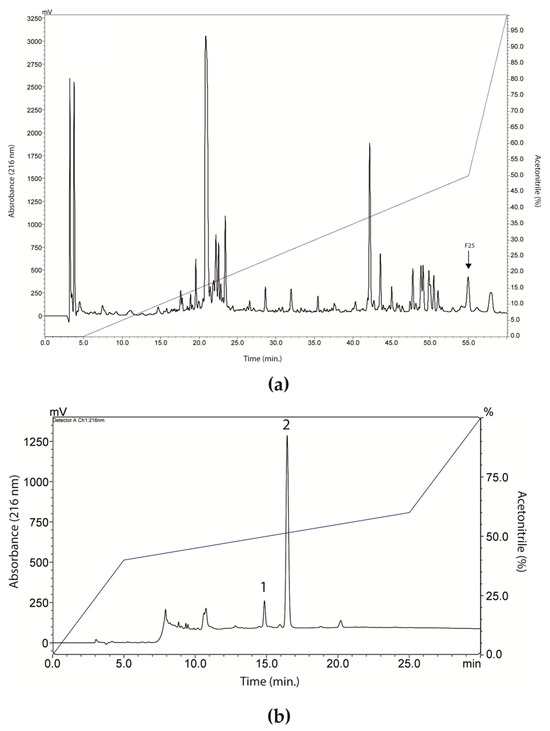
Figure 2.
Fractionation and isolation of peptides from Leptodactylus vastus skin secretion (LVS). (a) Chromatographic profile of LVS on a Shim-pack CLC-ODS column. The fraction 25 (indicated in the image) showed better antibacterial activity, as can be seen in Figure 3. (b) Chromatographic profile of fraction 25 on a Shim-pack CLC-ODS column. Peak 1 corresponded to ocellatin-VT.
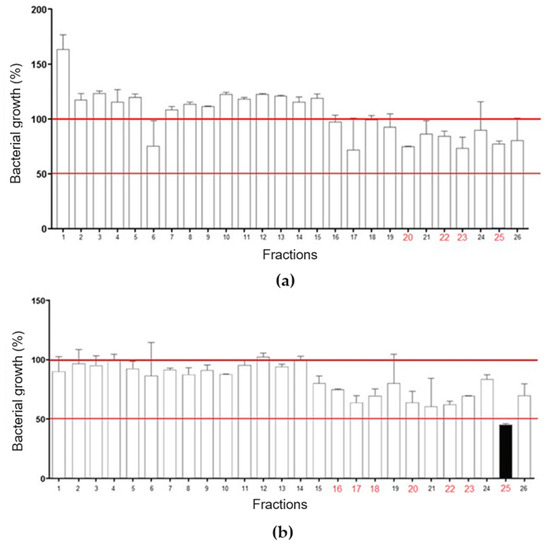
Figure 3.
Evaluation of chromatographic fractions from LVS for antibacterial activity. (a) Fractions 20, 22, 23, and 25 markedly inhibited the growth of Escherichia coli. (b) Fractions 16, 17, 18, 20, 22, 23, and 25 markedly inhibited the growth of Staphylococcus aureus. Fraction 25 reduced bacterial growth by more than 50%.
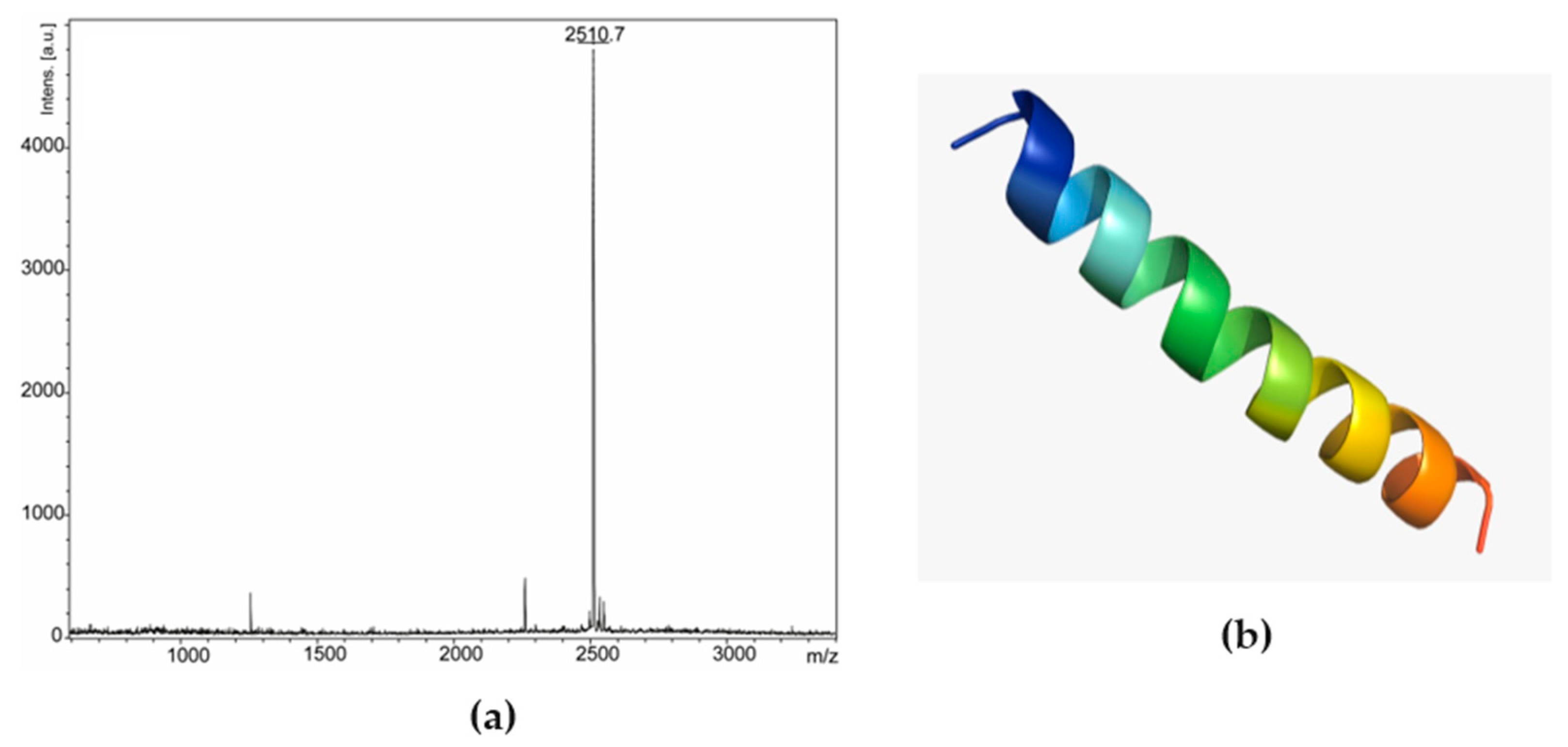
Based on these results, fraction 25 was chosen and went through the rechromatography process, being separated into two peaks (Figure 2b). Peak 1 of fraction 25 was analyzed by mass spectrometry, corresponding to a peptide with 2509.47 Da (calculated mass) and 2510.7 Da (observed mass) (Figure 4a). The primary structure of this peptide was obtained by Edman degradation, and the sequence obtained was GLLDVLKGAAKNVVGGLASKVMEKL. A prediction of its tridimensional structures can be seen in Figure 4b. The peptide corresponding to peak 2 (Figure 2b) will be the target of future studies.
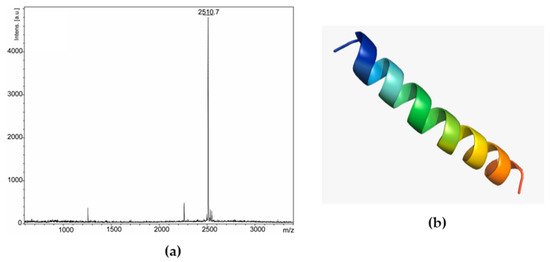
Figure 4.
Ocellatin-VT is from Leptodactylus vastus. (a) MALDI-TOF mass spectrometry spectrum of peak 1 (ocellatin-VT) from fraction 25 in reflected mode. (b) Three-dimensional structural model predictions of the ocellatin.
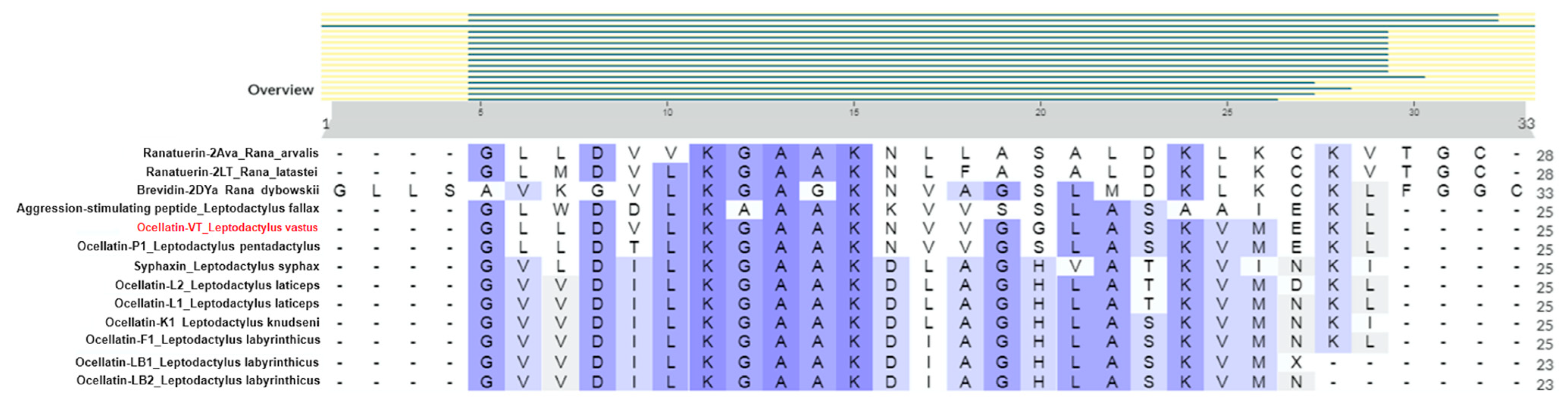
Through similarity searches using BLASTp, a high similarity was detected with peptides found in other anurans, including ocellatins from Leptodactylus species, ranatuerin, brevinin, syphaxin, and an aggression-stimulated peptide (Figure 5). The highest similarity was found with ocellatin-P1 from Leptodactylus pentadacthylus, presenting 100% query cover and 92% identity. Since the isolated peptide was not identical to any present in the database, we called it ocellatin-VT.
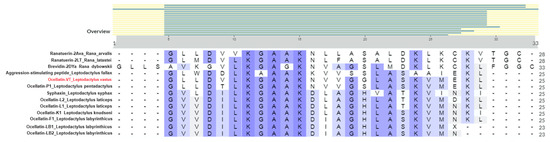
Figure 5.
Alignment of the sequence of ocellatin-VT from Leptodactylus vastus and other anuran peptides was conducted using the UniProt Clustal tool.
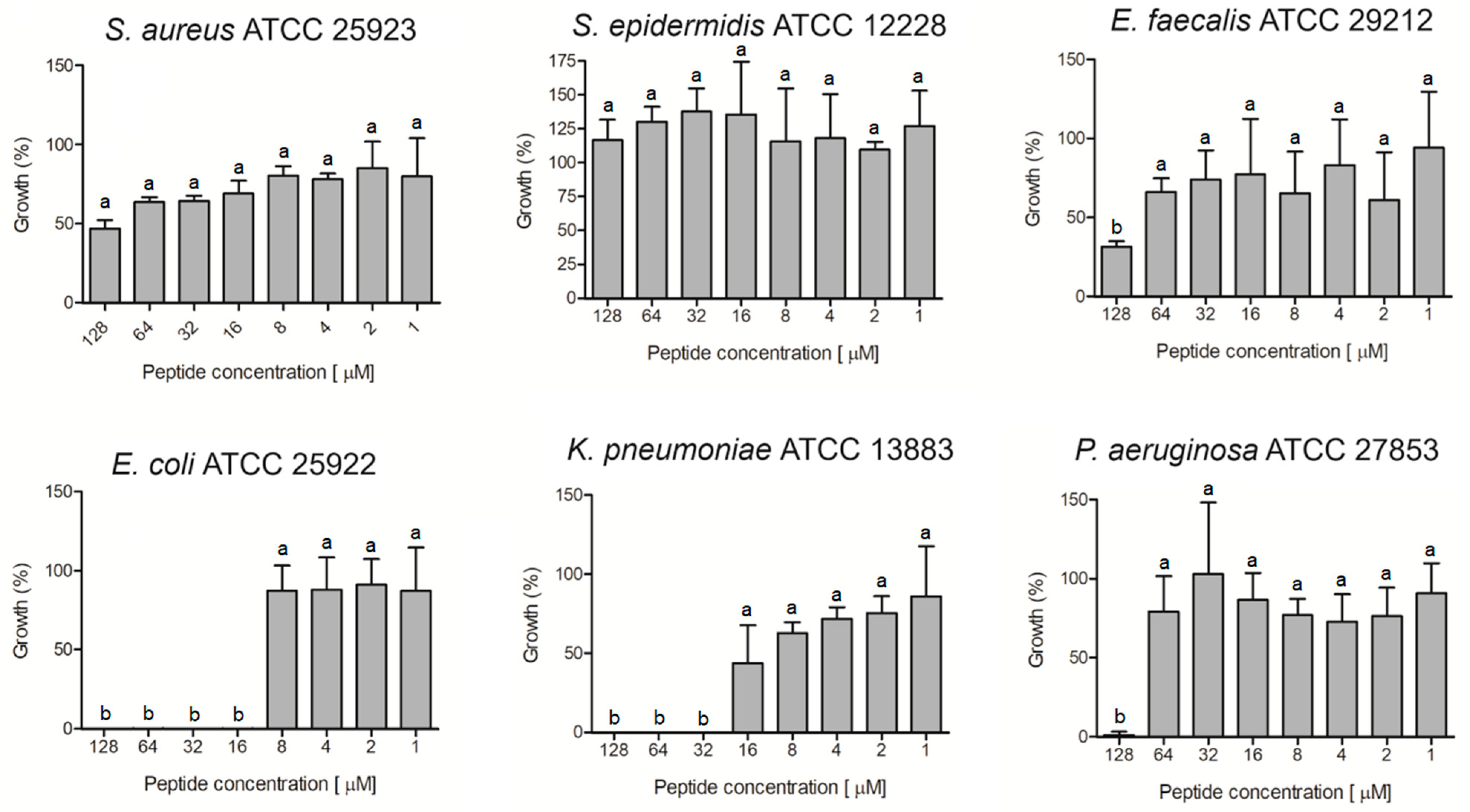
The ocellatin isolated from L. vastus was evaluated for antibacterial activity against non-resistant (Figure 6) and antibiotic-resistant (Figure 7) isolates. The results showed that this peptide was able to inhibit by more than 95% the growth of the non-resistant E. coli ATCC 25922, K. pneumoniae ATCC 13883, and P. aeruginosa ATCC 27853 at concentrations of 16, 32, and 128 µM, respectively (Figure 6). For S. aureus and E. faecalis, there was inhibition greater than 50% when the peptide was assessed at 128 µM. S. epidermidis was not inhibited at the tested concentrations.
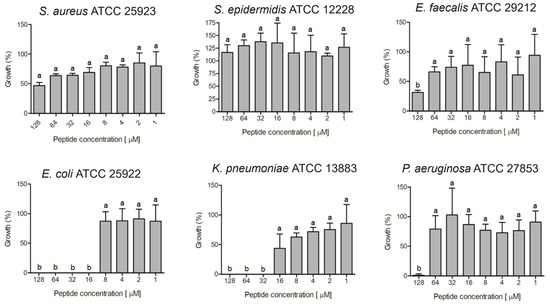
Figure 6.
Evaluation of antibacterial activity of ocellatin-VT from Leptodactylus vastus skin secretion against non-resistant isolates. Growth percentage was calculated for full growth control (100% growth). Different letters indicate significant differences (p < 0.05) between treatments.
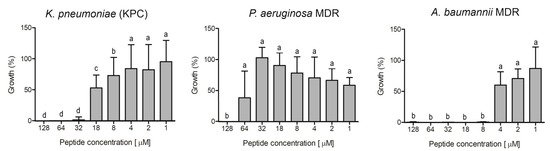
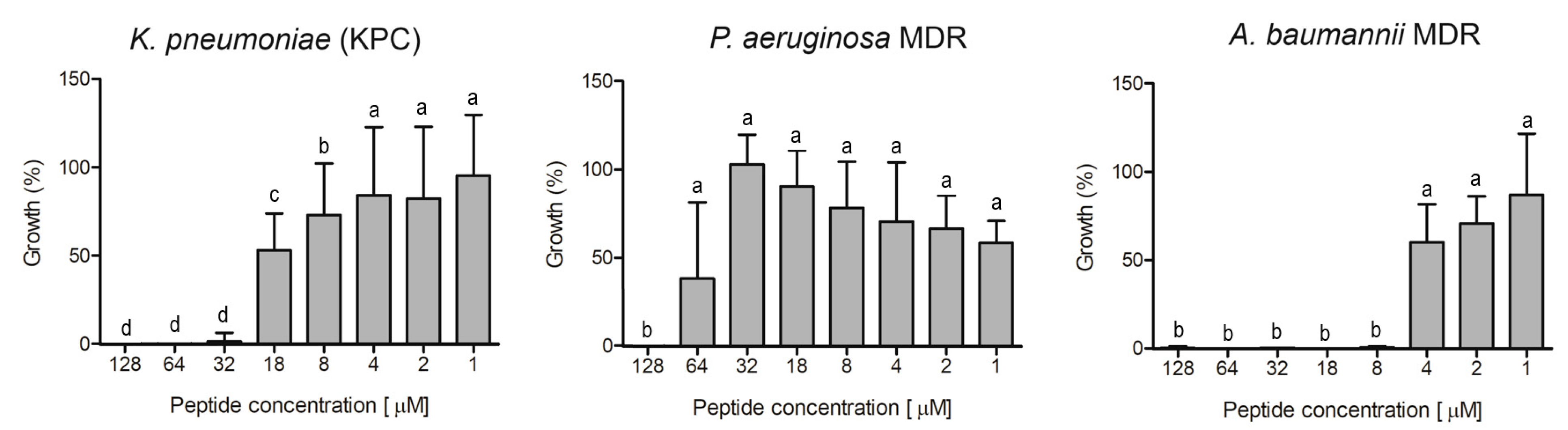
Figure 7.
Evaluation of antibacterial activity of ocellatin-VT from Leptodactylus vastus skin secretion against isolates resistant to antibiotics: a clinical isolate of carbapenemase-producing K. pneumoniae and multidrug-resistant (MDR) isolates of P. aeruginosa and A. baumannii. Growth percentage was calculated for full growth control (100% growth). Different letters indicate significant differences (p < 0.05) between treatments.
Ocellatin-VT was active against all the antibiotic-resistant isolates evaluated, causing inhibition higher than 95% of K. pneumoniae KPC, P. aeruginosa MDR, and A. baumannii MDR at concentrations of 32, 128, and 8 µM, respectively (Figure 7).
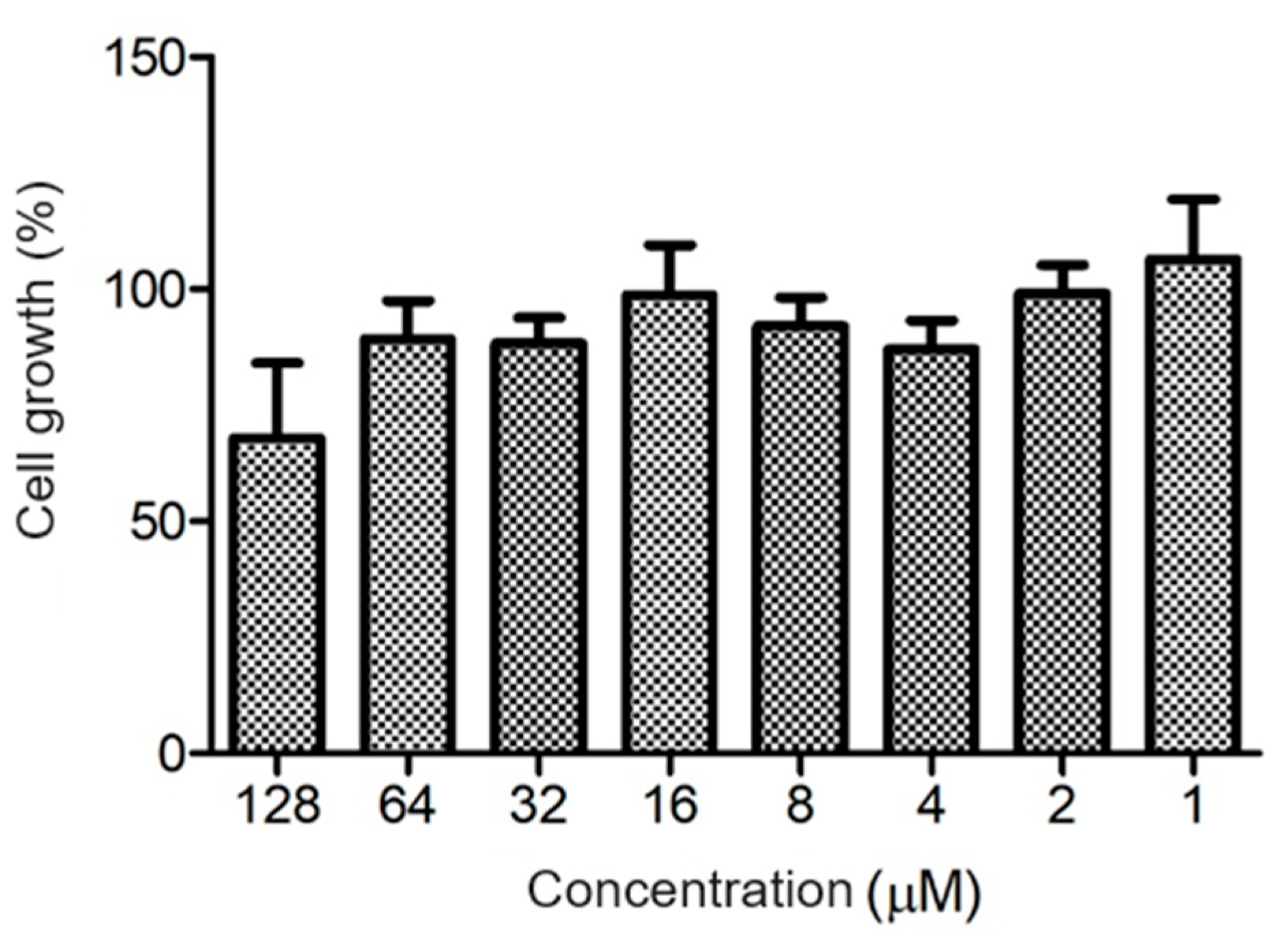
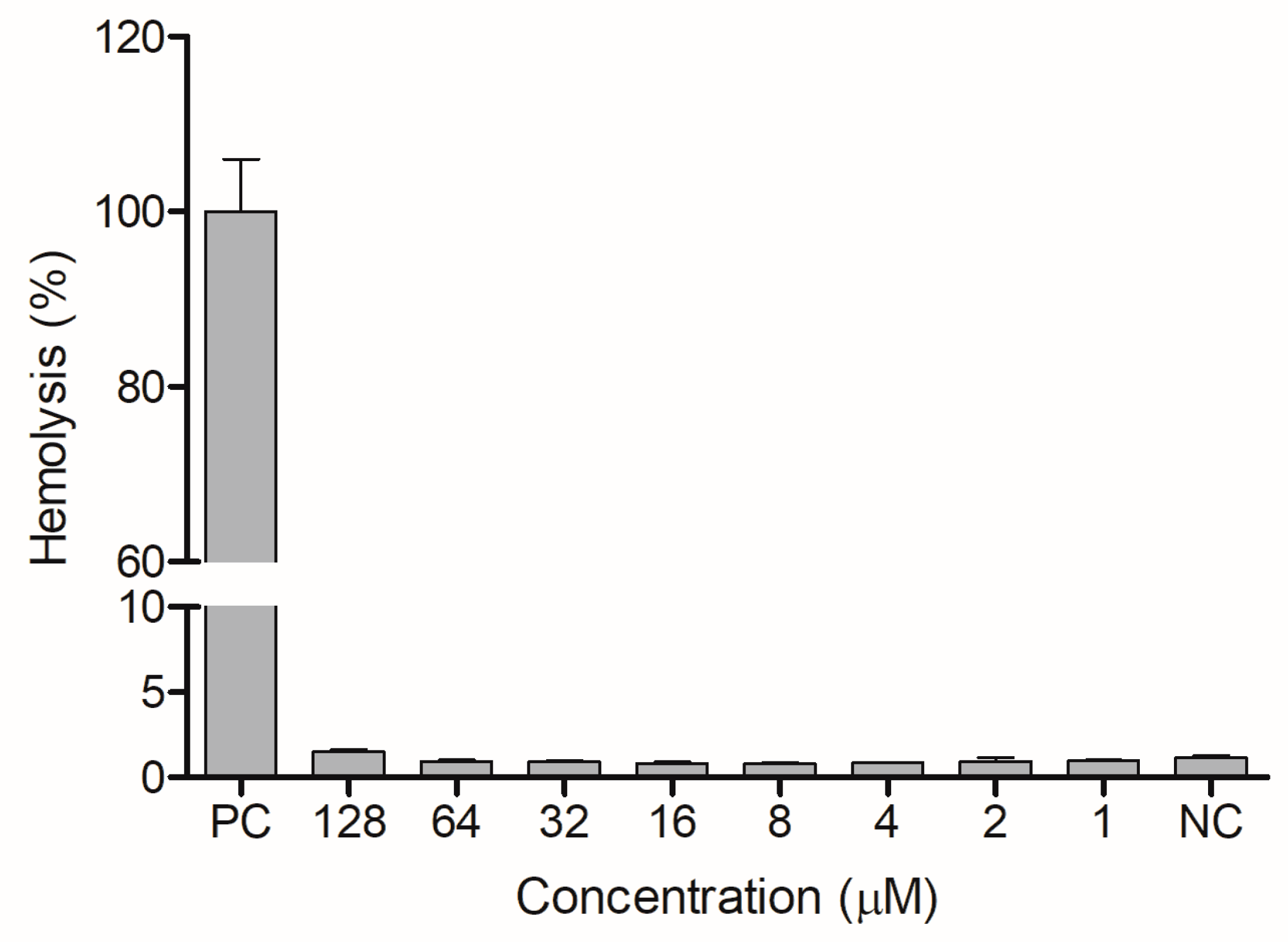
The ocellatin from L. vastus did not show cytotoxicity to B16-F10 melanoma murine cells when evaluated at a concentration of 64 µM. At 128 µM, it reduced the viability of these cells by ca. 40% (Figure 8). Ocellatin-VT showed low hemolytic activity even when assessed at high concentrations (Figure 9).
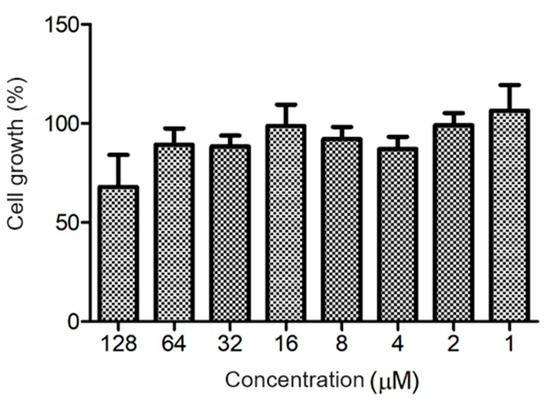
Figure 8.
Evaluation of cytotoxic activity of ocellatin-VT on B16-F10 murine melanoma cells. Growth percentage was calculated for the negative control (100% growth). There were no significant differences (p > 0.05) between treatments.
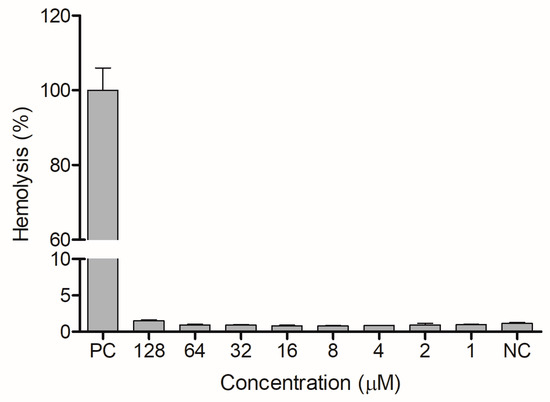
Figure 9.
Evaluation of hemolytic activity of ocellatin-VT on human erythrocytes. PC: positive control. NC: negative control. There were no significant differences (p > 0.05) between treatments with ocellatin and NC.
4. Discussion
AMPs from anurans have been studied for the last four decades, and interest in them has increased with the global health emergency of antimicrobial resistance. It has been shown that anuran AMPs are targets of patents that propose their development as new drugs, including through bioengineering involving amino acid modifications [20]. In this work, we performed the fractionation of skin secretion from L. vastus (LSV) from the Brazilian northeast and ended up isolating a new peptide belonging to the group of ocellatins.
The antimicrobial screening revealed that LSV contains different components with the ability to inhibit bacterial growth, being more active against Gram-positive S. aureus. Similarly to LSV, the growth of S. aureus was inhibited by the skin secretion of other leptodactylids such as Leptodactylus latrans [21], Leptodactylus insularum, Leptodactylus nesiot, and Leptodactylus labyrinthicus [22]. Regarding E. coli, other studies involving the secretion of other leptodactylids reported variable levels of growth inhibition, such as for the species Leptodactylus pentadactylus [23], L. latrans, with MIC of 15 µM [21], L. insularum [24], L. nesiot [24], and L. labyrinthicus, with MIC of 114.04 and 397.45 µM [22].
There are more than forty ocellatins described in the literature, and these peptides are the most abundant peptides found in the skin secretions of Leptodactylidae [10,12]. Glycine is a recurrent amino acid in multiple positions of these peptides [10], which can be observed in the ocellatin-VT from L. vastus. Carrillo et al. [10] also observed that leucine and lysine are often present, which also occurs in ocellatin-VT: leucine appears in five positions while there are four lysine residues.
The ocellatin-VT from L. vastus showed the highest similarity with ocellatin-P1, which is an antimicrobial peptide initially described as pentadactylin in the species Leptodactylus pentadactylus [11]. In 2008, Conlon et al. [25] proposed updating the nomenclature of ocellatins, so pentadactylin became ocellatin-P1 (P = pentadactylin). The sequences obtained for ocellatins VT and P1 showed the same size (25 amino acids) with differences in only two positions (Figure 5): in position 5, there is a valine (a non-polar aliphatic amino acid) in ocellatin-VT while a threonine (a polar uncharged amino acid) is present in ocellatin-P1; in position 16, a glycine (a non-polar amino acid that fits into both hydrophobic and hydrophilic environments) is found in ocellatin-VT, and a serine (a polar uncharged amino acid) is found in ocellatin-P1. This indicates that ocellatin-VT would be more hydrophobic than P1, and the impact of these differences in their molecular organization and bioactivities should be studied in the future.
Ocellatin-VT was evaluated against nine bacterial isolates, and the results showed that, interestingly, it was more active in Gram-negative bacteria. Indeed, ocellatin-VT was not able to achieve an inhibition level higher than 70% for none of the Gram-positive isolates, while it was able to reduce by more than 95% the growth of all Gram-negative isolates, including those resistant to antibiotics. These are distinct from the screening results or LSV, suggesting that this secretion probably contains other AMPs more active on Gram-positive bacteria.
Most ocellatins target predominantly Gram-negative bacteria, usually showing a weak antibacterial effect. It has been speculated that this specificity is linked to the fact that AMPs do not need to adopt a stabilized amphipathic conformation to be active on Gram-negative bacteria, while this seems to be a mechanism necessary for antibacterial action on Gram-positive species [24,26]. Ponnusamy and Ramalingam [12] showed that ocellatins P1, L1, and F1 are also more active against Gram-negative than Gram-positive bacteria. The outer membrane of Gram-negative bacteria is composed of phospholipids, lipopolysaccharides, and lipoproteins, which are known to provide heightened resistance against antibiotics [27], and the fact that ocellatin-VT was active on these microorganisms opens windows for future studies on possible synergistic potential with commercial drugs, for example. Another interesting observation regarding ocellatin-VT from L. vastus is that it was active on A. baumannii MDR and E. coli ATCC 25922 at 8 and 16 µM, respectively, while Carrillo et al. [10] reported that only six ocellatins showed antimicrobial activity at concentrations lower than 30 µM.
Ocellatin-P1 was reported as an antimicrobial against E. coli, Enterobacter cloacae, K. pneumoniae, P. aeruginosa, S. aureus, S. epidermidis, and E. faecalis [12]. The differences between this result and that found for ocellatin-VT can be associated with the differences in the primary structure. When studying the ocellatins (LB1, LB2, and F1) from L. labyrinthicus, Gusmão et al. [22] showed activity against E. coli, and specifically, ocellatin-F1 was active on S. aureus. Colon et al. [28] isolated ocellatin-3N from Leptodactylus nesiotus and reported its ability to inhibit the growth of A. baumannii and P. aeruginosa.
Ocellatins F1, LB1, and LB2 showed the ability to form membrane pores, all acquiring high helical conformation in the membrane environment [22]. It has been described that most amphibian AMPs tend to adopt an α-helix conformation when in a hydrophobic environment [29]. The distinct antimicrobial activities of ocellatins have been associated with variations in the cationic charge, hydrophobicity, helicity, and amphipathicity [12].
Ocellatin-VT was able to inhibit the growth of a KPC-producing K. pneumoniae isolate. Carbapenemases are defined as β-lactamase-type enzymes capable of hydrolyzing different classes of antibiotics, including carbapenems, cephalosporins, monobactams, and penicillins. These enzymes are taken as an example of the danger of gene transfer between bacteria since their presence in Enterobacteriaceae is the result of the widespread acquisition of genes from other bacterial families [30]. KPCs of class A constitute the most clinically relevant group of carbapenemases, being produced mainly by isolates of K. pneumoniae [31]. The blaKPC genes have been found extensively around the world [30].
The best activity of ocellatin-VT was found against the MDR A. baumannii isolate, which attracted attention. A. baumannii is responsible for several cases of nosocomial infections and has become one of the most common MDR pathogens, also causing great concern because of its resistance to the last resort drugs tigecycline and polymyxin [31]. Finding new potential antibiotics against this bacterium is a priority in biomedicine and biotechnology [32].
Ocellatin-VT showed weak cytotoxic activity on the cancer cells evaluated here. Conversely, Libério et al. [13] reported that the skin secretion of L. labyrinthicus, where pentadactylin (ocellatin-P1) is found, could reduce the viability of murine melanoma cell lines, causing morphological changes and even the disruption of their membranes.
The weak hemolytic effect found for ocellatin-VT agrees with the results observed for peptides from the secretion of L. labyrinticus (ocellatins LB1, LB2, and F1), which caused 1.0–13.0% hemolysis on rabbit blood [22] and from the secretion of L. pentadactylus (a leptoglycin that caused no hemolysis) [23]. On the other hand, ocellatins from the secretions of L. insularum and L. nesiotus showed moderate hemolytic activity against mouse erythrocytes [24], while the peptide P2-LI-1298 from L. latrans caused, at the highest concentration tested, 100% hemolysis [21]. These results demonstrate the variable cytotoxic potential of peptides from Leptodactylus species on red blood cells. The hemolytic activity of ocellatin-VT was determined to assess its effect on the membranes of human cells. However, it is important to highlight that red blood cells do not represent a complete eukaryotic cell model, so it is essential to evaluate the future cytotoxicity of ocellatin-VT on other cell types, such as lymphocytes.
5. Conclusions
The skin secretion of L. vastus contains bioactive peptides with antibacterial action. A new peptide from the ocellatin class (called ocellatin-VT) was isolated from L. vastus skin secretion, showing antibacterial activity mainly on Gram-negative species and being active against multidrug-resistant isolates, including a carbapenemase producer and an isolate of A. baumannii, one of the most concerning pathogens nowadays.
Author Contributions
Conceptualization, T.L.S., G.G.B., C.J.C.d.S., M.S.C. and T.H.N.; methodology, T.L.S., G.G.B., C.J.C.d.S., M.S.C. and T.H.N.; validation, T.L.S., G.G.B., C.J.C.d.S., P.M.G.P., M.S.C. and T.H.N.; formal analysis, T.L.S., G.G.B., C.J.C.d.S. and T.H.N.; investigation, T.L.S., G.G.B. and C.J.C.d.S.; resources, P.M.G.P., M.S.C. and T.H.N.; data curation, T.L.S., G.G.B. and C.J.C.d.S.; writing—original draft preparation, T.L.S. and C.J.C.d.S.; writing—review and editing, P.M.G.P., M.S.C. and T.H.N.; visualization, T.L.S., G.G.B., C.J.C.d.S., P.M.G.P., M.S.C. and T.H.N.; supervision, M.S.C. and T.H.N.; project administration, M.S.C. and T.H.N.; funding acquisition, P.M.G.P., M.S.C. and T.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 407192/2018-2; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number Finance Code 001; Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF), grant number 00193.00000930/2021-40; Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), grant numbers APQ-0108-2.08/14 and APQ-1491-2.08/22.
Data Availability Statement
Data are available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IUCN; Aquino, L.; Kwet, A.; Segalla, M.V.; Baldo, D. Rhinella crucifer. The IUCN Red List of Threatened Species 2004. 2004. Available online: https://www.iucnredlist.org/ (accessed on 31 August 2021).
- Haddad, C.F.B.; Toledo, L.F.; Prado, C.P.A.; Loebman, D.; Gasparini, J.L.E.; Sazima, I. Anfíbios da Mata Atlântica: Guia dos Anfíbios Anuros da Mata Atlântica; Editora Anolis Books: São Paulo, Brazil, 2013. [Google Scholar]
- Segalla, M.; Berneck, B.; Canedo, C.; Caramaschi, U.; Cruz, C.A.G.; Garcia, P.C.A.; Grant, T.; Haddad, C.F.B.; Lourenço, A.C.; Mangia, S.; et al. List of Brazilian Amphibians. Herpetol. Bras. 2021, 10, 121–216. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference, version 6.1; American Museum of Natural History: New York, NY, USA, 2021; Available online: https://research.amnh.org/herpetology/amphibia/index.html (accessed on 10 March 2024).
- Çömden, E.A.; Yenmiş, M.; Çakır, B. The complex bridge between aquatic and terrestrial life: Skin changes during development of amphibians. J. Dev. Biol. 2023, 11, 6. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; McGraw-Hill Book: New York, NY, USA, 1994. [Google Scholar]
- Antony, A.; Purayil, A.K.; Olakkaran, S.; Dhannura, S.; Shekh, S.; Gowd, K.H.; Gurushankara, H.P. Antimicrobial and antitumor properties of anuran peptide temporin-SHf induce apoptosis in A549 lung cancer cells. Amino Acids 2024, 56, 12. [Google Scholar] [CrossRef]
- Freitas, G.G.; Barbosa, J.M.; Santana, C.J.C.; Magalhães, A.C.M.; Macedo, K.W.R.; Souza, J.O.; Castro, J.S.; Vasconcelis, I.A.; Souza, A.A.; Freitas, S.M.; et al. Purification and biological properties of raniseptins-3 and -6, two antimicrobial peptides from Boana raniceps (Cope, 1862) skin secretion. Biomolecules 2023, 13, 576. [Google Scholar] [CrossRef]
- Loffredo, M.R.; Nencioni, L.; Mangoni, M.L.; Casciaro, B. Antimicrobial peptides for novel antiviral strategies in the current post-COVID-19 pandemic. J. Pept. Sci. 2024, 30, e3534. [Google Scholar] [CrossRef]
- Carrillo, J.F.C.; Boaretto, A.G.; Santana, D.J.; Silva, D.B. Skin secretions of Leptodactylidae (Anura) and their potential applications. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230042. [Google Scholar] [CrossRef]
- King, J.D.; Al-Ghaferi, N.; Abraham, B.; Sonnevend, A.; Leprince, J.; Nielsen, P.F.; Conlon, J.M. Pentadactylin: An antimicrobial peptide from the skin secretions of the South American bullfrog Leptodactylus pentadactylus. Comp. Biochem. Physiol. C 2005, 141, 393–397. [Google Scholar] [CrossRef]
- Ponnusamy, C.S.; Ramalingam, R. Therapeutic Efficacy of Antibacterial Ocellatin Peptides: A Comprehensive Review. Biointerf. Res. Appl. Chem. 2021, 12, 6804–6814. [Google Scholar]
- Libério, M.S.; Joanitti, G.A.; Azevedo, R.B.; Cilli, E.M.; Zanotta, L.C.; Nascimento, A.C.; Sousa, M.V.; Pires Junior, O.R.; Fontes, W.; Castro, M.S. Anti-proliferative and cytotoxic activity of pentadactylin isolated from Leptodactylus labyrinthicus on melanoma cells. Amino Acids 2011, 40, 51–59. [Google Scholar] [CrossRef]
- Kharat, A.S.; Makwana, N.; Nasser, M.; Gayen, S.; Yadav, B.; Kumar, D.; Veeraraghavan, B.; Mercier, C. Dramatic increase in antimicrobial resistance in ESKAPE clinical isolates over the 2010–2020 decade in India. Int. J. Antimicrob. Agents 2024, 63, 107125. [Google Scholar] [CrossRef]
- Tabarelli, M.; Pinto, L.P.; Silva, J.M.C.; Bede, L.C. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 2005, 1, 132–138. [Google Scholar]
- Apponyi, M.A.; Pukala, T.L.; Brinkworth, C.S.; Maselli, V.M.; Bowie, J.H.; Tyler, M.J.; Booker, G.W.; Wallace, J.C.; Carver, J.A.; Separovic, F.; et al. Host-defence peptides of Australian anurans: Structure, mechanism of action and evolutionary significance. Peptides 2004, 25, 1035–1054. [Google Scholar] [CrossRef] [PubMed]
- Bjellqvist, B.; Hughes, G.J.; Pasquali Ch Paquet, N.; Ravier, F.; Sanchez J-Ch Frutiger, S.; Hochstrasser, D.F. The focus positions of polypeptides in pH gradients immobilized materials can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Siever, S.F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- García, F.A.; Fuentes, T.F.; Alonso, I.P.; Bosch, R.A.; Brunetti, A.E.; Lopes, N.P. A comprehensive review of patented antimicrobial peptides from amphibian anurans. J. Nat. Prod. 2024, 87, 600–616. [Google Scholar] [CrossRef]
- Siano, A.; Humpola, M.V.; Oliveira, E.; Albericio, F.; Simonetta, A.C.; Lajmanovich, R.; Tonarelli, G.G. Leptodactylus latrans amphibian skin secretions as a novel source for the isolation of antibacterial peptides. Molecules 2018, 23, 2943. [Google Scholar] [CrossRef]
- Gusmão, K.A.G.; Santos, D.M.; Santos, V.M.; Cortés, M.E.; Reis, P.V.M.; Santos, V.L.; Piló-Veloso, D.; Verly, R.M.; Lima, M.E.; Resende, J.M. Ocellatin peptides from the skin secretion of the South American frog Leptodactylus labyrinthicus (Leptodactylidae): Characterization, antimicrobial activities and membrane interactions. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.; Berto, R.F.; Gois, E.G.; Fontenele-Cardi, N.C.; Honório-Junior, J.E.R.; Konno, K.; Richardson, M.; Rocha, M.F.G.; Camargo, A.A.C.M.; Pimenta, D.C.; et al. Leptoglycin: A new Glycine/Leucine-rich antimicrobial peptide isolated from the skin secretion of the South American frog Leptodactylus pentadactylus (Leptodactylidae). Toxicon 2009, 54, 23–32. [Google Scholar] [CrossRef]
- Barran, G.; Kolodziejek, J.; Coquet, L.; Leprince, J.; Jouenne, T.; Nowotny, N.; Conlon, J.M.; Mechkarska, M. Peptidomic analysis of skin secretions of the caribbean frogs Leptodactylus insularum and Leptodactylus nesiotus (Leptodactylidae) identifies an ocellatin with broad spectrum antimicrobial activity. Antibiotics 2020, 9, 718. [Google Scholar] [CrossRef]
- Conlon, J.M. A proposed nomenclature for antimicrobial peptides from frogs of the genus Leptodactylus. Peptides 2008, 29, 1631–1632. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic α-helical peptides. A systematic study of the effects of structural and physical properties on biological activity. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deber, C.M. Interaction of designed cationic antimicrobial peptides with the outer membrane of gram-negative bacteria. Sci. Rep. 2024, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Hunter, L.; Attoub, S.; Casciaro, B.; Mechkarska, M.; Abdel-Wahab, Y.H.A. Antimicrobial, cytotoxic, and insulin-releasing activities of the amphibian host-defense peptide ocellatin-3N and its L-lysine substituted analogs. J. Pept. Sci. 2022, 29, e3463. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.A.; Oliveira, G.A.; de Oliveira, A.P.; Lopes, A.L.F.; Iles, B.; Nogueira, K.M.; Araújo, T.S.; Souza, L.K.; Araújo, A.R.; Ramos-Jesus, J.; et al. Novel ocellatin peptides mitigate LPS-induced ROS formation and NF-kB activation in microglia and hippocampal neurons. Sci. Rep. 2020, 10, 2696. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, J.; Liu, S.; Zhu, Y.; Zhu, M. Acinetobacter baumannii: An evolving and cunning opponent. Front. Microbiol. 2024, 15, 1332108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).