Incorporating C3N5 and NiCo2S4 to Form a Novel Z-Scheme Heterojunction for Superior Photocatalytic Degradation of Norfloxacin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of C3N5
2.3. Synthesis of NiCo2S4
2.4. Synthesis of C3N5/NiCo2S4 Heterojunction
2.5. Characterization
2.6. Photocatalytic Activity
3. Results and Discussion
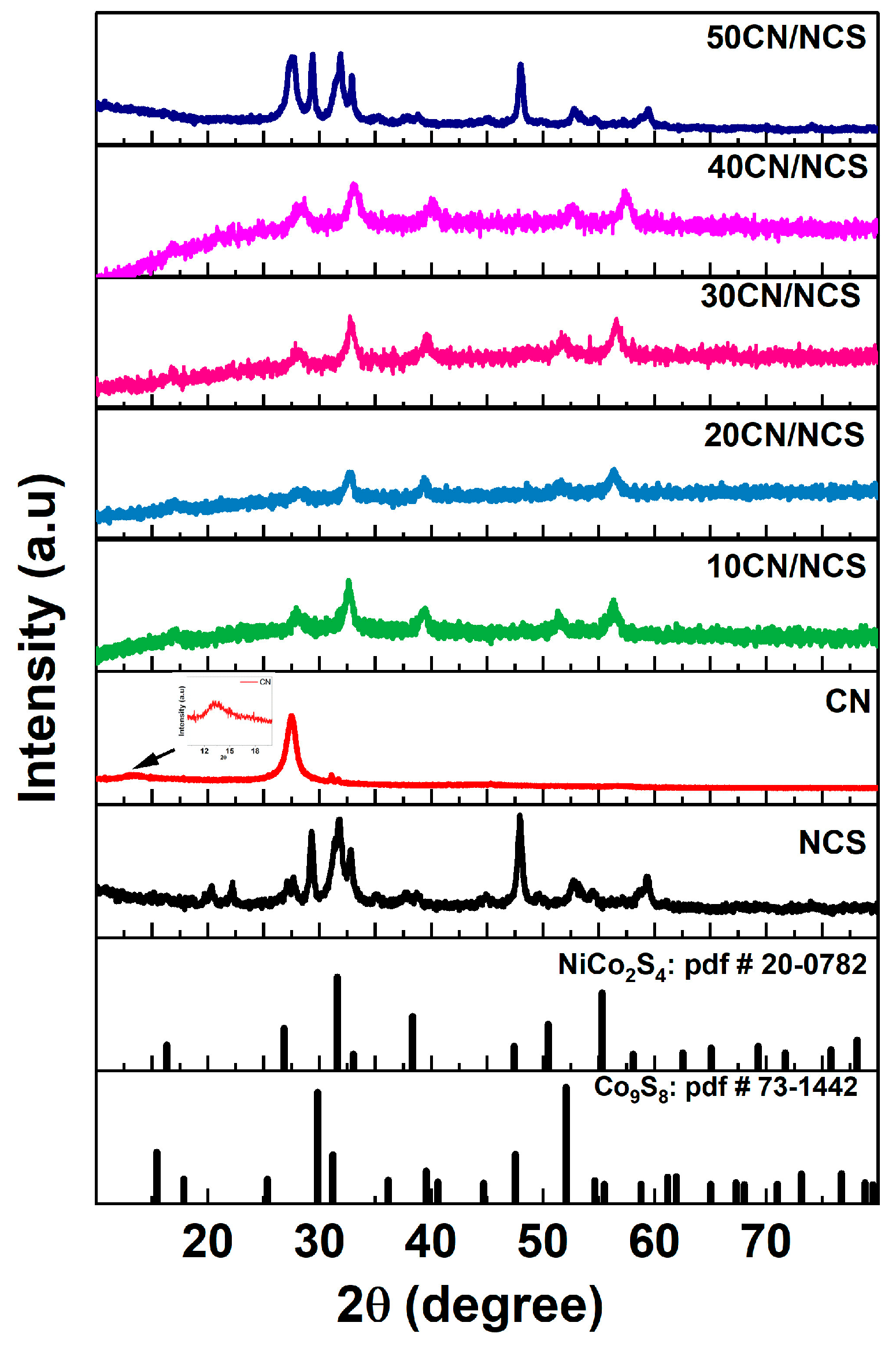
3.1. XRD Analysis
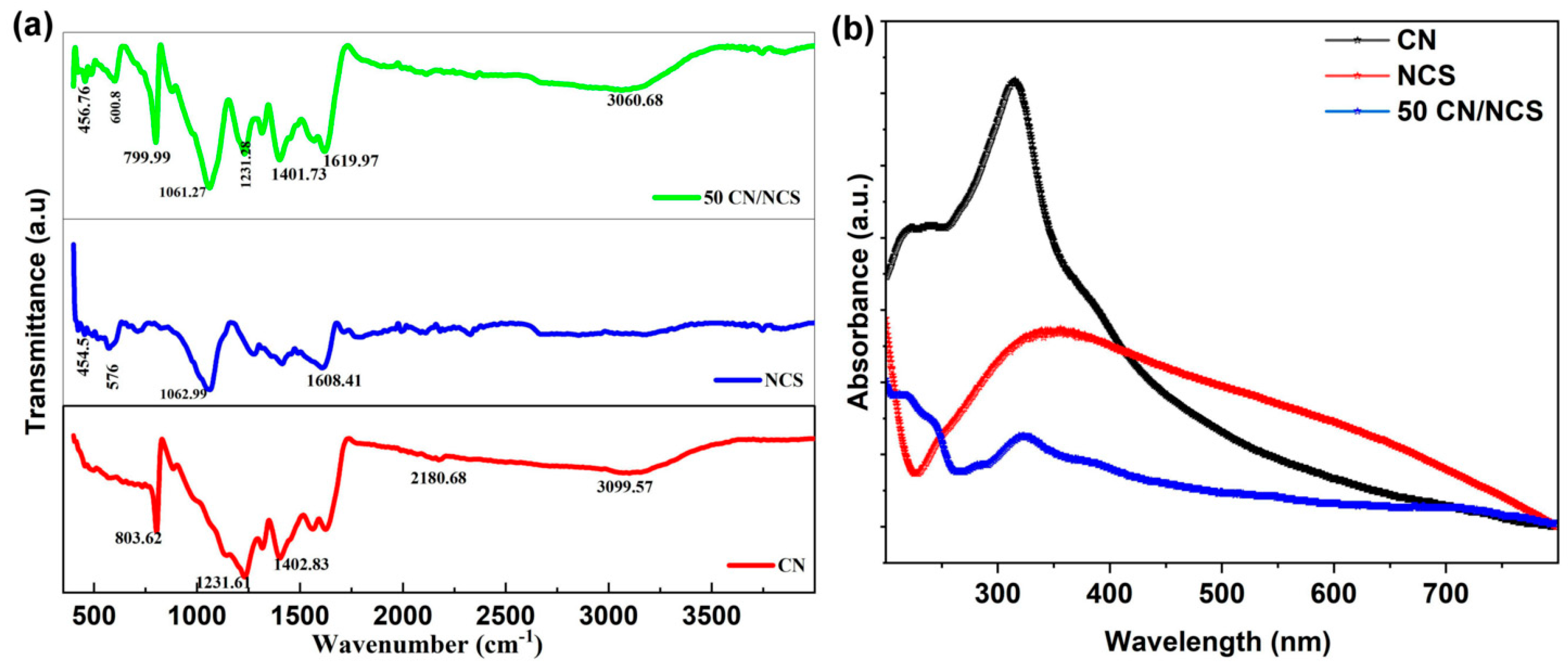
3.2. FTIR Analysis
3.3. UV–DRS Analysis
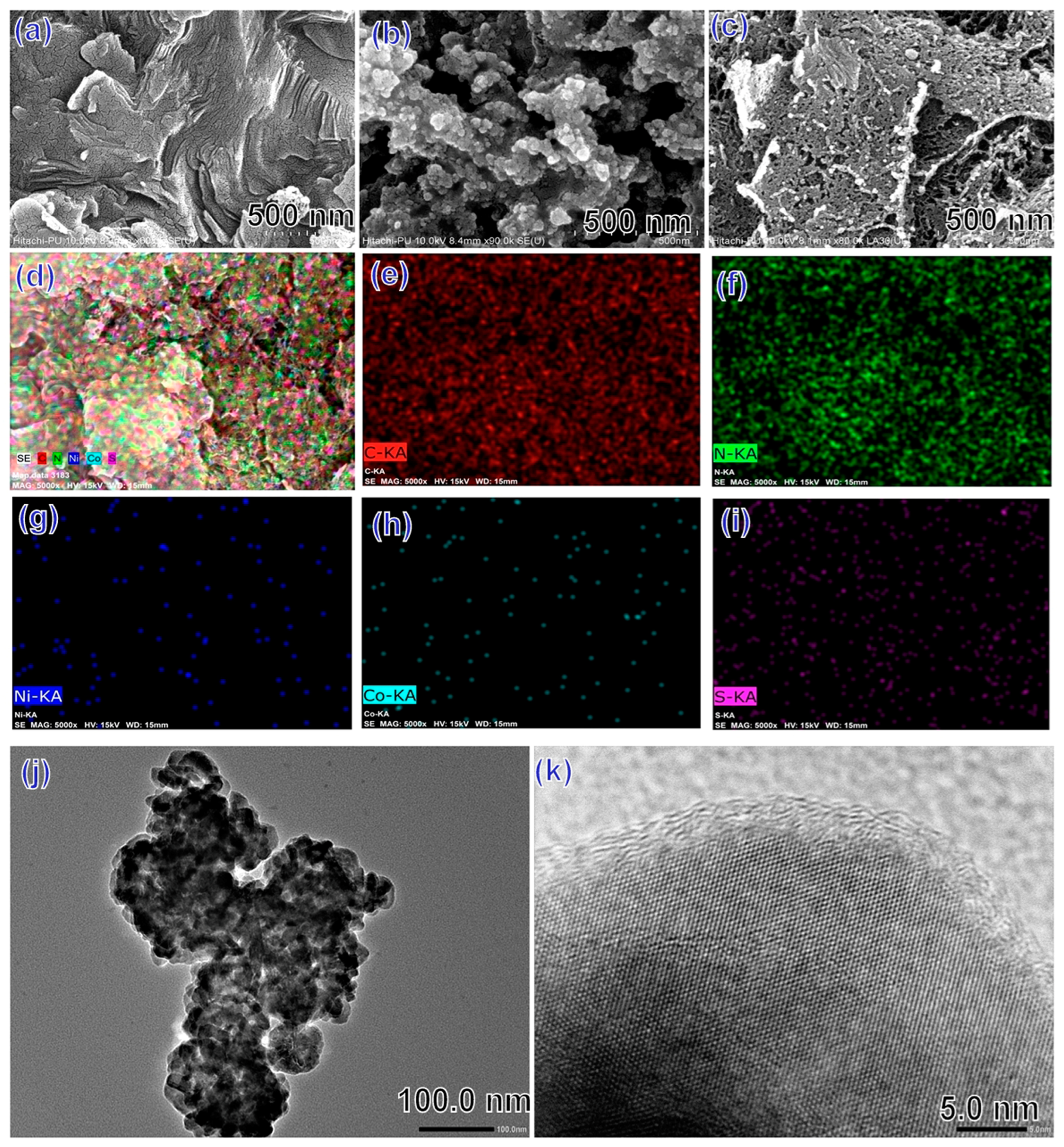
3.4. FESEM and TEM Analysis
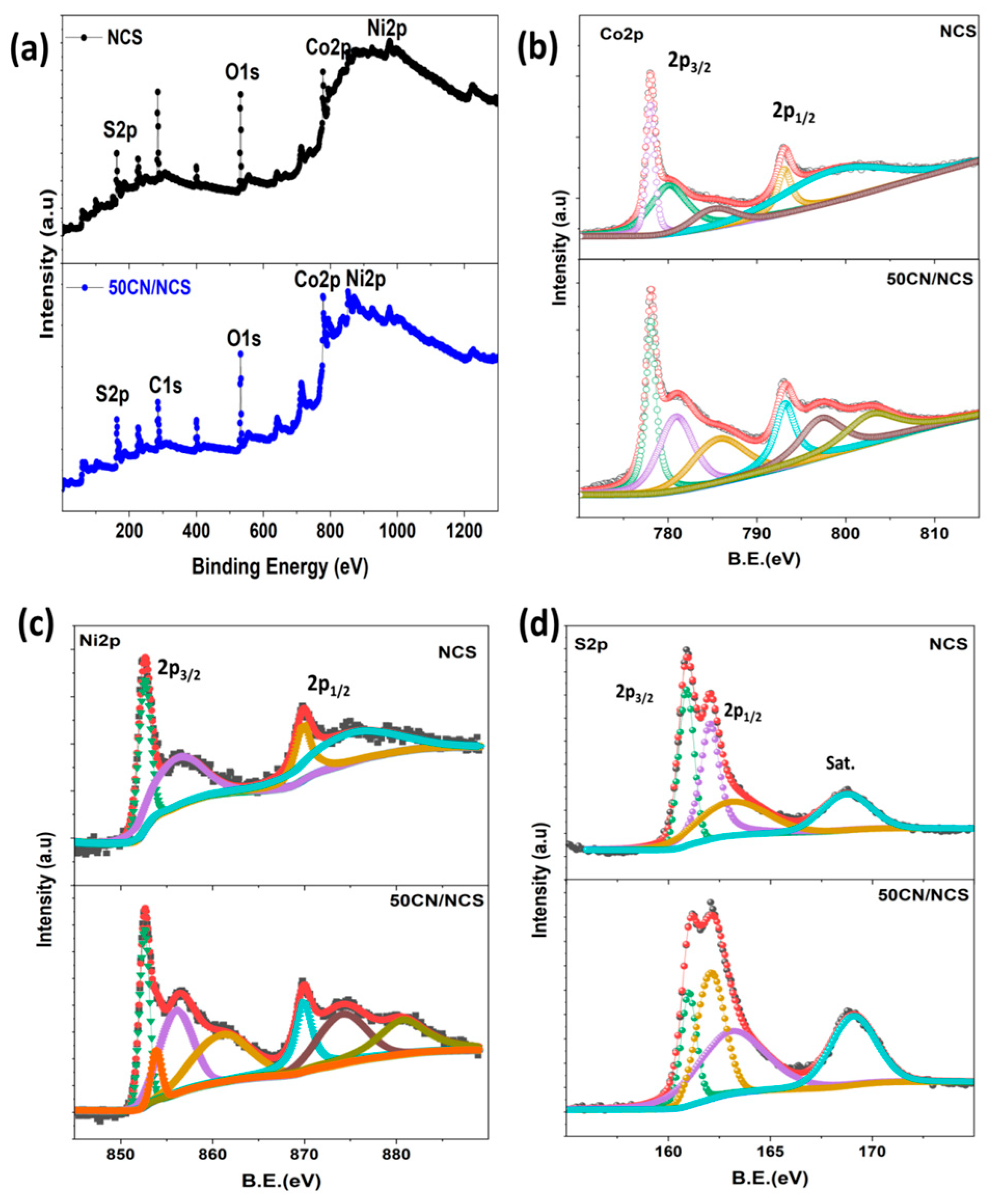
3.5. XPS Analysis
3.6. NOR Degradation Analysis
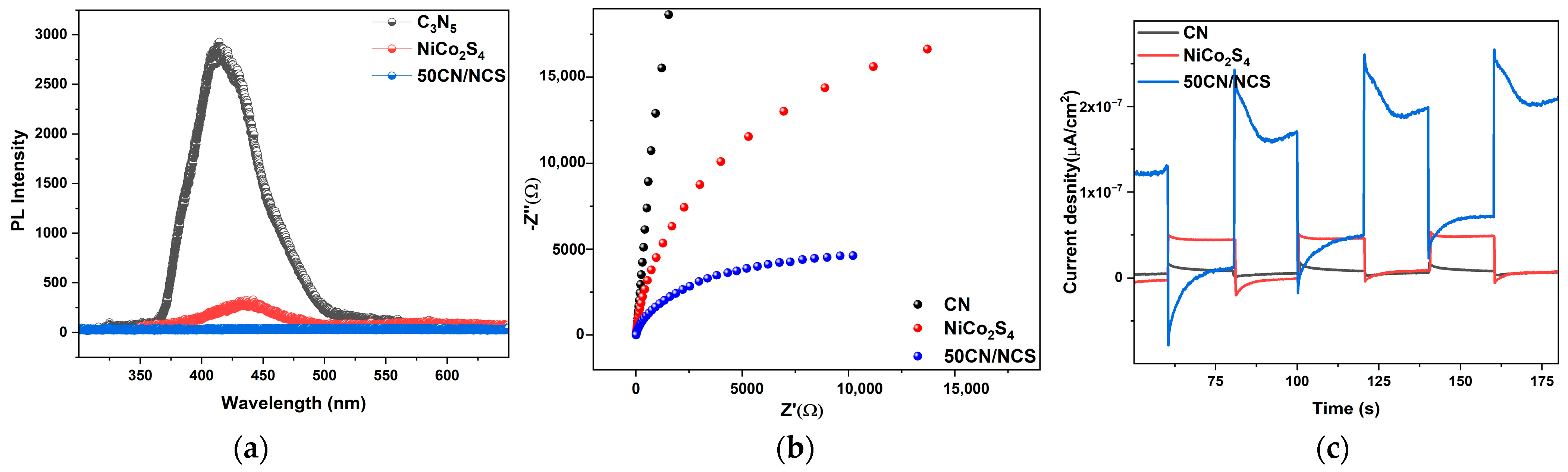
3.7. Photocatalytic Mechanism and Reusability Analysis
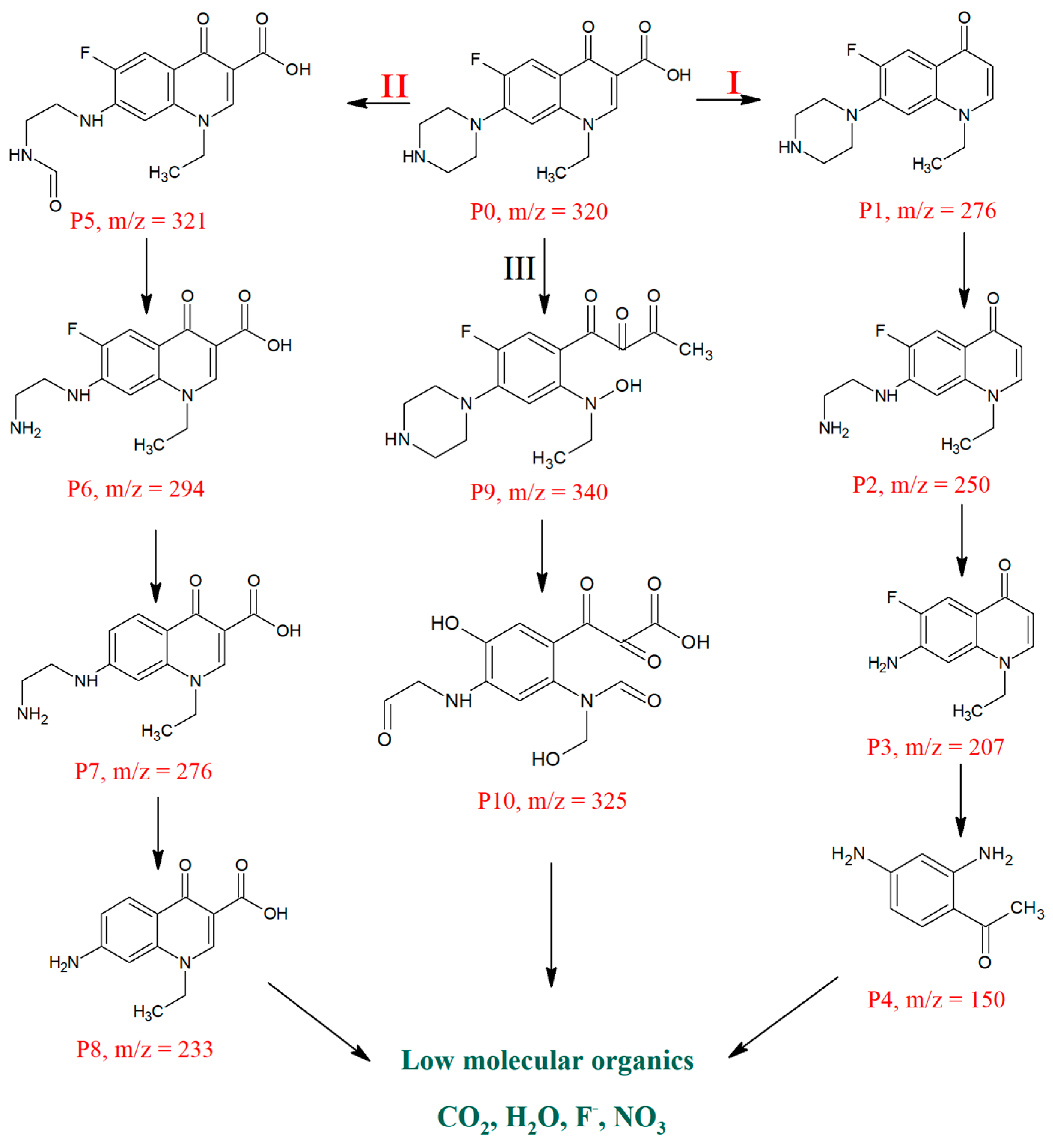
3.8. Degradation Pathway for Norfloxacin (NOR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Liu, Q.; Liu, W.; Lin, H. MoS2-based nanocomposites and aerogels for antibiotic pollutants removal from wastewater by photocatalytic degradation process: A review. Chemosphere 2024, 354, 141582. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Liu, W.; Zada, A.; Raziq, F.; Ali, S.; Shah, M.I.A.; Ateeq, M.; Khan, M.; Alei, D.; Fazil, P.; et al. Recent progress in emerging materials and hybrid nanocomposites for peroxymonosulfate and peroxydisulfate activation towards solar light-driven photocatalytic degradation of emerging pollutants. Coord. Chem. Rev. 2024, 499, 215466. [Google Scholar] [CrossRef]
- Mehrizad, A.; Gharbani, P. Removal of methylene blue from aqueous solution using nano-TiO2/UV process: Optimization by response surface methodology. Prog. Color Color. Coat. 2016, 9, 135–143. [Google Scholar]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Mehrizad, A.; Gharbani, P. Optimization of operational variables and kinetic modeling for photocatalytic removal of Direct Blue 14 from aqueous media by ZnS nanoparticles. J. Water Health 2017, 15, 955–965. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Wang, Y.; Jin, Z. Enhanced photocatalytic hydrogen evolution over coal-based carbon quantum dots modified CoMoO4/g-C3N4. Int. J. Hydrogen Energy 2024, 51, 16–30. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, L.; Hu, C. Chemical-bond conjugated BiO(OH)xI1-x-AgI heterojunction with high visible light activity and stability in degradation of pollutants. Appl. Catal. B Environ. 2017, 218, 443–451. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Lakhi, K.S.; Ilbeygi, H.; Ravon, U.; Al-Bahily, K.; Mori, T.; Park, D.H.; Vinu, A. Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2017, 56, 8481–8485. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kim, S.; Premkumar, S.; Yang, J.H.; Umapathy, S.; Vinu, A. Thermodynamically stable mesoporous C3N7 and C3N6 with ordered structure and their excellent performance for oxygen reduction reaction. Small 2020, 16, 1903572. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, D.; Li, B.; Guan, Y.; Wu, M.; Wu, J.; Guo, Y.; Sheng, L.; Liu, L.; Yao, T. An interfacial C-S bond bridged S-scheme ZnS/C3N5 for photocatalytic H2 evolution: Opposite internal-electric-field of ZnS/C3N4, increased field strength, and accelerated surface reaction. J. Colloid Interface Sci. 2024, 664, 960–971. [Google Scholar] [CrossRef]
- Jing, B.; Ao, Z.; Zhao, W.; Xu, Y.; Chen, Z.; An, T. Evaluation procedure of photocatalysts for VOCs degradation from the view of density functional theory calculations: gC3N4 dots/graphene as an example. J. Mater. Chem. A 2020, 8, 20363–20372. [Google Scholar] [CrossRef]
- Teymourinia, H.; Alshamsi, H.A.; Al-nayili, A.; Gholami, M. Photocatalytic degradation of chlorpyrifos using Ag nanoparticles-doped g-C3N5 decorated with dendritic CdS. Chemosphere 2023, 344, 140325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, Y.; Ma, X.; Liu, Y.; Zhang, L.; Wang, K.; Cao, S.; Xu, S. Constructing a Z-scheme heterojunction photocatalyst I-ABN-C3N4@CDs/BiOI by synergistically in situ modified g-C3N4 via carbon dots and copolymerization for BPA degradation. Mater. Res. Bull. 2024, 170, 112584. [Google Scholar] [CrossRef]
- Asoubar, S.; Mehrizad, A.; Behnajady, M.A.; Ramazani, M.E.; Gharbani, P. Hexavalent chromium reduction and Rhodamine B degradation by visible-light-driven photocatalyst of stannum indium sulfide-samarium vanadate. npj Clean Water 2023, 6, 27. [Google Scholar] [CrossRef]
- Cao, Y.; Guan, Y.; Zhang, Y.; Zhao, T.; Ding, W.; Hui, W.; Wu, J. Construction of P-C3N4/C3N5 isotypic heterojunction for effective degradation of organic pollutants. J. Taiwan Inst. Chem. Eng. 2023, 150, 105019. [Google Scholar] [CrossRef]
- He, S.; Liu, Y.; Wang, G.; Luo, L.; Tang, X.; Xiang, D.; Jiang, T.; Jing, J.; Wang, L. Heterojunction photocatalyst FeS2/g-C3N5 for activating sulfites to degrade tetracycline: A stable degradation system based on heterogeneous processes. Environ. Res. 2023, 237, 116939. [Google Scholar] [CrossRef]
- Xu, H.; Wang, D.; Ma, J.; Zhang, T.; Lu, X.; Chen, Z. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S. Appl. Catal. B Environ. 2018, 238, 557–567. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Wu, Z.; Liu, Y. NiCo2S4 hollow microsphere decorated by acetylene black for high-performance asymmetric supercapacitor. Electrochim. Acta 2015, 186, 562–571. [Google Scholar] [CrossRef]
- Li, J.; Wan, Y.; Li, Y.; Yao, G.; Lai, B. Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl. Catal. B Environ. 2019, 256, 117782. [Google Scholar] [CrossRef]
- Resende Leite, R.; Colombo, R.; Eduardo Bimbi, F., Jr.; Roberto de Vasconcelos Lanza, M.; da Silva Barud, H.; Ramos Moreira Afonso, C.; Inês Basso Bernardi, M. Precursor effect on the hydrothermal synthesis of pure ZnO nanostructures and enhanced photocatalytic performance for norfloxacin degradation. Chem. Eng. J. 2024, 496, 154374. [Google Scholar] [CrossRef]
- Liu, T.; Yang, G.; Wang, W.; Wang, C.; Wang, M.; Sun, X.; Xu, P.; Zhang, J. Preparation of C3N5 nanosheets with enhanced performance in photocatalytic methylene blue (MB) degradation and H2-evolution from water splitting. Environ. Res. 2020, 188, 109741. [Google Scholar] [CrossRef]
- Ito, K.; Noda, K. Highly efficient hydrogen production and selective CO2 reduction by the C3N5 photocatalyst using only visible light. Phys. Chem. Chem. Phys. 2024, 26, 153–160. [Google Scholar] [CrossRef]
- Golub, F.S.; Beloshapkin, S.; Gusel’nikov, A.V.; Bolotov, V.A.; Parmon, V.N.; Bulushev, D.A. Boosting Hydrogen Production from Formic Acid over Pd Catalysts by Deposition of N-Containing Precursors on the Carbon Support. Energies 2019, 12, 3885. [Google Scholar] [CrossRef]
- Chang, X.; Li, W.; Liu, Y.; He, M.; Zheng, X.; Bai, J.; Ren, Z. Hierarchical NiCo2S4@NiCoP core-shell nanocolumn arrays on nickel foam as a binder-free supercapacitor electrode with enhanced electrochemical performance. J. Colloid Interface Sci. 2019, 538, 34–44. [Google Scholar] [CrossRef]
- Wan, H.; Jiang, J.; Yu, J.; Xu, K.; Miao, L.; Zhang, L.; Chen, H.; Ruan, Y. NiCo2S4 porous nanotubes synthesis via sacrificial templates: High-performance electrode materials of supercapacitors. CrystEngComm 2013, 15, 7649–7651. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, B.; Tang, Z.; Ao, Z.; Xia, D.; Zhu, M.; Wang, S. Experimental and DFT insights into the visible-light driving metal-free C3N5 activated persulfate system for efficient water purification. Appl. Catal. B Environ. 2021, 289, 120023. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Liu, Y.; Dong, K.; Zhang, J. Designing oxygen vacancy mediated bismuth molybdate (Bi2MoO6)/N-rich carbon nitride (C3N5) S-scheme heterojunctions for boosted photocatalytic removal of tetracycline antibiotic and Cr(VI): Intermediate toxicity and mechanism insight. J. Colloid Interface Sci. 2022, 624, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; et al. C3N5: A Low Bandgap Semiconductor Containing an Azo-Linked Carbon Nitride Framework for Photocatalytic, Photovoltaic and Adsorbent Applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Y.; Zhou, Y.; Wang, K.; Yun, Y.; Chen, S.; Jiao, W.; Chen, L.; Zou, B.; Zhu, M. Dipole field in nitrogen-enriched carbon nitride with external forces to boost the artificial photosynthesis of hydrogen peroxide. Nat. Commun. 2023, 14, 5742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, G.; Hu, T.; He, Z.; Lu, Y.; Wang, G.; Guo, H.; Luo, J.; Lin, C.; Chen, Y. Synthesis of NiCo2S4-based nanostructured electrodes supported on nickel foams with superior electrochemical performance. J. Mater. Sci. 2016, 51, 1903–1913. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Fang, W.; Li, W.; He, X.; Zhang, F. Construction of hierarchical NiCo2S4 nanowires on 3D biomass carbon for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 9573–9581. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Zhang, M.; Yao, J.; Li, P.; Chen, S.; Liu, X. In situ synthesis of Ag3PO4/C3N5 Z-scheme heterojunctions with enhanced visible-light-responsive photocatalytic performance for antibiotics removal. Sci. Total Environ. 2021, 754, 141926. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shojaei, F.; Shahrokhi, M.; Azizi, M.; Rabczuk, T.; Shapeev, A.V.; Zhuang, X. Nanoporous C3N4, C3N5 and C3N6 nanosheets; novel strong semiconductors with low thermal conductivities and appealing optical/electronic properties. Carbon 2020, 167, 40–50. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Sharma, S.; Mishra, V.; Ghosh, T.K.; G, R.R. MoO3 thin layers on NiCo2S4 substrate for efficient electrochemical charge storage. Nanotechnology 2020, 31, 414003. [Google Scholar] [CrossRef]
- Li, C.; Liang, Y.; Lu, Z.; Xiang, X.; Ying, L.; Wang, H. NiCo2S4 decorated g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. J. Nanoparticle Res. 2020, 22, 41. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Chen, X.; Tao, H.; Zhou, Y.; Zhu, M. Upgraded charge transfer by an internal electric field in 2D/2D BiOCl/N-rich C3N5 heterojunctions for efficiently visible-light catalytic NO removal. Chem. Eng. J. 2023, 468, 143753. [Google Scholar] [CrossRef]
- Li, C.; Du, S.; Wang, H.; Naghadeh, S.B.; Allen, A.L.; Lin, X.; Li, G.; Liu, Y.; Xu, H.; He, C.; et al. Enhanced visible-light-driven photocatalytic hydrogen generation using NiCo2S4/CdS nanocomposites. Chem. Eng. J. 2019, 378, 122089. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Liu, Z.; Liu, X.; Xu, S.; Ma, Y. 2D NiCo2S4 decorated on ZnIn2S4 formed S-scheme heterojunction for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2023, 48, 3466–3477. [Google Scholar] [CrossRef]
- Wu, J.; Huang, X.; Xia, X. Exploring NiCo2S4 nanosheets arrays by hydrothermal conversion for enhanced high-rate batteries. J. Energy Chem. 2019, 35, 132–137. [Google Scholar] [CrossRef]
- Peng, J.; Xu, J.; Wang, Z.; Ding, Z.; Wang, S. Developing an efficient NiCo2S4 cocatalyst for improving the visible light H2 evolution performance of CdS nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 25919–25926. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Alshgari, R.A.; Kumar, A.; Sharma, G.; Naushad, M.; Alothman, Z.A. Magnetic Ni–Zn ferrite anchored on g-C3N4 as nano-photocatalyst for efficient photo-degradation of doxycycline from water. Environ. Res. 2023, 216, 114665. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; Farhat, L.B.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 heterojunction: An effective photocatalyst for photodegradation of rhodamine B dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Alsunbuli, M.M.; Sabit, D.A. Developing a magnetic bismuth-based quaternary semiconductor boosted by plasmonic action for photocatalytic detoxification of Cr(VI) and norfloxacin antibiotic under simulated solar irradiation: Synergistic work and radical mechanism. J. Alloys Compd. 2023, 958, 170521. [Google Scholar] [CrossRef]
- Ao, X.; Wang, W.; Sun, W.; Lu, Z.; Li, C. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: An investigation of the role of pH. Water Res. 2021, 203, 117458. [Google Scholar] [CrossRef]
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef]
- Dhiman, P.; Sharma, J.; Kumar, A.; Sharma, G.; Dawi, E.A. Photocatalytic degradation of malachite green by waste derived bio-char impregnated Bi5O7Br/Fe3O4 magnetic composite. Opt. Mater. 2023, 146, 114568. [Google Scholar] [CrossRef]
- Lan, Y.; Luo, Y.; Yu, S.; Ye, H.; Zhang, Y.; Xue, M.; Sun, Q.; Yin, Z.; Li, X.; Xie, C.; et al. Cornstalk hydrochar produced by phosphoric acid-assisted hydrothermal carbonization for effective adsorption and photodegradation of norfloxacin. Sep. Purif. Technol. 2024, 330, 125543. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Dhiman, P. Incorporating Bi nanodots into CuBi2O4/CuFe2O4 heterojunction for a wide spectral synchronous photoreduction of hexavalent chromium and degradation of imidacloprid. Opt. Mater. 2023, 140, 113876. [Google Scholar] [CrossRef]
- Dhiman, P.; Mehta, T.; Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Mola, G.T. Mg0.5NixZn0.5-xFe2O4 spinel as a sustainable magnetic nano-photocatalyst with dopant driven band shifting and reduced recombination for visible and solar degradation of Reactive Blue-19. Adv. Powder Technol. 2020, 31, 4585–4597. [Google Scholar] [CrossRef]
- Le, M.-V.; Nguyen, Q.-T.-N.; Huynh, N.-D.-T.; Pham, N.-D.; Truong, C.-H.; Tran, H.-T.; Le, P.-N.-M.; Ngo, T.-H.; Huynh, H.T. Direct Z-scheme CoTiO3/g-C3N4 nanoparticles: Fabrication and application as a photocatalyst for degradation of tetracycline hydrochloride assisted by peroxydisulfate or peroxymonosulfate under simulated sunlight. Nanotechnol. Environ. Eng. 2023, 8, 675–690. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, L.; Ban, Y.; Li, H. Hydrothermal synthesis of Step-scheme layer g-C3N4/columnar ZnZrO3 heterojunction photocatalyst toward efficient norfloxacin degradation under solar light. J. Solid State Chem. 2024, 337, 124815. [Google Scholar] [CrossRef]
- Lee, E.; Jagan, G.; Choi, J.U.; Cha, B.; Yoon, Y.; Saravanakumar, K.; Park, C.M. Peroxymonosulfate-activated photocatalytic degradation of norfloxacin via a dual Z-scheme g-C3N5/BiVO4/CoFe−LDH heterojunction: Operation and mechanistic insights. Chem. Eng. J. 2024, 494, 152961. [Google Scholar] [CrossRef]
- Tran, T.-H.; Le, P.-N.-M.; Ngo, T.-H.; Huynh, N.-D.-T.; Oh, W.-C.; Le, M.-V. An investigation on the visible light-driven Z-scheme BiVO4/g-C3N4 heterostructures: Performance, evaluation, and mechanism for dye and antibiotics degradation. Mater. Today Commun. 2024, 40, 109373. [Google Scholar] [CrossRef]
- Guan, Y.; Cao, Y.; Ma, S.; Yang, Y.; Zhao, T.; Zhang, Y.; Xin, B.; Wu, J.; Guo, Y. Metal-free g-C3N5 photocatalyst coupling MXenes Ti3C2 for tetracycline degradation: Insight for electron transfer mechanism, degradation mechanism and photothermal effect. J. Alloys Compd. 2023, 951, 169864. [Google Scholar] [CrossRef]
- Swedha, M.; Okla, M.K.; Al-amri, S.S.; Alaraidh, I.A.; Al-ghamdi, A.A.; Mohebaldin, A.; Abdel-Maksoud, M.A.; Aufy, M.; Studenik, C.R.; Thomas, A.M.; et al. Green synthesis of two-electron centre based ZnO/NiCo2S4 QDs-OVs using Punica granatum fruit peel extract for an exceptional visible light photocatalytic degradation of doxycycline and ciprofloxacin. Chemosphere 2022, 304, 135225. [Google Scholar] [CrossRef]
- Li, J.; Xia, Z.; Ma, D.; Liu, G.; Song, N.; Xiang, D.; Xin, Y.; Zhang, G.; Chen, Q. Improving photocatalytic activity by construction of immobilized Z-scheme CdS/Au/TiO2 nanobelt photocatalyst for eliminating norfloxacin from water. J. Colloid Interface Sci. 2021, 586, 243–256. [Google Scholar] [CrossRef]
- Gou, J.; Ma, Q.; Deng, X.; Cui, Y.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017, 308, 818–826. [Google Scholar] [CrossRef]
- Chen, Q.; Hao, Y.; Song, Z.; Liu, M.; Chen, D.; Zhu, B.; Chen, J.; Chen, Z. Optimization of photocatalytic degradation conditions and toxicity assessment of norfloxacin under visible light by new lamellar structure magnetic ZnO/g-C3N4. Ecotoxicol. Environ. Saf. 2021, 225, 112742. [Google Scholar] [CrossRef]
- Guo, J.; Shen, C.H.; Sun, J.; Xu, X.J.; Li, X.Y.; Fei, Z.H.; Liu, Z.T.; Wen, X.J. Highly efficient activation of peroxymonosulfate by Co3O4/Bi2MoO6 pn heterostructure composites for the degradation of norfloxacin under visible light irradiation. Sep. Purif. Technol. 2021, 259, 118109. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, M.; Xu, Z.; Xiong, W.; Yang, Z.; Cao, J.; Peng, H.; Xu, H.; Xiang, Y.; Jing, Y. Constructing 2D/2D N-ZnO/g-C3N4 S-scheme heterojunction: Efficient photocatalytic performance for norfloxacin degradation. Chem. Eng. J. 2022, 430, 132652. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, L.; Bai, M.; Ji, Z.; Li, J.; Huang, X. Oh mineralization of norfloxacin in the process of algae bloom water treatment in a drinking water treatment system Of 12,000 M3 Per Day. Chem. Eng. J. 2019, 360, 1355–1362. [Google Scholar] [CrossRef]
- Wang, T.; Shi, H.; Kumar, A.; Zhang, D.; Wang, H.; Wang, S.; Zheng, J. Efficient visible-light photocatalysis of chloramphenicol using novel engineered biochar-based Ti-doped Bi2WO6 composite: Mechanisms, degradation pathways, and applications. Sep. Purif. Technol. 2024, 332, 125780. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: Effects, mechanisms, actual wastewater treatment and disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef]





| Photocatalyst | Source of Illumination | Pollutant/Concentration | Catalyst Dose | Irradiation Time | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| CoTiO3/g-C3N4 | Low-power light source (26 W) | Tetracycline hydrochloride/10 ppm | 200 mg | 60 min | 79.6% | [54] |
| g–C3N4–ZnZrO3 | 500 W Xe lamp/400 nm optical filter | Norfloxacin | 20 mg | 180 min | 96% | [55] |
| g-C3N5/BiVO4/CoFe–LDH | Xenon lamp 300 W Xe lamp/400 nm optical filter | Norfloxacin/10 ppm | 600 mg + PMS | 120 min | 95.3% | [56] |
| BiVO4/g-C3N4 | 26 W Exo Terra Natural Light | Ciprofloxacin/10 ppm | 100 mg | 60 min | 78.2% | [57] |
| g-C3N5/Ti3C2 | 300 W Xe lap/400 nm optical filter | Tetracycline/10 ppm | 30 mg | 60 min | 81.6% | [58] |
| ZnO/NiCo2S4 | 1000 W halogen lamp/visible light | Doxycycline/40 ppm | 20 mgL−1 | 150 min | 99% | [59] |
| C3N5/NiCo2S4 (50CN/NCS) | 500 W Xe lamp/400 nm optical filter | Norfloxacin/20 ppm | 20 mg | 120 min | 86.5% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Kumar, A.; Wang, T.; Dhiman, P.; Sharma, G.; Shi, H. Incorporating C3N5 and NiCo2S4 to Form a Novel Z-Scheme Heterojunction for Superior Photocatalytic Degradation of Norfloxacin. Chemistry 2024, 6, 962-980. https://doi.org/10.3390/chemistry6050056
Rana S, Kumar A, Wang T, Dhiman P, Sharma G, Shi H. Incorporating C3N5 and NiCo2S4 to Form a Novel Z-Scheme Heterojunction for Superior Photocatalytic Degradation of Norfloxacin. Chemistry. 2024; 6(5):962-980. https://doi.org/10.3390/chemistry6050056
Chicago/Turabian StyleRana, Sahil, Amit Kumar, Tongtong Wang, Pooja Dhiman, Gaurav Sharma, and Hui Shi. 2024. "Incorporating C3N5 and NiCo2S4 to Form a Novel Z-Scheme Heterojunction for Superior Photocatalytic Degradation of Norfloxacin" Chemistry 6, no. 5: 962-980. https://doi.org/10.3390/chemistry6050056











