A Series of Zinc Mononuclear Complexes with Imidoyl Amidine Ligands: Syntheses, Crystal Structures, and Photoluminescence Properties
Abstract
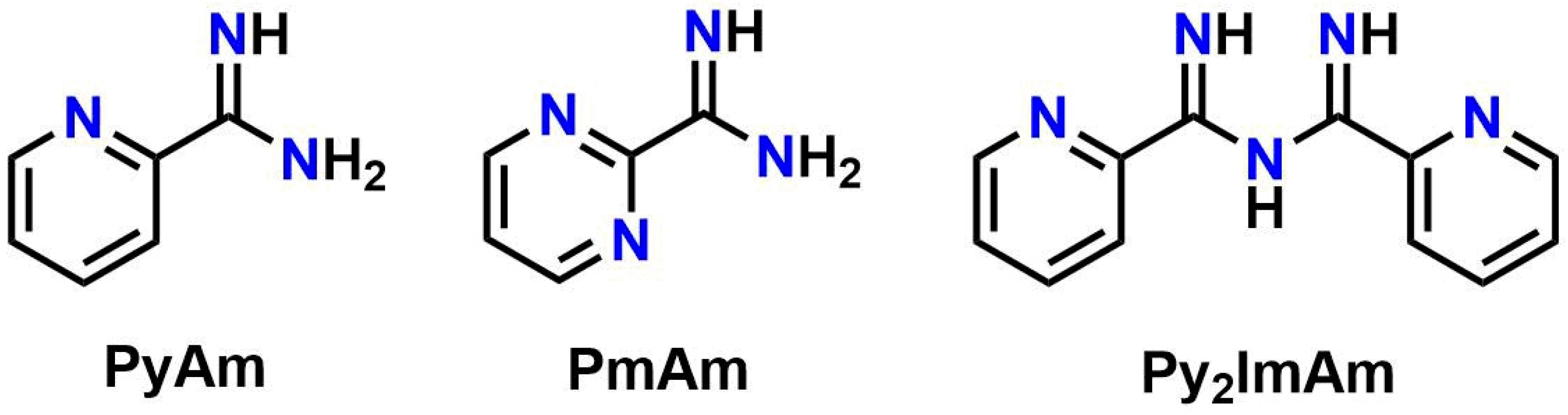
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
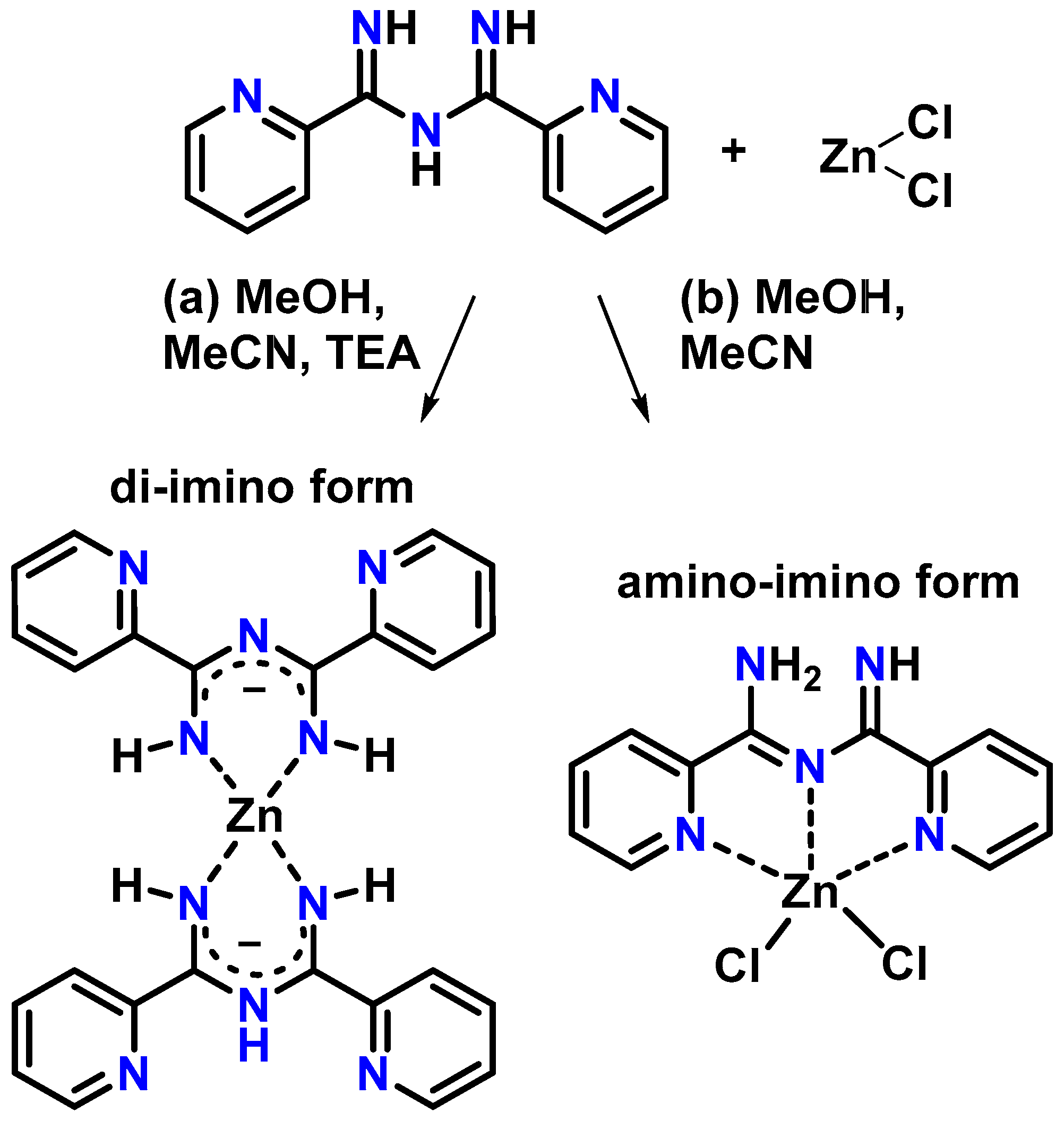
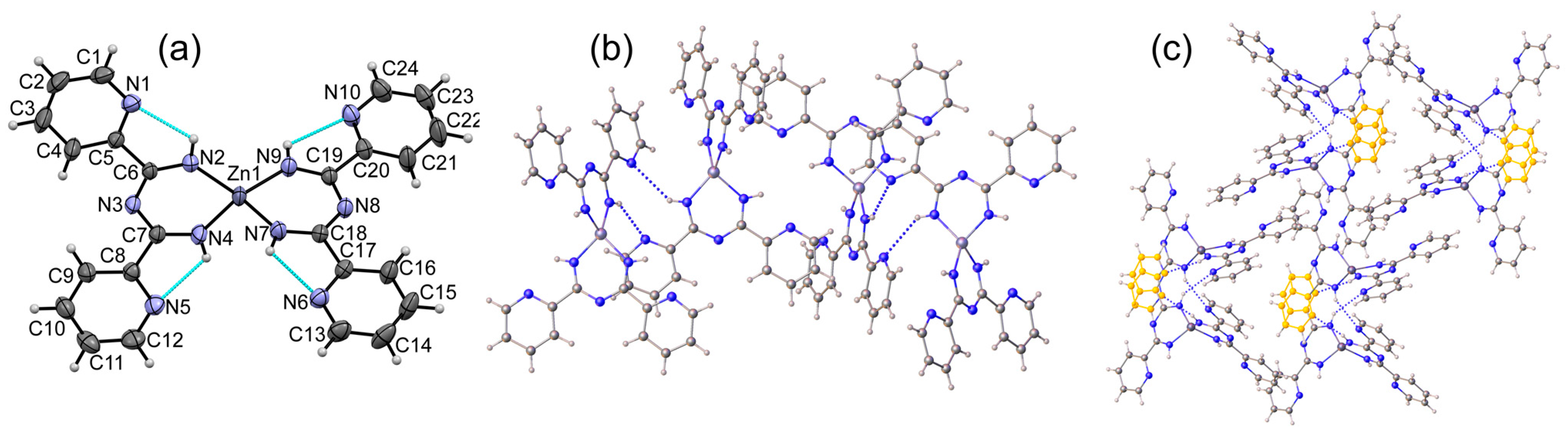
2.2.1. Synthesis of [Zn(Py2ImAm)2] (1)
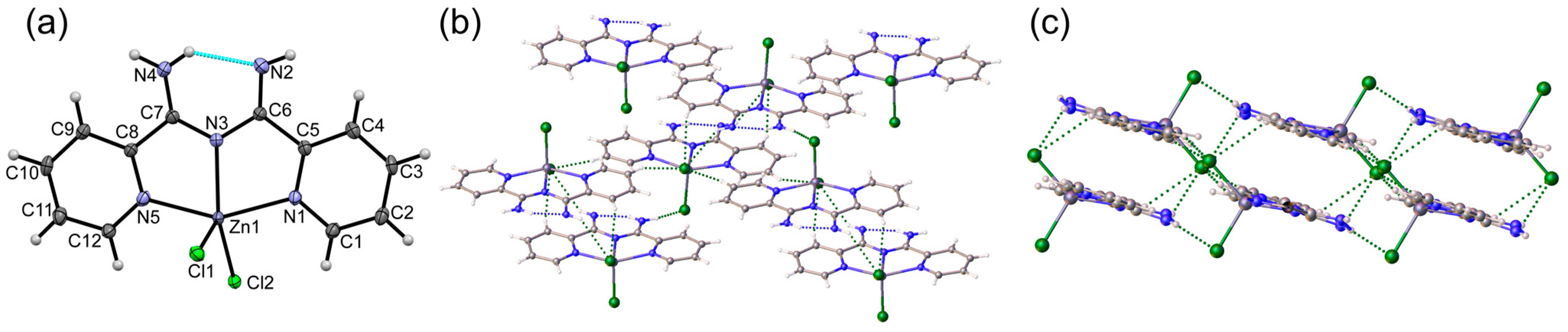
2.2.2. Synthesis of [ZnCl2(Py2ImAm)] (2)
2.2.3. Synthesis of [ZnCl(PyAm)2]Cl (3) and ZnCl(PyAm)2]2[ZnCl4]·C2H5OH (4)
2.2.4. Synthesis of [ZnCl(PyAm)2]2Cl·CH3OH (5)
2.2.5. Synthesis of [ZnCl(PmAm)2]2[ZnCl4] (6)
2.3. X-ray Single-Crystal Structure Determination
3. Results
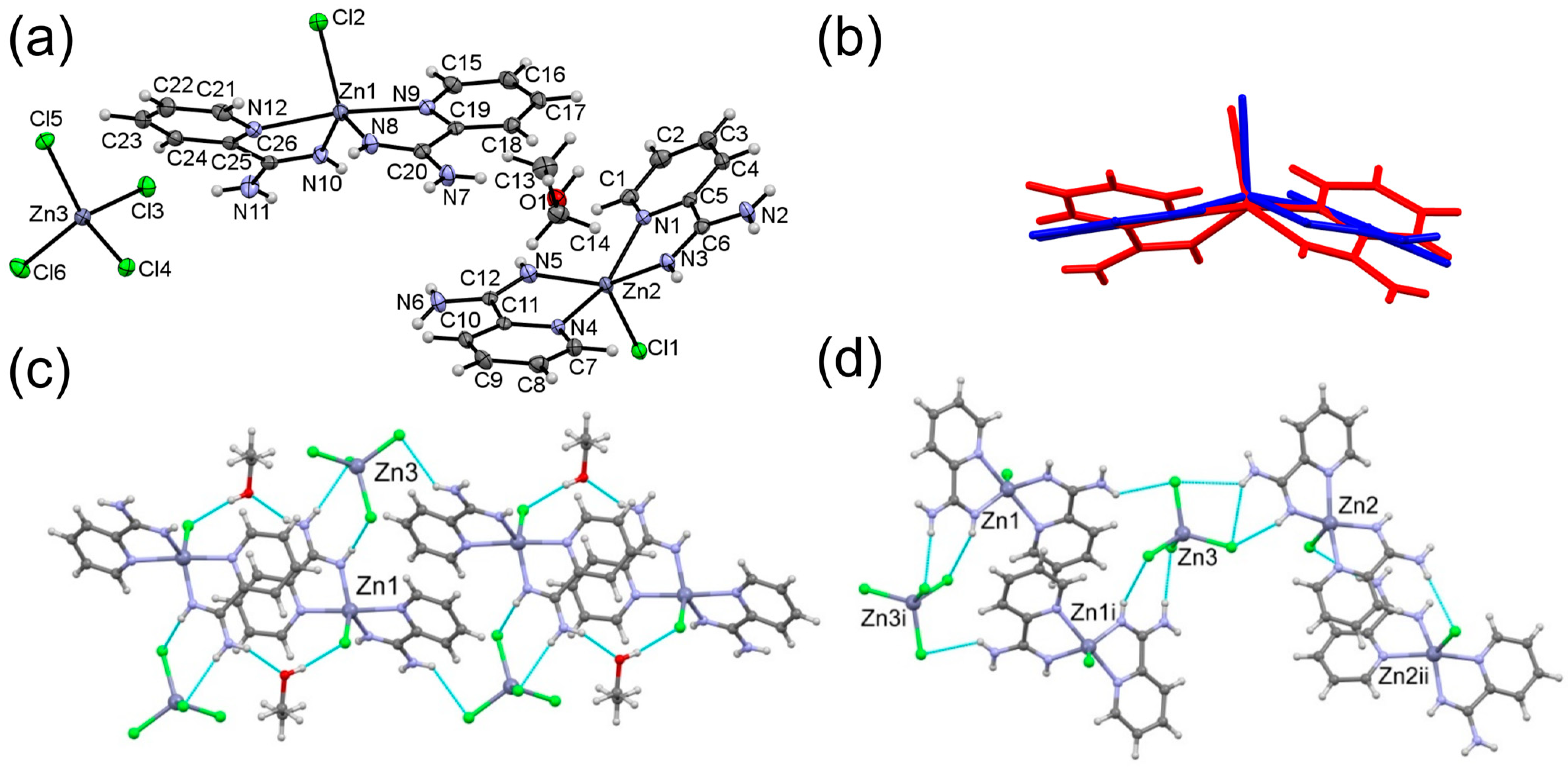
3.1. Crystal Structures of Complexes
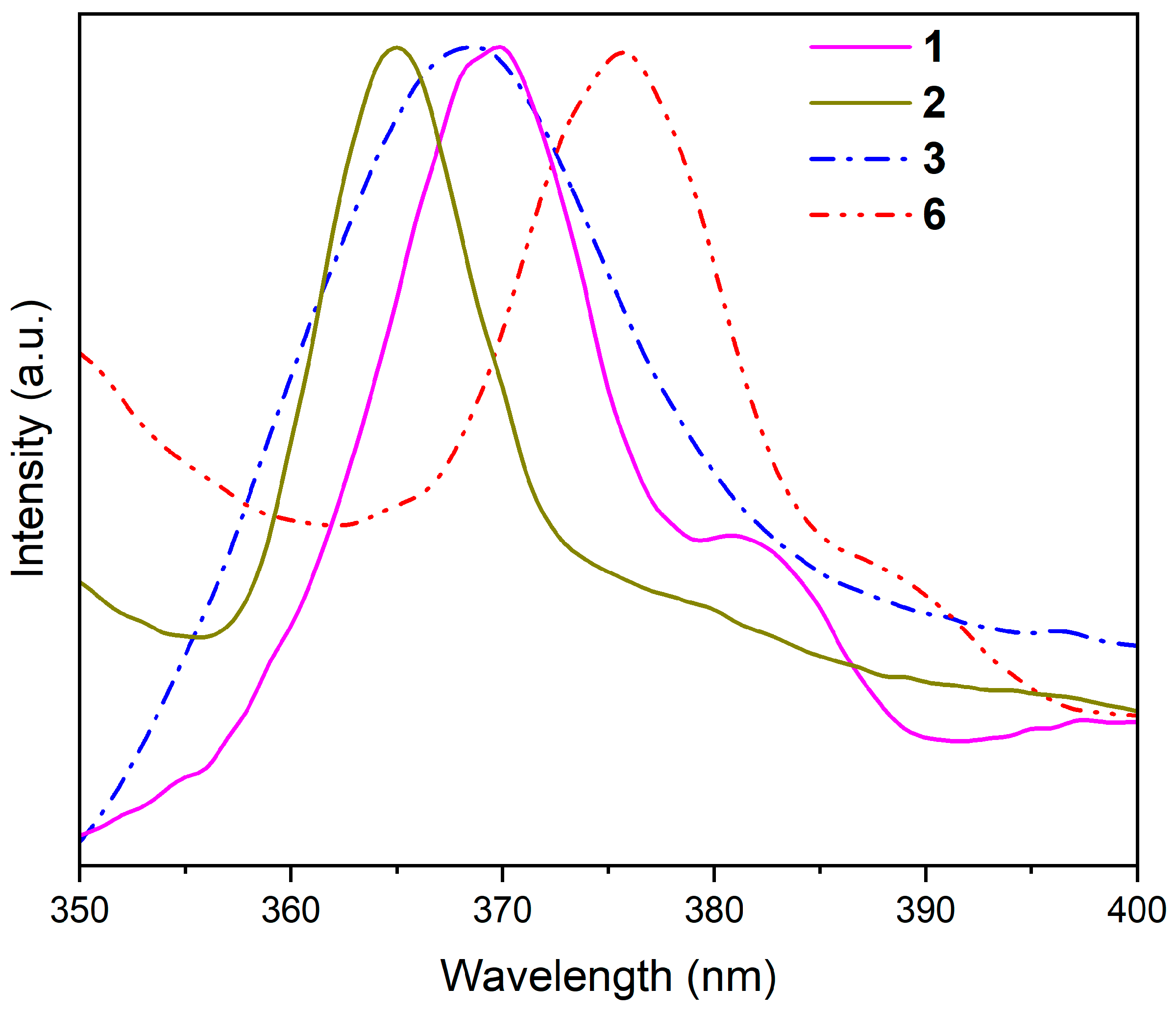
3.2. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drewry, J.A.; Gunning, P.T. Recent Advances in Biosensory and Medicinal Therapeutic Applications of Zinc(II) and Copper(II) Coordination Complexes. Coord. Chem. Rev. 2011, 255, 459–472. [Google Scholar] [CrossRef]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc Coordination Complexes as Anticancer Agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Gusev, A.; Braga, E.; Shul’gin, V.; Lyssenko, K.; Eremenko, I.; Samsonova, L.; Degtyarenko, K.; Kopylova, T.; Linert, W. Luminescent Properties of Zn and Mg Complexes on N-(2-Carboxyphenyl)Salicylidenimine Basis. Materials 2017, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Shul’gin, V.; Braga, E.; Zamnius, E.; Kryukova, M.; Linert, W. Luminescent Properties of Zn Complexes Based on Tetradentate N2O2-Donor Pyrazolone Schiff Bases. Dye. Pigment. 2020, 183, 108626. [Google Scholar] [CrossRef]
- Major, J.L.; Parigi, G.; Luchinat, C.; Meade, T.J. The Synthesis and in Vitro Testing of a Zinc-Activated MRI Contrast Agent. Proc. Natl. Acad. Sci. USA 2007, 104, 13881. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Roopa; Bhalla, V.; Kumar, M. Beyond zinc coordination: Bioimaging applications of Zn(II)-complexes. Coord. Chem. Rev. 2021, 427, 213550. [Google Scholar] [CrossRef]
- Li, T.; Schulz, S.; Roesky, P.W. Synthesis, Reactivity and Applications of Zinc–Zinc Bonded Complexes. Chem. Soc. Rev. 2012, 41, 3759–3771. [Google Scholar] [CrossRef]
- Dumur, F. Zinc Complexes in OLEDs: An Overview. Synth. Met. 2014, 195, 241–251. [Google Scholar] [CrossRef]
- Ho, K.-Y.; Yu, W.-Y.; Cheung, K.-K.; Che, C.-M. Blue luminescent zinc(II) complexes with polypyridylamine ligands: Crystal structures and luminescence properties. J. Chem. Soc. Dalton Trans. 1999, 10, 1581–1586. [Google Scholar] [CrossRef]
- Wang, S. Luminescence and electroluminescence of Al (III), B (III), Be (II) and Zn (II) complexes with nitrogen donors. Coord. Chem. Rev. 2001, 215, 79–98. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Two Aminobenzothiazole Derivatives for Pd(II) and Zn(II) Coordination: Synthesis, Characterization and Solid State Fluorescence. Inorg. Chem. Commun. 2011, 14, 46–48. [Google Scholar] [CrossRef]
- Gomes, C.S.B.; Gomes, P.T.; Duarte, M.T.; Di Paolo, R.E.; Maçanita, A.L.; Calhorda, M.J. Synthesis, Structure, and Photophysical Characterization of Blue-Green Luminescent Zinc Complexes Containing 2-Iminophenanthropyrrolyl Ligands. Inorg. Chem. 2009, 48, 11176–11186. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Chen, X.-M. Recent Advances in Luminescent Monomeric, Multinuclear, and Polymeric Zn(II) and Cd(II) Coordination Complexes. Aust. J. Chem. 2004, 57, 703–712. [Google Scholar] [CrossRef]
- Yang, W.; Schmider, H.; Wu, Q.; Zhang, Y.; Wang, S. Syntheses, Structures, and Fluxionality of Blue Luminescent Zinc(II) Complexes: Zn(2,2′,2″-tpa)Cl2, Zn(2,2′,2″-tpa)2(O2CCF3)2, and Zn(2,2′,3″-tpa)4(O2CCF3)2 (tpa = Tripyridylamine). Inorg. Chem. 2000, 39, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.P. Application of neutral amidines and guanidines in coordination chemistry. Dalton Trans. 2006, 8, 985–1001. [Google Scholar] [CrossRef]
- Peak, D.A. 43. Diamidides. Part II. 2:4-Diaryltriazapentadienes. J. Chem. Soc. 1952, 1952, 215–226. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Lasri, J.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Single-pot template transformations of cyanopyridines on a PdII centre: Syntheses of ketoimine and 2,4-dipyridyl-1,3,5-triazapentadiene palladium(II) complexes and their catalytic activity for microwaveassisted Suzuki−Miyaura and Heck reactions. Dalton Trans. 2009, 16, 3074–3084. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.A.; Korobkov, I.; Assoud, A.; Brusso, J.L. Noninnocent pyridyl nitrogens: Unprecedented interconversion of N-bridgehead-thiadiazolium salts and thiatriazine in the generation of thiatriazinyl. Chem. Commun. 2014, 50, 4934–4936. [Google Scholar] [CrossRef]
- Castañeda, R.; Hollingshead, A.; Gabidullin, B.; Brusso, J.L. Probing the Coordination Chemistry of N-2-Pyridylimidoyl-2-pyridylamidine: A Versatile Ligand with Multiple Coordination Sites. Cryst. Growth Des. 2017, 17, 6572–6578. [Google Scholar] [CrossRef]
- Castañeda, R.; Harriman, K.L.M.; Wong, J.W.L.; Gabidullin, B.; Murugesu, M.; Brusso, J.L. Rational Design of Tetranuclear Complexes Employing N-Imidoylamidine Based Ligands. Eur. J. Inorg. Chem. 2019, 2019, 963–972. [Google Scholar] [CrossRef]
- Castañeda, R.; Rouzières, M.; Clérac, R.; Brusso, J.L. Controlling the Nuclearity and Topology of Cobalt Complexes through Hydration at the Ppm Level. J. Mater. Chem. C 2020, 8, 4401–4407. [Google Scholar] [CrossRef]
- Maddison, K. Exploring Square Planar Complexes of Copper and Nickel by Employing the N-2-Pyridylimidoyl-2-Pyridylamidine Ligand Framework; University of Ottawa: Ottawa, ON, Canada, 2018. [Google Scholar]
- Kitos, A.A.; Gálico, D.A.; Castañeda, R.; Ovens, J.S.; Murugesu, M.; Brusso, J.L. Stark Sublevel-Based Thermometry with Tb(III) and Dy(III) Complexes Cosensitized via the 2-Amidinopyridine Ligand. Inorg. Chem. 2020, 59, 11061–11070. [Google Scholar] [CrossRef]
- Kitos, A.A.; Gálico, D.A.; Mavragani, N.; Castañeda, R.; Moilanen, J.O.; Brusso, J.L.; Murugesu, M. Probing Optical and Magnetic Properties via Subtle Stereoelectronic Effects in Mononuclear DyIII-Complexes. Chem. Commun. 2021, 57, 7818–7821. [Google Scholar] [CrossRef] [PubMed]
- Safin, D.A.; Tumanov, N.A.; Leitch, A.A.; Brusso, J.L.; Filinchuk, Y.; Murugesu, M. Elucidating the Elusive Crystal Structure of 2,4,6-tris(2-pyrimidyl)- 1,3,5-triazine (TPymT) through X-ray Powder Diffraction. CrystEngComm 2015, 17, 2190–2195. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Bruker/Siemens Area Detector Absorption Correction Program V.2.0.3; Bruker AXS: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXT − Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Dubrawski, Z.; Heidebrecht, J.; Puerta Lombardi, B.M.; Hyla, A.S.; Willkomm, J.; Radford, C.L.; Lin, J.-B.; Welch, G.C.; Ponnurangam, S.; Roesler, R.; et al. Ligand-centered electrochemical processes enable CO2 reduction with a nickel bis(triazapentadienyl) complex. Sustain. Energy Fuels 2019, 3, 1172. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kamiyama, A.; Ito, T. Complexed bridging ligand, {Cu(bptap)2}, as a ferromagnetic coupler. Chem. Comm. 2002, 12, 1256. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Dong, G.-H.; Xue, R.-D.; Li, J. Chlorido(pyridine-2-carboximidamide-κ2N1,N2)zinc(II) chloride dihydrate. Acta Crystallogr. E Struct. Rep. Online 2010, E66, m1557. [Google Scholar] [CrossRef] [PubMed]



| Compound | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Empirical formula | C24H20N10Zn | C12H11Cl2N5Zn | C12H14Cl2N6Zn | C26H34Cl6N12OZn3 | C13H18Cl2N6OZn | C20H24Cl6N16Zn3 |
| FW (g mol−1) | 513.87 | 361.53 | 378.56 | 939.46 | 410.60 | 897.36 |
| Temperature, K | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| Crystal system | monoclinic | monoclinic | triclinic | monoclinic | triclinic | monoclinic |
| Space group | P21/c | P21/n | P-1 | P21/n | P-1 | C2/c |
| a/Å | 10.6286(19) | 8.7118(4) | 7.8719(15) | 9.8693(8) | 7.0080(6) | 13.4968(10) |
| b/Å | 23.985(5) | 14.6347(5) | 8.5598(16) | 14.1886(12) | 9.6020(7) | 13.1249(11) |
| c/Å | 9.4821(16) | 10.6399(4) | 12.994(3) | 26.130(2) | 13.1569(10) | 19.6259(17) |
| α/deg | 90 | 90 | 96.765(3) | 90 | 91.723(6) | 90 |
| β/deg | 101.948(6) | 95.961(4) | 103.846(3) | 92.433(4) | 96.864(5) | 108.024(2) |
| γ/deg | 90 | 90 | 110.895(3) | 90 | 101.139(5) | 90 |
| V/Å3 | 2364.8(7) | 1349.19(9) | 774.0(3) | 3655.7(5) | 861.15(12) | 3306.0(5) |
| Z | 4 | 4 | 2 | 4 | 2 | 4 |
| Dcalcd g/cm3 | 1.443 | 1.780 | 1.624 | 1.707 | 1.584 | 1.803 |
| µ/mm−1 | 1.073 | 2.211 | 1.933 | 2.433 | 1.748 | 2.687 |
| Reflections collected | 28857 | 12768 | 19834 | 42016 | 26373 | 28609 |
| Independent reflections | 4642 [R(int) = 0.0362] | 3940 [R(int) = 0.0369] | 4312 [R(int) = 0.0313] | 7421 [R(int) = 0.0449] | 4922 [R(int) = 0.0334] | 4815 [R(int) = 0.0269] |
| GOF | 1.036 | 1.032 | 1.045 | 1.023 | 1.042 | 1.072 |
| R1, wR2 (I > 2σ(I)) | 0.0304, 0.0713 | 0.0428, 0.1101 | 0.0258, 0.0624 | 0.0298, 0.0626 | 0.0262, 0.0623 | 0.0257, 0.0665 |
| R1, wR2 (all data) | 0.0441, 0.0766 | 0.0633, 0.1260 | 0.0331, 0.0655 | 0.0446, 0.0673 | 0.0336, 0.0652 | 0.0332, 0.0688 |
| Largest diff. peak and hole/e·Å−3 | 0.245 and −0.282 | 1.550 and −1.012 | 0.435 and −0.294 | 0.818 and −0.457 | 0.431 and −0.405 | 0.723 and −0.312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijhuis, M.R.; Yamba, Z.; Novikov, E.; Fonari, M.S.; Timofeeva, T.V.; Castañeda, R. A Series of Zinc Mononuclear Complexes with Imidoyl Amidine Ligands: Syntheses, Crystal Structures, and Photoluminescence Properties. Chemistry 2024, 6, 760-772. https://doi.org/10.3390/chemistry6040045
Nijhuis MR, Yamba Z, Novikov E, Fonari MS, Timofeeva TV, Castañeda R. A Series of Zinc Mononuclear Complexes with Imidoyl Amidine Ligands: Syntheses, Crystal Structures, and Photoluminescence Properties. Chemistry. 2024; 6(4):760-772. https://doi.org/10.3390/chemistry6040045
Chicago/Turabian StyleNijhuis, Mart Ruben, Zaina Yamba, Egor Novikov, Marina S. Fonari, Tatiana V. Timofeeva, and Raúl Castañeda. 2024. "A Series of Zinc Mononuclear Complexes with Imidoyl Amidine Ligands: Syntheses, Crystal Structures, and Photoluminescence Properties" Chemistry 6, no. 4: 760-772. https://doi.org/10.3390/chemistry6040045
APA StyleNijhuis, M. R., Yamba, Z., Novikov, E., Fonari, M. S., Timofeeva, T. V., & Castañeda, R. (2024). A Series of Zinc Mononuclear Complexes with Imidoyl Amidine Ligands: Syntheses, Crystal Structures, and Photoluminescence Properties. Chemistry, 6(4), 760-772. https://doi.org/10.3390/chemistry6040045








