Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl: 2,2′;6′,2″-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [CoII(pytpy)2][BF4]2 (2)
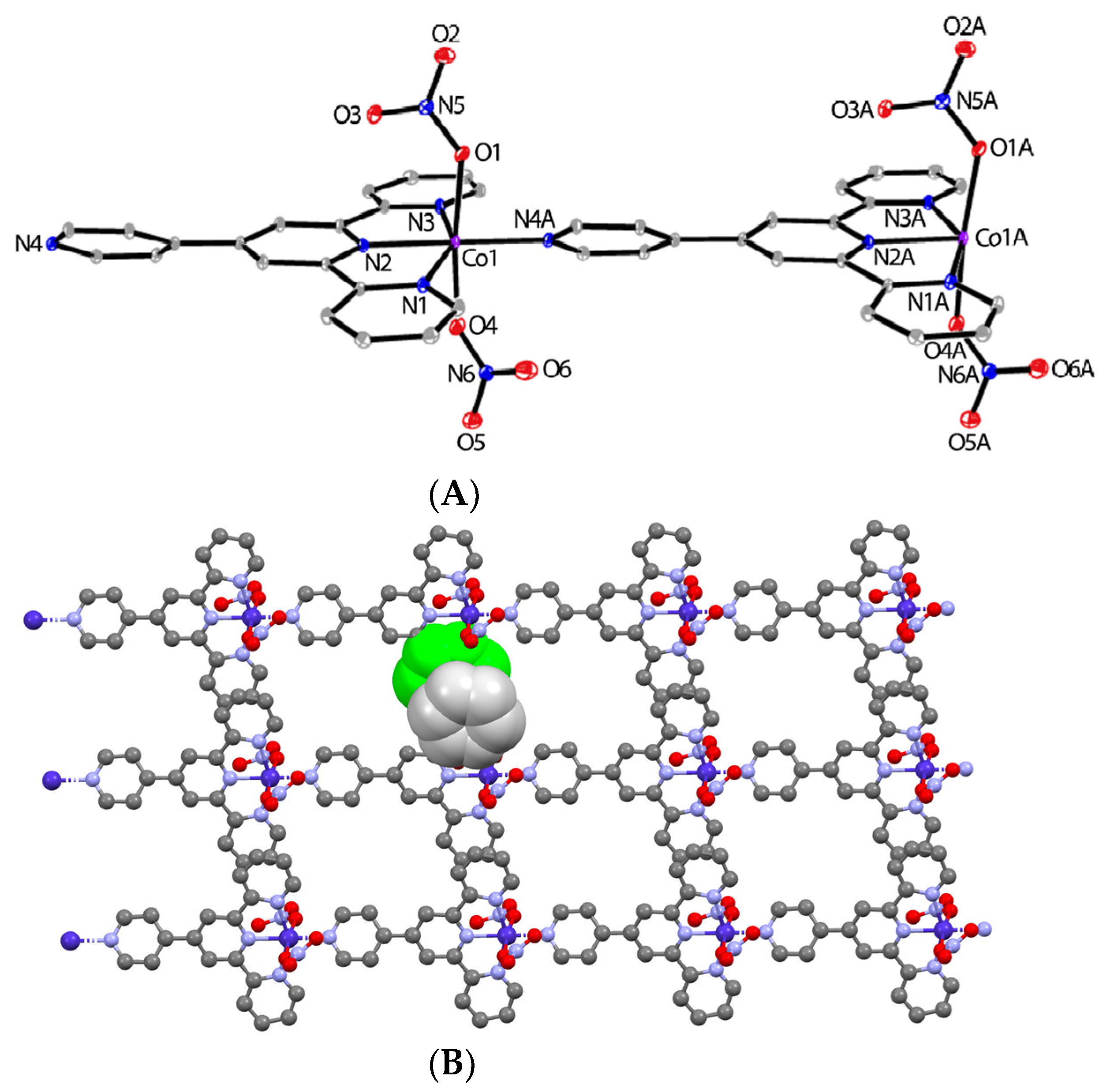
2.3. Synthesis of [CoII(pytpy)(NO3)2]n (5)
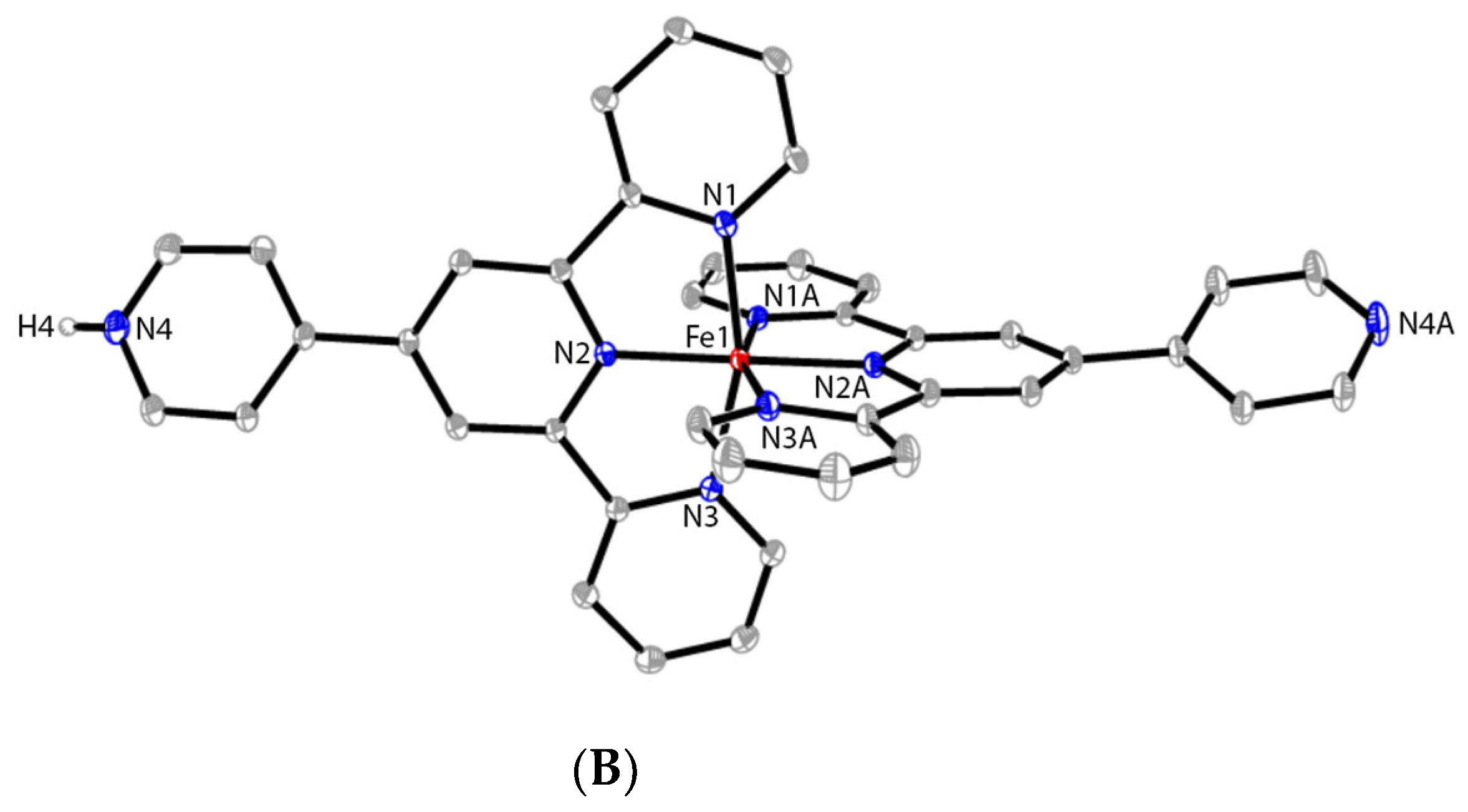
2.4. Synthesis of [FeII(pytpy)(H-pytpy)][BF4]3 (6)
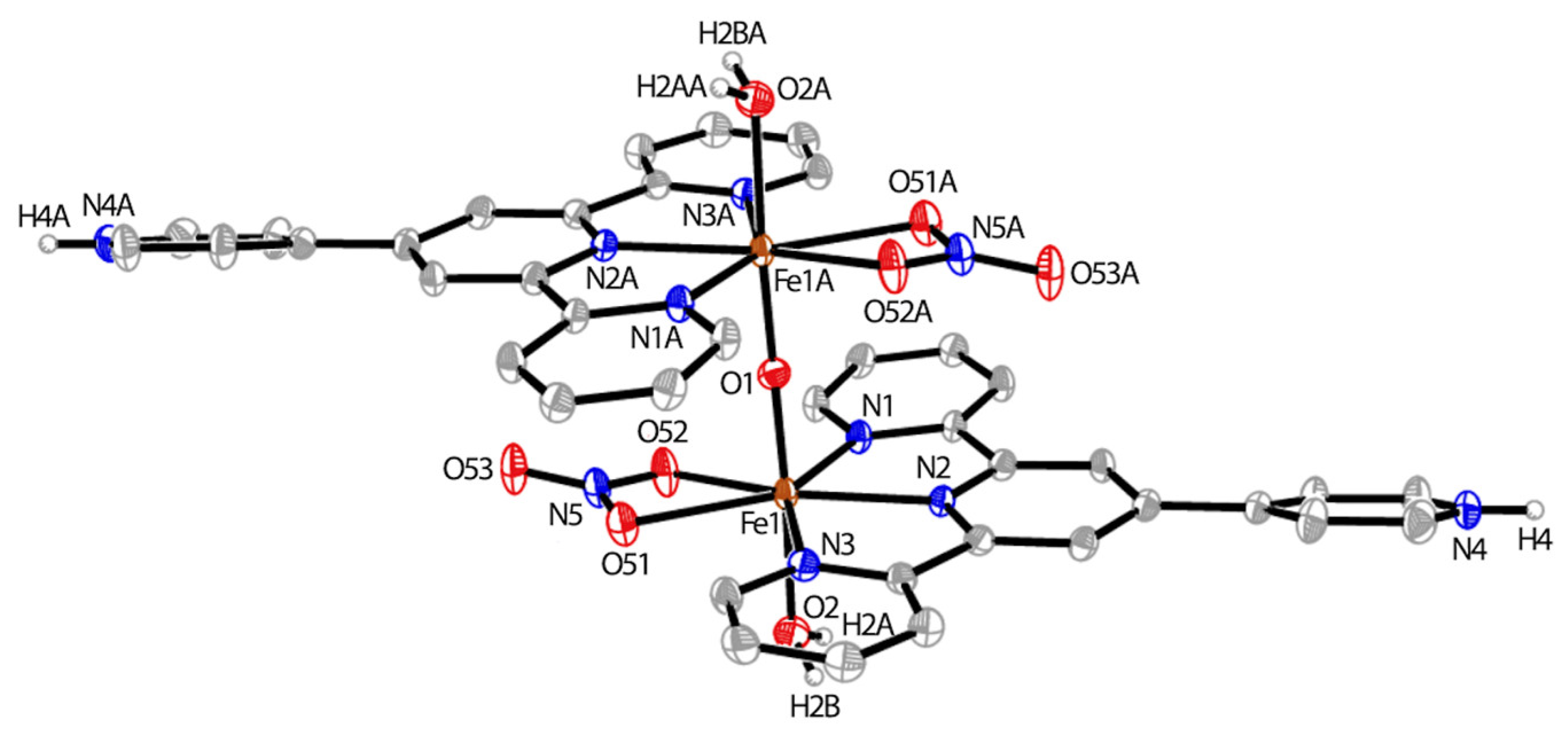
2.5. Synthesis of {[FeIII(H-pytpy)(OH2)(ONO2)]2(O)}[NO3]4 (8)
2.6. General Procedure for Catalytic Hydrosilylation of Styrene
2.7. Cytotoxicity Measurement
2.8. UV-Vis Absorption Measurement
2.9. X-ray Crystallography
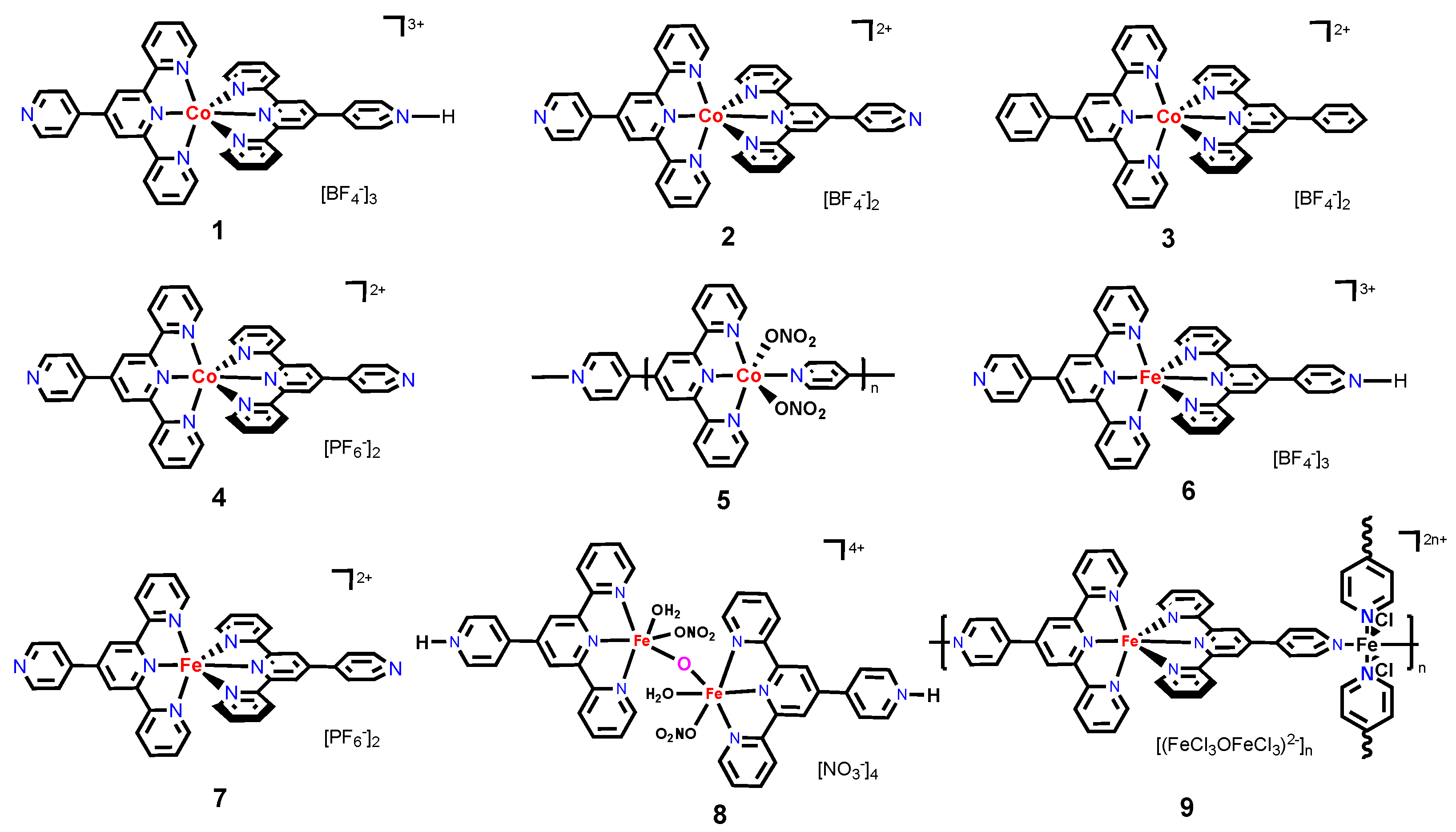
3. Results
3.1. Synthesis and Crystal Structures
3.2. Catalytic Properties
3.3. In Vitro Anticancer Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Momeni, B.Z.; Davarzani, N.; Janczak, J.; Ma, N.; Abd-El-Aziz, A.S. Progress in design and applications of supramolecular assembly of 2,2′:6′,2″-terpyridine-based first row d-block elements. Coord. Chem. Rev. 2024, 506, 215619. [Google Scholar] [CrossRef]
- Winter, A.; Newkome, G.R.; Schubert, U.S. The chemistry of the s-and p-block elements with 2,2′:6′,2″-terpyridine ligands. Inorg. Chem. Front. 2024, 11, 342–399. [Google Scholar] [CrossRef]
- Panicker, R.R.; Sivaramakrishna, A. Remarkably flexible 2,2′:6′,2″-terpyridines and their group 8–10 transition metal complexes–Chemistry and applications. Coord. Chem. Rev. 2022, 459, 214426. [Google Scholar] [CrossRef]
- Shi, J.; Wang, M. Self-Assembly Methods for Recently Reported Discrete Supramolecular Structures Based on Terpyridine. Chem.–Asian J. 2021, 16, 4037–4048. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Metal-Terpyridine Complexes in Catalytic Application—A Spotlight on the Last Decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar] [CrossRef]
- Abhijnakrishna, R.; Magesh, K.; Ayushi, A.; Velmathi, S. Advances in the biological studies of metal-terpyridine complexes: An overview from 2012 to 2022. Coord. Chem. Rev. 2023, 496, 215380. [Google Scholar] [CrossRef]
- Musiol, R.; Malecki, P.; Pacholczyk, M.; Mularski, J. Terpyridines as promising antitumor agents: An overview of their discovery and development. Exp. Opin. Drug Disc. 2022, 17, 259–271. [Google Scholar] [CrossRef]
- Elahi, S.M.; Raizada, M.; Sahu, P.K.; Konar, S. Terpyridine-Based 3D Metal–Organic-Frameworks: A Structure–Property Correlation. Chem. Eur. J. 2021, 27, 5858–5870. [Google Scholar] [CrossRef]
- Kainat, S.F.; Hawsawi, M.B.; Mughal, E.U.; Naeem, N.; Almohyawi, A.M.; Altass, H.M.; Hussein, E.M.; Sadiq, A.; Moussa, Z.; Abd-El-Aziz, A.S.; et al. Recent developments in the synthesis and applications of terpyridine-based metal complexes: A systematic review. RSC adv. 2024, 14, 21464–21537. [Google Scholar] [CrossRef]
- Attwood, M.; Turner, S.S. Back to back 2, 6-bis (pyrazol-1-yl) pyridine and 2,2′:6′,2″-terpyridine ligands: Untapped potential for spin crossover research and beyond. Coord. Chem. Rev. 2017, 353, 247–277. [Google Scholar] [CrossRef]
- Dickenson, J.C.; Haley, M.E.; Hyde, J.T.; Reid, Z.M.; Tarring, T.J.; Iovan, D.A.; Harrison, D.P. Fine-tuning metal and ligand-centered redox potentials of homoleptic bis-terpyridine complexes with 4′-aryl substituents. Inorg. Chem. 2021, 60, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Beves, J.E.; Bray, D.J.; Clegg, J.K.; Constable, E.C.; Housecroft, C.E.; Jolliffe, K.A.; Kepert, C.J.; Lindoy, L.F.; Neuburger, M.; Price, D.J.; et al. Expanding the 4, 4′-bipyridine ligand: Structural variation in {M(pytpy)2}2+ complexes (pytpy= 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine, M = Fe, Ni, Ru) and assembly of the hydrogen-bonded, one-dimensional polymer {[Ru(pytpy)(Hpytpy)]}n3n+. Inorg. Chim. Acta 2008, 361, 2582–2590. [Google Scholar] [CrossRef]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Neuburger, M.; Price, D.J.; Schaffner, S. The conjugate acid of bis{4′-(4-pyridyl)-2,2′:6′,2″-terpyridine} iron(II) as a self-complementary hydrogen-bonded building block. CrystEngComm 2007, 9, 1073–1077. [Google Scholar] [CrossRef]
- Beves, J.E.; Dunphy, E.L.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Neuburger, M.; Price, D.J.; Schaffner, S. Vectorial property dependence in bis {4′-(n-pyridyl)-2,2′:6′,2″-terpyridine} iron(II) and ruthenium(II) complexes with n = 2, 3 and 4. Dalton Trans. 2008, 3, 386–396. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.S.; Cheng, J.; Zheng, S.; Golen, J.A.; Manke, D.R.; Zhang, G. Cobalt (II) coordination polymer as a precatalyst for selective hydroboration of aldehydes, ketones, and imines. J. Org. Chem. 2018, 83, 9442–9448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, S.; Wu, J.; Zeng, H.; Mo, Z.; Davis, K.; Zheng, S. Highly efficient and selective hydroboration of terminal and internal alkynes catalysed by a cobalt (II) coordination polymer. Org. Chem. Front. 2019, 6, 3228–3233. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, S.; Neary, M.C. An ionic Fe-based metal–organic-framework with 4′-pyridyl-2,2′:6′,2″-terpyridine for catalytic hydroboration of alkynes. RSC Adv. 2023, 13, 2225–2232. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, J.; Li, S.; Cass, S.; Zheng, S. Markovnikov-Selective Hydroboration of Vinylarenes Catalyzed by a Cobalt(II) Coordination Polymer. Org. Lett. 2018, 20, 7893–7897. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Li, S.; Johnson, J.; Mo, Z.; Neary, M.C.; Zheng, S. 1-D manganese (II)-terpyridine coordination polymers as precatalysts for hydrofunctionalisation of carbonyl compounds. Dalton Trans. 2020, 49, 2610–2615. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Zadori, N.; Marino, C.; Zheng, S.; Neary, M.C. Homoleptic octahedral CoII complexes as precatalysts for regioselective hydroboration of alkenes with high turnover frequencies. RSC Adv. 2023, 13, 28089–28096. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Concepción Gimeno, M. The Therapeutic Potential in Cancer of Terpyridine-Based Metal Complexes Featuring Group 11 Elements. ChemMedChem 2024, 19, e202300645. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Gottschaldt, M.R.; Newkome, G.; Schubert, U.S. Terpyridines and their complexes with first row transition metal ions: Cytotoxicity, nuclease activity and self-assembly of biomacromolecules. Curr. Top. Med. Chem. 2012, 12, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Z.; Ge, Q.; Zheng, X.; Yang, Z. Antitumor activity of tridentate pincer and related metal complexes. Org. Biomol. Chem. 2021, 19, 5254–5273. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Combining inorganic chemistry and biology: The underestimated potential of metal complexes in medicine. ChemBioChem 2020, 21, 3044–3046. [Google Scholar] [CrossRef]
- Gasser, G. Metal complexes and medicine: A successful combination. Chimia 2015, 69, 442. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Karges, J.; Yempala, T.; Tharaud, M.; Gibson, D.; Gasser, G. A multi-action and multi-target RuII–PtIV conjugate combining cancer-activated chemotherapy and photodynamic therapy to overcome drug resistant cancers. Angew. Chem. Intern. Ed. 2020, 59, 7069–7075. [Google Scholar] [CrossRef]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A chemical perspective on the clinical use of platinum-based anticancer drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Gourdon, L.; Cariou, K.; Gasser, G. Phototherapeutic anticancer strategies with first-row transition metal complexes: A critical review. Chem. Soc. Rev. 2022, 51, 1167–1195. [Google Scholar] [CrossRef]
- Sen, S.; Won, M.; Levine, M.S.; Noh, Y.; Sedgwick, A.C.; Kim, J.S.; Sessler, J.L.; Arambula, J.F. Metal-based anticancer agents as immunogenic cell death inducers: The past, present, and future. Chem. Soc. Rev. 2022, 51, 1212–1233. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Kar, K.; Ghosh, D.; Kabi, B.; Chandra, A. A concise review on cobalt Schiff base complexes as anticancer agents. Polyhedron 2022, 222, 115890. [Google Scholar] [CrossRef]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef]
- Szlasa, W.; Gachowska, M.; Kiszka, K.; Rakoczy, K.; Kiełbik, A.; Wala, K.; Puchała, J.; Chorążykiewicz, K.; Saczko, J.; Kulbacka, J. Iron chelates in the anticancer therapy. Chem. Papers 2022, 76, 1285–1294. [Google Scholar] [CrossRef]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Basu, U.; Roy, M.; Chakravarty, A.R. Recent advances in the chemistry of iron-based chemotherapeutic agents. Coord. Chem. Rev. 2020, 417, 213339. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Twinabs; University of Göttingen: Göttingen, Germany, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXTL, an Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data; University of Göttingen: Göttingen, Germany, 1981. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.L.; Wei, R.J.; Zheng, L.S.; Tao, J. Spin transition and structural transformation in a mononuclear cobalt(II) complex. Inorg. Chem. 2015, 54, 7670–7672. [Google Scholar] [CrossRef]
- Bage, A.D.; Nicholson, K.; Hunt, T.A.; Langer, T.; Thomas, S.P. The hidden role of boranes and borohydrides in hydroboration catalysis. ACS Catal. 2020, 10, 13479–13486. [Google Scholar] [CrossRef]
- Gattuso, H.; Duchanois, T.; Besancenot, V.; Barbieux, C.; Assfeld, X.; Becuwe, P.; Gros, P.C.; Grandemange, S.; Monari, A. Interaction of Iron II Complexes with B-DNA. Insights from Molecular Modeling, Spectroscopy, and Cellular Biology. Front. Chem. 2015, 3, 67. [Google Scholar] [PubMed]

 | ||
| Entry | Precatalyst | Yield of 10/% b |
|---|---|---|
| 1 | 1 | 75 |
| 2 | 2 | 81 |
| 3 | 3 | 95 |
| 4 | 4 | 45 |
| 5 | 5 | 99 |
| 6 | 6 | <5 |
| 7 | 7 | <5 |
| 8 | 8 | <5 |
| 9 | 9 | <5 |
| 10 | [Co(pytpy)Cl2]n | 99 |
| Compound | MCF-7 | MDA-MB 468 | MCF-10A |
|---|---|---|---|
| 1 | 0.107 | 0.189 | 0.410 |
| 2 | 0.335 | 0.024 | 0.113 |
| 3 | 1.33 | 0.367 | 0.120 |
| 4 | 0.185 | 0.015 | 0.080 |
| 5 | 12.0 | 0.600 | 0.600 |
| 6 | 13.0 | 0.700 | 0.648 |
| 7 | 0.390 | 0.0001 | 0.004 |
| 8 | 169 | 2.90 | 0.287 |
| 9 | 0.698 | 2.56 | 20.57 |
| cisplatin | 11.46 | 0.309 | 17.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-Y.; Zhang, Q.; Tang, Q.; Neary, M.C.; Zheng, S. Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl: 2,2′;6′,2″-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities. Chemistry 2024, 6, 1099-1110. https://doi.org/10.3390/chemistry6050064
Cheng S-Y, Zhang Q, Tang Q, Neary MC, Zheng S. Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl: 2,2′;6′,2″-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities. Chemistry. 2024; 6(5):1099-1110. https://doi.org/10.3390/chemistry6050064
Chicago/Turabian StyleCheng, Shu-Yuan, Qinguo Zhang, Quan Tang, Michelle C. Neary, and Shengping Zheng. 2024. "Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl: 2,2′;6′,2″-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities" Chemistry 6, no. 5: 1099-1110. https://doi.org/10.3390/chemistry6050064







