Surface Thermodynamic Properties of Styrene–Divinylbenzene Copolymer Modified by Supramolecular Structure of Melamine Using Inverse Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent and Materials
2.2. Inverse Gas Chromatography
2.3. Thermodynamic Methods
2.3.1. Dispersive and Polar Energies, and Lewis Acid–Base Parameters
2.3.2. London Dispersive Surface Energy, and Lewis Acid–Base Surface Energies
3. Results
3.1. Variations in of Adsorbed Probes Against the Temperature
- where
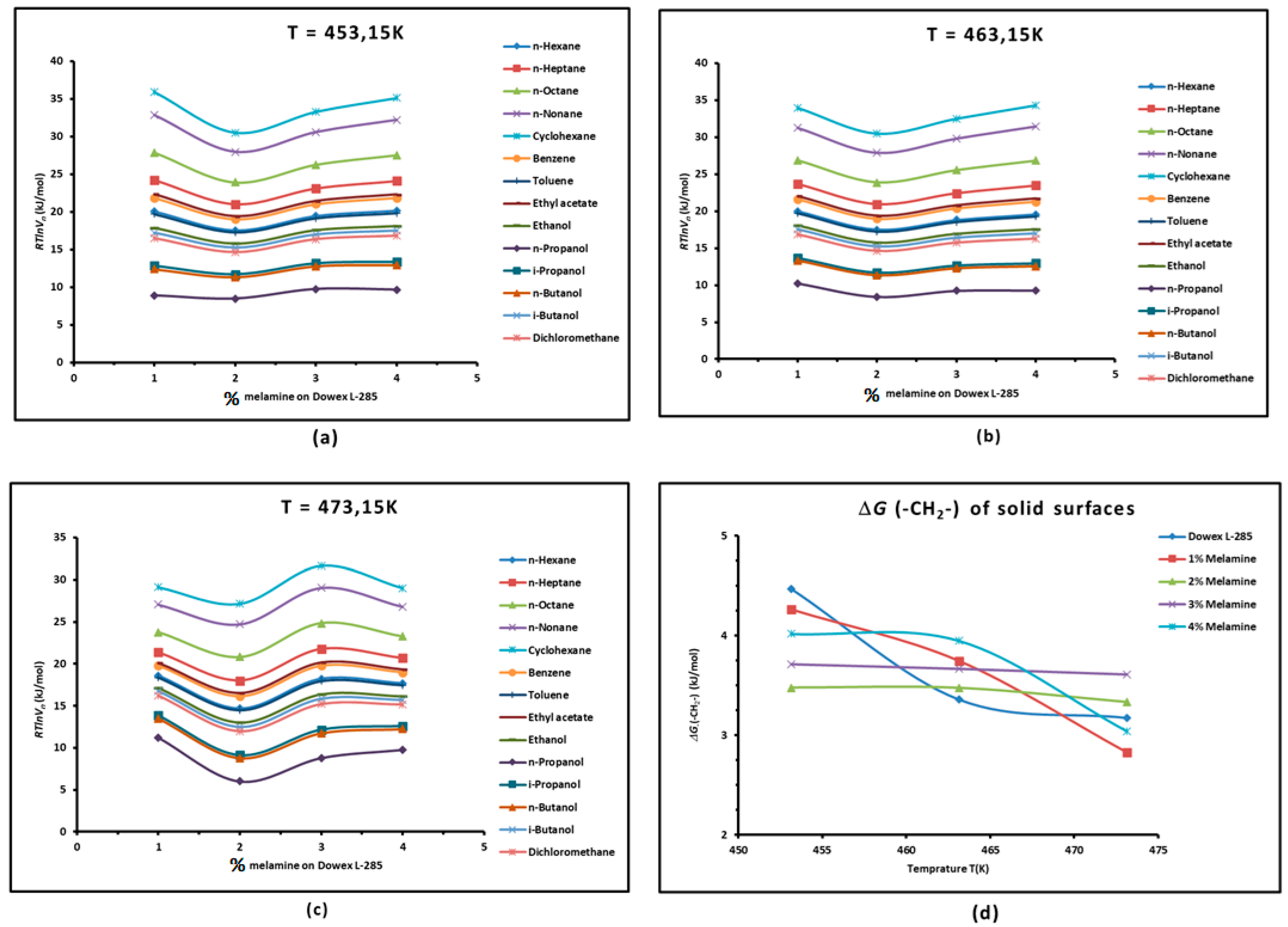
3.2. London Dispersive Surface Energy of Dowex L-285 with Different Melamine Percentages
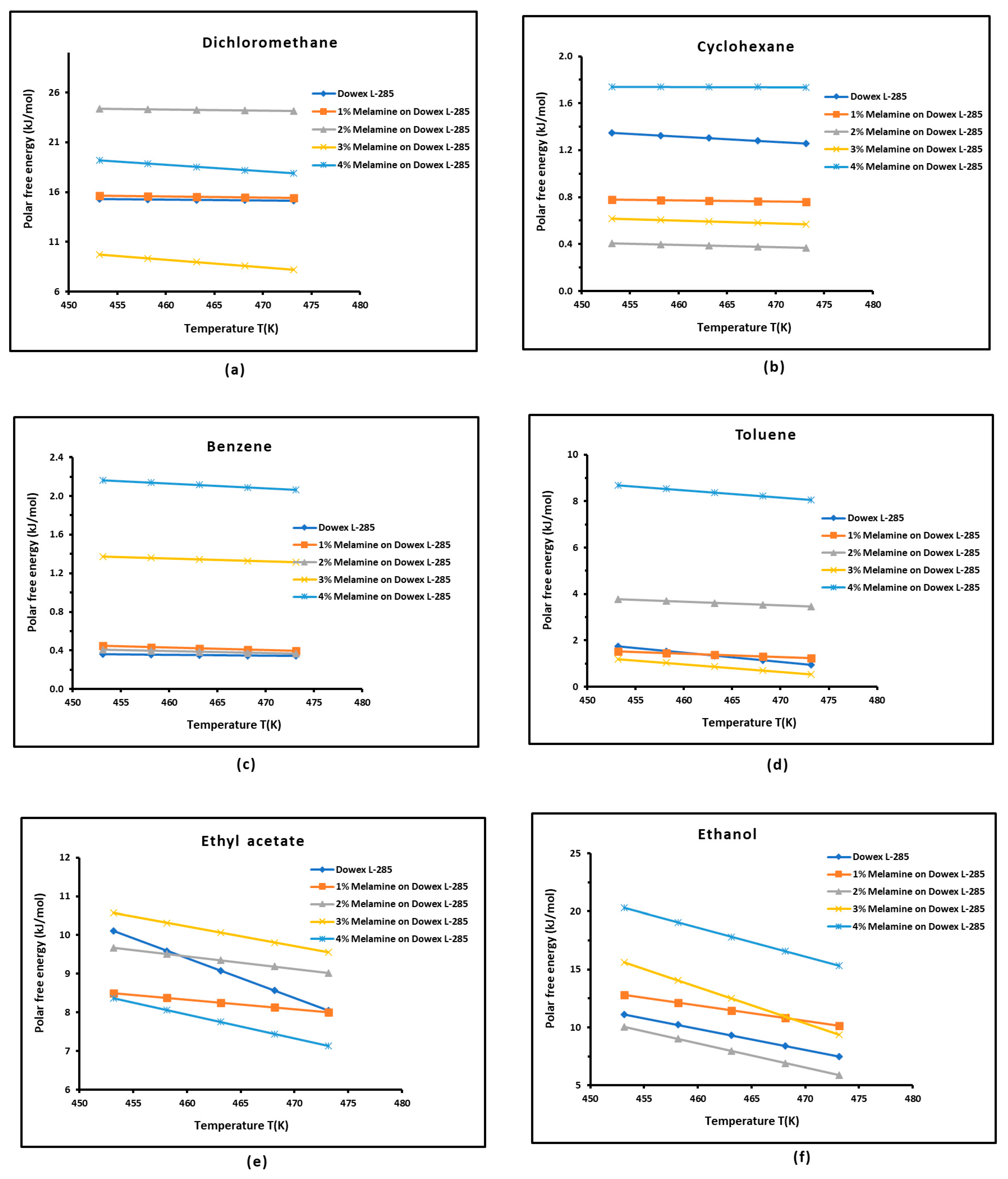
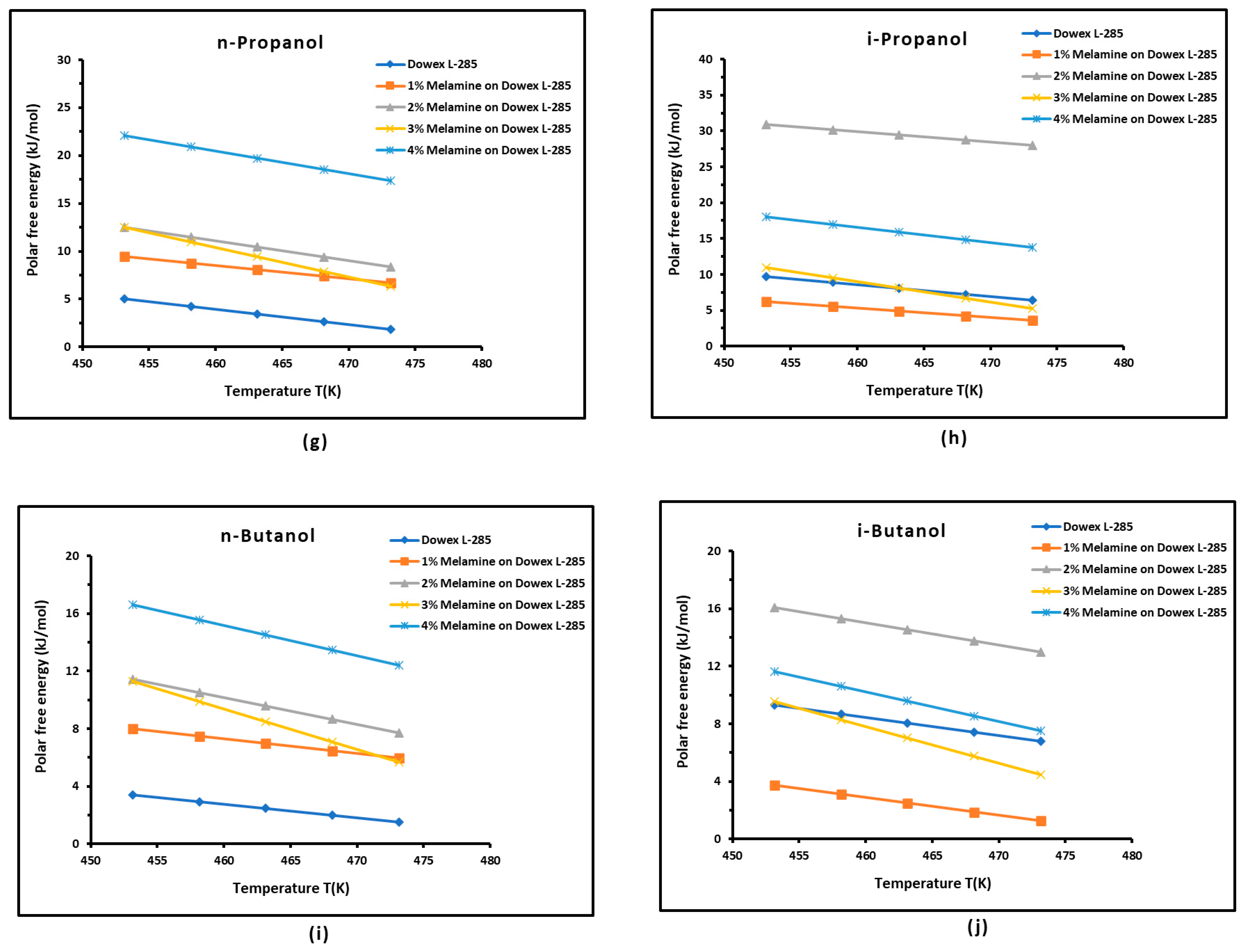
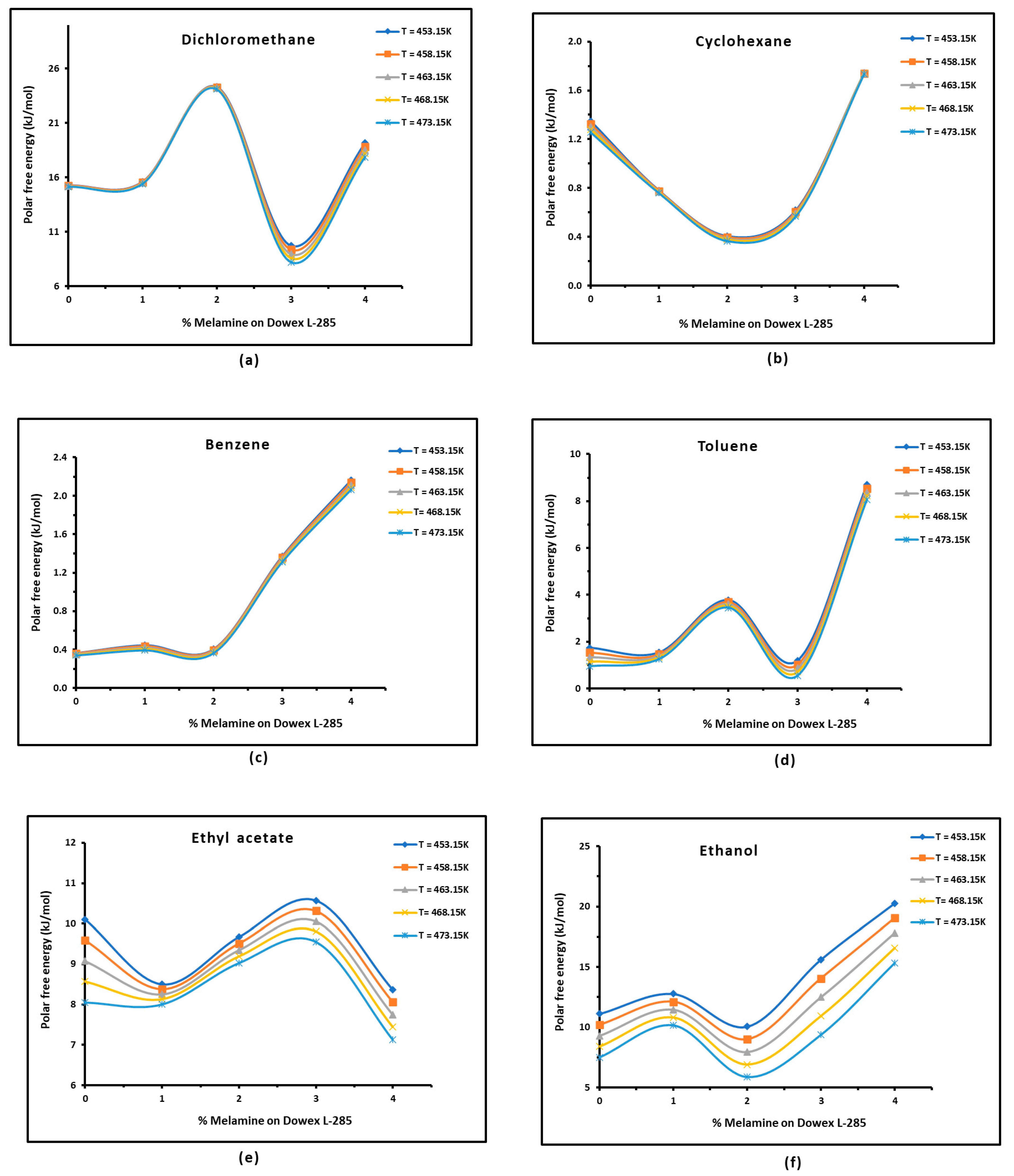
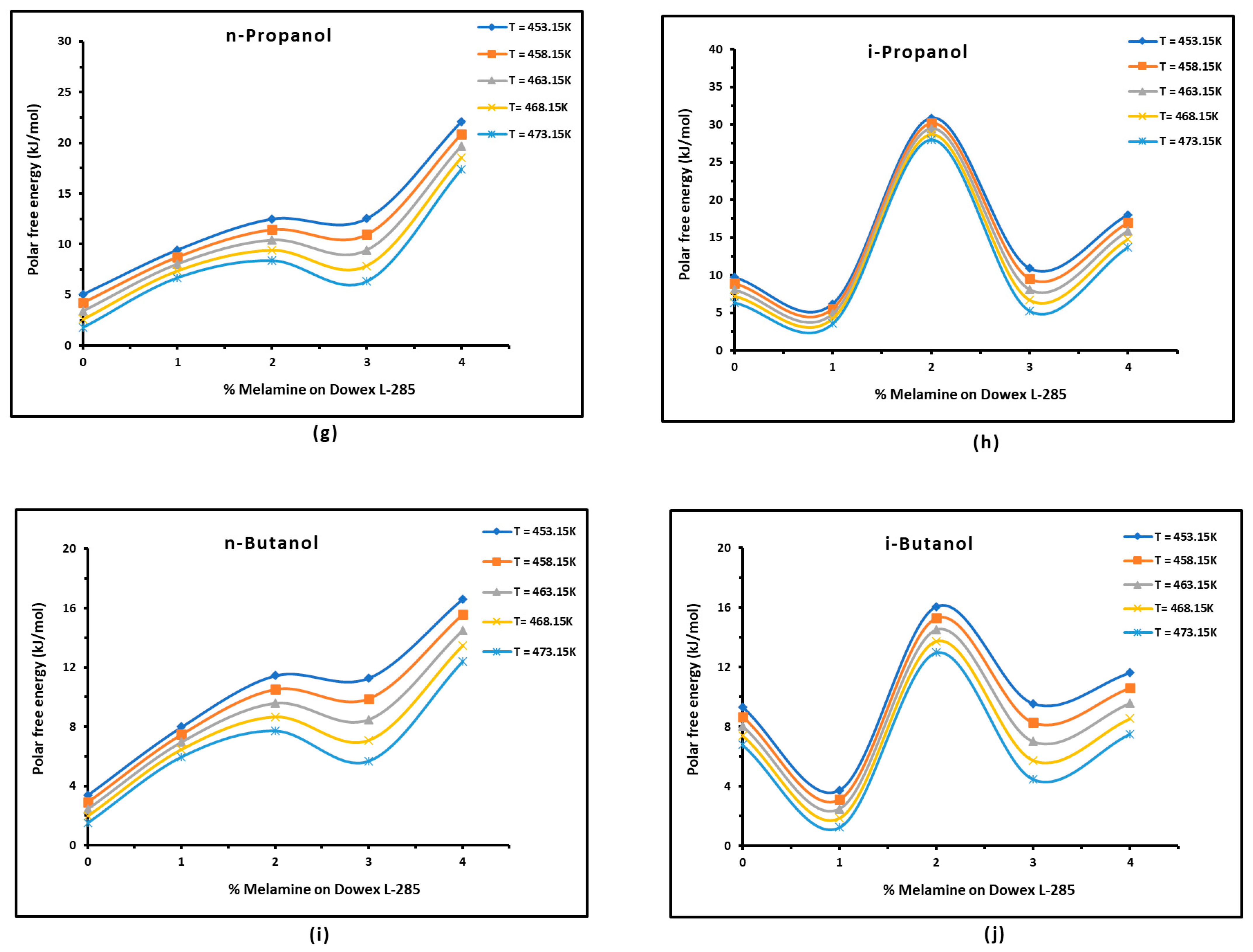
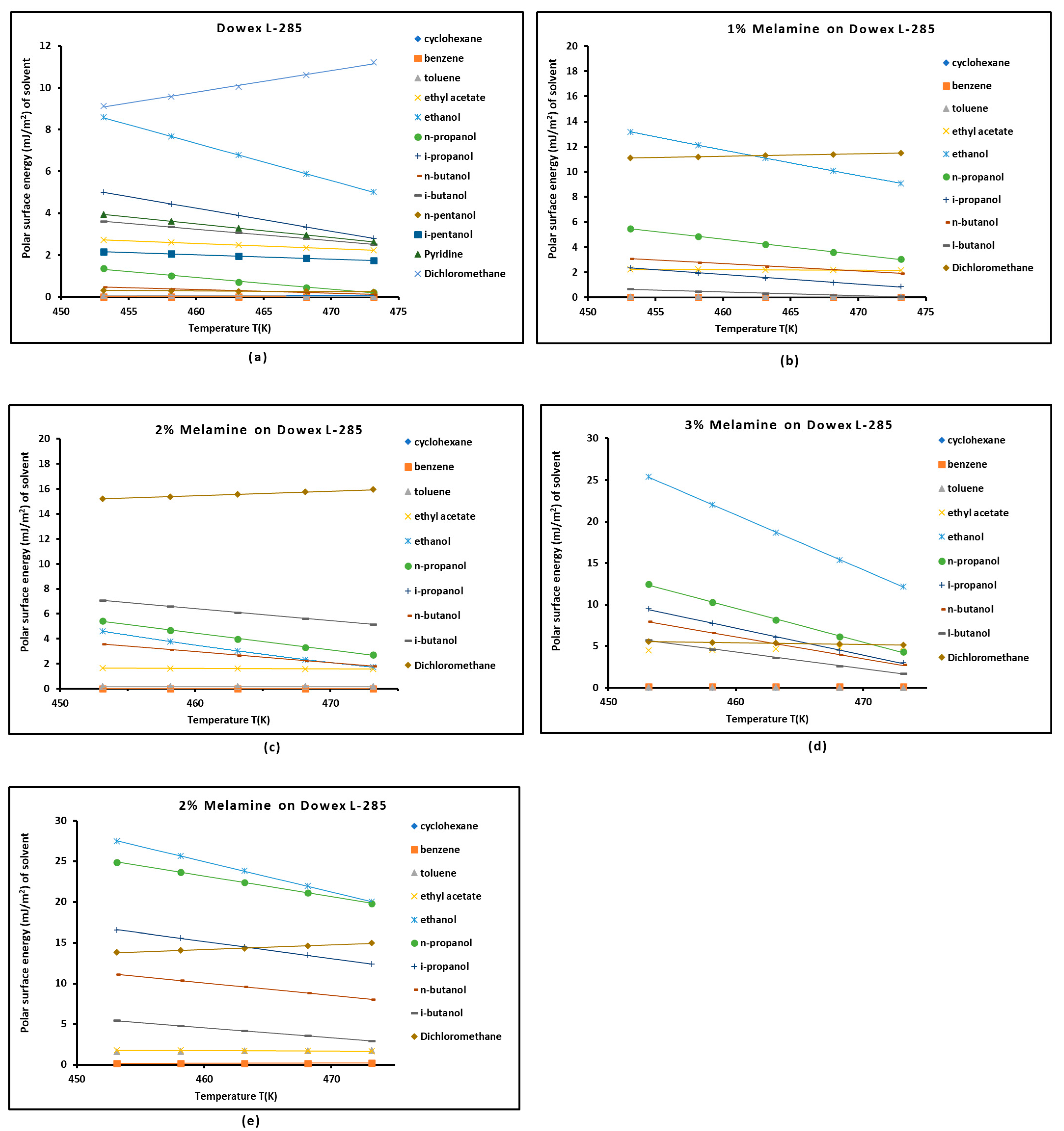
3.3. Polar Free Interaction Energy of Dowex L-285 Modified by Melamine with the Polar Probes
- The free polar energies, of interaction between the solids and the organic solvents can be globally classified in increasing order of interaction energy with the various polar probes at all temperatures: cyclohexane < benzene < toluene < ethyl acetate < i-butanol < n-butanol < ethanol < n-propanol < dichloromethane < i-propanol.
- The highest free interaction energy was obtained for 4% melamine on Dowex L-285 for the following polar molecules in increasing order: cyclohexane < benzene < toluene < n-butanol < ethanol < n-propanol.
- However, a maximum of free interaction energy was observed for 2% melamine on Dowex L-285 for the other polar solvents such as dichloromethane, i-propanol, and i-butanol.
- It was proved that the alcohol molecules exchanged the maximum free interaction energy with the various solid surfaces, and especially with 4% melamine, while the minimum interaction was observed in the case of adsorption on Dowex L-285.
- The above results show that the modification of the copolymer Dowex L-285 by melamine increased the polar free interaction.
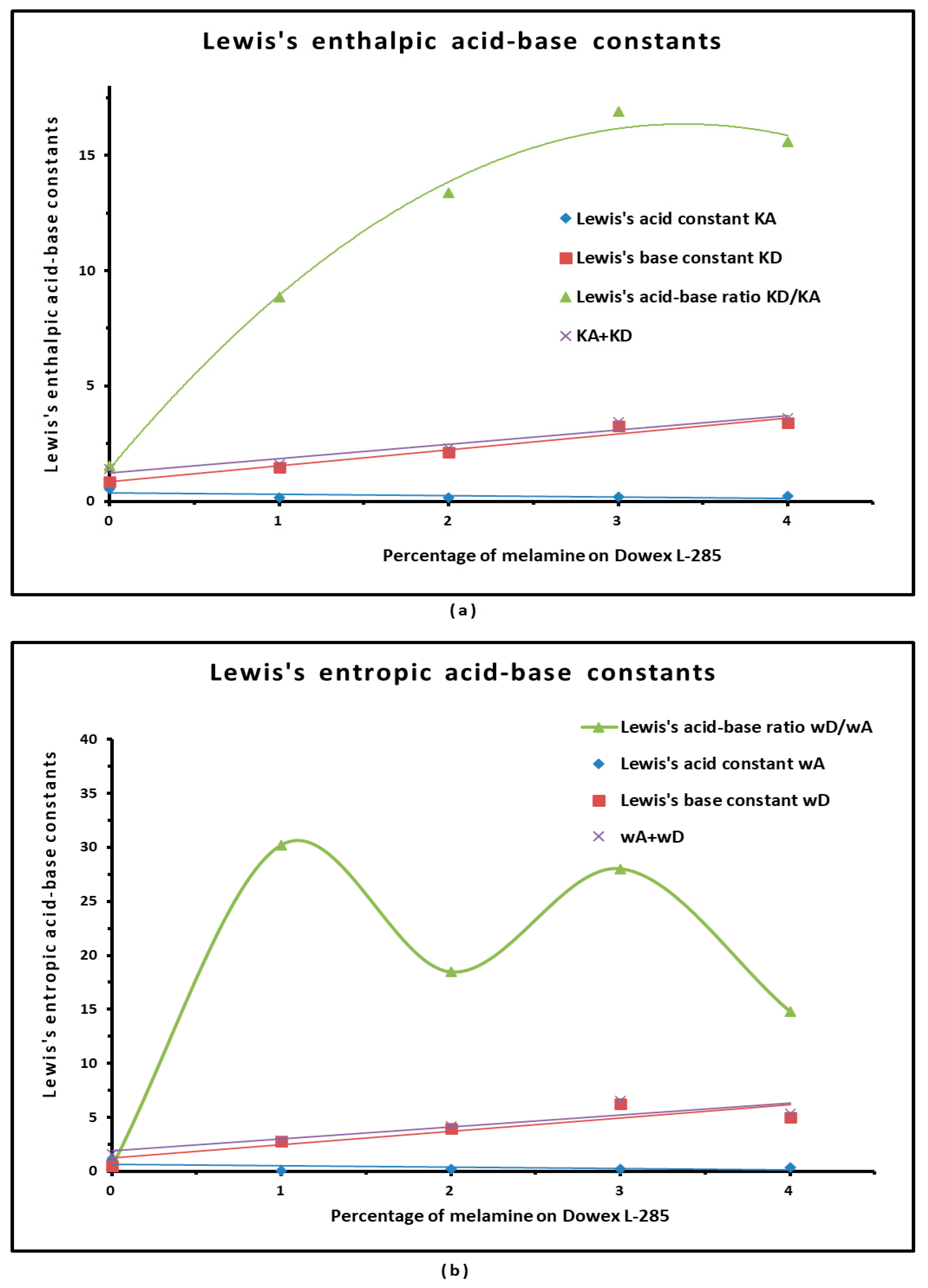
3.4. Polar Enthalpy and Entropy of Adsorption, and Lewis Acid–Base Parameters of Dowex L-285 Modified by Melamine
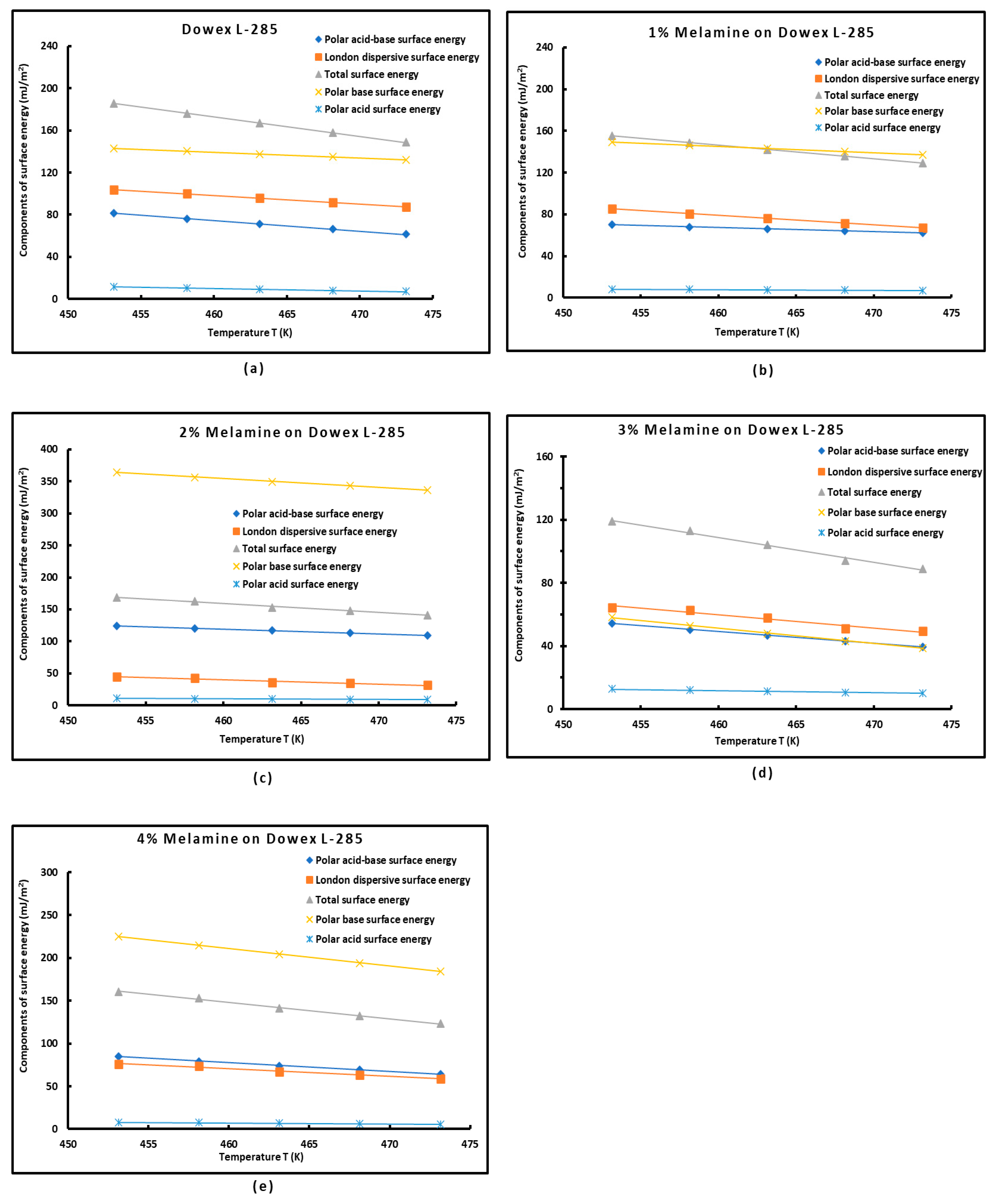
3.5. Polar Acid–Base Surface Energies of Dowex L-285 Modified by Melamine
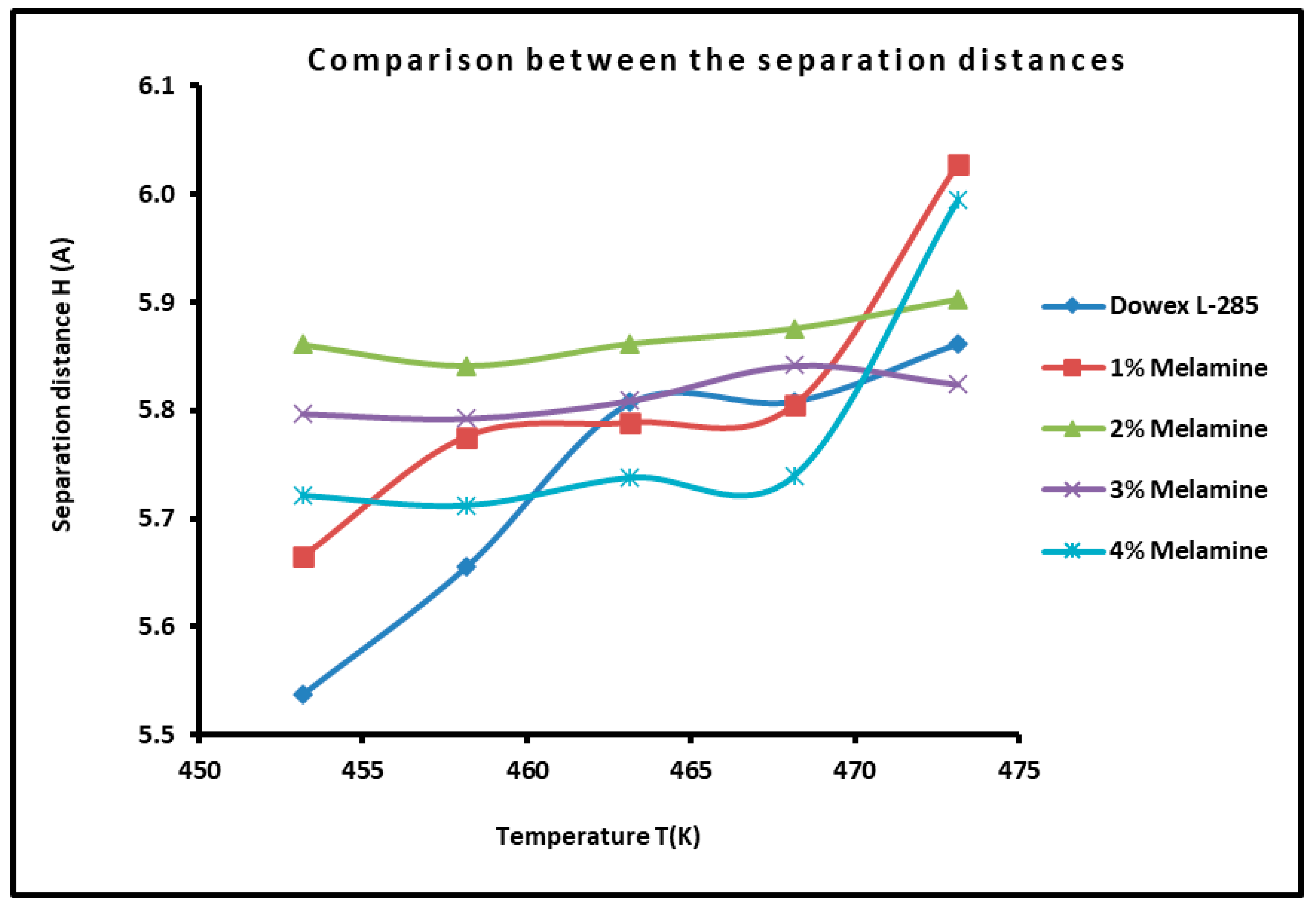
3.6. Determination of the Average Separation Distance H
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gus’kov, V.Y.; Bilalova, R.V.; Kudasheva, F.K. Adsorption of organic molecules on a melamine-modified porous polymer. Russ. Chem. Bull. 2017, 66, 857–861. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Vereschetin, V.P.; Gromov, S.P.; Fedorova, O.A.; Alfimov, M.V.; Huesmann, H.; Möbius, D. Photosensitive supramolecular systems based on amphiphilic crown ethers. Supramol. Sci. 1997, 4, 519–524. [Google Scholar] [CrossRef]
- Sukhareva, D.A.; Gus´kov, V.Y.; Karpov, S.I.; Kudasheva, F.K. Adsorption of organic molecules on the highly ordered MCM-41 sorbent modified by different amounts of melamine. Russ. Chem. Bull. 2017, 66, 958–962. [Google Scholar] [CrossRef]
- Gainullina, Y.Y.; Gus’kov, V.Y.; Timofeeva, D.V. Polarity of Thymine and 6-Methyluracil-Modified Porous Polymers, According to Data from Inverse Gas Chromatography. Russ. J. Phys. Chem. 2019, 93, 2477–2481. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Shaihitdinova, Y.F.; Zil’berg, R.A.; Kraikin, V.A.; Maistrenko, V.N. Intermolecular interactions between polyarylenephthalides surface and organic compounds of different nature by inverse gas chromatography. Sorpt. Chromatogr. Process 2019, 19, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Onuchak, L.A.; Kapralova, T.S.; Kuraeva, Y.G.; Belousova, Z.P.; Stepanova, R.F. Sorption and selective chromatographic properties of isomer-selective composite sorbent based on a eutectic mixture of nematic liquid crystals and perbenzoylated β-cyclodextrin. Russ. J. Phys. Chem. A 2015, 89, 2304–2312. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry. Concepts and Perspectives; VCH: Weinhiem, Germany, 1995; p. 271. [Google Scholar] [CrossRef]
- Guo, F.; Cheung, E.Y.; Harris, K.D.M.; Pedireddi, V.R. Contrasting Solid-State Structures of Trithiocyanuric Acid and Cyanuric Acid. Cryst. Growth Des. 2006, 6, 846–848. [Google Scholar] [CrossRef]
- Singh, U.P.; Kashyap, S.; Singh, H.J.; Mishra, B.K.; Roy, P.; Chakraborty, A. Effect of adenosine on the supramolecular architecture and activity of 5-fluorouracil. J. Mol. Struct. 2012, 1014, 47–56. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Gainullina, Y.Y.; Ivanov, S.P.; Kudasheva, F.K. Thermodynamics of organic molecule adsorption on sorbents modified with 5-hydroxy-6-methyluracil by inverse gas chromatography. J. Chromatogr. A 2014, 1356, 230–235. [Google Scholar] [CrossRef]
- Breckenridge, W.H.; Ayles, V.L.; Wright, T.G. Evidence for Emergent Chemical Bonding in Au+−Rg Complexes (Rg = Ne, Ar, Kr, and Xe). J. Phys. Chem. A 2008, 112, 4209–4214. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Ganieva, A.G.; Kudasheva, F.K. Monolayer capacity estimation by inverse gas chromatography at finite concentrations. Colloids Surf. A 2017, 513, 95–101. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Gainullina, Y.Y.; Ivanov, S.P.; Kudasheva, F.K. Properties of the surface of a porous polymer modified with 5-fluorouracil, according to data of gas chromatography. Russ. J. Phys. Chem. A 2014, 88, 1042–1046. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Gainullina, Y.Y.; Ivanov, S.P.; Kudasheva, F.K. Porous polymer adsorbents modified with uracil. Prot. Met. Phys. Chem. Surf. 2014, 50, 55–58. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Ivanov, S.P.; Khabibullina, R.A.; Garafutdinov, R.R.; Kudasheva, F.K. Gas chromatographic investigation of the properties of a styrene-divinylbenzene copolymer modified by 5-hydroxy-6-methyluracil. Russ. J. Phys. Chem. A 2012, 86, 475–478. [Google Scholar] [CrossRef]
- Godlewski, S.; Tekiel, A.; Piskorz, W.; Zasada, F.; Prauzner-Bechcicki, J.S.; Sojka, Z.; Szymonski, M. Supramolecular ordering of PTCDA molecules: The key role of dispersion forces in an unusual transition from physisorbed into chemisorbed state. ACS Nano 2012, 6, 8536–8545. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. Propriétés de surface des fibres de carbone déterminées par chromatographie gazeuse inverse. J. Chim. Phys. 1987, 84, 231–237. [Google Scholar] [CrossRef]
- Hamieh, T. Study of the temperature effect on the surface area of model organic molecules, the dispersive surface energy and the surface properties of solids by inverse gas chromatography. J. Chromatogr. A 2020, 1627, 461372. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T.; Ahmad, A.A.; Roques-Carmes, T.; Toufaily, J. New approach to determine the surface and interface thermodynamic properties of H-β-zeolite/rhodium catalysts by inverse gas chromatography at infinite dilution. Sci. Rep. 2020, 10, 20894. [Google Scholar] [CrossRef]
- Hamieh, T. New methodology to study the dispersive component of the surface energy and acid–base properties of silica particles by inverse gas chromatography at infinite dilution. J. Chromatogr. Sci. 2022, 60, 126–142. [Google Scholar] [CrossRef]
- Hamieh, T. Inverse Gas Chromatography to Characterize the Surface Properties of Solid Materials. Chem. Mater. 2024, 36, 2231–2244. [Google Scholar] [CrossRef]
- Hamieh, T. Some Irregularities in the Evaluation of Surface Parameters of Solid Materials by Inverse Gas Chromatography. Langmuir 2023, 39, 17059–17070. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. New Progress on London Dispersive Energy, Polar Surface Interactions, and Lewis’s Acid–Base Properties of Solid Surfaces. Molecules 2024, 29, 949. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. London Dispersive and Lewis Acid-Base Surface Energy of 2D Single-Crystalline and Polycrystalline Covalent Organic Frameworks. Crystals 2024, 14, 148. [Google Scholar] [CrossRef]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8–26. [Google Scholar] [CrossRef]
- Conder, J.R.; Young, C.L. Physical Measurements by Gas Chromatography; John Wiley & Sons: Chichester, NY, USA, 1979; p. 632. [Google Scholar]
- Sawyer, D.T.; Brookman, D.J. Thermodynamically based gas chromatographic retention index for organic molecules using salt-modified aluminas and porous silica beads. Anal. Chem. 1968, 40, 1847–1850. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Wang, S.; Li, M. Optimization for testing conditions of inverse gas chromatography and surface energies of various carbon fiber bundles. Carbon Lett. 2023, 33, 909–920. [Google Scholar] [CrossRef]
- Pal, A.; Kondor, A.; Mitra, S.; Thua, K.; Harish, S.; Saha, B.B. On surface energy and acid–base properties of highly porous parent and surface treated activated carbons using inverse gas chromatography. J. Ind. Eng. Chem. 2019, 69, 432–443. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. The role of the interface in carbon fibre-epoxy composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Saint-Flour, C.; Papirer, E. Gas-solid chromatography. A method of measuring surface free energy characteristics of short glass fibers. 1. Through adsorption isotherms. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 337–341. [Google Scholar] [CrossRef]
- Saint-Flour, C.; Papirer, E. Gas-solid chromatography: Method of measuring surface free energy characteristics of short fibers. 2. Through retention volumes measured near zero surface coverage. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 666–669. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Park, S.; Balard, H. Evaluation of specific interactions of solid surfaces by inverse gas chromatography. Chromatographia 1991, 31, 434–440. [Google Scholar] [CrossRef]
- Chehimi, M.M.; Pigois-Landureau, E. Determination of acid–base properties of solid materials by inverse gas chromatography at infinite dilution. A novel empirical method based on the dispersive contribution to the heat of vaporization of probes. J. Mater. Chem. 1994, 4, 741–745. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A New Topological Index for Molecular Probes Used in Inverse Gas Chromatography for the Surface Nanorugosity Evaluation. J. Colloid Interface Sci. 1997, 194, 207–216. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A New Topological Index for Molecular Probes Used in Inverse Gas Chromatography. J. Colloid Interface Sci. 1997, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewska, M.; Krzywania, A.; Zaborski, M.; Szynkowska, M.I. Surface properties of zinc oxide nanoparticles studied by inverse gas chromatography. J. Chromatogr. A 2009, 1216, 5284–5291. [Google Scholar] [CrossRef] [PubMed]
- Bakaoukas, N.; Sevastos, D.; Kapolos, J.; Koliadima, A.; Karaiskakis, G. Characterization of polymeric coatings in terms of their ability to protect marbles and clays against corrosion from sulfur dioxide by inverse gas chromatography. Int. J. Polym. Anal. Charact. 2013, 18, 401–4013. [Google Scholar] [CrossRef]
- Rückriem, M.; Inayat, A.; Enke, D.; Gläser, R.; Einicke, W.-D.; Rockmann, R. Inverse gas chromatography for determining the dispersive surface energy of porous silica. Colloids Surf. A Physicochem. Eng. Asp. 2010, 357, 21–26. [Google Scholar] [CrossRef]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Springer: New York, NY, USA, 1978; p. 279. [Google Scholar]
- Riddle, F.L.; Fowkes, F.M. Spectral shifts in acid-base chemistry. Van der Waals contributions to acceptor numbers, Spectral shifts in acid-base chemistry. 1. van der Waals contributions to acceptor numbers. J. Am. Chem. Soc. 1990, 112, 3259–3264. [Google Scholar] [CrossRef]
- Fowkes, F.M. Volume 1 Surface Chemistry and Physics. In Surface and Interfacial Aspects of Biomedical Polymers; Andrade, J.D., Ed.; Plenum Press: New York, NY, USA, 1985; pp. 337–372. [Google Scholar]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884. [Google Scholar] [CrossRef]
- Gus’kov, V.Y.; Gainullina, Y.Y.; Gabdulmanova, A.F.; Gareeva, A.N. Dispersive surface free energy of adsorbents modified by supramolecular structures of heterocyclic compounds. Chim. Techno Acta 2024, 11, 202411210. [Google Scholar] [CrossRef]
- Yamamoto, O.; Kambe, H. Thermal conductivity of cross-linked polymers. A comparison between measured and calculated thermal conductivities. Polym. J. 1971, 2, 623–628. [Google Scholar] [CrossRef]
- Hamieh, T. Thermal Surface Properties, London Dispersive and Polar Surface Energy of Graphene and Carbon Materials Using Inverse Gas Chromatography at Infinite Dilution. Molecules 2024, 29, 2871. [Google Scholar] [CrossRef] [PubMed]
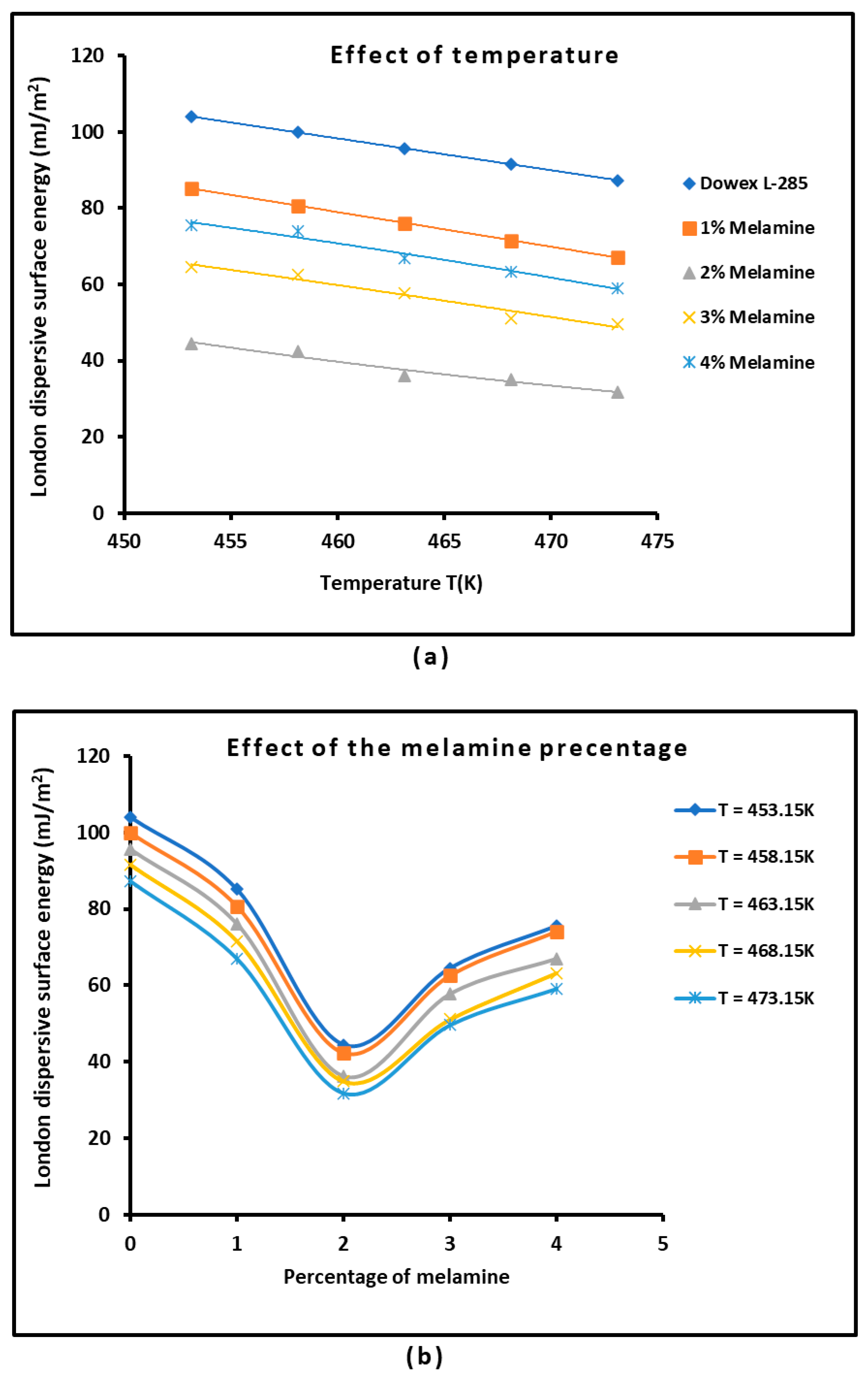


| Solid Material | (mJ/m2) | R2 | (mJ m−2 K−1) | (mJ/m2) | (mJ/m2) | (K) |
|---|---|---|---|---|---|---|
| Dowex L-285 | = −0.835 T + 482.43 | 0.9980 | −0.835 | 482.43 | 233.47 | 577.8 |
| 1% Melamine | = −0.907 T + 496.21 | 0.9972 | −0.907 | 496.21 | 225.79 | 547.1 |
| 2% Melamine | = −0.656 T + 341.59 | 0.9590 | −0.656 | 341.59 | 146.12 | 521.0 |
| 3% Melamine | = −0.827 T + 439.96 | 0.9608 | −0.827 | 439.96 | 193.51 | 532.3 |
| 4% Melamine | = −0.876 T + 473.64 | 0.9735 | −0.876 | 473.64 | 212.40 | 540.6 |
| Material | KA | KD | KD/KA | KA + KD | R2 | 10−3ωA | 10−3ωD | ωD/ωA | 10−3 (ωA + ωD) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dowex L-285 | 0.550 | 0.844 | 1.53 | 1.393 | 0.999 | 1.05 | 0.55 | 0.5 | 1.59 | 0.9968 |
| 1% Melamine on Dowex L-285 | 0.164 | 1.455 | 8.85 | 1.620 | 0.9783 | 0.09 | 2.81 | 30.2 | 2.90 | 0.9316 |
| 2% Melamine on Dowex L-285 | 0.160 | 2.137 | 13.38 | 2.297 | 0.9989 | 0.22 | 4.00 | 18.5 | 4.21 | 0.9520 |
| 3% Melamine on Dowex L-285 | 0.193 | 3.263 | 16.90 | 3.456 | 0.9938 | 0.22 | 6.30 | 28.0 | 6.52 | 0.9695 |
| 4% Melamine on Dowex L-285 | 0.218 | 3.401 | 15.58 | 3.619 | 0.9276 | 0.34 | 5.00 | 14.8 | 5.34 | 0.9702 |
| Dichloromethane | |||||
| T(K) | Dowex L-285 | 1% Melamine | 2% Melamine | 3% Melamine | 4% Melamine |
| 453.15 | 15.274 | 15.609 | 24.349 | 9.737 | 16.294 |
| 458.15 | 15.239 | 15.559 | 24.294 | 9.352 | 16.452 |
| 463.15 | 15.204 | 15.509 | 24.239 | 8.967 | 16.012 |
| 468.15 | 15.169 | 15.459 | 24.184 | 8.582 | 15.989 |
| 473.15 | 15.134 | 15.409 | 24.129 | 8.197 | 12.318 |
| Ethyl Acetate | |||||
| T(K) | Dowex L-285 | 1% Melamine | 2% Melamine | 3% Melamine | 4% Melamine |
| 453.15 | 10.106 | 8.503 | 9.667 | 10.567 | 39.887 |
| 458.15 | 9.593 | 8.378 | 9.507 | 10.314 | 38.842 |
| 463.15 | 9.079 | 8.253 | 9.347 | 10.060 | 37.077 |
| 468.15 | 8.566 | 8.128 | 9.187 | 9.807 | 35.814 |
| 473.15 | 8.052 | 8.003 | 9.027 | 9.553 | 30.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamieh, T.; Gus'kov, V.Y. Surface Thermodynamic Properties of Styrene–Divinylbenzene Copolymer Modified by Supramolecular Structure of Melamine Using Inverse Gas Chromatography. Chemistry 2024, 6, 830-851. https://doi.org/10.3390/chemistry6050050
Hamieh T, Gus'kov VY. Surface Thermodynamic Properties of Styrene–Divinylbenzene Copolymer Modified by Supramolecular Structure of Melamine Using Inverse Gas Chromatography. Chemistry. 2024; 6(5):830-851. https://doi.org/10.3390/chemistry6050050
Chicago/Turabian StyleHamieh, Tayssir, and Vladimir Yu Gus'kov. 2024. "Surface Thermodynamic Properties of Styrene–Divinylbenzene Copolymer Modified by Supramolecular Structure of Melamine Using Inverse Gas Chromatography" Chemistry 6, no. 5: 830-851. https://doi.org/10.3390/chemistry6050050







