Coordination Modes of Ortho-Substituted Benzoates Towards Divalent Copper Centres in the Presence of Diimines
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Measurements
2.2. X-Ray Crystallographic Details
2.3. Synthesis of the Complexes
3. Results and Discussion
3.1. Structure Elucidation
3.2. X-Ray-Structures
3.3. Computational Verification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, K.; Mathivathanan, L.; Herchel, R.; Boudalis, A.K.; Raptis, R.G. Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry 2020, 2, 626–644. [Google Scholar] [CrossRef]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating the Conformation of 3,2′:6′,3″-Terpyridine in [Cu2(μ-OAc)4(3,2′:6′,3″-tpy)]n 1D-Polymers. Chemistry 2021, 3, 182–198. [Google Scholar] [CrossRef]
- Antenucci, A.; Dughera, S. C-N, C-O and C-S Ullmann-Type Coupling Reactions of Arenediazoniumo-Benzenedisulfonimides. Reactions 2022, 3, 300–311. [Google Scholar] [CrossRef]
- Shaban, S.Y.; Ramadan, A.E.M.A.; Ibrahim, M.M.; Elshami, F.I.; Eldik, R. Square planar versus square pyramidal copper(II) complexes containing N3O moiety: Synthesis, structural characterization, kinetic and catalytic mimicking activity. Inorg. Chim. Acta 2019, 486, 608–616. [Google Scholar] [CrossRef]
- Andrejević, T.P.; Aleksic, I.; Počkaj, M.; Kljun, J.; Milivojevic, D.; Stevanović, N.L.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B.D. Tailoring copper(ii) complexes with pyridine-4,5-dicarboxylate esters for anti-Candida activity. J. Chem. Soc. Dalton Trans. 2021, 50, 2627–2638. [Google Scholar] [CrossRef]
- Wang, C.; Tang, R.; Wan, C.; Qin, Z.; Chen, S.; Xu, K. Two novel metal complexes based on 2,2′-bipyridine and 2,4-dihydroxybenzoic acid: Synthesis, crystal structure and catalytic performance. J. Mol. Struct. 2023, 1291, 136066. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Dial, M.T.; Salem, N.N.M.H.; Fischer, R.C.; Torvisco, A.; Mautner, F.A.; Nakashima, K.; Handa, M.; Mikuriya, M. Magnetostructural Properties of Some Doubly-Bridged PhenoxidoCopper(II) Complexes. Molecules 2023, 28, 2648. [Google Scholar] [CrossRef]
- Veber, S.L.; Tumanov, S.V.; Fokin, S.V.; Tolstikov, S.E.; Sobenina, L.N.; Romanenko, G.V.; Bogomyakov, A.S.; Morozov, V.A.; Trofimov, B.A.; Ovcharenko, V.I.; et al. Five-Spin Copper(II) Nitroxide Complex with Apparently Compressed Octahedral Geometry: Design, Synthesis, and Magnetostructural Studies. Cryst. Growth Des. 2023, 23, 1057–1065. [Google Scholar] [CrossRef]
- Maldonado, N.; Perles, J.; Martínez, J.I.; Gómez-García, C.J.; Marcos, M.L.; Amo-Ochoa, P. Experimental and Theoretical Study of Dynamic Structural Transformations between Sensing Copper(II)-Uracil Antiferromagnetic and Metamagnetic Coordination Compounds. Cryst. Growth Des. 2020, 20, 5097–5107. [Google Scholar] [CrossRef]
- Rigamonti, L.; Carlino, S.; Halibi, Y.; Demartin, F.; Castellano, C.; Ponti, A.; Pievo, R.; Pasini, A. Copper 1D coordination polymers and dimers: Role of the carboxylate and the ammonium cation, crystal structures and magnetic studies. Polyhedron 2013, 53, 157–165. [Google Scholar] [CrossRef][Green Version]
- Sk, S.; Biswas, S.; Dutt, N.; Das, A.; Suryadevara, N.; Vijaykumar, G.; Bhunia, P.; Ruben, M.; Mandal, S.; Bera, M. Structures and Properties of a Series of High-Spin [CoII2] Complexes Supported by Ancillary Benzoate, Ortho-Hydroxybenzoate, and Para-Hydroxybenzoate Ligands. Cryst. Growth Des. 2023, 23, 5925–5940. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Pou, R.; Bayés-García, L.; Font-Bardia, M.; Pons, J.; Ayllón, J.A. Benzoate substituents effects on the structure of Zn(II) complexes and 1D 4,4′-bipyridine derived coordination polymers. Inorg. Chim. Acta 2020, 500, 119218. [Google Scholar] [CrossRef]
- Ayllón, J.A.; Vallcorba, O.; Domingo, C. Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives. Inorganics 2024, 12, 24. [Google Scholar] [CrossRef]
- Eom, G.H.; Park, H.M.; Hyun, M.Y.; Jang, S.P.; Kim, C.; Lee, J.H.; Lee, S.J.; Kim, S.J.; Kim, Y. Anion effects on the crystal structures of ZnII complexes containing 2,2′-bipyridine: Their photoluminescence and catalytic activities. Polyhedron 2011, 30, 1555–1564. [Google Scholar] [CrossRef]
- Katzsch, F.; Münch, A.S.; Mertens, F.O.R.L.; Weber, E. Copper(II) benzoate dimers coordinated by different linear alcohols—A systematic study of crystal structures. J. Mol. Struct. 2014, 1064, 122–129. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Systems, Inc. Apex2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Siemens Industrial Automation, Inc. SADABS: Area–Detector Absorption Correction; Siemens Industrial Automation, Inc.: Plano, TX, USA, 1996. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Watkin, D.J.; Prout, C.K.; Pearce, L.J. X-Ray, CAMERON, Chemical Crystallography Laboratory; Oxford University: Oxford, UK, 1996. [Google Scholar]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Jorgensen, C.K. Studies of Absorption Spectra. III. Absorption Bands as Gaussian Error Curves. Acta Chem. Scand. 1954, 8, 1495–1501. [Google Scholar] [CrossRef][Green Version]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem.Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Computational Chemistry, Colorado Springs, CO, USA. Available online: http://OpenMOPAC.net (accessed on 10 October 2019).
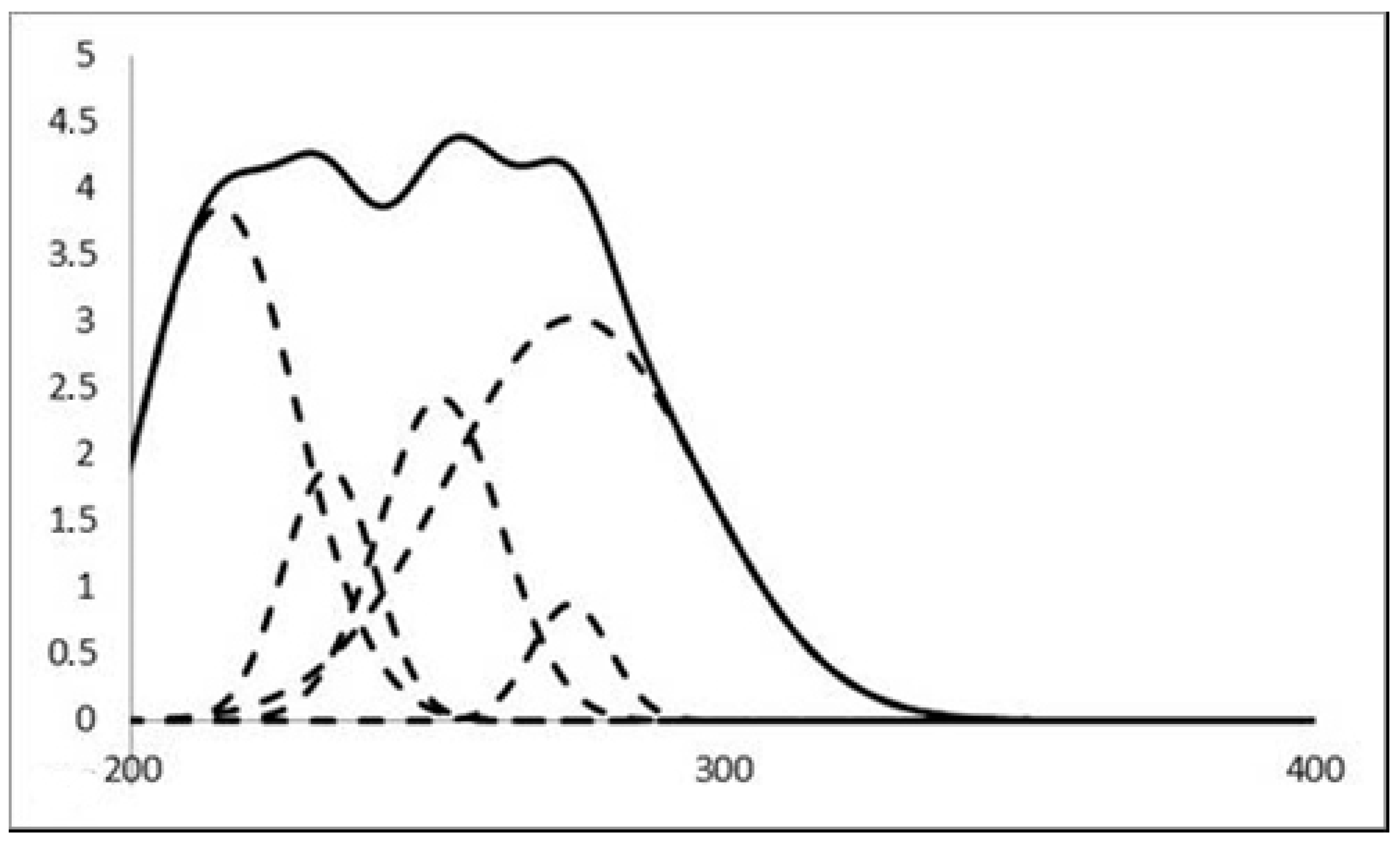
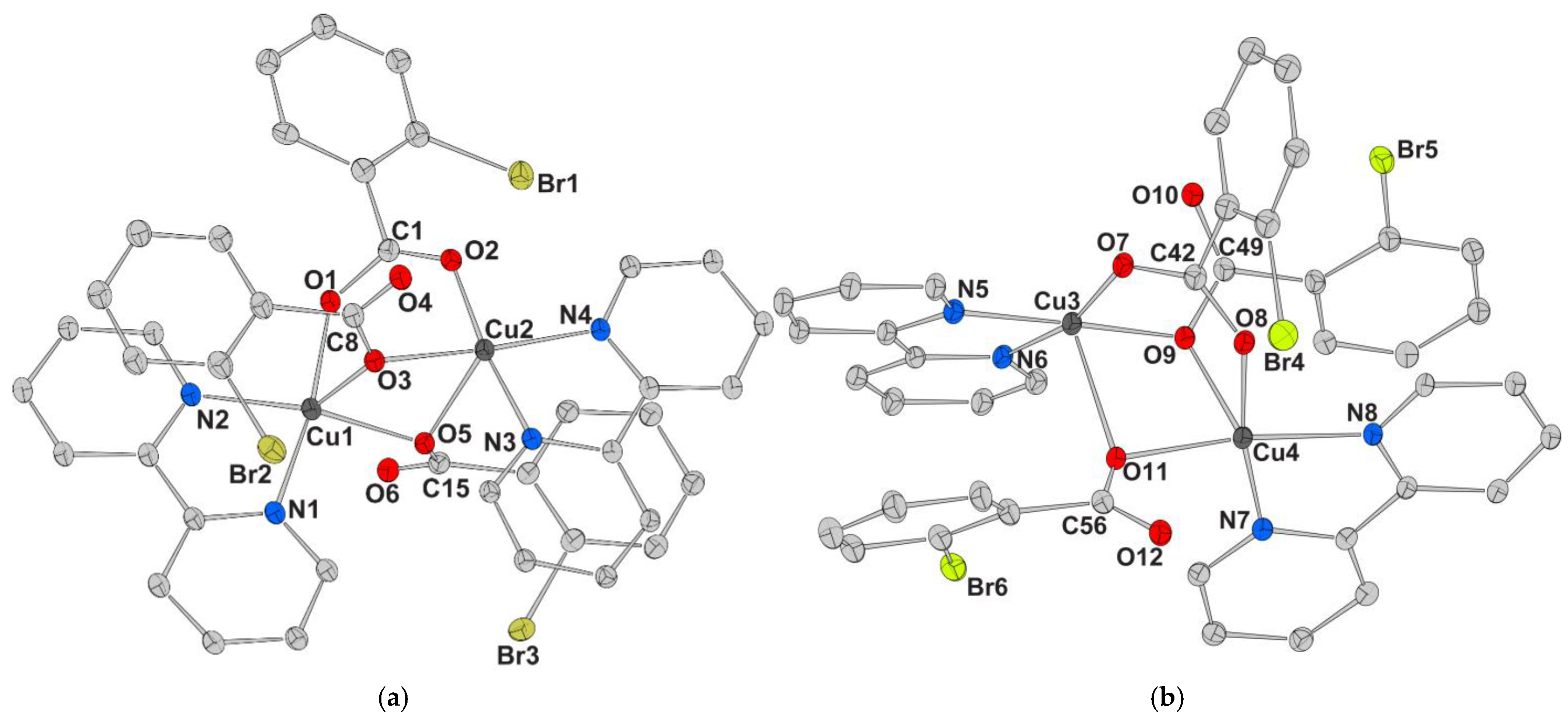
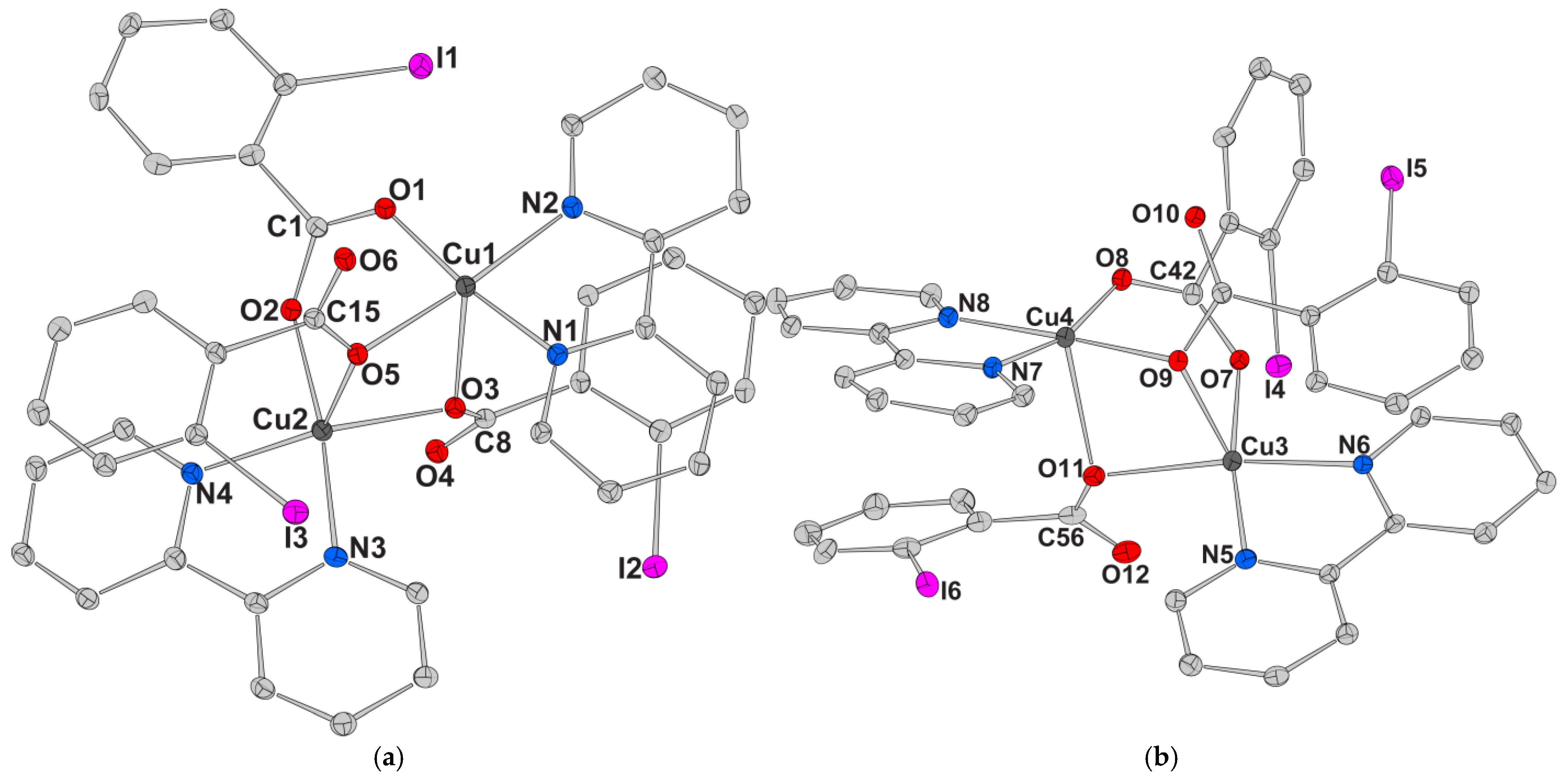
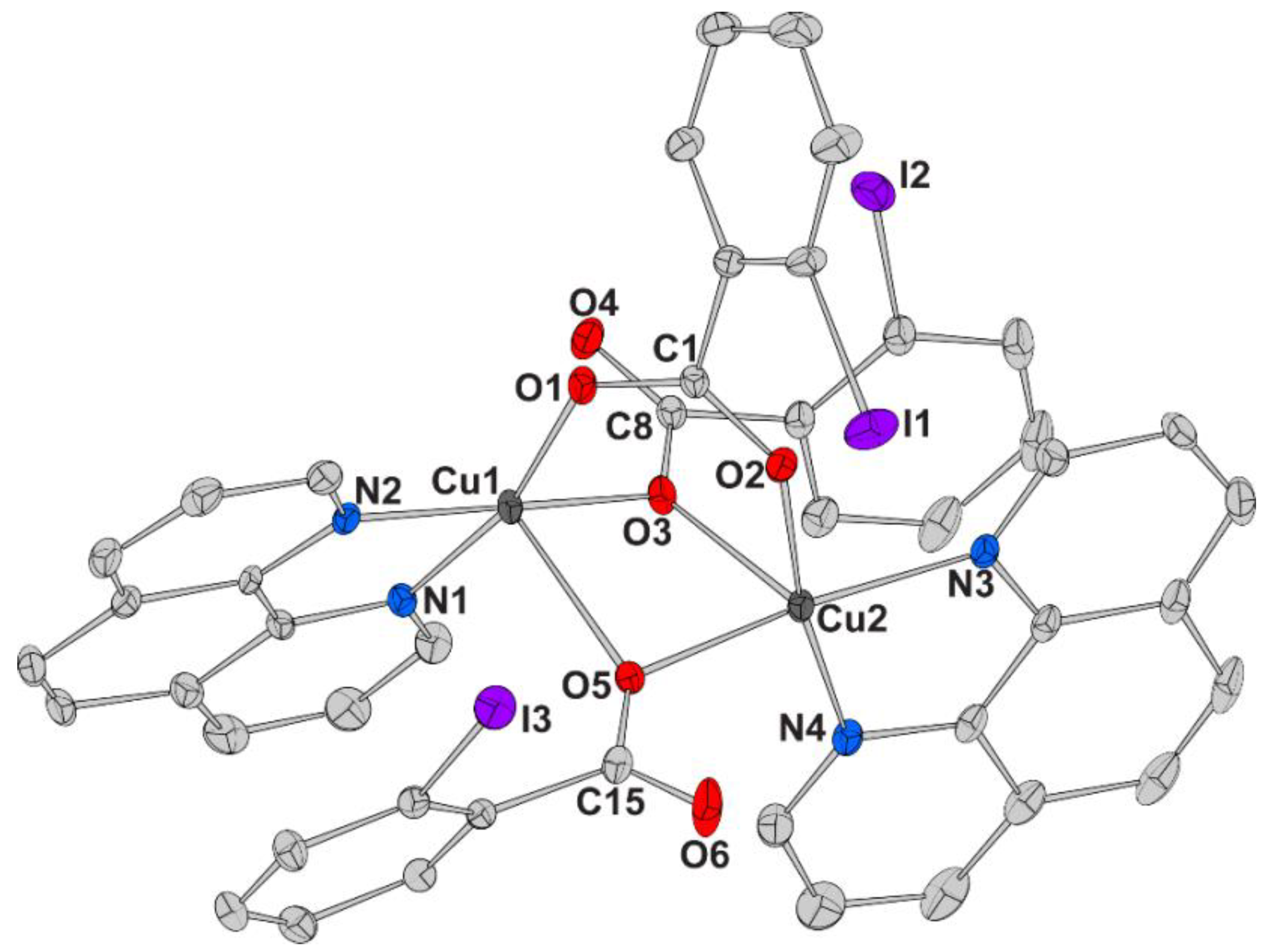
| Chemical formula sum | C41.50H31Br3Cu2N5O10 | C41.50H32Cu2I3N5O10.50 | C45.75H32.50ClCu2I3N4O11.50 |
| Chemical formula moiety | Cu2(C7H3O2Br)3(C10H8N2)2 . NO3 . 0.5(CH3OH). 0.5(H2O) | Cu2(C7H3O2I)3(C10H8N2)2 . NO3 . 0.5(CH3OH) . H2O | [Cu2(C7H3O2I)3(C10H8N2)2] . ClO4 . 0.75(CH3OH) . 0.75(H2O) |
| Mr | 1126.54 | 1276.55 | 1365.54 |
| Crystal system Space group | Triclinic Pī | Triclinic Pī | Triclinic Pī |
| Temperature (K) | 295 | 295 | 295 |
| a (Å) b (Å) c (Å) | 14.933 (2) 16.939 (3) 19.285 (3) | 14.911 (2) 17.044 (2) 19.322 (3) | 10.7073 (6) 13.0072 (8) 18.2613 (10) |
| α (°) β (°) γ (°) | 80.552 (4) 83.179 (5) 71.468 (4) | 81.219 (4) 84.200 (4) 72.181 (4) | 79.937 (2) 74.2319 (19) 75.857 (2) |
| V (Å3) | 4551.0 (12) | 4612.5 (11) | 2357.3 (2) |
| Z | 4 | 4 | 2 |
| Radiation type | MoKα | MoKα | MoKα |
| µ (mm−1) | 3.63 | 2.99 | 2.99 |
| Crystal size (mm) | 0.27 × 0.26 × 0.18 | 0.26 × 0.15 × 0.14 | 0.17 × 0.16 × 0.12 |
| Data collection | |||
| Diffractometer | Bruker Kappa Apex2 | ||
| Tmin, Tmax | 0.39, 0.52 | 0.64, 0.66 | 0.62, 0.70 |
| No. of reflections measured independent observed [I > 2.0σ(I)] | 63,702 15,751 11,081 | 71,115 18,100 12,696 | 34,902 9026 7074 |
| Rint | 0.033 | 0.030 | 0.031 |
| (sinθ/λ)max (Å−1) | 0.595 | 0.619 | 0.616 |
| Refinement | |||
| R[F2 > 2σ(F2)] wR(F2) S | 0.049 0.067 1.00 | 0.048 0.090 1.00 | 0.051 0.093 1.00 |
| No. of reflections | 11,081 | 12,696 | 7074 |
| No. of parameters | 1106 | 1216 | 607 |
| No. of restraints | 24 | 40 | 22 |
| H-atom treatment | H-atom parameters constrained | ||
| Δρmax, Δρmin (e Å−3) | 0.82, −0.61 | 1.70, −1.19 | 1.30, −1.32 |
| Complex 1a | Complex 1b | ||
| Cu1—Cu2 | 3.2376 (8) | Cu3—Cu4 | 3.1989 (8) |
| Cu1—O1 | 1.957 (3) | Cu3—O7 | 1.947 (3) |
| Cu1—O3 | 2.294 (3) | Cu3—O9 | 1.956 (3) |
| Cu1—O5 | 1.952 (3) | Cu3—O11 | 2.308 (3) |
| Cu1—N1 | 2.001 (4) | Cu3—N5 | 1.965 (3) |
| Cu1—N2 | 2.006 (3) | Cu3—N6 | 1.963 (3) |
| Cu2—O2 | 1.967 (3) | Cu4—O8 | 1.945 (3) |
| Cu2—O3 | 1.953 (3) | Cu4—O9 | 2.330 (3) |
| Cu2—O5 | 2.303 (3) | Cu4—O11 | 1.968 (3) |
| Cu2—N3 | 1.988 (4) | Cu4—N7 | 1.971 (3) |
| Cu2—N4 | 1.986 (3) | Cu4—N8 | 1.990 (3) |
| O1—Cu1—O3 | 87.34 (11) | O7—Cu3—O9 | 93.48 (11) |
| O1—Cu1—O5 | 93.44 (12) | O7—Cu3—O11 | 90.83 (11) |
| O3—Cu1—O5 | 76.11 (10) | O9—Cu3—O11 | 78.61 (11) |
| O1—Cu1—N1 | 171.85 (13) | O7—Cu3—N5 | 91.21 (13) |
| O3—Cu1—N1 | 94.88 (12) | O9—Cu3—N5 | 175.15 (13) |
| O5—Cu1—N1 | 94.70 (13) | O11—Cu3—N5 | 102.56 (13) |
| O1—Cu1—N2 | 91.15 (14) | O7—Cu3—N6 | 166.19 (13) |
| O3—Cu1—N2 | 116.50 (12) | O9—Cu3—N6 | 94.49 (13) |
| O5—Cu1—N2 | 166.80 (13) | O11—Cu3—N6 | 101.79 (12) |
| N1—Cu1—N2 | 80.84 (15) | N5—Cu3—N6 | 80.67 (15) |
| O2—Cu2—O3 | 91.37 (12) | O8—Cu4—O9 | 87.10 (11) |
| O2—Cu2—O5 | 90.27 (11) | O8—Cu4—O11 | 94.25 (11) |
| O3—Cu2—O5 | 75.88 (10) | O9—Cu4—O11 | 77.84 (11) |
| O2—Cu2—N3 | 172.71 (13) | O8—Cu4—N7 | 170.10 (13) |
| O3—Cu2—N3 | 95.85 (13) | O9—Cu4—N7 | 94.14 (12) |
| O5—Cu2—N3 | 90.54 (12) | O11—Cu4—N7 | 95.62 (13) |
| O2—Cu2—N4 | 91.47 (13) | O8—Cu4—N8 | 89.83 (13) |
| O3—Cu2—N4 | 170.31 (13) | O9—Cu4—N8 | 109.52 (12) |
| O5—Cu2—N4 | 113.37 (12) | O11—Cu4—N8 | 171.79 (12) |
| N3—Cu2—N4 | 81.56 (14) | N7—Cu4—N8 | 80.49 (14) |
| Complex 2a | Complex 2b | ||
| Cu1—Cu2 | 3.2327 (11) | Cu3—Cu4 | 3.2183 (11) |
| Cu1—O1 | 2.011 (4) | Cu3—O7 | 1.960 (4) |
| Cu1—O3 | 2.315 (4) | Cu3—O9 | 2.350 (4) |
| Cu1—O5 | 1.964 (4) | Cu3—O11 | 2.112 (5) |
| Cu1—N1 | 1.954 (5) | Cu3—N5 | 1.992 (5) |
| Cu1—N2 | 1.954 (5) | Cu3—N6 | 1.985 (5) |
| Cu2—O2 | 1.947 (4) | Cu4—O8 | 1.953 (4) |
| Cu2—O3 | 1.966 (4) | Cu4—O9 | 1.975 (4) |
| Cu2—O5 | 2.327 (4) | Cu4—O11 | 2.294 (5) |
| Cu2—N3 | 1.989 (5) | Cu4—N7 | 1.996 (5) |
| Cu2—N4 | 1.992 (5) | Cu4—N8 | 1.958 (6) |
| O1—Cu1—O3 | 90.39 (16) | O7—Cu3—O9 | 86.52 (17) |
| O1—Cu1—O5 | 90.92 (18) | O7—Cu3—O11 | 95.73 (17) |
| O3—Cu1—O5 | 77.48 (16) | O9—Cu3—O11 | 76.54 (16) |
| O1—Cu1—N1 | 172.4 (2) | O7—Cu3—N5 | 170.5 (2) |
| O3—Cu1—N1 | 90.12 (19) | O9—Cu3—N5 | 94.02 (19) |
| O5—Cu1—N1 | 96.6 (2) | O11—Cu3—N5 | 93.6 (2) |
| O1—Cu1—N2 | 91.9 (2) | O7—Cu3—N6 | 89.68 (19) |
| O3—Cu1—N2 | 113.04 (19) | O9—Cu3—N6 | 109.45 (18) |
| O5—Cu1—N2 | 169.1 (2) | O11—Cu3—N6 | 172.22 (19) |
| N1—Cu1—N2 | 80.9 (2) | N5—Cu3—N6 | 81.2 (2) |
| O2—Cu2—O3 | 92.52 (18) | O8—Cu4—O9 | 93.85 (18) |
| O2—Cu2—O5 | 86.65 (17) | O8—Cu4—O11 | 93.09 (18) |
| O3—Cu2—O5 | 77.15 (16) | O9—Cu4—O11 | 80.54 (17) |
| O2—Cu2—N3 | 172.5 (2) | O8—Cu4—N7 | 165.7 (2) |
| O3—Cu2—N3 | 95.0 (2) | O9—Cu4—N7 | 93.6 (2) |
| O5—Cu2—N3 | 95.72 (19) | O11—Cu4—N7 | 100.2 (2) |
| O2—Cu2—N4 | 92.2 (2) | O8—Cu4—N8 | 91.3 (2) |
| O3—Cu2—N4 | 167.6 (2) | O9—Cu4—N8 | 174.8 (2) |
| O5—Cu2—N4 | 114.61 (19) | O11—Cu4—N8 | 100.09 (19) |
| N3—Cu2—N4 | 80.3 (2) | N7—Cu4—N8 | 81.2 (2) |
| Cu1—Cu2 | 3.1884 (9) | N1—Cu1—O5 | 98.13 (17) |
| Cu1—N1 | 2.003 (5) | N2—Cu1—O5 | 104.07 (16) |
| Cu1—N2 | 2.009 (5) | O1—Cu1—O5 | 94.79 (16) |
| Cu1—O1 | 1.955 (4) | O3—Cu1—O5 | 77.96 (16) |
| Cu1—O3 | 1.939 (4) | N3—Cu2—N4 | 82.4 (2) |
| Cu1—O5 | 2.267 (4) | N3—Cu2—O2 | 88.74 (19) |
| Cu2—N3 | 2.000 (5) | N4—Cu2—O2 | 171.06 (19) |
| Cu2—N4 | 1.993 (5) | N3—Cu2—O3 | 110.85 (16) |
| Cu2—O2 | 1.950 (4) | N4—Cu2—O3 | 92.82 (18) |
| Cu2—O3 | 2.354 (4) | O2—Cu2—O3 | 89.36 (16) |
| Cu2—O5 | 1.950 (4) | N3—Cu2—O5 | 173.09 (18) |
| N1—Cu1—N2 | 82.3 (2) | N4—Cu2—O5 | 95.08 (18) |
| N1—Cu1—O1 | 166.32 (18) | O2—Cu2—O5 | 93.86 (17) |
| N2—Cu1—O1 | 90.20 (18) | O3—Cu2—O5 | 75.62 (15) |
| N1—Cu1—O3 | 94.72 (19) | N1—Cu1—O5 | 98.13 (17) |
| N2—Cu1—O3 | 176.61 (18) | N2—Cu1—O5 | 104.07 (16) |
| O1—Cu1—O3 | 92.33 (17) | O1—Cu1—O5 | 94.79 (16) |
| O3—Cu1—O5 | 77.96 (16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukas, I.; Frantzana, E.; Hatzidimitriou, A.; Tzimopoulos, D.; Akrivos, P. Coordination Modes of Ortho-Substituted Benzoates Towards Divalent Copper Centres in the Presence of Diimines. Chemistry 2024, 6, 1374-1384. https://doi.org/10.3390/chemistry6060081
Loukas I, Frantzana E, Hatzidimitriou A, Tzimopoulos D, Akrivos P. Coordination Modes of Ortho-Substituted Benzoates Towards Divalent Copper Centres in the Presence of Diimines. Chemistry. 2024; 6(6):1374-1384. https://doi.org/10.3390/chemistry6060081
Chicago/Turabian StyleLoukas, Ioannis, Eirini Frantzana, Antonios Hatzidimitriou, Demetrios Tzimopoulos, and Pericles Akrivos. 2024. "Coordination Modes of Ortho-Substituted Benzoates Towards Divalent Copper Centres in the Presence of Diimines" Chemistry 6, no. 6: 1374-1384. https://doi.org/10.3390/chemistry6060081
APA StyleLoukas, I., Frantzana, E., Hatzidimitriou, A., Tzimopoulos, D., & Akrivos, P. (2024). Coordination Modes of Ortho-Substituted Benzoates Towards Divalent Copper Centres in the Presence of Diimines. Chemistry, 6(6), 1374-1384. https://doi.org/10.3390/chemistry6060081







