Extended Chalcones: Synthesis, In Vitro Analysis, and In Vivo Testing Against a Drosophila melanogaster Alzheimer’s Disease Model
Abstract
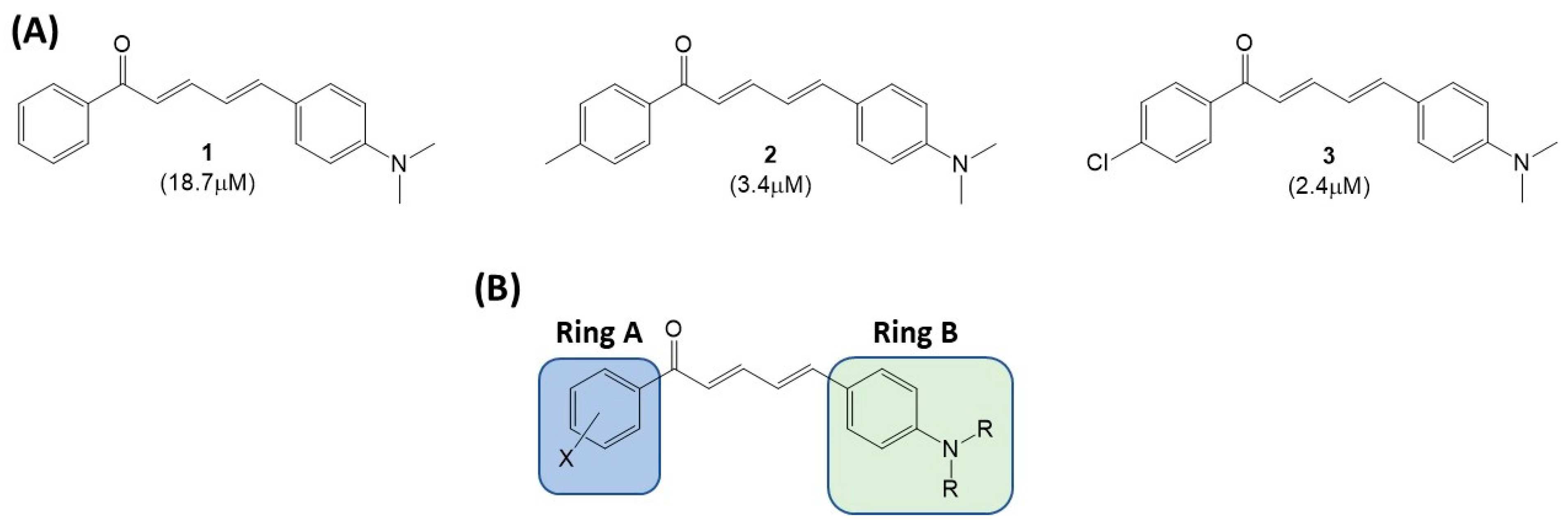
1. Introduction
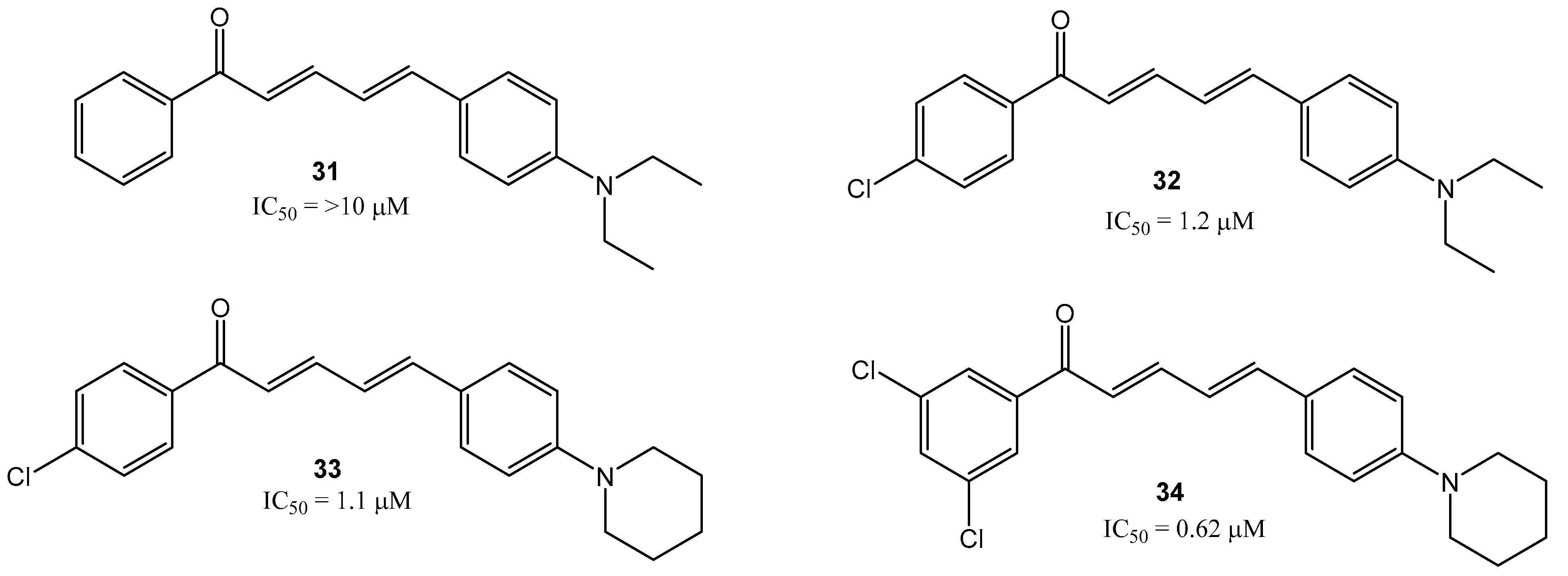
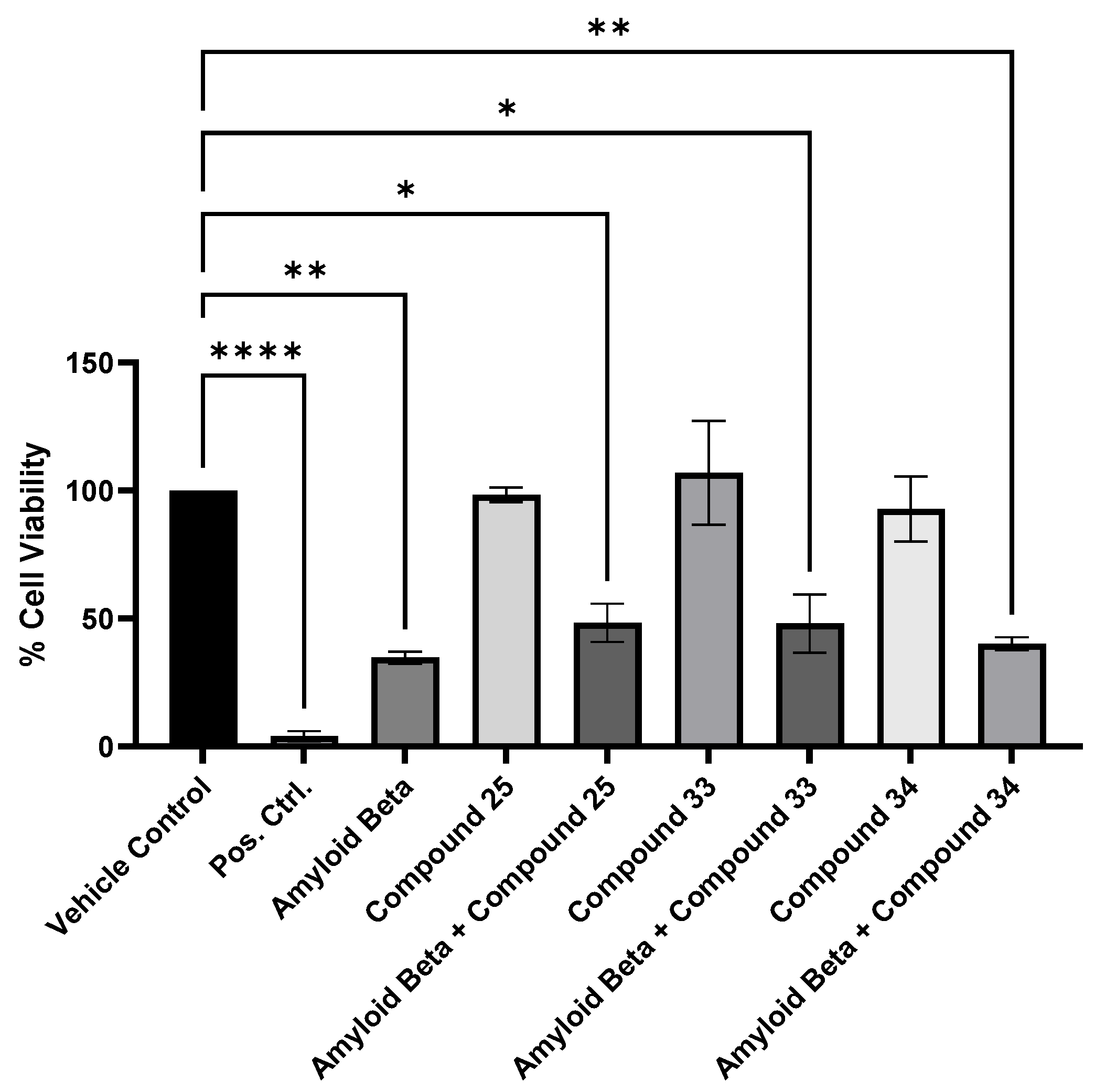
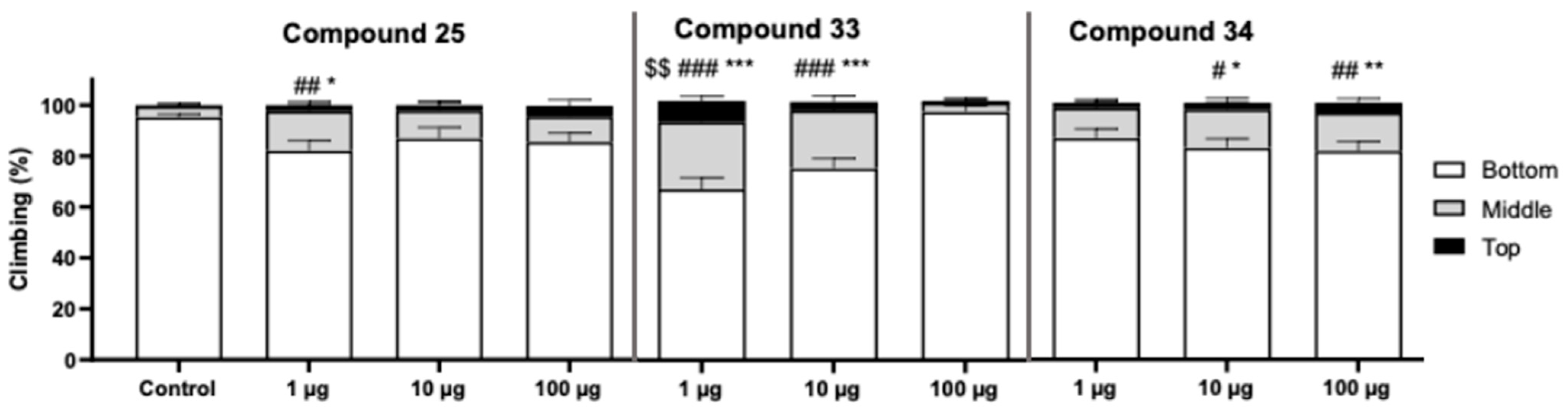
2. Results and Discussion
3. Conclusions
4. Experimental
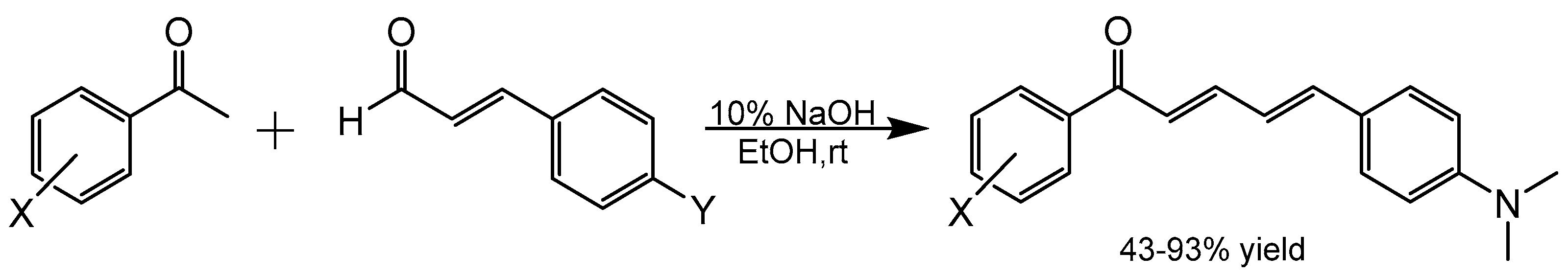
4.1. Chemistry
4.1.1. General Information
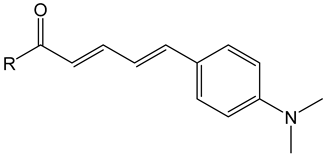
4.1.2. Example Aldol Condensation
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-ethylphenyl)penta-2,4-dien-1-one (4)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-propylphenyl)penta-2,4-dien-1-one (5)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-isopropylphenyl)penta-2,4-dien-1-one (6)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-isobutylphenyl)penta-2,4-dien-1-one (7)
- (2E,4E)-1-(4-cyclohexylphenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (8)
- (2E,4E)-1-(4-bromophenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (9)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-iodophenyl)penta-2,4-dien-1-one (10)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-(trifluoromethyl)phenyl)penta-2,4-dien-1-one (11)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-nitrophenyl)penta-2,4-dien-1-one (12)
- 4-((2E,4E)-5-(4-(dimethylamino)phenyl)penta-2,4-dienoyl)benzonitrile (13)
- (2E,4E)-1-(4-acetylphenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (14)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-methoxyphenyl)penta-2,4-dien-1-one (15)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-ethoxyphenyl)penta-2,4-dien-1-one (16)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(4-(methylthio)phenyl)penta-2,4-dien-1-one (18)
- (2E,4E)-1-(4-(1H-imidazol-1-yl)phenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (19)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(3-(trifluoromethyl)phenyl)penta-2,4-dien-1-one (20)
- (2E,4E)-1-(3-chlorophenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (21)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(2-(trifluoromethyl)phenyl)penta-2,4-dien-1-one (22)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(o-tolyl)penta-2,4-dien-1-one (23)
- (2E,4E)-1-(2-chlorophenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (24)
- (2E,4E)-1-(3,5-dichlorophenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (25)
- (2E,4E)-1-(2,4-dichlorophenyl)-5-(4-(dimethylamino)phenyl)penta-2,4-dien-1-one (26)
- (2E,4E)-1-(2-chloro-5-(trifluoromethyl)phenyl)-5-(4-(dimethylamino)phenyl)penta-2,4- dien-1-one (27)
- (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(2,5-dimethylphenyl)penta-2,4-dien-1-one (28)
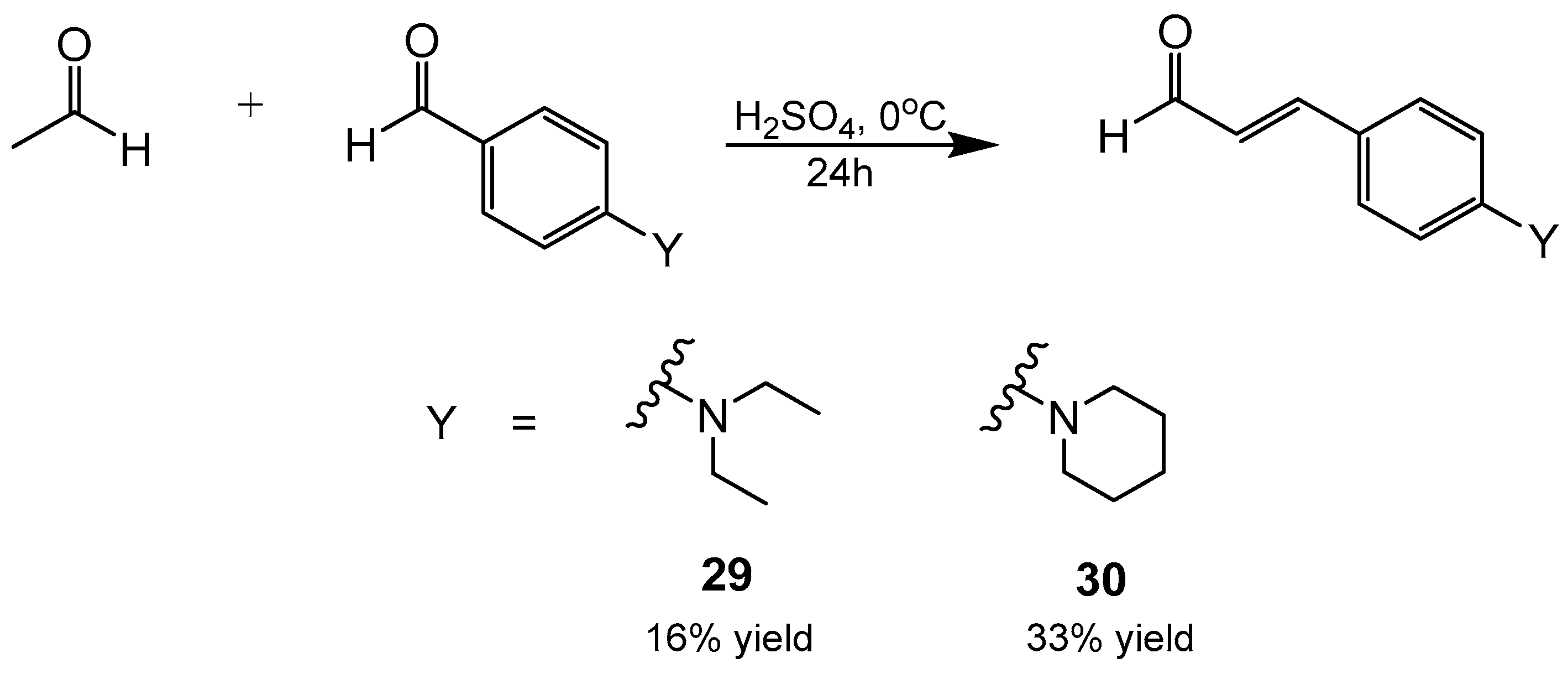
- 4-(diethylamino)cinnamaldehyde (29)
- 4-(piperdin-1-yl)cinnamaldehyde (30)
- (2E,4E)-5-(4-(diethylamino)phenyl)-1-phenylpenta-2,4-dien-1-one (31)
- (2E,4E)-1-(4-chlorophenyl)-5-(4-(piperidin-1-yl)phenyl)penta-2,4-dien-1-one (33)
- (2E,4E)-1-(4-chlorophenyl)-5-(4-(piperidin-1-yl)phenyl)penta-2,4-dien-1-one (34)
4.2. In Vitro Studies
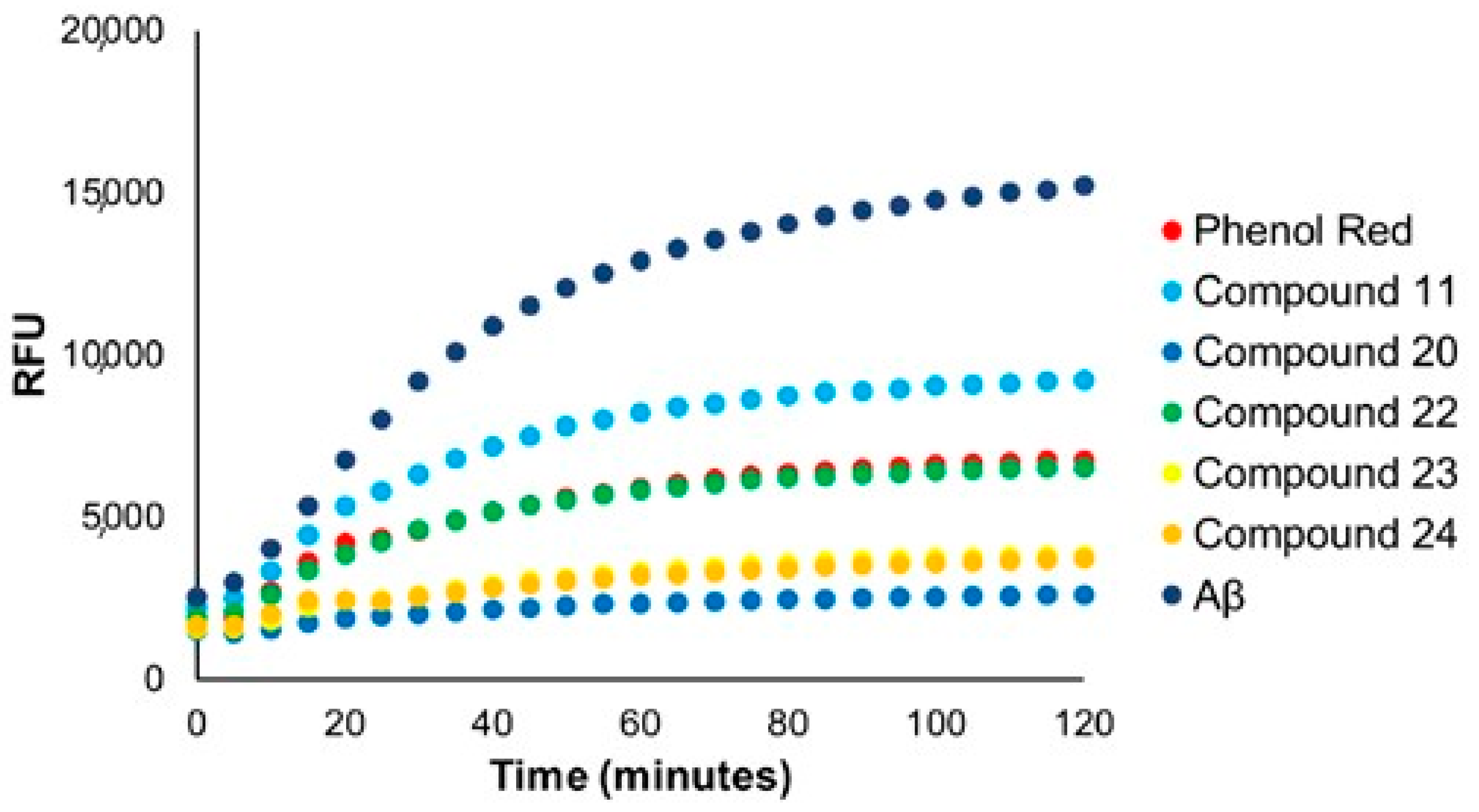
4.2.1. Thioflavin T (ThT) Fluorescence Assay
4.2.2. Cell Viability Assay
Cell Culture and Exposure
MTT Assay and Cell Viability
4.3. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarpini, E.; Schelterns, P.; Feldman, H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s Facts and Figures Report; Alzheimer’s Association: Chicago, IL, USA, 2022; Available online: https://www.alz.org/alzheimers-dementia/facts-figures (accessed on 19 February 2024).
- Panza, F.; Seripa, D.; D’Onofrio, G.; Frisardi, V.; Solfrizzi, V.; Mecocci, P.; Pilotto, A. Neuropsychiatric Symptoms, Endophenotypes, and Syndromes in Late-Onset Alzheimer′s Disease: Focus on APOE Gene. Int. J. Alzheimer’s Dis. 2011, 2011, 721457. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Quiroz, J.P.; Zeng, A.; Young, M.; Gordon, P.; Jaipuria, A.; Reed, K.J.; Landry, G.M.; Yang, S.; Asher, S.; Zhang, S.R.C.; et al. Homotaurine and Curcumin Analogues as Potential Anti-Amyloidogenic Agents. Chemistry 2023, 5, 223–241. [Google Scholar] [CrossRef]
- Müller, U.C.; Zheng, H. Physiological Functions of APP Family Proteins. Cold Spring Harb. Perspect. Med. 2012, 2, a006288. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Olsson, F.; Schmidt, S.; Althoff, V.; Munter, L.M.; Jin, S.; Rosqvist, S.; Lendahl, U.; Multhaup, G.; Lundkvist, J. Characterization of Intermediate Steps in Amyloid Beta (Aβ) Production under Near-native Conditions. J. Biol. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Madav, Y.; Wairkar, S.; Prabhakar, B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res. Bull. 2019, 146, 171–184. [Google Scholar] [CrossRef]
- Takami, M.; Nagashima, Y.; Sano, Y.; Ishihara, S.; Morishima-Kawashima, M.; Funamoto, S.; Ihara, Y. γ-Secretase: Successive Tripeptide and Tetrapeptide Release from the Transmembrane Domain of β-Carboxyl Terminal Fragment. J. Neurosci. 2009, 29, 13042–13052. [Google Scholar] [CrossRef]
- Roeters, S.J.; Iyer, A.; Pletikapić, G.; Kogan, V.; Subramaniam, V.; Woutersen, S. Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy. Sci. Rep. 2017, 7, 41051. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.M.; Schilling, S.; Cynis, H.; Silva, A.; Swanson, E.; Wangsanut, T.; Tayler, K.; Wiltgen, B.; Hatami, A.; Rönicke, R.; et al. Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-β. Nature 2012, 485, 651–655. [Google Scholar] [CrossRef]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.J.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.W.; Paulsen, O.; et al. Tau Protein Is Required for Amyloid -Induced Impairment of Hippocampal Long-Term Potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Fan, Y.-G.; Zhao, L.-X.; Zhang, Q.; Wang, Z.-Y. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 2022, 131, 106301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ganeshpurkar, A.; Kumar, D.; Modi, G.; Gupta, S.K.; Singh, S.K. Secretase inhibitors for the treatment of Alzheimer’s disease: Long road ahead. Eur. J. Med. Chem. 2018, 148, 436–452. [Google Scholar] [CrossRef]
- de la Torre, J.C.; Gonzalez-Lima, F. The FDA Approves Aducanumab for Alzheimer’s Disease, Raising Important Scientific Questions1. J. Alzheimer’s Dis. 2021, 82, 881–882. [Google Scholar] [CrossRef]
- Verger, A.; Yakushev, I.; Albert, N.L.; van Berckel, B.; Brendel, M.; Cecchin, D.; Fernandez, P.A.; Fraioli, F.; Guedj, E.; Morbelli, S.; et al. FDA approval of lecanemab: The real start of widespread amyloid PET use?—The EANM Neuroimaging Committee perspective. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1553–1555. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/index.php (accessed on 15 March 2024).
- Alvarez, J.; Alvarez-Illera, P.; Santo-Domingo, J.; Fonteriz, R.I.; Montero, M. Modeling Alzheimer’s Disease in Caenorhabditis elegans. Biomedicines 2022, 10, 288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, D.E.; Cook, K.R.; Arvanitakis, A.V.; Hawley, R.S. Third Chromosome Balancer Inversions Disrupt Protein-Coding Genes and Influence Distal Recombination Events in Drosophila melanogaster. G3 2017, 6, 1959–1967, Correction in G3 2017, 7, 3837. [Google Scholar] [CrossRef]
- Sekiya, M.; Iijima, K.M. Phenotypic analysis of a transgenic Drosophila model of Alzheimer’s amyloid-β toxicity. STAR Protoc. 2022, 2, 100501. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.; Sheng, X.B.; Liu, X.H.; Chen, F.H. Design, synthesis and SARs of novel telomerase inhibitors based on BIBRA1532. Bioorg Chem. 2020, 102, 104007. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Doi, Y.; Watanabe, H.; Ihara, M.; Ozaki, A.; Saji, H. Structure-activity relationships of radioiodinated diphenyl derivatives with different con-jugated double bonds as ligands for α-synuclein aggregates. RSC Adv. 2016, 6, 44305–44312. [Google Scholar] [CrossRef]
- Niu, C.; Tuerxuntayi, A.; Li, G.; Kabas, M.; Dong, C.-Z.; Aisa, H.A. Design, synthesis and bioactivity of chalcones and its analogues. Chin. Chem. Lett. 2017, 28, 1533–1538. [Google Scholar] [CrossRef]
- Le, V.-H.; Lien, V.T.K.; Pham, V.T.; Tran, Q.T.; Thuy, P.T.; Ha, C.V.; Doanh, V.V.; Ha, L.T.; Hanh, C.H.; Thao, P.N.; et al. Effect of extended π-conjugation on photophysical characteristics of chalcone and cinnamyli-deneacetophenone. Mater. Sci. Semicond. Process. 2023, 162, 107507. [Google Scholar] [CrossRef]
- Alphonsa, A.T. (E)-1-(benzo [d] [1,3] dioxol-5-yl)-3-phenyl prop 2-en-1-ones: Design, synthesis, anti-cancer activity and molec-ular docking studies. Chem. Data Coll. 2020, 25, 100310. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, P.; Lu, J.; Xing, C. Characterization of the Fluorescence Properties of 4-Dialkyaminochalcones and Investigation of the Cytotoxic Mechanism of Chalcones. Arch. Pharm. 2016, 349, 539–552. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Compound | R | IC50 (µM) | Compound | R | IC50 (µM) |
| 4 | 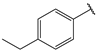 | 1.6 | 17 | 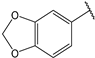 | 2.7 |
| 5 | 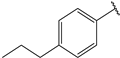 | 1.5 | 18 | 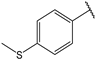 | 1.5 |
| 6 | 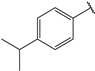 | 2.1 | 19 | 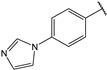 | 2.3 |
| 7 |  | 1.1 | 20 | 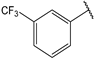 | 2.0 |
| 8 | 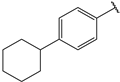 | >10 | 21 |  | 1.3 |
| 9 | 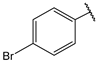 | 3.5 | 22 | 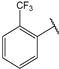 | 10 |
| 10 | 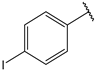 | 9.3 | 23 |  | 2.5 |
| 11 | 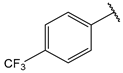 | >10 | 24 |  | 1.6 |
| 12 |  | 20.0 | 25 | 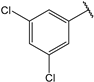 | 0.91 |
| 13 | 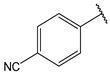 | 8.8 | 26 | 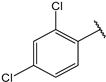 | 1.9 |
| 14 | 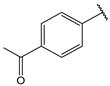 | 1.3 | 27 |  | 1.6 |
| 15 | 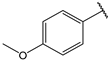 | 3.2 | 28 | 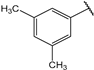 | 2.2 |
| 16 | 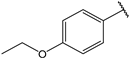 | 1.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaipuria, A.; Castillo, M.; Boksanski, J.; Landry, G.; Beak, J.H.; Young, M.; Priefer, D.T.; Guessab, K.; Ellis, C.N.; Priefer, R. Extended Chalcones: Synthesis, In Vitro Analysis, and In Vivo Testing Against a Drosophila melanogaster Alzheimer’s Disease Model. Chemistry 2024, 6, 1477-1494. https://doi.org/10.3390/chemistry6060089
Jaipuria A, Castillo M, Boksanski J, Landry G, Beak JH, Young M, Priefer DT, Guessab K, Ellis CN, Priefer R. Extended Chalcones: Synthesis, In Vitro Analysis, and In Vivo Testing Against a Drosophila melanogaster Alzheimer’s Disease Model. Chemistry. 2024; 6(6):1477-1494. https://doi.org/10.3390/chemistry6060089
Chicago/Turabian StyleJaipuria, Aadya, Madison Castillo, James Boksanski, Greg Landry, Ji Hyung Beak, Michelle Young, David T. Priefer, Kaïs Guessab, Crystal N. Ellis, and Ronny Priefer. 2024. "Extended Chalcones: Synthesis, In Vitro Analysis, and In Vivo Testing Against a Drosophila melanogaster Alzheimer’s Disease Model" Chemistry 6, no. 6: 1477-1494. https://doi.org/10.3390/chemistry6060089
APA StyleJaipuria, A., Castillo, M., Boksanski, J., Landry, G., Beak, J. H., Young, M., Priefer, D. T., Guessab, K., Ellis, C. N., & Priefer, R. (2024). Extended Chalcones: Synthesis, In Vitro Analysis, and In Vivo Testing Against a Drosophila melanogaster Alzheimer’s Disease Model. Chemistry, 6(6), 1477-1494. https://doi.org/10.3390/chemistry6060089








